Abstract
Except for the essential amino acids (AAs), much of the focus on adequate dietary protein intake has been on total nitrogen and caloric intake rather than AA composition. Recent data, however, demonstrate that “amino-acid sensing” can occur through either intracellular or extracellular nutrient-sensing mechanisms. In particular, members of the class 3 G-protein coupled receptor family, like the calcium-sensing receptor are known to preferentially bind specific AAs, which then modulate receptor activation by calcium ions and thus potentially impact bone turnover. In pursuing the possibility of direct nutrient effects on bone cells, we examined individual AA effects on osteoprogenitor/bone marrow stromal cells (BMSCs), a key target for bone anabolism. We demonstrate that BMSCs express both intracellular and extracellular nutrient sensing pathways and that AAs are required for BMSC survival. In addition, certain AA types, like members of the aromatic AAs, can potently stimulate increases in intracellular calcium and ERK phosphorylation/activation. Further, based on the in vitro data, we examined the effect of specific AAs on bone mass. To better evaluate the impact of specific AAs, we added these to a low-protein diet. Our data demonstrate that a low-protein diet itself is associated with a significant drop in bone mineral density (BMD) in the older mice, related, at least in part, to an increase in osteoclastic activity. This drop in BMD in mice on the low-protein diet is prevented by addition of AAs from the aromatic group. Taken together our data show that AAs function as specific and selective signaling molecules in bone cells.
INTRODUCTION
Dietary protein is known to be an important nutrient for maintaining musculoskeletal health. Both bone and muscle are lost with aging (sarcopenia and osteoporosis), up to 1% per year after age 50, and it has been recommended that dietary protein intake increase with age to ameliorate this loss(1). However, there has been some debate on whether increasing dietary protein intake could instead result in bone loss by producing an increased acid load and thus promoting bone breakdown. More recent data have shown that dietary protein increases intestinal calcium absorption and is beneficial for bone(2). Nevertheless, the major effect of dietary protein on bone is thought to be related to its anabolic effects through increasing nitrogen intake and providing a rich energy source and substrate for protein synthesis. There has been some discussion on whether the source of dietary protein matters with respect to bone (animal versus vegetable sources) with data being inconclusive to date(3). The actual AA composition of the various forms of protein however has not been generally discussed as a potential source of variability in these results.
There are nine essential amino acids, meaning that they cannot be synthesized in the body. We have previously shown that low protein diets negatively impact older but not younger mouse bone mass, suggesting that “nutrient sensing” could decrease with age(4). Data from a number of laboratories suggest that an underappreciated mechanism of nutrient action is directly on bone cells, a paradigm in which nutrients act as signaling molecules. Work by Conigrave et al.(5) showed that a number of tissues express the calcium-sensing receptor and that these receptors preferentially bind aromatic amino acids to serve as nutrient sensors. These data suggested that AAs might have other mechanisms of action besides just changing the cellular energetics.
Whether the main amino acid sensor is the calcium-sensing receptor (CaSR) or the closely related GPRC6A has been unclear. GPRC6A has a variety of ligands and in fact has also been proposed to be the osteocalcin receptor. In addition, increased extracellular calcium still modulates osteoblastic function in CaSR knockout mice(6–9).
The present study addressed the question of whether amino acids could have a direct effect on bone cells and if so, could select amino-acids provide beneficial effects on bone mass in vivo.
MATERIALS AND METHODS
METHODS
Progenitor Cell Isolation and Culture
Bone marrow cells were isolated as previously described(10). Briefly, femora and tibiae from C57BL/6 mice were dissected free of soft tissue, cut open at both ends and flushed with complete isolation media (CIM, consisting of RPMI-1640 supplemented with 9% FBS, 9% horse serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 12 μM L-glutamine, 2 mL/mouse)(11) using a 22-gauge syringe followed by filtration through a 70-μm nylon mesh filter. Marrow aspirates were pooled and used for bone marrow stromal cell (BMSC) isolation. The single-cell suspension was plated in 175-cm2 flasks at a density of 2 × 107 cells/flask. After a 3-hr incubation at 37 °C in 5% CO2, the media containing non-adherent cells were removed, and the adherent cells were washed 2 times gently with PBS to reduce the degree of hematopoietic lineage cell contamination. BMSCs were then isolated by negative and positive immunoselection as described in Zhang et al. (10). BMSCs were cultured in either DMEM (Corning Cellgro, low glucose #10-014CV) or M199 (Corning Cellgro # 10-060-CV).
RT-PCR for amino acid sensors
Total RNA was extracted from BMSC cells with the RNeasy mini kit (Qiagen). cDNA was synthesized using 1 μg total RNA (SuperScript III First-Strand Synthesis System, Invitrogen). For PCR reactions the “Platinum PCR SuperMix” (Invitrogen) was used (94°C, 30 sec; 57° C, 30 sec; 72° C, 1 min for 35 cycles). Primers used are listed in Table 1.
