Abstract
Background: The apolipoprotein E epsilon4 (ApoE ε4) allele and female gender may be important risk factors for the development of Alzheimer’s disease and amnestic mild cognitive impairment (aMCI). Novelty mismatch negativity (MMN) represents the pre-attentive index of deviance detection and P3a represents the attention orienting response. Furthermore, MMN and P3a components have been reported to be potential markers in aMCI. Therefore, this study will investigate the effects of gender and ApoE on auditory novelty MMN and P3a and their relationship to neuropsychological performance in aMCI.
Methods: Thirty nine aMCI subjects and 44 controls underwent neuropsychological assessment and ApoE genotyping. Novelty MMN and P3a components were investigated during an auditory novelty oddball task.
Results: Firstly, novelty MMN latency was significantly shorter in aMCI than in healthy control (HC) group. Secondly, novelty MMN latency was negatively correlated with episodic memory in aMCI, but not in HC. Novelty P3a latency was negatively correlated with information processing speed in all subjects. For gender effect, novelty MMN latency was shorter in aMCI females than in HC females. Moreover, novelty P3a amplitudes were lower in males than in females in both aMCI and HC. For the effect of ApoE status, novelty MMN latency was shorter in aMCI ApoE ε4- than HC ApoE ε4-.
Conclusion: aMCI presents altered pre-attentive processing indexed by novelty MMN components. Furthermore, there may be a compensatory mechanism on the impaired processing in aMCI. It further suggests that aMCI female and ApoE ε4- recruited the compensatory mechanism.
Keywords: apolipoprotein E, gender, event-related potential, amnestic mild cognitive impairment, novelty mismatch negativity, novelty P3a
Introduction
Amnestic mild cognitive impairment (aMCI) is thought to reflect a transitional state between normal aging and dementia due to Alzheimer’s disease (AD) (Petersen and Negash, 2008; Albert et al., 2011). Behaviorally, both AD and aMCI are traditionally characterized and diagnosed in association with disruption in higher-level brain functions such as memory, perception, executive function, and attention. More specifically, abnormalities of episodic memory and attentional functions are the earliest clinical symptoms of aMCI and AD (Sperling et al., 2011; Simon et al., 2012; Bourrelier et al., 2015). Therefore, it is important to determine whether the abnormal mechanism of attentional function can contribute to the search for markers of the disease process early in the course of AD.
Event-related potential (ERP) measurements, a powerful non-invasive approach with a time resolution of milliseconds, is widely utilized to assess information processing of different cognitive functions in individuals (Kim et al., 2013). Mismatch negativity (MMN) and P300 subcomponent (P3a) are thought to be indices of the pre-attentive information processing (Naatanen et al., 2012) and attention orienting process (Jaworska et al., 2013), respectively. It was reported that both MMN and P3a components can be simultaneously elicited by any discriminable changes (including novel sounds) in a passive oddball experimental paradigm (Fisher et al., 2014). The novelty P3a component is considered to follow MMN and derives from fronto-central lobes during task processing and the negative novelty MMN component is thought to be located in the frontal and temporal regions and be associated with a mismatch between a trace in perceptual and sensory memory inputs (Naatanen et al., 2007). Recently, several studies suggested the combination of MMN and P3a (MMN/P3a complex) might be an underlying index of fundamental perceptual and pre-attentive information processing in the auditory pathway (Hermens et al., 2010; Chen et al., 2015). Furthermore, a recent study has indicated that novelty MMN is a more sensitive biomarker for aMCI than conventional MMN (Lindin et al., 2013). Therefore, the novelty MMN/P3a complex may be a useful brain marker to estimate cortical markers in the underlying neurobiology of aMCI.
Growing evidence from diverse aspects suggests that female gender may be an important risk factor for the development of AD and aMCI. Evidence from epidemiological studies has indicated a higher prevalence of AD in females than in age-matched males (Mielke et al., 2014). Previous studies have reported a faster rate of general cognitive function decline in females compared to males, with an average additional decline of 3.8 points over 5 years (Tschanz et al., 2011). Furthermore, a cross-sectional research study has reported females with AD had smaller hippocampal volumes than males (Apostolova et al., 2006) and a longitudinal study showed that the temporal lobe atrophic rate was about 1–1.5% faster in AD women than in men (Hua et al., 2010). Several studies investigated gender effects on components in auditory oddball paradigms in healthy subjects. For MMN, compared to males, females exhibited larger MMN amplitude in healthy young subjects (Barrett and Fulfs, 1998). In contrast, some studies reported no gender effects on the amplitude or latency of MMN in healthy young and old subjects (Kasai et al., 2002; Tsolaki et al., 2015; Yang et al., 2016). For P3, compared to males, females exhibited larger P3b amplitude and shorter latency in healthy young subjects (Yuan et al., 2008). In contrast, one study reported no gender effects on the amplitude or latency of P3b in healthy young and old subjects (Tsolaki et al., 2015). However, gender effect on novelty MMN and P3a in aMCI was not reported.
Apolipoprotein E (ApoE) is a glycoprotein of 299 amino acids with a molecular mass of ∼34 kDa and is encoded by a polymorphic gene localized on chromosome 19. The ApoE gene exists as three alleles (ε2, ε3, and ε4), which encode for ApoE2, ApoE3, and ApoE4, respectively (Kanekiyo et al., 2014). The apolipoprotein E epsilon4 (ApoE ε4) allele is the major genetic susceptibility factor for the development of AD (Teter et al., 2002). Individuals with the ε4 allele have an increased risk of developing AD and decreased age of onset (Raber et al., 2004). ApoE ε4 carriers have a greater rate of cognitive decline compared with non-carriers (Olofsson et al., 2010). There were two studies regarding the effects of ApoE status on components in auditory oddball paradigms in mild cognitive impairment patients. One study showed no significant differences in MMN, P3a and P3b indices between ApoE ε4 carriers and non-carriers in mildly cognitively impairment patients (Reinvang et al., 2005). The subjects of this research combined MCI and subjective cognitive complaints without neuropsychological deficits. Another study revealed that ApoE ε4 carriers with MCI had longer P3b latency than non-carriers (Barcin and Cintra, 2018). The effect of ApoE on novelty MMN and P3a in pure aMCI has not been reported.
