Abstract
OBJECTIVES
The present study evaluated the relationship between the 2011 American College of Rheumatology fibromyalgia (FM) survey criteria and quantitative sensory testing (QST).
METHODS
Patients with knee osteoarthritis scheduled to undergo knee arthroplasty completed the FM survey criteria and self-report measures assessing clinical symptoms. Patients also underwent a battery of QST procedures at the surgical knee and remote body sites, including pressure algometry, conditioned pain modulation (CPM), and temporal summation (TS). All assessments were completed prior to surgery. FM survey criteria were used to calculate a continuous FM score indicating FM severity.
RESULTS
129 patients were analyzed. Of these, 52.7% were female, 93.8% were Caucasian, and 3.8% met the FM survey criteria for FM classification. Mean age for females (63.57 years) and males (64.74 years) was similar. Females and males differed significantly in nearly every outcome, including FM severity, clinical pain, anxiety, depression, and pressure pain sensitivity. In females, FM scores significantly correlated with pressure pain sensitivity, but not CPM or TS, such that increased sensitivity was associated with greater FM severity at all body sites examined. Additionally, as FM scores increased, the association between pain sensitivity at the surgical knee and that at remote body sites also increased. No relationship between FM score and QST was observed in males.
DISCUSSION
We demonstrated an association between diffuse hyperalgesia as measured by QST and FM severity in females with knee osteoarthritis. These results suggest that the FM survey criteria may represent a marker of pain centralization in females with potential utility in clinical decision making.
Keywords: Osteoarthritis, Fibromyalgia, Chronic Pain, Centralized Pain, Quantitative Sensory Testing
Introduction
The construct of “centralized pain” refers to central nervous system mechanisms associated with augmented pain and sensory processing. Clinically, it manifests as widespread chronic pain often independent of nociceptive input, combined with fatigue, sleep, cognitive, and mood problems 1. Laboratory-based quantitative sensory testing (QST) has shown that individuals with centralized pain exhibit global hypersensitivity to painful and non-painful sensory stimulation,2,3 facilitated temporal summation (TS) of pain,4 and decreased endogenous analgesia5 compared to pain-free controls. Neuroimaging studies in centralized pain states substantiate these findings showing evidence of augmented sensory-evoked activity,6,7 as well as alterations in connectivity, structure, and neurochemistry in pain processing brain regions.8,9 Individuals with centralized pain respond preferentially to centrally acting treatments (tricyclics, serotonin-norepinephrine reuptake inhibitors, gabapentinoids, cognitive behavioral therapy) and show poor response to opioids and peripheral interventions such as surgery.10-13
Fibromyalgia (FM) is the prototypical centralized pain condition.1 FM was previously diagnosed on the presence of widespread pain at multiple discrete “tender points.” 14 In 2010, the American College of Rheumatology (ACR) released new diagnostic criteria for FM that eliminated tender point palpation but added assessments of somatic symptoms and pain distribution.15 A modified version of self-report criteria for research that did not require physician interview was released in 2011.16 The 2011 criteria can be used in a dichotomous manner for FM classification or as a continuous “FM score,” with higher scores indicating increased symptom severity and more painful body sites.
We previously reported that FM scores predicted perioperative and postsurgical outcomes in patients with pain conditions other than FM. For every 1-point increase in FM score, there was an adjusted 7–9 mg oral morphine equivalent increase in opioid requirement during the inpatient postoperative admission,13 and an adjusted 18% increased risk of failing to meet the threshold for surgical pain improvement.12 FM scores also predicted postoperative opioid consumption in females with chronic pelvic pain undergoing hysterectomy with a similar effect size.12
That the FM survey criteria was independently associated with suboptimal analgesic responses to opioids and peripheral inventions suggests that it may represent a marker of centralized pain, with potential utility across various chronic pain conditions. The present study sought to provide convergent validity for this hypothesis by examining the relationship between FM scores and QST. Patients with knee osteoarthritis (OA) scheduled for total knee arthroplasty (TKA) underwent QST prior to surgery. We hypothesized that QST outcomes would be associated with FM scores, with higher FM scores corresponding to increased pressure pain sensitivity at multiple body sites and dysfunctional pain modulation measured by temporal summation (TS) and conditioned pain modulation (CPM). We further hypothesized that pain sensitivity at the surgical knee and at remote body sites would be more strongly associated in OA patients with higher FM scores.
Materials and Methods
Approval was attained from the University of Michigan Institutional Review Board. Our reporting conforms to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement.
Study Participants
Patients ≥ 18 years old scheduled for TKA at the University of Michigan were recruited from a knee surgery informational workshop between January 17, 2012 and August 25, 2016, as a subset of the parent Analgesic Outcomes Study (AOS).12,13 AOS light phenotyping consisted of a battery of self-report measures acquired on the day of surgery. Patients in the present study enrolled in an additional deep phenotyping visit of QST completed within 1 month prior to surgery (i.e. these patients completed both light and deep phenotyping protocols).
Exclusion criteria included: severe physical impairment (e.g. bilateral amputation, blindness, or deafness); severe psychiatric illnesses (e.g. schizophrenia, major depression, suicidality, psychotic features, or substance abuse within two years); alcohol consumption exceeding 7 drinks/week for females or 14 drinks/week for males within 6 months of screening; treatment with an investigational drug within 30 days prior to testing; analgesic consumption within 8 hours of QST; if the principal investigator determined that the patient was unlikely to be able to complete the study; previous TKA; current pregnancy; current incarceration; life expectancy < 1 year; and inability to provide written informed consent.
