Abstract
Immune checkpoint inhibitors targeting the programmed cell death PD-1 receptor have dramatically changed the landscape of metastatic melanoma treatment. Still, these immuno-modulatory agents have associated side effects, including dermatologic manifestations. To this end, we report a patient with metastatic melanoma that was treated with PD-1 inhibitors, and subsequently developed inflammation of existing seborrheic keratosis lesions and new verrucous keratoses, a cutaneous side effect that has not been previously reported to our knowledge. The etiology of seborrheic and verrucous keratoses is not well understood, though their physical and histopathologic similarities to chronic viral-derived lesions, such as human papilloma virus, suggest a potential viral association. Chronic viral infections are known to result in T-cell tolerance due to persistent antigen stimulation. PD-1 inhibition is able to reinvigorate exhausted T-cells, which are accordingly able to decrease viral load. Thus, the inflammatory reaction, seen in our patient, may be the result of PD-1 inhibition reactivating virally driven T lymphocytes.
Keywords: Metastatic Melanoma, PD-1 Inhibition, Nivolumab, Seborrheic Keratosis
Immune checkpoint inhibitors directed against the programmed cell death (PD)-1 receptor have dramatically improved outcomes for metastatic melanoma patients. Specifically, PD-1 has been identified as a major regulator of T-cell exhaustion, thus PD-1 inhibition has demonstrated successful ability to restore T-cell function and participate in tumor eradication.1 PD-1 inhibitors, Pembrolizumab and Nivolumab, have been associated with a wide array of dermatologic adverse effects, including morbiliform rash, pruritus, vitiligo, lichenoid skin/mucosal reactions, sarcoid-like reactions, and disappearance of pigmented lesions.2,3 Here, we present a patient with metastatic melanoma that was treated with PD-1 inhibitors, and subsequently developed inflammation of existing seborrheic keratosis lesions and new verrucous keratoses.
Report of Case
A 74 year old male presented for evaluation with multiple inflamed pink, tan and brown verrucous and stuck-on pruritic papules with surrounding erythema on the scalp, trunk, and extremities. The patient reported that the lesions intermittently arose during combination ipilimumab/nivolumab therapy and continued while on single agent nivolumab therapy. Notably, the patient did not ever receive BRAF inhibition therapy. Histologic examination of the verrucous lesions revealed papillomatosis of benign appearing keratinocytes with coarse keratohyalin granules, consistent with an inflamed verrucous keratosis. This dermatologic effect of PD-1 inhibition, in the context of metastatic melanoma, has not been previously reported. Treatment consisted of use of medium potency topical steroids and cryotherapy to symptomatic lesions with some effect.
Seborrheic keratosis (SK) is a common benign epidermal tumor known to classically occur on the face or trunk of middle-aged to older adults. The etiology of seborrheic and verrucal keratoses is not well characterized. The clinical and histologic similarities to virally driven lesions, such as verruca, illustrate a potential viral association, including human papillomavirus (HPV), in seborrheic keratosis development.4 Studies have detected the presence of epidermodysplasia verruciformis HPV DNA in non-genital SKs, further illustrating a potential role for HPV in SK evolution.1
At the cellular level, T-cell’s are involved in viral clearance, though prolonged immune activation from chronic viral infections, results in T-cell exhaustion. This further contributes to viral persistence.5 PD-1 has been identified as a major regulator of chronically activated T-cells, as it is induced on both CD4+ and CD8+ T cells. When its physiological ligand PD-L1 or PD-L2, which are classically up-regulated on cancer cells, binds, PD-1 suppresses the activation and function of T cells through the recruitment of downstream effector molecules. This results in the dephosphorylation and inactivation of Zap70, Zeta-chain-associated protein kinase 70, a major integrator of TCR-mediated signaling.6 The PD-1/PD-L1 pathway is also known to be up-regulated in chronic viral infections including HBV, HCV, and HIV.7,8 Thus, PD-1 signaling is able to induce T-cell tolerance via inhibition of TCR signaling pathway, ultimately attenuating anti-viral and anti-tumor immune responses, allowing for persistence of chronic viral infections.6 Pre-clinical studies have demonstrated that PD-1 inhibition is able to reinvigorate exhausted T-cells, thereby restoring effector functions of virus specific CD8 T-cells, and accordingly decreasing viral load.5
We postulate that the seborrheic and verrucal keratosis inflammation seen in our patient are the result of reactivation of effector T-cells specific to HPV and/or another uncharacterized virus, resulting in a classic inflammatory cascade that is enacted to clear the virus. The viral-induced inflammatory response may impact existing SK lesions, and result in our patients’ presentation. This dermatologic adverse event (DAE) of Nivolumab therapy for metastatic melanoma has not been previously reported to our knowledge.