The objective of the present study was thus to assess the diagnostic value of novelty MMN and P3a, and investigate the effect of gender and ApoE ε4 on auditory novelty MMN and P3a in healthy control (HC) and aMCI.
Materials and Methods
Subjects
The present study recruited 83 elderly individuals (all of whom were Chinese Han; right-handed; age range: 55–80 years old; ≥8-year education), including 39 aMCI patients and 44 HC, through newspaper advertisements, normal community health screening, and a hospital outpatient service. Written informed consent was obtained from all participants, and the study was approved by the responsible Human Participants Ethics Committee of Affiliated Zhongda Hospital, Southeast University (2016ZDSYLL011.0). All aMCI-multiple domain subjects met the diagnostic criteria proposed by Petersen (2004) and others’ recommendations (Albert et al., 2011) including: (1) subjective memory impairment corroborated by subject and an informant; (2) objective memory performances documented by an Auditory Verbal learning Test-delayed recall (AVLT-DR) score that is ≤1.5 standard deviation (SD) of age- and education-adjusted norms; (3) normal general cognitive function evaluated by a Mini-Mental State Examination (MMSE) score of 24 or higher; (4) a Clinical Dementia Rating of 0.5, with at least a 0.5 score in the memory domain; (5) no or minimal impairment in daily living activities; and (6) absence of dementia, or does not meet the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria and the National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroups on diagnostic guidelines for AD. Exclusion criteria were as follows: (1) history of stroke, alcohol, or drug abuse/dependence, traumatic brain injury, Parkinson’s disease, epilepsy; (2) major medical or psychiatric illnesses (e.g., cancer, anemia, thyroid dysfunction, and major depression); and (3) severe visual or hearing loss.
Healthy control were required to have a clinical dementia rating of zero, an MMSE score of ≥26, and an AVLT-DR score >4. These participants were matched for age, gender, and level of education by subject to aMCI. The inclusion and exclusion assessment was performed by two experienced neuropsychiatric physicians who administered a structured interview to subjects and their informants (Chen et al., 2016c).
Neuropsychological Assessment
All subjects underwent a standardized clinical interview and comprehensive neuropsychological assessments performed by neuropsychologists (Drs. Gu, Gao, and Yan). The neuropsychological assessments included cognitive function as follows (Chen et al., 2016a,c): (1) general cognitive function: MMSE and Mattis Dementia Rating Scale 2 (MDRS-2); (2) episodic memory: Auditory Verbal Learning Test (AVLT), Rey-Osterrieth Complex Figure Test-Delayed Recall (ROCFT-DR), and Logical Memory Test-Delayed Recall (LMT-DR); (3) information processing speed: Trail-Making Test A (TMT-A), Digital Symbol Substitution Test (DSST), Stroop Color and Word Tests A and B (Stroop-A and Stroop-B); (4) executive function: Verbal Fluency Test (VFT), Digit Span Test (DST), Trail-Making Test B (TMT-B), Stroop Color and Word Test C (Stroop-C), and Semantic Similarity Test (Similarity); and (5) visuospatial function: Clock Drawing Test (CDT) and Rey-Osterrieth Complex Figure Test (ROCFT).
ApoE Genotyping
A DNA purification kit (Tiangen, China) was used to extract genomic DNA from EDTA-anticoagulated blood. ApoE genotypes were determined by polymerase chain reaction-based restriction fragment length polymorphism (PCR-RFLP) (Chen et al., 2016b).
Procedure and Stimuli
After preparation for electroencephalogram (EEG) recording, participants were presented, via headphones, with 280 binaural pure tones (80 dB SPL, 10 ms rise/fall) at a 200 ms stimulus onset asynchrony; this comprised a pseudo-random sequence of 224 (80%) 1000 Hz standard tones, 28 (10%) 1050 Hz deviant tones, and 28 (10%) novel stimuli. Tones were presented while participants watched a silent video of a comedy movie. Participants were asked to report back the storyline of the movie at the end of the task.
EEG Acquisition and Analysis
EEG measurements of 64 scalp locations based on the 10–20 system were recorded using a BrainAmp MR portable ERP system (Brain Products GmbH; Munich, Germany). Data was referenced to a common average reference. Blinks and eye movement artifacts were monitored by recording the vertical and horizontal electro-oculogram located above and below the midpoint of the right eye and the outer canthus of each eye. Ocular artifact correction was performed using independent component analysis (Jung et al., 2000; Neuhaus et al., 2011). The EEG was band-pass filtered from 0.1 to 100 Hz with a gain of 20,000. EEG signals were analyzed with Brain Vision Analyzer software (Brain Products GmbH). In offline analyses, the EEG signal was band-pass filtered at 0.01–30 Hz (Chen et al., 2014a,b, 2015). EEG signals with an amplitude larger than ±100 μV were rejected (Chen et al., 2015). Mismatched difference waveforms were acquired by subtracting ERP waveforms induced by the novel auditory stimulus from those of the standard auditory stimulus. To calculate the ERP, epochs of EEG were averaged offline from 100 ms pre-stimulus to 700 ms post-stimulus relative to a 100 ms pre-stimulus baseline. The times were relative to the stimulus onset. The peak amplitudes and latencies of novelty MMN and P3 were identified within the established epochs of 150–250 and 250–450 ms, respectively (Jia et al., 2015; Weigl et al., 2016) and acquired with peak detection process. Novelty MMN and P3 variables were acquired at fronto-central (Fz, FCz, Cz) electrodes.