Table 1.
| Gene | Primer |
|---|---|
| CAT1 | ACT GGA ACA CAA GGC TGT ATC CGT ACA GAT CAC GTC AGT GGC CAG AAA |
| CAT2 | TGC TAT CTC AGT GAC CGT GCC TTT CCA ATC CCT GCA CAA ACA CAC ACA |
| CAT3 | TAT GGG CTC TGA GGT TTC ATG CCT TCT CCG GTG GTT GCA ATA CAG TCA |
| CaSR | AGC AAC CGG CTC CGG TGT CT CGT CGT TGG GAA TGG TGC GGA |
| T1R1 | TGT CTG ACA ATC CGC TCC TTC CAA ACA GAG GAA CAA ATG GAC CGT GGA |
| T1R3 | AAT GCG GAG TTG GAT CTG GAG GAA TAG GTT CTG CAA CAG CCC AGA TGA |
| GPRC6A | CCT GCA GCG TGA CGT CTT CAT CA ACA GCT GTG TTG GCT TCC TGT GG |
Western blotting
For experiments examining extracellular signal-regulated kinase (ERK) phosphorylation, BMSCs were grown in 60 mm2 dishes in DMEM + 10% fetal calf serum to ~ 80% confluency. Antibodies directed against both total ERK and phospho-ERK were utilized. Primary (1:750, 4°C, overnight) and HRP-coupled secondary (1:2000, room temperature, 1 hour) antibodies were obtained from Cell Signaling Technology (Danvers, MA).
Intracellular Calcium Measurements
BMSCs isolated from C57BL/6 mice were plated on cover-slips and loaded at 37° C for 40 min with 5 μM Fura-2 AM (Thermo Fisher Scientific, Waltham, MA). Cells were then washed three times with Hepes buffered saline (HBS), and bathed in HBS for 15 min at 37° C before Ca2+ imaging at room temperature. Cells were viewed with a Zeiss Axiovert S100 microscope using a 40x oil immersion objective, and sequentially illuminated with a 100 W Xenon monochrometer at 0.1s intervals using alternating 340/380 nm wavelengths. Images were collected with a CCD camera (Imago QE) connected to a Dell workstation. Fluorescence emission at 510 nm was monitored for each excitation wavelength, and analyzed with TillVision software (TillPhotonics, Germany). Pixel intensities within selected areas of the images (with each area corresponding to a single cell) were digitized for both wavelengths at each time point. The first ten data points were averaged to establish F0, the baseline ratio of emission detected at 510 nm following excitation at 340 and 380 nm (F340/F380). Changes in F340/F380 with time were plotted as F−F0/F0. Different cells/coverslips were used for each concentration tested (n=9 samples/concentration).
Mice
18- and 24-month-old mice were obtained from the aging colony of the National Institutes on Aging. Mice were kept in cages of four animals/cage at 25°C with a 12/12h light/dark cycle. Diets were prepared by Dr. Barbara Mickelson and mice had free access to the indicated diets [Harlan TakLad Rodent Diet (W) 8604] and water ad libitum during the entire experiment. All experiments, which were approved by the Institutional Animal Care and Use Committee at Medical College of Georgia (Augusta, GA), were performed with male mice.
Bone densitometry and body structure measurements
Bone mineral density (BMD) bone mineral content (BMC), scanned area, and lean and fat percentage were measured by dual-energy X-ray absorptiometry (DXA) (Piximus system; GE LUNAR, Madison, WI) between 9:00 AM and 12:00 PM as previously reported (12).
ELISA
Mice were sacrificed and bloos samples collected from each mouse by cardiac puncture and serum samples prepared, aliquoted and stored at −80C. The levels of pyridinoline crosslinks (PYD) a marker of bone breakdown, in the samples were measured with an ELISA kit from Quidel Corporation (San Diego, CA) according to the manufacturers instructions as previously reported(12).
Biomechanical Testing
Bone biomechanical measurements were performed as previously described (12). Briefly, biomechanical properties were evaluated using the left femora of mice using a three point bending test. Femora were placed in a mechanical testing machine on two supports separated by a distance of 5 mm and load was applied to the middle of the shaft. The mechanical resistance to failure was tested using a servo-controlled electromechanical system (Instron Corp., High Wycombe, England). The actuator was displaced at a rate of 100μm/s. Both displacement and load were recorded.
Micro-computed tomography (μ-CT)
Micro-CT was performed as previously described (4). L4 and L5 vertebrae or distal femora were scanned with an ex vivo μ-CT system (Skyscan 1174; Skyscan, Aartlesaar, Belgium). The three-dimensional morphometric parameters of bone microarchitecture were calculated using CTAn (Skyscan) software and the parameters measured included bone volume fraction [bone volume/total volume (BV/TV)] and trabecular thickness (Tb.Th), number (Tb.N), and separation (Tb.Sp).
Statistics
Results are expressed as mean ± SEM. Experiments were performed three separate times except where noted. Data were analyzed using either ANOVA with Bonferroni post-hoc testing or unpaired t tests, using a commercial statistical package (Instat, Graphpad Inc, San Diego, CA).
RESULTS
BMSCs cannot grow in culture medium with low amino acid levels
Effects of AA on BMSC proliferation have not been previously reported. Most published studies have used DMEM (or α-MEM) as tissue culture medium for BMSC. AA levels in DMEM are substantially higher than in Medium 199, mimicking the postprandial state(13), whereas AA levels in Medium 199 approximate the fasting state.