Previous studies have reported the development of “low-grade” DAE with Nivolumab therapy, including rash and vitiligo, both of which were significantly associated with increased progression-free survival and overall survival.9 It has been speculated that the development of DAE’s in these patients may serve as a surrogate marker for reinvigoration of anti-tumor immune response, and potentially treatment efficacy. Given the changing landscape of targeted immunotherapies for metastatic melanoma, it is important to understand the DAE profile of PD-1 inhibitors and how they may potentially interact with chronic viral infections existing in metastatic melanoma patients. Further studies are needed to better characterize the impact of PD-1 blockade on chronic viral infections and their role in existing skin lesions, such as seborrheic keratoses.
Figure I.
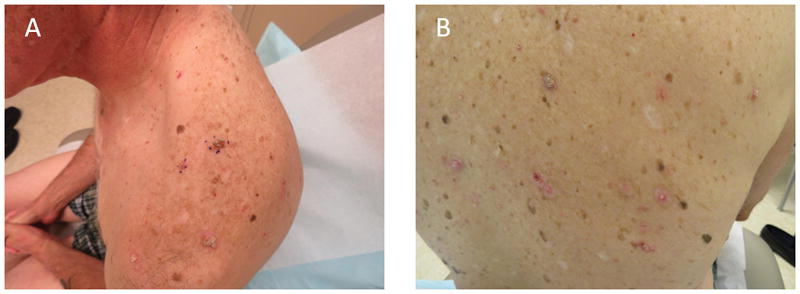
(A–B) Clinical photos. Multiple inflamed pink, tan and brown verrucous and stuck-on pruritic papules with surrounding erythema on the back.
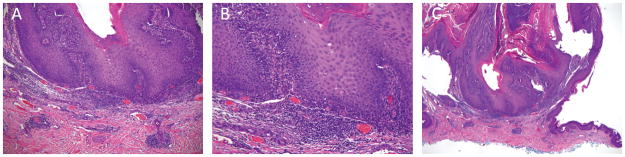
Figure II.
Scanning magnification. Histologic examination of the verrucous lesions revealed papillomatosis of benign appearing keratinocytes with coarse keratohyalin granules, consistent with an inflamed verrucous keratosis. (A) 4x, (B) 10x, (C) 20x.
Acknowledgments
Funding Source: PHR is supported by the NIH 5 T32 AR 7569-22 National Institute of Health T32 grant. JA is supported by the Dermatology Foundation Medical Dermatology Career Development Award and the NCI K12 CA076917 Clinical Oncology Research Career Development Program (CORP)
Abbreviations
- PD-1
programmed death receptor-1
- TCR
t-cell receptor
- SK
sebhorrheic keratosis
- HPV
human papilloma virus
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- Zap70
Zeta-chain-associated protein kinase 70
- DAE
dermatologic adverse event
Footnotes
IRB status: None required
Conflict of Interest Disclosure: None declared
References
- 1.Li Y-H, Chen G, Dong X-P, Chen H-D. Detection of epidermodysplasia verruciformis-associated human papillomavirus DNA in nongenital seborrhoeic keratosis. Br J Dermatol. 2004;151(5):1060–1065. doi: 10.1111/j.1365-2133.2004.06244.x. [DOI] [PubMed] [Google Scholar]
- 2.Wolner ZJ, Marghoob AA, Pulitzer MP, Postow MA, Marchetti MA. A case report of disappearing pigmented skin lesions associated with pembrolizumab treatment for metastatic melanoma. Br J Dermatol. 2017 Jan; doi: 10.1111/bjd.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gushi A, Kanekura T, Kanzaki T, Eizuru Y. Detection and sequences of human papillomavirus DNA in nongenital seborrhoeic keratosis of immunopotent individuals. J Dermatol Sci. 2003;31(2):143–149. doi: 10.1016/s0923-1811(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 5.Gardiner D, Lalezari J, Lawitz E, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8(5):e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. 2013;14(12):1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 7.Wang XF, Lei Y, Chen M, Chen CB, Ren H, Shi TD. PD-1/PDL1 and CD28/CD80 pathways modulate natural killer T cell function to inhibit hepatitis B virus replication. J Viral Hepat. 2013;20(Suppl 1):27–39. doi: 10.1111/jvh.12061. [DOI] [PubMed] [Google Scholar]
- 8.Davar D, Wilson M, Pruckner C, Kirkwood JM. PD-1 Blockade in Advanced Melanoma in Patients with Hepatitis C and/or HIV. Case Rep Oncol Med. 2015;2015:737389. doi: 10.1155/2015/737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 2016;22(4):886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]