Statistical Analysis
Statistical analyses were conducted with SPSS 18.0 software. The Student t-test and chi-square test were performed to compare the demographic data and neuropsychological performances between aMCI group and HC group.
Mean peak amplitudes and latencies were subjected to repeated measures ANOVA (RMANOVA) with Group (aMCI and HC) as one between-subjects factor, and Electrode location (Fz, FCz, Cz) as one within-subject factor for novelty MMN and P3a. Years of education were included as covariates. To investigate the effect of gender on novelty MMN and P3, mean peak amplitudes and latencies were subjected to ANOVA among four groups (aMCI female, aMCI male, HC female, HC male) in Fz, FCz, Cz electrode sites. To investigate the effect of ApoE status on novelty MMN and P3, mean peak amplitudes and latencies were subjected to ANOVA among four groups (aMCI ApoE ε4+, aMCI ApoE ε4-, HC ApoE ε4+, HC ApoE ε4-) in Fz, FCz, Cz electrode sites. Degrees of freedom were corrected for non-sphericity using the Greenhouse–Geisser adjustment. Post hoc comparisons were analyzed by LSD test.
Partial correlation analyses were applied between amplitudes and latencies of novelty MMN and P3a and the neuropsychological performances in aMCI, HC and all subjects, controlling for the effects of age, gender, education years, ApoE status, and group. To increase statistical power by reducing random variability, as previously described (Shu et al., 2016), we grouped the neuropsychological tests into four cognitive domains and transformed the raw scores into four composite Z scores.
Results
Demographic and Neuropsychological Characteristics
The demographic and neuropsychological characteristics for all subjects are shown in Table 1 and Supplementary Table S1. No significant differences in age, gender or ApoE status were found between aMCI and HC (p > 0.05). Compared with HC, aMCI showed significant declines in years of education, MMSE, MDRS-2, information processing speed function, executive function, episodic memory and visuospatial function (p < 0.05).
Table 1.
Demographics and clinical measures of subjects with aMCI and HC subjects.
| Items | HC (N = 44) | aMCI (N = 39) | t-Values (χ2) | p-Values |
|---|---|---|---|---|
| Age (years) | 69.93 (5.58) | 71.28 (5.98) | -1.06 | 0.290 |
| Gender (males/females) | 21/23 | 25/14 | 2.24 | 0.134 |
| Education (years) | 12.23 (2.95) | 10.81 (2.93) | 2.19 | 0.031∗ |
| ApoE ε4 carriers (%) | 18 (40.91%) | 12 (30.77%) | 0.92 | 0.337 |
| MMSE score | 28.41 (1.31) | 27.08 (2.11) | 3.49 | 0.001∗ |
| MDRS-2 score | 137.59 (3.17) | 134.85 (5.35) | 2.80 | 0.007∗ |
| Episodic memory Z score | 0.49 (0.53) | -0.54 (0.61) | 8.19 | 0.000∗ |
| Information processing speed Z score | 0.21 (0.82) | -0.21 (0.64) | 2.61 | 0.011∗ |
| Executive function Z score | 0.25 (0.64) | -0.24 (0.50) | 3.83 | 0.000∗ |
| Visuospatial function Z score | 0.26 (0.45) | -0.31 (0.82) | 3.81 | 0.000∗ |
aMCI, amnestic mild cognitive impairment; HC, healthy controls; MMSE, Mini Mental State Exam; MDRS-2, Mattis Dementia Rating Scale 2. ∗Significant differences were found between aMCI patients and HC subjects. p-Values were obtained by Student’s t-test except for gender and ApoE ε4 carriers (chi square test).
Novelty MMN
For novelty MMN latency, RMANOVA showed significant effects of the Group factor [F(2,79) = 4.796, p = 0.031], but no significant Electrode Sites factor [F(1,80) = 0.331, p = 0.681]. Compared with HC (mean = 204.33 ± 27.82 ms), aMCI (mean = 190.72 ± 26.45 ms) had shorter novelty MMN latency. For novelty MMN amplitude, RMANOVA showed significant effects of the Electrode Sites factor [F(2,79) = 3.673, p = 0.042], but there was no significant Group factor [F(1,80) = 0.000, p = 0.996].
Effect of Gender
There was a significant effect of gender with shorter novelty MMN latency observed for aMCI female compared with HC female in Cz site (Table 2). No significant gender effect was found for novelty MMN amplitude (Table 2).
Table 2.
Event-related potential (ERP) data for HC and aMCI with different gender.