When we cultured BMSCs in Medium 199 + 10% FCS, the cells unexpectedly died within 3 days after seeding. In contrast, BMSCs that were seeded in DMEM + 10% FCS under the same conditions rapidly proliferated, reaching 80% confluence within the same time frame. To determine whether these striking differences in proliferation rates and cell viability were effected by different levels of amino acids, we seeded BMSCs in Medium 199 then analyzed the proliferative effects of increasing concentrations (0.3, 0.5 and 1.0x) of a commercially available 50X MEM amino acid solution (alanine, arginine, aspartic acid, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryosine, tryptophan and valine; 0.5 μmol/mL with the exception of cystine, which was 25 μmol/mL (Sigma-Aldrich, St. Louis, MO). The 1x concentration mimicked amino acid levels present in DMEM. As shown in figure 1A, increasing concentrations of AAs were correlated with increased BMSC survival rates, with over 80% survival at the 1x concentration. Lower AA concentrations (0.3x) were only partially effective in restoring BMSC growth. However, BMSCs grown with the two highest AA concentrations (0.5 and 1x) were fully capable of proliferation and differentiation into mature osteoblasts (as measured by either Von Kossa or Alizarin Red staining) following addition of differentiation medium (containing 50 μg/mL ascorbic acid and 10mM sodium β-glycerophosphate; data not shown). These data suggested that elevated AA concentrations in DMEM are important for BMSC survival.
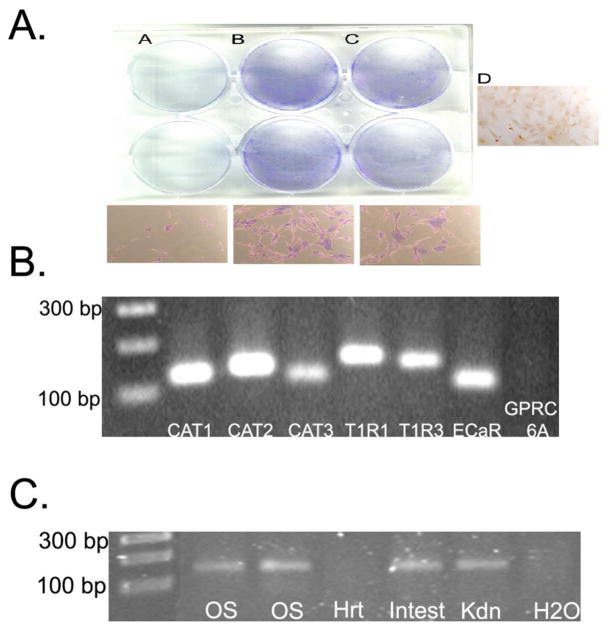
Figure 1. Amino Acid Effects on BMSCs.
Panel A: (A) Effect of low amino acid levels on BMSC survival or of amino acid supplementation at a 1x (B) or 0.5x (C) level for three days followed by cell staining with Coomassie Blue. Higher magnification images are shown in the panels below. Essentially no BMSCs survived at the end of the three-day culture in M199 (A). Increasing concentrations of the amino acid mixture resulted in increased cell survival (B,C). Images representative of fifteen independent experiments. (D) Unstained cells grown in 1x amino-acid mixture.
Panel B: RT-PCR for amino acid sensors: Total RNA was extracted from BMSC cells with the RNeasy mini kit (Qiagen). cDNA was synthesized using 1 μg total RNA (SuperScript III First-Strand Synthesis System, Invitrogen) and PCR performed as described in Methods.
Panel C: RT-PCR for GPRC6A: To evaluate GPRC6A expression in bone cells we ran additional controls using the same conditions as in Panel B. As seen, we could detect GPRC6A in primary calvarial osteoblasts. Additional mouse tissues were used as positive and negative controls. OS= primary mouse calvarial osteoblasts; Hrt= mouse heart; Intest= mouse intestine; Kdn=mouse kidney, H20 was a negative control
BMSCs express multiple amino acid transporters
Cellular amino acid sensing can occur through members of the class 3, seven transmembrane domain, G-protein receptor superfamily including: 1) extracellular calcium sensing receptor (CaSR)(14); 2) taste receptors T1R1 and T1R3(15); and 3) member 6A of the G-protein coupled receptor family C (GPRC6A)(16). These extracellular AA sensing receptors are stereoselective, binding L-type but not D-type amino acids. Initial experiments were designed to determine which, if any, of these amino acid sensors were expressed in BMSCs. As shown in figures 1B, PCR analysis of BMSC mRNA using primer sets for various genes detected the presence of the extracellular AA sensors CaSR and T1R1/T1R3, but not GPRC6A. An alternative mechanism for amino acid effects on cells is for AAs to be taken up and thus alter energy balance through “energy sensors” such as adenosine 5-monophosphate-activated protein kinase (AMPK). One such transporter class are the family of cationic amino-acid transporters (CAT), members of solute carrier family 7 (SLC7); this family consists of several members including CAT-1, -2A, -2B and -3. All have affinity for cationic L-amino acids, which are taken up and metabolized. As shown in Figure 1B, CAT-1, -2 and -3 are all expressed in BMSCs.