Light Phenotyping
Fibromyalgia Survey Criteria
The 2011 FM survey criteria,16 is comprised of the Widespread Pain Index (WPI) and the Symptom Severity score (SS score). The WPI (extrapolated from the Michigan Body Map17) assesses 19 body locations to determine the spatial distribution of pain in the past week, with scores ranging 0–19. The SS score evaluates the severity of three cardinal FM symptoms (fatigue, trouble thinking or remembering, and waking up tired/unrefreshed), as well as the presence of three additional somatic symptoms (pain or cramps in lower abdomen, depression, and headache). The SS score ranges 0–12. The WPI and SS score are combined to calculate an overall index of FM severity score (referred to here as the “FM score”) ranging 0–31. A score of ≥ 13 is required for FM positive classification.
Brief Pain Inventory (BPI) measures clinical pain using a 4-item subscale assessing overall body pain at its “worst,” “least,” “current,” and “average” in the last week, each on scale of 0-10.18 A composite measure of pain severity is computed from the average of the above four items. We also administered a modified version that focused on pain at the surgical knee, ignoring the rest of the body.
Western Ontario and McMaster Universities Arthritis Index (WOMAC) is a 24-item survey with pain, stiffness, and function subscales for patients with knee or hip OA.19 The pain subscale ranges 0–20, with higher values indicating greater pain. The stiffness subscale ranges 0–8, with higher values indicating greater joint stiffness. The function subscale ranges 0–68, with higher values indicating greater functional impairment. The total score is the sum total of the above three subscales.
PainDETECT is used to screen for neuropathic pain.20 Total score ranges from 0-38. Higher scores indicate an increasing likelihood of a neuropathic component, with scores > 18 suggesting a neuropathic component to the patient’s pain is likely.
Coping Strategies Questionnaire (CSQ) contains a catastrophizing subscale that is used to measure a patient’s ability to cope with their pain and the extent to which they view their pain as threatening.21 It contains six items with a total score of 0–36. Higher scores indicate greater pain catastrophizing.
Hospital Anxiety and Depression Scale (HADS) is composed of depression (0–21), anxiety (0–21), and positive affect (0–18) subscales.22 Lower scores indicate less depression and anxiety and a more positive affect.
Quantitative Sensory Testing (QST)
Patients completed deep AOS QST phenotyping within one month prior to surgery. Patients were read scripted instructions and completed practice testing before data collection to reduce testing-related anxiety.
Pressure Pain Sensitivity
Pressure pain sensitivity was assessed at the thumbnail following well-described procedures23,24 using the Multimodal Automated Sensory Testing (MAST) system (Arbor Medical Innovations, Ann Arbor, MI).25 The MAST system delivered computer-controlled pressure to the thumbnail with a mechanically-driven 1 cm2 rubber-tipped probe. A series of 5-s pressures were delivered in ascending order to the dominant thumbnail at 4 kgf/cm2/s, starting at 0.50 kgf/cm2 and increasing in 0.50 kgf/cm2 steps (20-s inter-stimulus interval). Patients rated pain intensity after each stimulus using a digital 0–100 numerical rating scale (NRS) displayed on a touchscreen (0 = no pain; 100 = worst pain imaginable). The test was completed when: 1) the patient reached his/her pain tolerance, 2) the patient reported a pain intensity of ≥ 80/100, or 3) a maximum possible pressure of 10 kgf/cm2 was delivered. Data were fit to a linear model, from which Pain50—defined as the pressure that evoked a moderate amount pain (i.e. 50/100)—was interpolated. Additional derived variables include: 1) pressure pain threshold (PPT), which is the first pressure in a series of at least two consecutive pressures that produced a NRS > 0; and 2) pressure pain tolerance, defined as the last pressure collected.
PPTs were also assessed bilaterally using a hand-held algometer with a 1-cm2 flat rubber probe (FPX 50, Wagner Instruments, Greenwich, CT) at the knee scheduled for TKA (surgical knee), the contralateral knee (non-surgical knee), and several sites distant from the knees. Knees were stimulated 2 cm lateral to the midpoint of the lateral edge of the patella, a site previously studied in knee OA patients.26 A site on the lower leg lateral to the tibial tuberosity and anterior to the tibial crest was used to assess secondary hyperalgesia relative to the knees. The wrist, serving as a secondary joint site, was tested at the first metacarpophalangeal joint. The midpoint of the upper trapezius was tested as a remote muscle site. All patients were tested at all sites in the same order, counter-balanced between the left and right body side. Pressure was applied manually at a rate of 0.5 kgf/cm2/s until the patient indicated an initial sensation of pain, with this being recorded as the PPT. Pressures did not exceed 10 kgf/cm2. This procedure was repeated three times at each location with the mean PPT used for analysis.
Conditioned Pain Modulation (CPM)
CPM was performed according to validated methods.24,27 Pressures were delivered via two MAST actuators positioned on the dominant thumb as a test stimulus and the non-dominant thumb as a conditioning stimulus. Continuous test pressure was applied for 30-s at an intensity individually calibrated for each patient to evoke a moderate level of pain (Pain30–50). Patients rated the pain intensity of the pressure 3 times, 10-s apart (i.e. at time 10, 20, and 30-s) using a 0–100 NRS. CPM was induced 10-15 minutes later by applying 60-s of continuous Pain30–50 pressure to the non-dominant thumbnail. Parallel to the last 30-s of conditioning, the test stimulus was reapplied to the dominant thumbnail for 30-s and rated 3 times, 10-s apart (corresponding to the 40, 50, and 60-s time points of the conditioning stimulus). CPM magnitude was calculated as the difference between the mean of the pain ratings given to the test stimulus prior to the conditioning stimuli and the mean of the pain ratings of the test stimulus given during the conditioning stimulus. Negative values imply intact inhibitory CPM; positive values reflect deficient CPM.