| Items | Site | HC | aMCI | F-Values | p-Values | ||
|---|---|---|---|---|---|---|---|
| Male (N = 21) | Female (N = 23) | Male (N = 25) | Female (N = 14) | ||||
| Novelty MMN latency | Fz | 197.24 (28.35) | 203.39 (32.98) | 197.44 (34.16) | 199.71 (32.06) | 0.18 | 0.908 |
| FCz | 200.86 (35.51) | 216.87 (25.49) | 201.20 (34.96) | 187.43 (42.51) | 2.22 | 0.092 | |
| Cz | 201.43 (39.88) | 205.04 (33.43) | 180.56 (44.80) | 172.43 (43.44)d | 2.93 | 0.039* | |
| Novelty MMN amplitude | Fz | -3.10 (1.78) | -4.01 (1.97) | -3.33 (1.76) | -3.30 (2.03) | 1.04 | 0.379 |
| FCz | -2.65 (1.67) | -3.86 (1.56) | -3.02 (2.13) | -2.50 (1.87) | 2.17 | 0.098 | |
| Cz | -1.93 (1.17) | -2.77 (1.21) | -2.73 (2.52) | -2.21 (2.00) | 1.62 | 0.191 | |
| Novelty P3 latency | Fz | 364.47 (35.66) | 358.87 (32.88) | 370.88 (34.65) | 377.71 (29.64) | 1.47 | 0.230 |
| FCz | 385.14 (39.81) | 358.78 (28.15) | 358.16 (47.23) | 370.29 (33.88) | 2.03 | 0.116 | |
| Cz | 390.57 (43.77) | 387.57 (39.13) | 381.60 (51.33) | 397.29 (44.48) | 0.40 | 0.755 | |
| Novelty P3 amplitude | Fz | 3.52 (1.65) | 5.56 (2.86)a | 3.57 (2.45) | 5.60 (2.88)b | 4.44 | 0.006* |
| FCz | 3.22 (1.47) | 5.07 (2.93)a | 3.23 (3.05) | 5.16 (2.59)b | 3.79 | 0.014* | |
| Cz | 2.49 (1.08) | 3.78 (2.31) | 2.74 (2.89) | 3.83 (2.32) | 2.36 | 0.078 | |
aMCI, amnestic mild cognitive impairment; HCs, healthy controls. ∗Significant differences were found among the four groups. p-Values were obtained by ANOVA analysis. a,b,dPost hoc analysis (LSD test) further revealed the source of ANOVA difference (a, HC male vs. HC female; b, aMCI male vs. aMCI female; d, aMCI female vs. HC female).
Effect of ApoE Status
There was a significant effect of ApoE status with shorter novelty MMN latency observed for aMCI ApoE ε4- compared with HC ApoE ε4- (Table 3). No significant ApoE status effect was found for novelty MMN amplitude (Table 3).
Table 3.
Event-related potential (ERP) data for HC and aMCI subjects with different ApoE status.
| Items | Site | HC | aMCI | F-Values | p-Values | ||
|---|---|---|---|---|---|---|---|
| ApoE ε4+ (N = 18) | ApoE ε4- (N = 26) | ApoE ε4+ (N = 12) | ApoE ε4- (N = 27) | ||||
| Novelty MMN latency | Fz | 206.67 (29.85) | 196.15 (31.04) | 190.00 (42.07) | 201.93 (28.25) | 0.81 | 0.490 |
| FCz | 215.78 (28.25) | 204.69 (33.13) | 198.50 (30.26) | 195.26 (41.31) | 1.34 | 0.266 | |
| Cz | 203.67 (35.83) | 203.08 (37.26) | 187.00 (31.75) | 173.48 (48.31)d | 3.11 | 0.031* | |
| Novelty MMN amplitude | Fz | -4.01 (2.20) | -3.27 (1.67) | -3.50 (1.71) | -3.24 (1.91) | 0.74 | 0.534 |
| FCz | -3.83 (2.17) | -2.90 (1.21) | -2.93 (1.84) | -2.80 (2.14) | 1.28 | 0.288 | |
| Cz | -2.50 (1.29) | -2.28 (1.24) | -2.65 (1.69) | -2.49 (2.60) | 0.12 | 0.945 | |
| Novelty P3 latency | Fz | 351.89 (27.84) | 368.23 (36.64) | 378.17 (30.40) | 371.19 (34.01) | 1.85 | 0.144 |
| FCz | 365.78 (35.05) | 375.23 (37.37) | 368.83 (42.24) | 359.70 (43.62) | 0.69 | 0.562 | |
| Cz | 379.67 (47.44) | 395.46 (35.31) | 384.50 (50.53) | 388.44 (49.21) | 0.47 | 0.706 | |
| Novelty P3 amplitude | Fz | 3.99 (2.00) | 4.99 (2.84) | 4.28 (3.24) | 4.31 (2.58) | 0.58 | 0.629 |
| FCz | 3.11 (1.92) | 4.93 (2.62) | 3.02 (2.39) | 4.33 (3.21) | 2.38 | 0.076 | |
| Cz | 2.40 (1.71) | 3.70 (1.91) | 2.71 (2.09) | 3.32 (2.98) | 1.32 | 0.275 | |
aMCI, amnestic mild cognitive impairment; HCs, healthy controls. ∗Significant differences were found among the four groups. p-Values were obtained by ANOVA analysis. dPost hoc analysis (LSD test) further revealed the source of ANOVA difference (d, aMCI ApoE ε4- vs. HC ApoE ε4-).
Novelty P3a
For novelty P3a amplitude, RMANOVA showed significant effects of the Electrode Sites factor [F(2,79) = 19.840, p < 0.000], but no significant Group factor [F(1,80) = 0.029, p = 0.864]. For novelty P3a latency, RMANOVA showed no significant effects of the Electrode Sites factor [F(2,79) = 1.469, p = 0.234] and Group factor [F(1,80) = 0.250, p = 0.619].
Effect of Gender
There was a significant effect of gender with lower novelty P3a amplitudes observed for males compared with females in both aMCI and HC in FCz and Fz sites (Table 2). No significant gender effect was found for novelty P3a latency (Table 2).
Effect of ApoE Status
No significant ApoE status effect was found for novelty P3a amplitudes and latencies (Table 3).