GPRC6A has also been reported to be the osteocalcin receptor but our screening did not find this receptor to be expressed in BMSCs. However. the ability of our primers to detect GPRC6A was confirmed using mRNA isolated from several positive controls including primary mouse calvarial osteoblasts, kidney and liver, all of which are known to express this receptor(6) (Fig. 1C). Interestingly, we found that GPRC6A was expressed in osteoblasts, but not osteoprogenitor cells(6). Our findings confirm these data and provide new evidence that osteoprogenitor cells also express messages for the taste receptors,T1R1 and T1R3, as well as three known amino acid transporters (CAT1, 2, and 3).
Aromatic (but not aliphatic) amino acids increase intracellular calcium in BMSCs
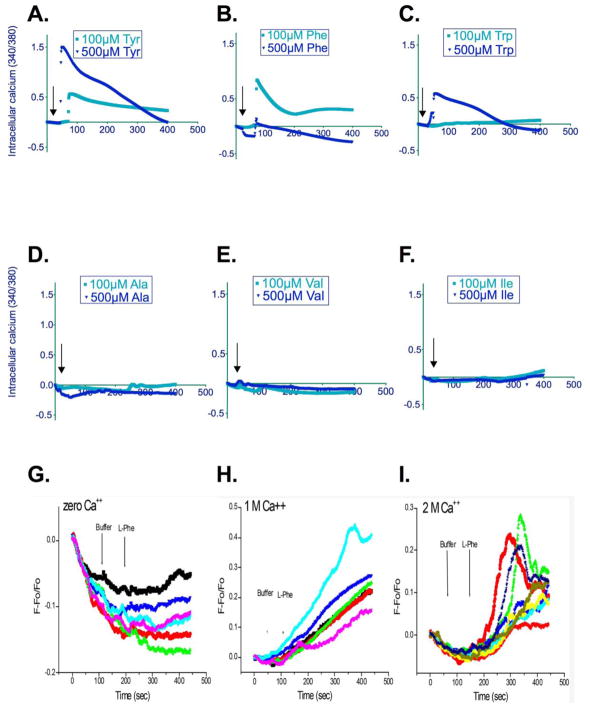
Because our initial observations suggested that AAs play an important role in the regulation of BMSC function, we next tested potential signaling pathways used by AAs. The aromatic AAs (tyrosine, Tyr; phenylalanine, Phe; and tryptophan, Trp) have been reported to bind to the calcium-sensing receptor and raise intracellular calcium. Thus, aromatic AAs were tested for their effects on intracellular calcium in a single cell setup as measured using fura-2 (Figure 2 A, B, C). Previous studies from our group(17,18) have shown that aromatic AA in the μM range increase ERK phosphorylation/activation. As shown, single aromatic AAs at concentrations between 100 and 500 μM significantly increased intracellular calcium levels. In contrast no change in intracellular calcium was detected when aliphatic AAs (Ala; Val; Ile) were tested (Figure 2 D, E, F).
Figure 2. Amino acid effects on intracellular calcium.
Panels A–F: Aromatic amino acids increase intracellular calcium levels, as shown by traces from individual cells. Each trace represents the average change in fluorescence from three different cells. Different cells/coverslips were used for each concentration tested (n=9 samples/concentration). Agonists were added at the indicated times (arrows). For statistical analyses, areas under the curve of stimulated vs. control samples were compared (A. Tyr, 100 and 500 μM; P<0.0002; B. Phe, 100 and 500 μM; P<0.0001; C. Trp, 100 and 500 μM; P<0.0001). Interestingly, the dose response relationship for Phe appeared to be biphasic with higher levels of this AA having a reduced response as compared to lower (100–200 μM) doses. In contrast, the aliphatic amino acids, Ala, Val and Ile had no effect on intracellular calcium (Panels D–F).
Panels G–I: Phenylalanine increases intracellular calcium. Calcium measurements were performed under nominally calcium-free conditions (G) and in the presence of 1 (H) and 2 mM (I) extracellular calcium. Cells were stimulated with phenylalanine (500 μM) or buffer at the indicated times. Shown are individual calcium transients from three experiments using three different cell preparations.
AAs that activate the CaSR are also known to act by allosteric mechanisms to increase the sensitivity of the receptor to extracellular calcium, thereby potentiating intracellular calcium mobilization. Aromatic AA effects on intracellular calcium were dependent on extracellular calcium (2G) since phenylalanine at a concentration of 100 μM failed to increase intracellular calcium in the nominal absence of calcium in the medium. Interestingly, as shown in Figure 2 H and I, when extracellular calcium concentrations are increased from 1 (H) to 2 mM (I), the profile of the calcium transient is altered, possibly because elevation of extracellular calcium concentrations itself activates phospholipase C which further elevates intracellular calcium.