Temporal Summation (TS)
Twelve pressure stimuli of 1-s duration and equal intensity were delivered in succession to the thumbnail at 1-s intervals using the MAST system. Stimulus intensity was individually calibrated to be 20% above the patient’s thumbnail PPT. Patients rated their pain on a 0–100 NRS following each stimulus presentation. The pain rating of the first stimulus was subtracted from the 12th and final stimulus to calculate TS scores, where larger numbers indicate increased pain summation.
Statistical Analysis
Patients without complete FM survey criteria were excluded from analysis. Missing item-level data in all other self-report measures were handled according to instructions provided by the author(s) of each instrument. When individual self-report measures were missing in their entirety or could not be scored due to excessive missing items, those measures were not analyzed but all other usable data from that patient remained in the analysis. Technical errors, procedural deviations, and patient refusal to complete portions of the QST battery resulted in missing QST data in some analyses.
Continuous measures are reported as mean ± standard deviation, and categorical variables are reported as frequency (%). Normality was assessed via histograms and q-q plots; nonparametric statistics were used when violations in normality were detected. Bivariate analyses were conducted to determine significant differences between females and males; chi-square tests (X2) and independent samples t-tests were used for categorical and continuous variables, respectively. Associations between FM score and self-report measures were assessed by Pearson correlation. The relationship between FM score and QST was examined using Pearson partial correlations adjusted for age28 and conducted separately for females and males. The a priori decision to stratify the analysis by sex was based on considerable literature demonstrating significant female-male differences in pain processing and FM, with females generally demonstrating increased clinical and experimental pain,29,30 and a higher prevalence of FM.31,32 Preliminary visual analysis of the present data also showed increased FM severity in females and a different distribution of FM scores between sexes. Significant correlations were further explored by subgrouping participants into FM score tertiles, consistent with previous assessments of this instrument: Females (Low = 0–4; Moderate = 5–8; High = 9–20) and Males (Low = 0–2; Moderate = 3–5; High = 6–16).11-13 These cut-points were calculated from this cohort’s distribution of FM scores. Analysis of covariance (ANCOVA) adjusted for age was used to assess for significant differences in QST outcomes between FM score tertiles. Planned comparisons (unadjusted) assessed for differences in estimated marginal means between QST outcomes at each tertile. Spearman’s rho was used to examine the relationship between PPT at the surgical knee and PPT at other body sites across FM score tertiles. Statistical analysis was conducted using IBM SPSS version 24 (IBM Corp, Armonk, NY). All analyses were two-tailed with significance set at p < 0.05.
Results
Participant Demographics
187 patients were consented for deep phenotyping (Figure 1). Cancelled surgeries, patient withdrawals, and missing data resulted in a final dataset of 129 (68 female; 61 male) patients (Table 1). They were primarily Caucasian and older (females 63.57 ± 8.62 years; males 64.74 ± 8.68 years). Males were more likely to be Caucasian than females (p = 0.044). There were no significant differences in FM positive classification between sexes.
Figure 1.
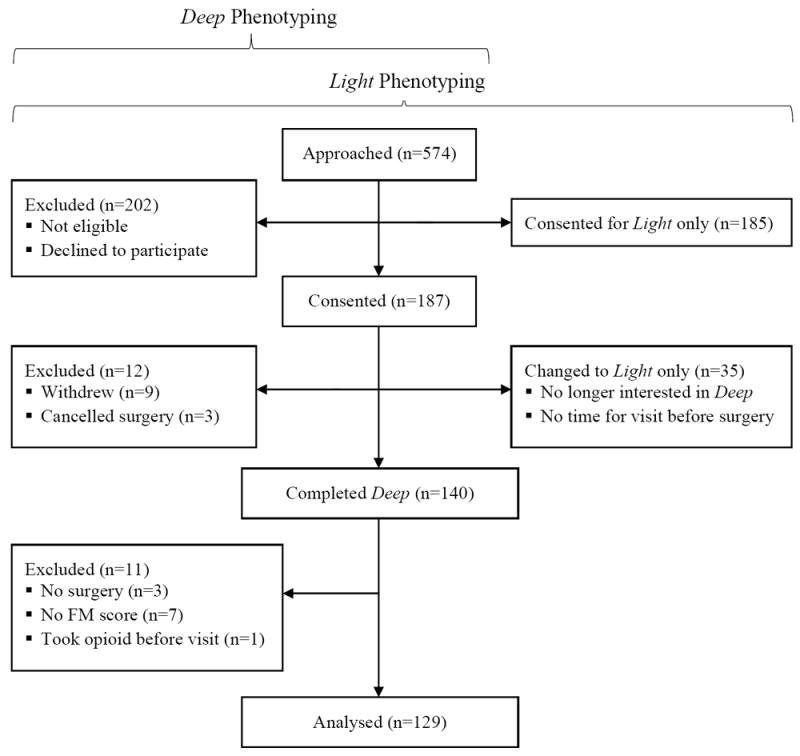
CONSORT flow diagram of patient enrollment patterns. Patients analyzed here completed both baseline Light phenotyping as well as Deep phenotyping, which included quantitative sensory testing.