The Relationship Between Key Clinical Variables, Neuropsychological Performance, and ERP Indices
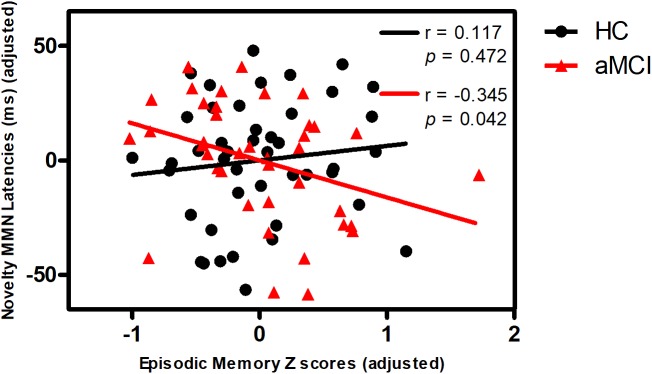
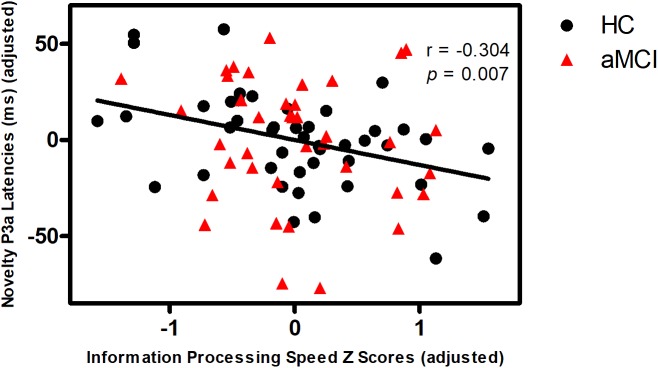
Partial correlation analyses demonstrated that novelty MMN latency was negatively correlated with episodic memory in aMCI, but not in HC (Figure 1). Novelty P3a latency was negatively correlated with information processing speed in all subjects (Figure 2). No correlations were found between MMN and P3a amplitudes and latencies and other neuropsychological performances in aMCI and HC (p > 0.05).
FIGURE 1.
Correlation of novelty MMN latency with episodic memory in aMCI and HC. Scattergram represents correlation between episodic memory Z score (adjusted scores) and novelty MMN latency (adjusted scores) in HC (black line) and aMCI (red line). Note that the data of the latency is mean latency from the Fz, FCz, Cz electrodes. The figure used the adjusted data controlling for the effects of age, gender, education years, ApoE status, and group.
FIGURE 2.
Correlation of novelty P3a latency with information processing speed in all subjects. Scattergram represents correlation between information processing speed Z score (adjusted scores) and novelty P3a latency (adjusted scores) in all subjects (black line). Note that the data of the latency is mean latency from the Fz, FCz, Cz electrodes. The figure used the adjusted data controlling for the effects of age, gender, education years, ApoE status, and group.
Discussion
The current study is the first to examine the effect of gender and ApoE status on novelty MMN and P3a in pure aMCI. In summary, novelty MMN latency was significantly shorter in aMCI than in the HC group. Novelty MMN latency was negatively correlated with episodic memory in aMCI, but not in HC. Novelty P3a latency was negatively correlated with information processing speed in all subjects. For gender effect, novelty MMN latency was shorter in aMCI female than in HC female. Moreover, novelty P3a amplitudes were lower in male than in female in both aMCI and HC. For the effect of ApoE status, novelty MMN latency was shorter in aMCI ApoE ε4- than HC ApoE ε4-.
Novelty MMN
Novelty MMN latency was significantly shorter in aMCI than in the HC group, which is consistent with one previous study (Lindin et al., 2013). This may represent a compensatory mechanism in aMCI. Studies have reported increased activity in the prefrontal lobe in MCI group (Browndyke et al., 2013), and increased functional connectivity between the right prefrontal regions and other regions in aMCI group (Bai et al., 2009). In addition, MCI has shown significantly higher EEG synchronization in the alpha and beta frequency ranges, but are no longer effective once AD develops, which indicated compensational mechanisms in MCI (Pijnenburg et al., 2004). Therefore, shorter novelty MMN latency may suggest a compensation mechanism of frontal cortex in aMCI. However, Ji et al. (2015) observed longer MMN latency in aMCI than in HC group, which may be attributed to relatively low years of education (aMCI mean = 3.88 ± 2.80, HC mean = 4.90 ± 2.76) of patients in the study, while years of education were higher in this research (aMCI mean = 10.81 ± 2.93, HC mean = 12.23 ± 2.95) and the research of Lindin et al. (2013) (aMCI mean = 10.15 ± 4.7, HC mean = 9.4 ± 4.4), respectively. One study has observed that glucose metabolism was positively associated with years of education in frontal lobe in MCI patients, suggesting a compensatory increase (Arenaza-Urquijo et al., 2017). Therefore, novelty MMN latency showed inconsistent changes in aMCI patients in these studies.
In addition, Novelty MMN latency was negatively associated with episodic memory in aMCI, indicating that novelty MMN latency may predict episodic memory performance in aMCI. Functional magnetic resonance imaging studies have reported association between episodic memory and functional connectivity between the prefrontal regions and other regions in aMCI group (Xie et al., 2012; Yuan et al., 2016). Although shorter novelty MMN latency represents a compensatory mechanism, shorter novelty MMN latency still reflects better episodic memory in aMCI.
Interestingly, this study showed that novelty MMN latency was shorter in aMCI female than in HC female, but not male, indicating that there is a gender effect on the altered MMN indices, that is, only females with aMCI present the altered pre-attentive processing. Accordingly, the findings further corroborated previous studies (Tschanz et al., 2011; Mielke et al., 2014), which suggest that females with aMCI may be more susceptible to AD pathology than males. One reason is that the effects of the ε4 genotype are more pronounced in women than in men. Some studies have reported that women with one ε4 allele have about a fourfold risk of AD (Farrer et al., 1997). Second reason is that the ApoE ε4 allele also has a greater deleterious effect in women than in men. Some studies have reported that, compared with men at different stages of AD, female with ApoE ε4 allele showed a greater deleterious effect on memory performance, cortical thickness, hippocampal pathology, functional connectivity changes in the default mode network (Fleisher et al., 2005). Furthermore, a large autopsy study found that women with ε4 carriers had greatest amyloid plaque and neurofibrillary tangle pathology (Corder et al., 2004). Collectively, based on the above-mentioned findings in aMCI, it further suggests that aMCI female recruit a compensatory mechanism, and female gender should clinically be considered as an important risk factor for the development of AD and aMCI, especially in clinical trials.