Aromatic Amino Acids Stimulate ERK phosphorylation
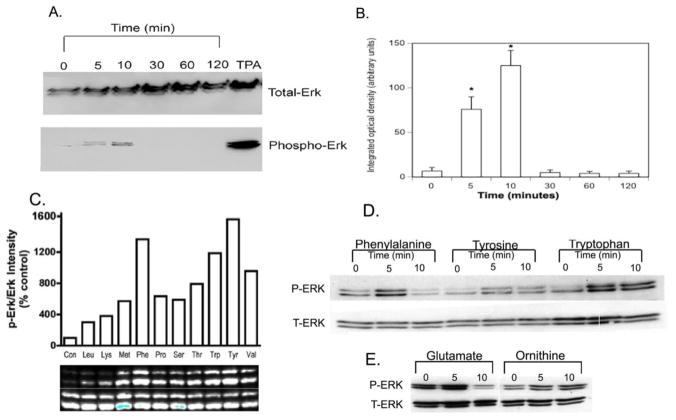
AA binding to the CaSR is known to activate the ERK1/2 signaling pathway(19). The ERKs are members of the mitogen-activated protein kinase (MAPK) family and are known to play an important role in transducing extracellular proliferation signals. When phosphorylated, ERKs are activated and can phosphorylate transcription factors. Thus, effects of AAs on ERK phosphorylation/activation were examined next. Initially meat extract (Sigman-Aldrich, St. Louis, MO) an AA mixture was used to to stimulate ERK phosphorylation. As shown in figure 3A, time course experiments demonstrated that a 0.3% meat extract solution did, indeed, increase ERK phosphorylation with peak phosphorylation occurring at ~10 min post stimulation and declining thereafter. An amino acid mixture at a concentration of 0.3x AA was used because initial experiments determined this to be an effective concentration (data not shown). The changes in ERK phosphorylation were quantified by densitometry (Figure 3B) and demonstrated a significant rise at 5 minutes, continuing at 10 minutes, and returning to baseline by 30 minutes. This pattern of rapid, transient ERK phosphorylation has been described for a wide range of agonists including growth factors such as EGF and proteases such as urokinase type plasminogen activator(20). Thus, our preliminary findings are consistent with AAs selectively activating specific signaling cascades (MAPK/ERK) in BMSCs. To further test the ability of AAs to stimulate ERK phosphorylation individual AAs were then tested (Figure 3C). Interestingly the time course of ERK phosphorylation varied among the AAs (Figure 3D). Glutamate, which activates the T1R1 receptor (500 μM) induced transient ERK phosphorylation that peaked within five minutes (Figure 3E). In contrast, ornithine (500 μM), which activates GPRC6A, had no effect (Figure 3E).
Figure 3. Effects of amino acids on ERK phosphorylation.
Panels A and B: Western blot demonstrating the time course of ERK phosphorylation elicited by meat extract a mixture of amino acids. BMSCs from C57BL/6 mice were grown in 60 mm2 dishes in DMEM + 10% fetal calf serum to ~ 80% confluency, then switched to KRB buffer +0.2% BSA. After an overnight incubation, cells were stimulated with a 0.3% meat extract for the indicated times. Cellular protein was extracted and 50 μg/lane was separated by electrophoresis on SDS-PAGE gels. For Western blotting with ECL detection, antibodies directed against both total ERK and phospho-ERK were utilized. Primary (1:750, 4°C, overnight) and HRP-coupled secondary (1:2000, room temperature, 1 hour) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Phorbol ester (TPA, 1 μM, 10 min) was used as a positive control. (A) Representative blot. (B) Quantitation by densitometric analysis of six different experiments from three different cell preparations. Values are means±SEMs. *P<0.001.
Panels C–E: Comparison of time courses for phenylalanine and glutamate-stimulated ERK phosphorylation. BMSCs from C57BL/6 mice were stimulated with phenylalanine (500 μM) to activate CaSR or glutamate (500 μM) to activate T1R1/T1R3. Samples were analyzed for ERK phosphorylation by Western blotting as described above. There was no response to ornithine (500 μM), which activates GPRC6A. Images are representative blots from six independent experiments.
While low dietary protein content negatively impacts bone mass in the aging animal, high dietary protein is not beneficial
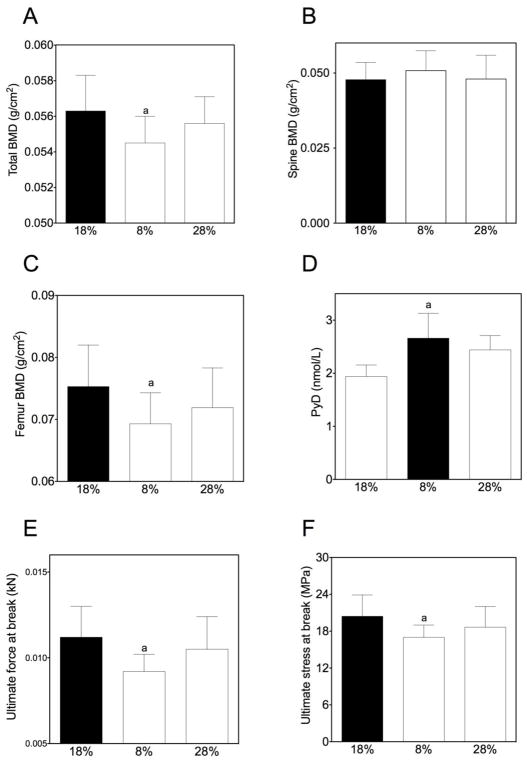
Our in vitro data suggested that alterations in dietary protein (e.g. AA composition and levels) could impact bone formation in vivo. We have previously shown that low dietary protein negatively impacts bone mass in aged mice but has no impact on bone mass in mature mice(4). This would suggest that increasing dietary protein might have a beneficial effect on bone mass. To address this question we tested the impact of high and low dietary protein diets on 18 month-old mice. Standard dietary protein is 18 grams of protein/100 g chow, low protein is 8 g/100g and high protein is 28 g/100 g, prepared by Dr. Barbara Mickelson (Harlan Teklad). A previously characterized C57BL/6 aging mouse model (21) was used for these experiments. Ten mice, 18 months of age, were placed on low, high or standard protein diets for eight weeks (ending at age 20 months). Measurements were performed at the beginning and end of the study period (except where noted) as follows: 1) bone density and body composition were measured by DXA; 2) blood was collected for measurement of markers of bone turnover; and 3) after sacrifice, bones were used for biomechanical testing. We were initially concerned that a high dietary protein might lead to weight loss, but no significant differences in body weight, lean or fat body mass were found between the groups (data not shown). Similar to our previous findings, low dietary protein content resulted in a significantly lower bone mass (Figure 4A, C), higher bone breakdown (Pyd; Figure 4D) and weaker bones (Figure 4 E, F). However, mice fed the higher dietary content did not have a significant change in bone mass (Figure 4B).