Table 1.
Patient demographics and self-report measures presented as mean (standard deviation)
| Females (n = 68) | Males (n = 61) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age | 63.57 (8.62) | 64.74 (8.68) | 0.447 |
| Ethnicity (% Hispanic) | 0 | 3.3 | 0.132 |
| Race (% Caucasian) | 89.7 | 98.3 | 0.044 |
| Opioids (% currently taking) | 32.4 | 26.2 | 0.446 |
| Oral Morphine Equivalents | 15.93 (16.40) | 142.06 (428.57) | 0.291 |
|
| |||
| Clinical Pain Measures | |||
| FM score | 6.88 (4.12) | 4.67 (3.31) | 0.001 |
| FM (% meeting criteria) | 5.9 | 1.6 | 0.213 |
| BPI (overall body pain) | 4.74 (2.08) | 3.94 (1.72) | 0.020 |
| BPI (surgical knee pain) | 4.83 (2.06) | 4.14 (1.61) | 0.042 |
| WOMAC Pain | 11.25 (3.03) | 9.71 (3.09) | 0.009 |
| WOMAC Stiffness | 5.02 (1.57) | 4.39 (1.82) | 0.043 |
| WOMAC Function | 35.94 (11.05) | 32.03 (10.93) | 0.053 |
| WOMAC Total | 52.13 (14.47) | 46.16 (14.86) | 0.027 |
| painDETECT | 11.53 (6.18) | 10.18 (5.98) | 0.235 |
|
| |||
| Psychological Measures | |||
| CSQ Catastrophizing | 5.62 (4.81) | 3.82 (4.57) | 0.041 |
| HADS Anxiety | 6.71 (4.41) | 4.83 (4.35) | 0.019 |
| HADS Depression | 5.13 (3.76) | 3.85 (3.25) | 0.046 |
| HADS Positive Affect | 14.56 (3.13) | 15.15 (2.95) | 0.285 |
FM = Fibromyalgia; BPI = Brief Pain Inventory; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; CSQ= Coping Strategies Questionnaire; HADS = Hospital Anxiety and Depression Scale
Sex Differences in Clinical Pain and Symptoms
Females had significantly higher FM scores (p = 0.001), overall body pain (p = 0.020), and pain at their surgical knee (p = 0.042) compared to males as measured by the BPI (Table 1). Females had significantly higher pain (p = 0.009), stiffness (p = 0.043), and total scores (p = 0.027) on the WOMAC. Females reported greater pain catastrophizing (p = 0.041), anxiety (p = 0.019) and depression (p = 0.046) as measured by HADS.
Sex Differences in QST
Compared to males, females showed significantly greater suprathreshold pressure pain sensitivity at the thumbnail as demonstrated by lower Pain50 (p = 0.017) and tolerance (p = 0.001) values (see table, Supplemental Digital Content 1). Additionally, females were significantly more sensitive at all locations measured by hand-held algometry (all p ≤ 0.013; see table, Supplemental Digital Content 1). PPTs at the lower leg and trapezius showed no difference between left and right sides of the body in either sex (paired t-tests, all p > 0.05). Laterality in wrist PPT was detected in males (p = 0.022), but not females (p = 0.339). Mean CPM and TS values were positive in males and females, indicating deficient pain inhibition and the presence of pain facilitation at the group level; however, no sex differences were found for either measure: CPM (p = 0.306), TS (p = 0.334).
Correlation between FM Score and Symptoms
In females, FM scores correlated significantly with all symptom measures (all p ≤ 0.037) except BPI (see table, Supplemental Digital Content 2). FM scores correlated with all symptom measures in males (all p ≤ 0.043).
Correlation between FM score and QST
In females, FM scores correlated with all measures of pressure pain sensitivity (all |r| ≥ 0.27, all p ≤ 0.021) except thumbnail PPT (Table 2), such that higher FM scores were associated with increased pain sensitivity (i.e. lower thresholds) throughout the body. These correlations remained significant (all |r| ≥ 0.33, all p ≤ 0.015), with the exception of thumbnail pain50 (r = -0.243, p = 0.057) and tolerance (r = -0.224, p = 0.08), after excluding patients that satisfied survey criteria for FM positive classification. A series of secondary partial correlations showed that the relationship between FM Score and QST was substantially unchanged when controlling for symptoms that could potentially influence QST responses, including clinical pain severity (BPI, WOMAC), anxiety, and catastrophizing (Supplemental Digital Content 3).
Table 2.
Partial correlations (r) between pre-surgical fibromyalgia scores and quantitative sensory testing outcomes
| Females | P-value | Males | P-value | |
|---|---|---|---|---|
| Pain Modulation | ||||
| Temporal Summation | 0.22 | 0.078 | 0.03 | 0.820 |
| Conditioned Pain Modulation | 0.01 | 0.945 | 0.02 | 0.871 |
|
| ||||
| Pressure Pain Sensitivity (kgf/cm2) | ||||
| Thumbnail PPT (dominant) | -0.18 | 0.144 | -0.11 | 0.397 |
| Thumbnail Pain50 (dominant) | -0.31 | 0.013 | -0.02 | 0.897 |
| Thumbnail Pain Tolerance (dominant) | -0.27 | 0.027 | 0.07 | 0.620 |
| Left Lower Leg PPT | -0.38 | 0.004 | 0.02 | 0.889 |
| Right Lower Leg PPT | -0.38 | 0.003 | -0.02 | 0.908 |
| Left Trapezius PPT | -0.36 | 0.006 | -0.15 | 0.347 |
| Right Trapezius PPT | -0.44 | 0.001 | -0.10 | 0.539 |
| Left Wrist PPT | -0.42 | 0.001 | -0.02 | 0.914 |
| Right Wrist PPT | -0.43 | 0.001 | -0.02 | 0.905 |
| Surgical Knee PPT | -0.44 | 0.001 | 0.01 | 0.973 |
| Non-Surgical Knee PPT | -0.33 | 0.013 | 0.02 | 0.887 |
PPT = pressure pain threshold
In addition to static threshold measures of pain sensitivity, females also showed a positive but non-significant correlation between TS and FM score (r = 0.22, p = 0.078), with higher FM scores associated with increased pain facilitation. No QST outcomes correlated with FM score in males (all p > 0.05; Table 2). CPM was not related to FM score in either sex.