Additionally, novelty MMN latency was shorter in aMCI ApoE ε4- than HC ApoE ε4-, but not ApoE ε4+, suggesting that aMCI ApoE ε4- recruit a compensatory mechanism. One previous study also indicated that aMCI ApoE ε4- are able to compensate for reduced processing efficiency by recruiting distributed cortical areas through alpha and beta oscillatory cortical networks while aMCI ApoE ε4+ do not recruit compensatory mechanisms (Prieto del Val et al., 2015). No significant effect of ApoE status was found on MMN amplitude or latency in aMCI. The findings were consistent with a previous study (Reinvang et al., 2005), which showed no significant difference of novelty MMN potentials among ApoE gene status in mildly cognitive impaired patients, although subjects of the study combined MCI and subjective cognitive complaints without neuropsychological deficits. Furthermore, the current study demonstrated no significant difference of novelty MMN potentials between ApoE ε4+ and ApoE ε4- in HC. These results suggest that ApoE status does not affect novelty MMN potentials in HC or aMCI.
Novelty P3a
This study showed no significant difference in novelty P3a amplitude or latency between aMCI and HC, which indicates that aMCI have no deficits on attention orienting response. However, novelty P3a latency was negatively correlated with information processing speed in all subjects. According to the theoretical models of attention, attention has many aspects, including arousal, selection, strategic control, and processing speed (Ponsford et al., 2014). Furthermore, several studies have reported that P3a represents the attention orienting response (Jaworska et al., 2013). Therefore, the findings suggest that novelty P3a latency can reflect the performance of information processing speed.
For gender effect, novelty P3a amplitudes were lower in male than in female in both aMCI and HC, indicating that female recruited more attention orienting process than male during the novelty condition and the gender effect was not influenced by disease state. Furthermore, no significant effect of ApoE status was found on novelty P3a amplitude or latency in HC and aMCI, which corroborated previous finding that no significant differences in P3a indices between ApoE ε4+ and ApoE ε4- in mildly cognitively impairment patients (Reinvang et al., 2005). The result indicates that ApoE status does not affect novelty P3a potentials in HC or aMCI.
Limitations
Although this is the first and largest study of novelty MMN/P3a in aMCI, the sample size was still relatively small, which might influence the explanation of the results. Due to the small sample size and only three ApoE ε2 carriers in aMCI, this study did not explore ApoE ε2 and ε3 carriers, respectively, and the ApoE alleles-by-gender interaction in aMCI. Future study will enlarge the sample size and further explore the effect of the three alleles (ε2, ε3, ε4) on novelty MMN/P3a in aMCI and the ApoE alleles-by-gender interaction in aMCI. In addition, the cross-sectional study design itself has limitations. The follow-up of this sample and an additional AD group will help to clarify whether these changes of pre-attentive information processing in aMCI represent a transitional state between normal aging and AD.
Conclusion
The aMCI patients present altered pre-attentive processing indexed by novelty MMN components. Furthermore, there may be a compensatory mechanism on the impaired processing in aMCI. It further suggests that aMCI female and ApoE ε4- recruited the compensatory mechanism. Collectively, the present study may aid to focus attention to MMN and P3a indices when developing new potential biomarkers for the early identification of AD. Moreover, these findings underline the importance of taking into account the effects of sex and ApoE genotype in future clinical trials. The present results could help in identifying different aMCI phenotypes, and then to select possible target groups for prevention, and to design rational strategies for therapeutic trials.
Author Contributions
LjG, LhG, and JC were in charge of EEG recording and ERP data acquisition and analysis. HS, ZW, DL, and YY were in charge of patient enrollment and neuropsychological assessments. LjG and ZZ had the major responsibility for preparing the paper and manuscript writing. ZZ and JC contributed to the design and plan of the study. ZZ supervised the project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (Nos. 81420108012, 81701675), the Foundation for Distinguished Medical Scholars of Jiangsu Province (No. JCRCA2016006), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX17_0178).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00256/full#supplementary-material
References
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova L. G., Dinov I. D., Dutton R. A., Hayashi K. M., Toga A. W., Cummings J. L., et al. (2006). 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer’s disease. Brain 129(Pt 11), 2867–2873. 10.1093/brain/awl274 [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo E. M., Bejanin A., Gonneaud J., Wirth M., La Joie R., Mutlu J., et al. (2017). Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol. Aging 59 72–79. 10.1016/j.neurobiolaging.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Bai F., Watson D. R., Yu H., Shi Y., Yuan Y., Zhang Z. (2009). Abnormal resting-state functional connectivity of posterior cingulate cortex in amnestic type mild cognitive impairment. Brain Res. 1302 167–174. 10.1016/j.brainres.2009.09.028 [DOI] [PubMed] [Google Scholar]
- Barcin E., Cintra M. T. G. (2018). Increased N200 and P300 latencies in cognitively impaired elderly carrying ApoE epsilon-4 allele. Neurol. Sci. 33 e221–e227. 10.1007/s10072-017-3106-3 [DOI] [PubMed] [Google Scholar]