Figure 4. A low-protein diet negatively impacted bone density.
C57BL/6 mice fed a low dietary protein diet had lower total and femoral bone mass (Panels A, C) and higher levels of a marker of bone breakdown, Pyd (Panel D). The bones from these mice were also weaker with lower ultimate force at break (Panel E) and ultimate stress at break (Panel F; measurements are expressed as the means ± SEM, n=10 mice/condition, a=p<0.05).
Selective AA replacement blunts or enhances the negative impact of low dietary protein on bone mass in the aging animal
We have previously reported that individual AAs varied in their potency for stimulation of osteoclastic activity(22). We have also reported that some AAs (e.g. aromatic) have greater anabolic effects than other AAs in vitro (17,18). These data suggest that some AAs could negatively impact bone mass while others could be anabolic. Thus, in the next set of experiments we placed mice on a low-protein diet and then selectively restored AAs to the diet to the same level as if they were ingesting 18% dietary protein. AA combinations selected were either the triad of serine, valine and threonine (SVT) or phenylalanine, tyrosine and tryptophan (PTT). The former triad had significant stimulatory potency in the osteoclastic assay and the latter triad were the aromatic AAs. As shown in Figure 5, neither AA triad impacted animal weight or body composition. In contrast, the SVT triad negatively impacted bone mass compared to the low dietary protein by itself. In contrast diets supplemented with aromatic AAs did not impact bone mass compared to mice receiving the standard 18% dietary protein.
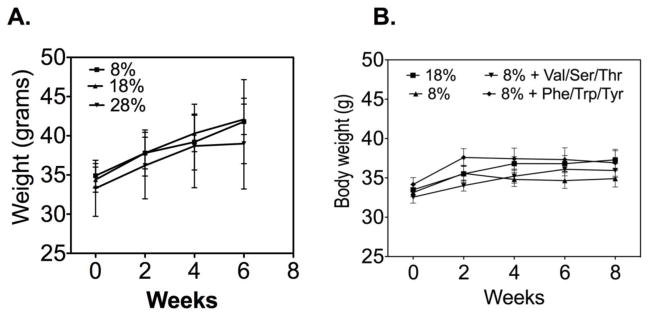
Figure 5. A high protein diet or low-protein diet with or without additional amino acid supplementation had no effect on body weight.
C57BL/6 mice were fed either a high protein (28%), standard protein (18%) (Panel A) or a low-protein (8%), low-protein with supplemented serine, valine or threonine (SVT) or low-protein supplemented with phenylalanine, tyrosine or tryptophan (PTT) diets (Panel B) for eight weeks. Neither the dietary protein content or AA supplements had any significant impact on body weight (measurements are expressed as the means ± SEM, n=10 mice/condition).
DISCUSSION
Taken together, our data demonstrate that BMSCs express specific transporters and extracellular receptors for AAs suggesting that they are important signaling molecules for normal cell function. Decreased AA concentration in the culture medium (as in medium 199) leads to BMSC death even if additional fetal calf serum (up to 20%) is added to the medium. However, if BMSCs are kept in medium 199 supplemented with additional AAs, the cells now survive and proliferate. Further, binding of aromatic (but not aliphatic) AAs leads to an increase in intracellular calcium. In addition, increasing extracellular calcium results in enhanced changes in Fura-2 fluorescence, consistent with an effect mediated through the CaSR. Nevertheless, our experiments do not prove that this effect is not mediated through the GPRC6A receptor or a closely related receptor. It is also possible that receptor expression can vary depending on the differentiation state of the BMSCs with GPRC6A becoming a more important signaling pathway once the BMSCs commit to the osteoblastic pathway. Once binding of the AA occurs, the AA stimulates (to varying degrees) an increase in ERK phosphorylation/activation. Differences in the time course of activation and duration of ERK activation could result in different patterns of osteoblastic proliferation/differentiation depending on the mixture of amino acids present in the particular type of protein ingested. Moreover, stimulation of ERK phosphorylation by glutamate is consistent with umami taste receptors being present in BMSCs, as has been shown by others(23,24). Work by a number of investigators suggests that these receptors play a role as cellular energy sensors, and activation or knocking out of these receptors can alter both adiposity and bone mass in vivo(15,23,25). How these taste receptors interact with other AA sensors on BMSCs is not known. However these receptors have also been recently shown to be present in osteoclasts suggesting a role in bone turnover(26).