Pain Sensitivity across FM Score Tertiles in Females
To further explore the relationship between FM score and QST, females were separated into FM score tertiles (Low, Moderate, and High). This analysis was not conducted in males given their lack of association between FM score and QST (Table 2). Because left- and right-sided PPTs were not statistically different in females, left and right PPTs for each location except the knees were collapsed together for clarity. ANCOVA revealed that female PPTs measured at the surgical knee (p = 0.037), lower leg (p = 0.007), trapezius (p = 0.025), and wrist (p = 0.005) significantly differed between FM tertiles, demonstrating a step-wise increase in pain sensitivity by tertile (Figure. 2). Pairwise comparisons showed that this was primarily driven by the differences between Low and High tertiles (all p ≤ 0.011). ANCOVA also revealed differences in thumbnail Pain50 (p = 0.052) and tolerance (p = 0.058), and non-surgical knee PPT (p = 0.088) across FM tertiles that approached significance, with significant pairwise comparisons between Low and High FM tertiles for Pain50 (p = 0.018), tolerance (p = 0.021), and non-surgical knee PPT (p = 0.030).
Figure 2.
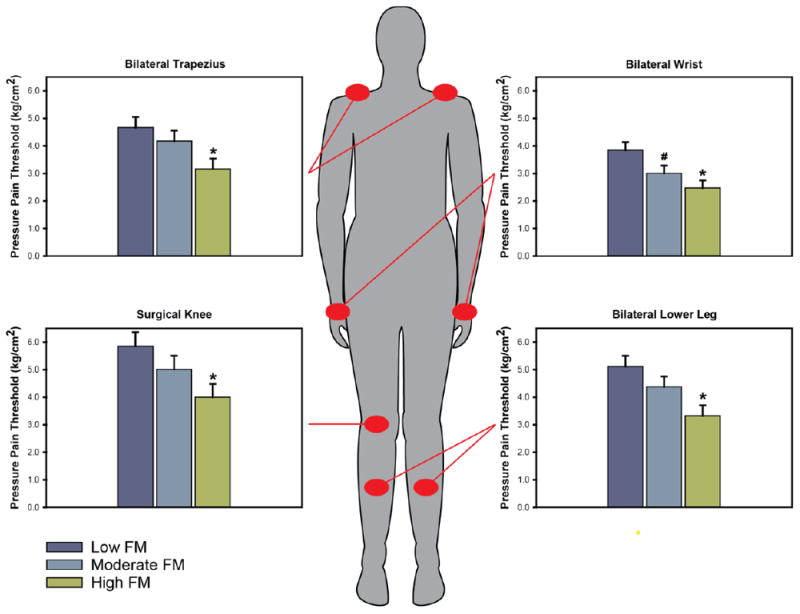
Pressure pain thresholds (PPT) assessed at four body sites exhibit a stepwise increase in pain sensitivity (lower thresholds) across fibromyalgia (FM) score tertiles. * ≤ 0.011 compared to Low FM. # = 0.042 compared to Low FM.
Correlation between Surgical Knee and Remote Body Site Pain Sensitivity in Females
We then examined the correlation between PPT at the surgical knee with PPTs at other body sites. As above, this analysis was not conducted in males given their lack of association between FM score and QST. Results showed that as FM scores increased, the associations between PPT at the surgical knee with PPTs at remote sites became stronger in a step-wise fashion (Figure 3). Spearman’s correlations between surgical knee PPT and bilateral trapezius PPT increased from 0.58 (p = 0.012) to 0.71 (p = 0.001) to 0.78 (p < 0.001) from Low to Moderate to High tertiles, respectively. Likewise, correlations with bilateral wrist increased from 0.40 (p = 0.107) to 0.45 (p = 0.056) to 0.68 (p = 0.001). Lastly, correlations between surgical knee and bilateral lower leg increased from 0.52 (p = 0.029) to 0.54 (p = 0.016) to 0.73 (p < 0.001). The correlation between PPT at the surgical knee and contralateral knee which was also likely affected by OA was consistently strong across FM score tertiles.
Figure 3.
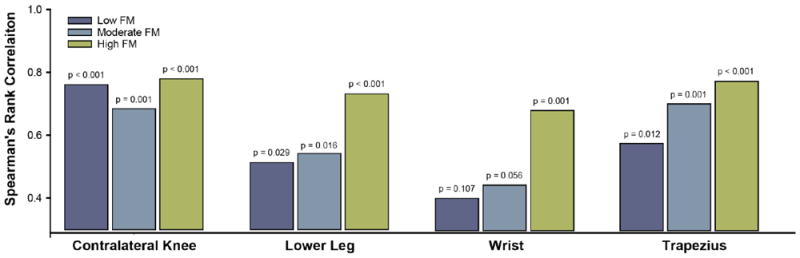
Associations between pressure pain thresholds (PPT) as a function of FM score tertile. Spearman’s correlations between surgical knee PPT and PPTs measured at the lower leg, wrist, and trapezius become stronger as FM score increases.