- Barrett K. A., Fulfs J. M. (1998). Effect of gender on the mismatch negativity auditory evoked potential. J. Am. Acad. Audiol. 9 444–451. [PubMed] [Google Scholar]
- Bourrelier J., Kubicki A., Rouaud O., Crognier L., Mourey F. (2015). Mental rotation as an indicator of motor representation in patients with mild cognitive impairment. Front. Aging Neurosci. 7:238. 10.3389/fnagi.2015.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browndyke J. N., Giovanello K., Petrella J., Hayden K., Chiba-Falek O., Tucker K. A., et al. (2013). Phenotypic regional functional imaging patterns during memory encoding in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement 9 284–294. 10.1016/j.jalz.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Duan X., Shu H., Wang Z., Long Z., Liu D., et al. (2016a). Differential contributions of subregions of medial temporal lobe to memory system in amnestic mild cognitive impairment: insights from fMRI study. Sci. Rep. 6:26148. 10.1038/srep26148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Shu H., Wang Z., Liu D., Shi Y., Xu L., et al. (2016b). Protective effect of APOE epsilon 2 on intrinsic functional connectivity of the entorhinal cortex is associated with better episodic memory in elderly individuals with risk factors for Alzheimer’s disease. Oncotarget 7 58789–58801. 10.18632/oncotarget.11289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Shu H., Wang Z., Zhan Y., Liu D., Liao W., et al. (2016c). Convergent and divergent intranetwork and internetwork connectivity patterns in patients with remitted late-life depression and amnestic mild cognitive impairment. Cortex 83 194–211. 10.1016/j.cortex.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Chen J., Ma W., Zhang Y., Wu X., Wei D., Liu G., et al. (2014a). Distinct facial processing related negative cognitive bias in first-episode and recurrent major depression: evidence from the N170 ERP component. PLoS One 9:e109176. 10.1371/journal.pone.0109176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Ma W., Zhang Y., Yang L. Q., Zhang Z., Wu X., et al. (2014b). Neurocognitive impairment of mental rotation in major depressive disorder: evidence from event-related brain potentials. J. Nerv. Ment. Dis. 202 594–602. 10.1097/NMD.0000000000000167 [DOI] [PubMed] [Google Scholar]
- Chen J., Zhang Y., Wei D., Wu X., Fu Q., Xu F., et al. (2015). Neurophysiological handover from MMN to P3a in first-episode and recurrent major depression. J. Affect. Disord. 174 173–179. 10.1016/j.jad.2014.11.049 [DOI] [PubMed] [Google Scholar]
- Corder E. H., Ghebremedhin E., Taylor M. G., Thal D. R., Ohm T. G., Braak H. (2004). The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann. N. Y. Acad. Sci. 1019 24–28. 10.1196/annals.1297.005 [DOI] [PubMed] [Google Scholar]
- Farrer L. A., Cupples L. A., Haines J. L., Hyman B., Kukull W. A., Mayeux R., et al. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA 278 1349–1356. 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- Fisher D. J., Smith D. M., Labelle A., Knott V. J. (2014). Attenuation of mismatch negativity (MMN) and novelty P300 in schizophrenia patients with auditory hallucinations experiencing acute exacerbation of illness. Biol. Psychol. 100 43–49. 10.1016/j.biopsycho.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Fleisher A., Grundman M., Jack C. R., Jr., Petersen R. C., Taylor C., Kim H. T. (2005). Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch. Neurol. 62 953–957. 10.1001/archneur.62.6.953 [DOI] [PubMed] [Google Scholar]
- Hermens D. F., Ward P. B., Hodge M. A., Kaur M., Naismith S. L., Hickie I. B. (2010). Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog. Neuropsychopharmacol. Biol. Psychiatry 34 822–829. 10.1016/j.pnpbp.2010.03.019 [DOI] [PubMed] [Google Scholar]
- Hua X., Hibar D. P., Lee S., Toga A. W., Jack C. R., Jr., Weiner M. W., et al. (2010). Sex and age differences in atrophic rates: an ADNI study with n = 1368 MRI scans. Neurobiol. Aging 31 1463–1480. 10.1016/j.neurobiolaging.2010.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska N., De Somma E., Blondeau C., Tessier P., Norris S., Fusee W., et al. (2013). Auditory P3 in antidepressant pharmacotherapy treatment responders, non-responders and controls. Eur. Neuropsychopharmacol. 23 1561–1569. 10.1016/j.euroneuro.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L. L., Zhang Y. Y., Zhang L., He B., Lu G. H. (2015). Mismatch negativity latency as a biomarker of amnestic mild cognitive impairment in chinese rural elders. Front. Aging Neurosci. 7:22. 10.3389/fnagi.2015.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., Tsang Y. K., Huang J., Chen H. C. (2015). Processing Cantonese lexical tones: evidence from oddball paradigms. Neuroscience 305 351–360. 10.1016/j.neuroscience.2015.08.009 [DOI] [PubMed] [Google Scholar]
- Jung T. P., Makeig S., Westerfield M., Townsend J., Courchesne E., Sejnowski T. J. (2000). Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin. Neurophysiol. 111 1745–1758. 10.1016/S1388-2457(00)00386-2 [DOI] [PubMed] [Google Scholar]
- Kanekiyo T., Xu H., Bu G. (2014). ApoE and Abeta in Alzheimer’s disease: accidental encounters or partners? Neuron 81 740–754. 10.1016/j.neuron.2014.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K., Nakagome K., Iwanami A., Fukuda M., Itoh K., Koshida I., et al. (2002). No effect of gender on tonal and phonetic mismatch negativity in normal adults assessed by a high-resolution EEG recording. Brain Res. Cogn. Brain Res. 13 305–312. 10.1016/S0926-6410(01)00125-2 [DOI] [PubMed] [Google Scholar]