The in vivo studies focused on the potential clinical significance of AA composition on bone mass. We have previously shown that a low dietary protein resulta in bone loss in the aged but not the young mouse(4). Further, our previous publications reported that some AAs promote osteoblastic function while others promote osteoclastic activity(17,18,22). Based on these in vitro data we added back selective AA to the low-protein diet provided to aging C57BL/6 mice. The AAs were supplemented only to levels equivalent to an 18% dietary protein level (i.e., these AAs were not added in excess of those they normally ingest with an 18% protein diet). Our data demonstrated that if certain AAs are added to a low protein diet they induce further drops in bone mass (SVT); however, other AA groups (PTT) revert the negative effect of low dietary protein. These data support the specificity of individual AA induced cellular responses and suggest that when dietary protein is ingested, a mixture of AAs are taken in, with some causing beneficial effects on bone mass and others promoting bone breakdown. The net effect depends on the balance of the various AAs in the dietary protein and this composition varies according to the source of the protein. Dietary protein restriction has been shown to be a countermeasure effective in extending life span. However, restricting dietary protein in the aged organism can negatively impact the musculoskeletal system. These studies raise the possibility that dietary protein restriction with selective AA supplementation might be an effective strategy to obtain the lifespan benefits of a low protein diet without the negative effects on bone.
In summary, the present study demonstrates that BMSCs respond to AAs as specific signaling molecules by increasing intracellular calcium and ERK phosphorylation/activation. These data also suggest that the impact of AAs is most noticeable with age and thus are consistent with an age-dependent loss of nutrient sensing. However, our data indicates that age related loss of nutrient sensing can be modulated by selective AA replacement. Finally, these data suggest that perhaps AAs should not simply be viewed as nutrients but also as selective signaling molecules.
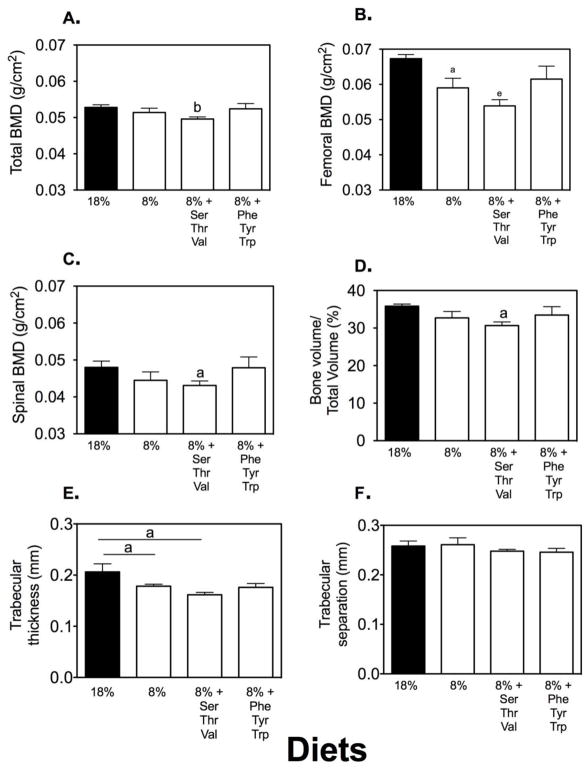
Figure 6. Supplemental SVT but not PTT negatively impacted bone mass.
C57BL/6 mice fed a low-protein diet had a decreased femoral bone mass (Panel B) as measured by DXA. This decrease in BMD was enhanced by addition of SVT but blunted by addition of PTT. By micro-CT, BV/TV showed a similar decrease in response to low dietary protein and this was further enhanced by SVT but reversed by PTT. Shown are means ± SEM (a=p<0.05; b=p<0.01, e=p<0.001, n=10/condition).
Acknowledgments
Funding Sources: This work was supported by a program project award from the National Institutes on Aging (P01AG036675 to CMI) and in part by 1I01CX000930 from the Department of Veterans Affairs (WDH). The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
Funding Sources: This work was supported by funding from the National Institutes of Aging (P01AG036675).