Missing Data
The number of participants with missing self-report data was: 2 (1.6% of the analyzed n = 129) for ethnicity and race; 5 (3.9%) for HADS depression and positive affect; 6 (4.7%) for HADS anxiety and all WOMAC scales; 7 (5.4%) for painDETECT; 8 (6.2%) for surgical knee BPI; 13 (10.0%) for CSQ catastrophizing. For the following QST outcomes, the number of participants with missing data was: 2 (1.6%) for MAST-derived thumbnail PPT, Pain50, tolerance; 7 (5.4%) for TS; 12 (9.3%) for CPM; and 31 (24.0%) for algometer-derived PPT variables. The high number of missing PPT data was due to algometer malfunction.
Discussion
FM scores were previously shown to predict perioperative and postsurgical pain outcomes,12,13 leading us to suggest that the FM survey criteria may help to identify patients with centralized pain. A critical component of the centralized pain construct is diffuse hyperalgesia measured by QST. In this study, we examined the relationship between FM scores and QST in patients with knee OA. Results showed that, in females only, higher FM scores were associated with increased pressure pain sensitivity at the surgical knee as well as at several remote body sites. This is consistent with other studies showing a relationship between pain sensitivity at distal or asymptomatic sites and OA symptoms.33,34 The present results represent the first demonstration to our knowledge of a relationship between QST and FM scores, providing convergent support that the FM survey criteria may represent a self-report measure of pain centralization.
Pain Centralization in Osteoarthritis
Knee OA is traditionally conceptualized as a peripheral nociceptive pain condition resulting from damage and inflammation in the knee. However, a significant disparity exists between reported pain in OA and identifiable joint pathology on radiographic imaging:35,36 some patients reporting pain have minimal findings on imaging, whereas others have extensive joint pathology on imaging but report little pain, suggesting that at least a subset of knee OA patients have predominantly centralized pain.37 This hypothesis is supported by extensive QST findings demonstrating abnormal pain processing in OA subgroups consistent with centralized pain,26,38,39 including multisite hyperalgesia and impaired endogenous pain modulation. This is corroborated further by neuroimaging data showing altered pain-related processing and morphology in the OA brain.40,41 Here, less than 6% of female OA patients met criteria for FM. To ensure that our findings were not driven by these patients, we performed a secondary analysis using only patients who were subthreshold for being classified as “FM positive.” In agreement with our primary findings, non-FM female patients with higher FM scores also demonstrated increased sensitivity throughout the body, suggesting that the FM score is useful for identifying OA patients with centralized pain regardless of their FM classification.
Sex Differences in Centralized Pain
The association between FM score and QST was only observed in females, suggesting that the FM survey criteria probe a dimension of centralized pain that is unique to females. While not anticipated,33 this finding is supported by a literature demonstrating significant sex differences in clinical and experimental pain,28,30,42,43 and in fMRI studies of activation and connectivity patterns within and between brain regions involved in pain processing.44,45 Consistent with these studies, females here showed higher FM scores, pain intensity, catastrophizing, anxiety, depression, and pressure pain sensitivity compared to males. Importantly, the association between FM score and QST observed in females was not driven by their higher levels of clinical pain and psychological symptoms. Beyond the aforementioned sex differences in neural pain processing, it is also possible that hormonal (e.g., estrogen level, meonopausal status) and genetic factors contributed to the female-specific associations observed in the present study. Future studies examining the influence of these factors are warranted.30,46 Moreover, studies examining sex and FM score with other QST modalities (e.g., thermal) may produce different results, especially given recent findings demonstrating unique sensory profiles within knee OA patients.47 Regardless, it remains unclear why the relationship between pain sensitivity and FM score was observed only in females. It should be noted, however, that the ability of the FM score to predict postsurgical outcomes was generalizable to both males and females.11,13
FM Score Associated with Pressure Sensitivity but Not CPM or TS
Across the body, nearly all measures of pressure pain sensitivity in females were related to FM score (Table 2), supporting the association between diffuse hyperalgesia and pain centralization. This suggests that the FM survey criteria are at least partially evaluating the static “tone” of CNS pain systems. However, CPM and TS, both considered dynamic measures of pain modulation, failed to show a similar relationship. CPM is an experimental method used to evaluate the integrity of endogenous pain inhibitory systems, whereas TS assesses facilitative pain mechanisms. Both CPM and TS have been shown to be dysfunctional in various pain syndromes and predictive of postsurgical outcomes.4,5,48,49 Failure to observe associations between these measures and the FM score suggests either that these tests are not measuring what has been postulated, or that the FM survey criteria are not probing pain modulatory pathways. Several studies have also failed to demonstrate a relationship between CPM and clinical outcomes, including self-reported pain intensity36,50 and number of painful body sites51 – a significant component of the FM score. It is also becoming evident that different CPM paradigms can yield conflicting results, even in the same individuals.27,52 Therefore, it’s possible that other CPM and TS paradigms, including those assessed with neuroimaging53 would show a relationship with the FM score.