- Kim E. Y., Lee S. H., Park G., Kim S., Kim I., Chae J. H., et al. (2013). Gender difference in event related potentials to masked emotional stimuli in the oddball task. Psychiatry Investig. 10 164–172. 10.4306/pi.2013.10.2.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindin M., Correa K., Zurron M., Diaz F. (2013). Mismatch negativity (MMN) amplitude as a biomarker of sensory memory deficit in amnestic mild cognitive impairment. Front. Aging Neurosci. 5:79. 10.3389/fnagi.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. M., Vemuri P., Rocca W. A. (2014). Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin. Epidemiol. 6 37–48. 10.2147/clep.s37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R., Kujala T., Escera C., Baldeweg T., Kreegipuu K., Carlson S., et al. (2012). The mismatch negativity (MMN)–a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin. Neurophysiol. 123 424–458. 10.1016/j.clinph.2011.09.020 [DOI] [PubMed] [Google Scholar]
- Naatanen R., Paavilainen P., Rinne T., Alho K. (2007). The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin. Neurophysiol. 118 2544–2590. 10.1016/j.clinph.2007.04.026 [DOI] [PubMed] [Google Scholar]
- Neuhaus A. H., Popescu F. C., Grozea C., Hahn E., Hahn C., Opgen-Rhein C., et al. (2011). Single-subject classification of schizophrenia by event-related potentials during selective attention. Neuroimage 55 514–521. 10.1016/j.neuroimage.2010.12.038 [DOI] [PubMed] [Google Scholar]
- Olofsson J. K., Nordin S., Wiens S., Hedner M., Nilsson L. G., Larsson M. (2010). Odor identification impairment in carriers of ApoE-varepsilon4 is independent of clinical dementia. Neurobiol. Aging 31 567–577. 10.1016/j.neurobiolaging.2008.05.019 [DOI] [PubMed] [Google Scholar]
- Petersen R. C. (2004). Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256 183–194. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Petersen R. C., Negash S. (2008). Mild cognitive impairment: an overview. CNS Spectr. 13 45–53. 10.1017/S1092852900016151 [DOI] [PubMed] [Google Scholar]
- Pijnenburg Y. A., v d Made Y., van Cappellen van Walsum A. M., Knol D. L., Scheltens P., Stam C. J. (2004). EEG synchronization likelihood in mild cognitive impairment and Alzheimer’s disease during a working memory task. Clin. Neurophysiol. 115 1332–1339. 10.1016/j.clinph.2003.12.029 [DOI] [PubMed] [Google Scholar]
- Ponsford J., Bayley M., Wiseman-Hakes C., Togher L., Velikonja D., McIntyre A., et al. (2014). INCOG recommendations for management of cognition following traumatic brain injury, part II: attention and information processing speed. J. Head Trauma Rehabil. 29 321–337. 10.1097/HTR.0000000000000072 [DOI] [PubMed] [Google Scholar]
- Prieto del Val L., Cantero J. L., Atienza M. (2015). APOE varepsilon4 constrains engagement of encoding-related compensatory networks in amnestic mild cognitive impairment. Hippocampus 25 993–1007. 10.1002/hipo.22422 [DOI] [PubMed] [Google Scholar]
- Raber J., Huang Y., Ashford J. W. (2004). ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol. Aging 25 641–650. 10.1016/j.neurobiolaging.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Reinvang I., Espeseth T., Gjerstad L. (2005). Cognitive ERPs are related to ApoE allelic variation in mildly cognitively impaired patients. Neurosci. Lett. 382 346–351. 10.1016/j.neulet.2005.03.024 [DOI] [PubMed] [Google Scholar]
- Shu H., Shi Y., Chen G., Wang Z., Liu D., Yue C., et al. (2016). Opposite neural trajectories of apolipoprotein e 4 and 2 alleles with aging associated with different risks of Alzheimer’s disease. Cereb. Cortex 26 1421–1429. 10.1093/cercor/bhu237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. S., Yokomizo J. E., Bottino C. M. (2012). Cognitive intervention in amnestic mild cognitive impairment: a systematic review. Neurosci. Biobehav. Rev. 36 1163–1178. 10.1016/j.neubiorev.2012.01.007 [DOI] [PubMed] [Google Scholar]
- Sperling R. A., Aisen P. S., Beckett L. A., Bennett D. A., Craft S., Fagan A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7 280–292. 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter B., Raber J., Nathan B., Crutcher K. A. (2002). The presence of apoE4, not the absence of apoE3, contributes to AD pathology. J. Alzheimers. Dis. 4 155–163. 10.3233/JAD-2002-4305 [DOI] [PubMed] [Google Scholar]
- Tschanz J. T., Corcoran C. D., Schwartz S., Treiber K., Green R. C., Norton M. C., et al. (2011). Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the cache county dementia progression study. Am. J. Geriatr. Psychiatry 19 532–542. 10.1097/JGP.0b013e3181faec23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolaki A., Kosmidou V., Hadjileontiadis L., Kompatsiaris I. Y., Tsolaki M. (2015). Brain source localization of MMN, P300 and N400: aging and gender differences. Brain Res. 1603 32–49. 10.1016/j.brainres.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Weigl M., Mecklinger A., Rosburg T. (2016). Transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates auditory mismatch negativity. Clin. Neurophysiol. 127 2263–2272. 10.1016/j.clinph.2016.01.024 [DOI] [PubMed] [Google Scholar]
- Xie C., Bai F., Yu H., Shi Y., Yuan Y., Chen G., et al. (2012). Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. Neuroimage 63 320–327. 10.1016/j.neuroimage.2012.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Chen L., Sun H., Qiao Z., Qiu X., et al. (2016). Gender differences in pre-attentive change detection for visual but not auditory stimuli. Clin. Neurophysiol. 127 431–441. 10.1016/j.clinph.2015.05.013 [DOI] [PubMed] [Google Scholar]
- Yuan B., Chen J., Gong L., Shu H., Liao W., Wang Z., et al. (2016). Mediation of episodic memory performance by the executive function network in patients with amnestic mild cognitive impairment: a resting-state functional MRI study. Oncotarget 7 64711–64725. 10.18632/oncotarget.11775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., He Y., Qinglin Z., Chen A., Li H. (2008). Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology 45 986–993. 10.1111/j.1469-8986.2008.00693.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.