Footnotes
All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaffney-Stomberg E, Insogna KL, Rodriguez NR, Kerstetter JE. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J Am Geriatr Soc. 2009;57(6):1073–9. doi: 10.1111/j.1532-5415.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 2.Kerstetter JE, O’Brien K, Insogna K. Dietary protein and intestinal calcium absorption. Am J Clin Nutr. 2001;73(5):990–2. doi: 10.1093/ajcn/73.5.990. [DOI] [PubMed] [Google Scholar]
- 3.Kerstetter JE, Bihuniak JD, Brindisi J, et al. The Effect of a Whey Protein Supplement on Bone Mass in Older Caucasian Adults. J Clin Endocrinol Metab. 2015;100(6):2214–22. doi: 10.1210/jc.2014-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Refaey ME, McGee-Lawrence ME, Fulzele S, et al. Kynurenine, a Tryptophan Metabolite That Accumulates With Age, Induces Bone Loss. J Bone Miner Res. 2017;32(11):2182–93. doi: 10.1002/jbmr.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conigrave AD, Brown EM, Rizzoli R. Dietary protein and bone health: roles of amino acid-sensing receptors in the control of calcium metabolism and bone homeostasis. Annu Rev Nutr. 2008;28:131–55. doi: 10.1146/annurev.nutr.28.061807.155328. [DOI] [PubMed] [Google Scholar]
- 6.Pi M, Chen L, Huang MZ, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008;3(12):e3858. doi: 10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pi M, Nishimoto SK, Quarles LD. GPRC6A: Jack of all metabolism (or master of none) Mol Metab. 2017;6(2):185–93. doi: 10.1016/j.molmet.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26(7):1680–3. doi: 10.1002/jbmr.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pi M, Zhang L, Lei SF, et al. Impaired osteoblast function in GPRC6A null mice. J Bone Miner Res. 2010;25(5):1092–102. doi: 10.1359/jbmr.091037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Ou G, Hamrick M, et al. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J Bone Miner Res. 2008;23(7):1118–28. doi: 10.1359/JBMR.080304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103(5):1662–8. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 12.Xie D, Cheng H, Hamrick M, et al. Glucose-dependent insulinotropic polypeptide receptor knockout mice have altered bone turnover. Bone. 2005;37(6):759–69. doi: 10.1016/j.bone.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Rudman D, Abbasi AA, Chaudry F, Mattson DE. Delayed plasma clearance of phenylalanine and tyrosine in elderly men. J Am Geriatr Soc. 1991;39(1):33–8. doi: 10.1111/j.1532-5415.1991.tb05903.x. [DOI] [PubMed] [Google Scholar]
- 14.Conigrave AD, Franks AH, Brown EM, Quinn SJ. L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur J Clin Nutr. 2002;56(11):1072–80. doi: 10.1038/sj.ejcn.1601463. [DOI] [PubMed] [Google Scholar]
- 15.Kokabu S, Lowery JW, Toyono T, Sato T, Yoda T. On the Emerging Role of the Taste Receptor Type 1 (T1R) Family of Nutrient-Sensors in the Musculoskeletal System. Molecules. 2017;22(3) doi: 10.3390/molecules22030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conigrave AD, Hampson DR. Broad-spectrum L-amino acid sensing by class 3 G-protein-coupled receptors. Trends Endocrinol Metab. 2006;17(10):398–407. doi: 10.1016/j.tem.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 17.El Refaey M, Watkins CP, Kennedy EJ, et al. Oxidation of the aromatic amino acids tryptophan and tyrosine disrupts their anabolic effects on bone marrow mesenchymal stem cells. Mol Cell Endocrinol. 2015 doi: 10.1016/j.mce.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Refaey M, Zhong Q, Hill WD, et al. Aromatic amino Acid activation of signaling pathways in bone marrow mesenchymal stem cells depends on oxygen tension. PLoS One. 2014;9(4):e91108. doi: 10.1371/journal.pone.0091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HJ, Mun HC, Lewis NC, et al. Allosteric activation of the extracellular Ca2+-sensing receptor by L-amino acids enhances ERK1/2 phosphorylation. Biochem J. 2007;404(1):141–9. doi: 10.1042/BJ20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi T, Kar S, Wang M, Carr BI. Transient and sustained ERK phosphorylation and nuclear translocation in growth control. J Cell Physiol. 2002;192(2):151–9. doi: 10.1002/jcp.10124. [DOI] [PubMed] [Google Scholar]
- 21.Hamrick MW, Ding KH, Pennington C, et al. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006;39(4):845–53. doi: 10.1016/j.bone.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Refaey ME, Zhong Q, Ding KH, et al. Impact of dietary aromatic amino acids on osteoclastic activity. Calcif Tissue Int. 2014;95(2):174–82. doi: 10.1007/s00223-014-9878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon BR, Learman BS, Parlee SD, et al. Sweet taste receptor deficient mice have decreased adiposity and increased bone mass. PLoS One. 2014;9(1):e86454. doi: 10.1371/journal.pone.0086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon BR, Parlee SD, Learman BS, et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem. 2013;288(45):32475–89. doi: 10.1074/jbc.M113.514034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaton MS, Weinstein N, Newby JB, et al. Loss of the nutrient sensor TAS1R3 leads to reduced bone resorption. J Physiol Biochem. 2017 doi: 10.1007/s13105-017-0596-7. [DOI] [PubMed] [Google Scholar]
- 26.Eaton MS, Weinstein N, Newby JB, et al. Loss of the nutrient sensor TAS1R3 leads to reduced bone resorption. J Physiol Biochem. 2018;74(1):3–8. doi: 10.1007/s13105-017-0596-7. [DOI] [PubMed] [Google Scholar]