Females were divided into FM score tertiles (Low, Moderate, and High) to better examine the relationship between QST and FM scores. We observed a step-wise increase in pain sensitivity as FM score tertile increased, with the greatest differences observed between High and Low tertiles. This suggests that individuals with the highest FM scores may represent a unique pain phenotype compared to those with lower scores. This distinction may have important clinical significance for pain management: in patients with knee OA, higher FM scores would indicate the potential for improved outcomes with centrally-acting therapies over peripheral interventions.
FM scores were also associated with the degree to which pain sensitivities correlated across the body. In individuals with purely localized nociceptive pain, such as that following acute injury, sensitivity at the injured site is normally expected to be increased (hyperalgesia), with uninjured body sites maintaining a baseline level of pain sensitivity. The correlation between sensitivity at the injured site and sensitivity at distant uninjured sites would therefore be low. In contrast, in individuals with centralized pain, augmented CNS pain processing is presumed to impart a global increase in pain sensitivity that would manifest as widespread hyperalgesia. In support of this, we showed that as FM scores increased, not only did pain sensitivity increase, but there was an increased association of pain sensitivities between remote body sites. Although speculative, this coupling may reflect an altered CNS process that effectively unifies pain signals from distant and distinct locations. This suggests that the FM score reflects not only a CNS process of pain augmentation, but also one of widespread pain integration and unification.
Limitations
This study has several limitations. Though our overall sample was larger than most studies examining these issues, it yielded relatively small subgroups when divided by sex and FM score tertiles. Patients were older surgical OA candidates; it is unclear if these findings would be applicable to younger individuals or those with OA of lesser severity. All QST measures were pressure-based, and other stimulus modalities were not assessed. Furthermore, as this is a single-center university study with mostly Caucasian patients, the generalizability of our findings to other populations is unknown.
Conclusions
We demonstrated a relationship between QST and FM score in females with knee OA suggesting that the 2011 FM survey criteria may provide a useful measure of centralized pain. As a brief, self-report tool that does not require administration by a healthcare provider or the resource burden of other measures of central pain processing (e.g., neuroimaging and QST), there is potential for its clinical implementation in pain management decision making.
Supplementary Material
Supplemental Digital Content 1.docx, table
Supplemental Digital Content 2.docx, table
Supplemental Digital Content 3.docx, table
Acknowledgments
We would like to acknowledge Andrew Schrepf, Ph.D., for providing critical comments and analytical recommendations.
Source of Funding:
This project was supported by the Department of Anesthesiology at the University of Michigan. Dr. Harte has received research funding from Aptinyx, Cerephex, Forest Laboratories, Eli Lily and Merck; he has served as a consultant for Pfizer, Analgesic Solutions, Aptinyx, and deCode Genetics. He and Dr. Clauw are inventors of the MAST system used in this study. Dr. Harte is a member of Arbor Medical Innovations, Ann Arbor, MI. Dr. Clauw has received research funding from Cerephex, Forest, Merck, and Pfizer, and serves as a consultant for Tonix, Pfizer, Depomed, Sammumed, Aptinyx, and Zynerba. Chad Brummet reports the following current disclosures: Patent for Peripheral Perineural Dexmedetomidine (no royalties), as well as research funding from Neuros Medical Inc., UM Michigan Genomics Initiative, NIDA (R01 DA038261-05), MDHHS (Sub K Michigan OPEN), NIH-DHHS (P50 AR070600-05 CORT), NIH- DHHS-US (K23 DA038718-04), NIH0DHHS-US-16 PAF 07628 (R01 NR017096-05), NIH-DHHS-US-16- PAF06270 (R01 HD088712-05), and IH-DHHS-US-17-PAF02680 (R01 DA042859-05).
Footnotes
Conflicts of Interest
Stephen Neville, Andrew Urquhart, Andrew Clauw, and Stephanie Moser report no competing interests to declare.
- We confirm that each author listed has participated in this study to a significant extent.
- We confirm that the manuscript is not being simultaneously submitted elsewhere, and that no portion of the data has been or will be published elsewhere.
References
- 1.Clauw DJ. Fibromyalgia and related conditions. Mayo Clin Proc. 2015;90(5):680–692. doi: 10.1016/j.mayocp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Harte SE, Ichesco E, Hampson JP, et al. Pharmacologic attenuation of cross-modal sensory augmentation within the chronic pain insula. Pain. 2016 doi: 10.1097/j.pain.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollins M, Harper D, Gallagher S, et al. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis. Pain. 2009;141(3):215–221. doi: 10.1016/j.pain.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91(1-2):165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 5.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13(10):936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Sola M, Woo CW, Pujol J, et al. Towards a neurophysiological signature for fibromyalgia. Pain. 2017;158(1):34–47. doi: 10.1097/j.pain.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain. 2013;14(6):579–589. doi: 10.1016/j.jpain.2013.01.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kutch JJ, Ichesco E, Hampson JP, et al. Brain signature and functional impact of centralized pain: a Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Network Study. Pain. 2017 doi: 10.1097/j.pain.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris RE, Clauw DJ. Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy. Neurosci Lett. 2012;520(2):192–196. doi: 10.1016/j.neulet.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Experimental and clinical psychopharmacology. 2008;16(5):405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015;67(5):1386–1394. doi: 10.1002/art.39051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janda AM, As-Sanie S, Rajala B, et al. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015;122(5):1103–1111. doi: 10.1097/ALN.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 13.Brummett CM, Janda AM, Schueller CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology. 2013;119(6):1434–1443. doi: 10.1097/ALN.0b013e3182a8eb1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and rheumatism. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis care & research. 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. The Journal of rheumatology. 2011;38(6):1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 17.Brummett CM, Bakshi RR, Goesling J, et al. Preliminary validation of the Michigan Body Map. Pain. 2016;157(6):1205–1212. doi: 10.1097/j.pain.0000000000000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Bellamy N. Version X. Brisbane, Australia: 2012. WOMAC Osteoarthritis Index User Guide. [Google Scholar]
- 20.Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 21.Swartzman LC, Gwadry FG, Shapiro AP, Teasell RW. The factor structure of the Coping Strategies Questionnaire. Pain. 1994;57(3):311–316. doi: 10.1016/0304-3959(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Petzke F, Gracely RH, Park KM, Ambrose K, Clauw DJ. What do tender points measure? Influence of distress on 4 measures of tenderness. The Journal of rheumatology. 2003;30(3):567–574. [PubMed] [Google Scholar]
- 24.Henry NL, Conlon A, Kidwell KM, et al. Effect of estrogen depletion on pain sensitivity in aromatase inhibitor-treated women with early-stage breast cancer. J Pain. 2014;15(5):468–475. doi: 10.1016/j.jpain.2014.01.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harte SE, Mitra M, Ichesco EA, et al. Development and validation of a pressure-type automated quantitative sensory testing system for point-of-care pain assessment. Medical & biological engineering & computing. 2013;51(6):633–644. doi: 10.1007/s11517-013-1033-x. [DOI] [PubMed] [Google Scholar]
- 26.Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Schoen CJ, Ablin JN, Ichesco E, et al. A novel paradigm to evaluate conditioned pain modulation in fibromyalgia. Journal of pain research. 2016;9:711–719. doi: 10.2147/JPR.S115193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115(3):410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain. 2012;153(3):602–618. doi: 10.1016/j.pain.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. British journal of anaesthesia. 2013;111(1):52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones GT, Atzeni F, Beasley M, Fluss E, Sarzi-Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol. 2015;67(2):568–575. doi: 10.1002/art.38905. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis and rheumatism. 1995;38(1):19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 33.Goode AP, Shi XA, Gracely RH, Renner JB, Jordan JM. Associations between pressure-pain threshold, symptoms, and radiographic knee and hip osteoarthritis. Arthritis care & research. 2014;66(10):1513–1519. doi: 10.1002/acr.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suokas AK, Walsh DA, McWilliams DF, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20(10):1075–1085. doi: 10.1016/j.joca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. The Journal of rheumatology. 2000;27(6):1513–1517. [PubMed] [Google Scholar]
- 36.Finan PH, Buenaver LF, Bounds SC, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis and rheumatism. 2013;65(2):363–372. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13(2):211. doi: 10.1186/ar3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YC, Lu B, Bathon JM, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis care & research. 2011;63(3):320–327. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(7):1043–1056. doi: 10.1016/j.joca.2015.02.163. [DOI] [PubMed] [Google Scholar]
- 40.Gwilym SE, Keltner JR, Warnaby CE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis and rheumatism. 2009;61(9):1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 41.Harvey AK, Taylor AM, Wise RG. Imaging Pain in Arthritis: Advances in Structural and Functional Neuroimaging. Current Pain and Headache Reports. 2012;16(6):492–501. doi: 10.1007/s11916-012-0297-4. [DOI] [PubMed] [Google Scholar]
- 42.Sorge RE, Totsch SK. Sex Differences in Pain. Journal of neuroscience research. 2017;95(6):1271–1281. doi: 10.1002/jnr.23841. [DOI] [PubMed] [Google Scholar]
- 43.Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang G, Erpelding N, Davis KD. Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain. 2014;155(4):755–763. doi: 10.1016/j.pain.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Henderson LA, Gandevia SC, Macefield VG. Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. NeuroImage. 2008;39(4):1867–1876. doi: 10.1016/j.neuroimage.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 46.Craft RM. Modulation of pain by estrogens. Pain. 2007;132(Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 47.Frey-Law LA, Bohr NL, Sluka KA, et al. Pain sensitivity profiles in patients with advanced knee osteoarthritis. Pain. 2016;157(9):1988–1999. doi: 10.1097/j.pain.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138(1):22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 49.Weissman-Fogel I, Granovsky Y, Crispel Y, et al. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain. 2009;10(6):628–636. doi: 10.1016/j.jpain.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Martel MO, Wasan AD, Edwards RR. Sex differences in the stability of conditioned pain modulation (CPM) among patients with chronic pain. Pain medicine (Malden, Mass) 2013;14(11):1757–1768. doi: 10.1111/pme.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerhardt A, Eich W, Treede RD, Tesarz J. Conditioned pain modulation in patients with nonspecific chronic back pain with chronic local pain, chronic widespread pain, and fibromyalgia. Pain. 2017;158(3):430–439. doi: 10.1097/j.pain.0000000000000777. [DOI] [PubMed] [Google Scholar]
- 52.Imai Y, Petersen KK, Morch CD, Arendt Nielsen L. Comparing test-retest reliability and magnitude of conditioned pain modulation using different combinations of test and conditioning stimuli. Somatosensory & motor research. 2016;33(3-4):169–177. doi: 10.1080/08990220.2016.1229178. [DOI] [PubMed] [Google Scholar]
- 53.Piche M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(45):14236–14246. doi: 10.1523/JNEUROSCI.2341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1.docx, table
Supplemental Digital Content 2.docx, table
Supplemental Digital Content 3.docx, table


