Abstract
Hypoglycin A (HGA) and methylenecyclopropylglycine (MCPG) are naturally-occurring amino acids known to cause hypoglycemia and encephalopathy. Exposure to one or both toxins through the ingestion of common soapberry (Sapindaceae) fruits are documented in illness outbreaks throughout the world. Jamaican Vomiting Sickness (JVS) and seasonal pasture myopathy (SPM, horses) are linked to HGA exposure from unripe ackee fruit and box elder seeds, respectively. Acute toxic encephalopathy is linked to HGA and MCPG exposures from litchi fruit. HGA and MCPG are found in several fruits within the soapberry family and are known to cause severe hypoglycemia, seizures, and death. HGA has been directly quantified in horse blood in SPM cases and in human gastric juice in JVS cases. This work presents a new diagnostic assay capable of simultaneous quantification of HGA and MCPG in human plasma, and it can be used to detect patients with toxicity from soapberry fruits. The assay presented herein is the first quantitative method for MCPG in blood matrices.
Keywords: Soapberry, Sapindaceae, Acute Toxic Encephalopathy, Hypoglycin A (HGA), Methylenecyclopropylglycine (MCPG)
1. Introduction
Many common soapberry fruits contain toxins known to cause hypoglycemia and encephalopathy [1–3]. In fact, there are a number of international illness-outbreaks linked to soapberry ingestion, including Jamaican Vomiting Sickness (JVS), acute toxic encephalopathy, and seasonal pasture myopathy (SPM, horses) [3–5]. JVS, for example, is linked to the ingestion of unripe ackee fruit containing high concentrations of hypoglycin A (HGA) [3, 6–9]. Investigations of JVS have led to the regulation of imported ackee into the United States in order to prevent ackee poisoning [10]. HGA is also the causative agent of seasonal pasture myopathy (SPM), or atypical myopathy (AM), in horses, resulting from ingestion of box elder seeds [5, 11–18]. Recently, HGA and methylenecyclopropylglycine (MCPG) exposures were identified in cases of acute toxic encephalopathy in Asia [4]. Both toxins were also detected in litchi fruit obtained from the region of the illness outbreak [4]. Public health investigations into acute toxic encephalopathy led to the following public health recommendations in the outbreak region: reduce litchi consumption among children, ensure children eat evening meals throughout outbreak period(s), and countermeasure suspected cases with rapid glucose correction [4].
Confirmation of HGA and MCPG exposure in illness outbreaks has been reported using the detection of urinary metabolites, HGA in gastric juices, elevated acylglycines in urine, and elevated acylcarnitines in blood products [3, 4, 6, 19]. In horses, analyses of SPM cases have also included the detection of HGA in blood, where the toxin has been quantified in serum at concentrations up to 8.5 × 103 ng/mL [11, 12, 18, 20]. Similar methods for the detection of HGA in blood have been applied to human serum in the case of a healthy adult consuming meals of ackee (1g/kg body weight) and litchi (5 g/kg body weight) [17]. HGA was present in serum at 79.1 ng/mL (560 nmol/L) and 2.09 ng/mL (14.8 nmol/L) up to ten hours after ingestion of ackee and litchi, respectively [17].
In the same case of a healthy adult consuming meals of ackee and litchi fruit, metabolic products of exposure to HGA and MCPG were also identified [17]. The specific urinary metabolites of HGA and MCPG are methylenecyclopropylacetyl-glycine (MCPA-Gly) and methylenecyclopropylformyl-glycine (MCPF-Gly), respectively. Both metabolites were detected in urine after ingestion of ackee as well as litchi fruit. These results created a renewed interest in cases of JVS, with respect to whether MCPG may be a causative agent along with HGA. To date, MCPG exposure has not been evaluated in cases of JVS [3, 6–8].
The metabolites of HGA and MCPG, MCPA-Gly and MCPF-Gly, have both been detected in the urine of acute toxic encephalopathy cases, and MCPA-Gly has been indirectly detected, after hydrolysis, in the urine of JVS patients [3, 19]. HGA has been detected in the blood of horses suffering from SPM as well as in the gastric juice of patients with JVS. However, neither HGA nor MCPG have been detected in human blood products in illness outbreaks [7]. The direct detection of HGA and MCPG in cases of JVS, acute toxic encephalopathy, and other illness outbreaks would provide additional support of direct exposure to the toxins. The assay presented herein will allow for the simultaneous quantification of HGA and MCPG in human plasma and can be applied to cases of JVS, acute toxic encephalopathy, and other illness outbreaks where HGA and MCPG exposures are suspected. This assay utilizes the same instrument platform as the urinary metabolites and the fruit toxins methods previously published [19, 21]. This pairing with earlier methodology allows for a swifter workflow during the evaluation of multiple matrices received for analysis during an illness outbreak. Combined with previous clinical and agricultural assays, this assay expands current laboratory capabilities during a public health investigation to evaluate both HGA and MCPG content regardless of whether the specimen received is urine, fruit, or blood.
2. Materials and Methods
2.1. Safety
Universal precautions were followed, and personal protective equipment, including gloves, safety glasses, and laboratory coats were used when handling biological fluids such as human serum and plasma. Biological hazards were handled in a biological safety cabinet.
2.2. Materials
Isotopically-labeled standards (ISTD) (≥99.5%, 15N13C2 –HGA-TFA and 13C3 –MCPG-TFA) and unlabeled standards (≥ 97%, HGA-TFA and MCPG-TFA) were synthesized by IsoSciences, LLC (King of Prussia, PA). Individual and pooled plasma samples were purchased from Tennessee Blood Services (Memphis, TN). Protein precipitation plates were purchased from Pierce (Rockford, IL). Dansyl chloride (98%), HPLC-grade acetonitrile, methanol, and water were purchased from Fisher Scientific (St. Louis, MO). Phosphate buffered saline (10X PBS), sodium hydroxide (0.1 N), and formic acid (98%), were purchased from Sigma-Aldrich (Pittsburgh, PA). Laboratory deionized water (18 MΩ·cm) was filtered in-house using an Aqua Solutions Water Purification system (Jasper, GA). Oasis HLB 96-well, 60 mg per well, 60 μm particle size solid phase extraction plates were purchased from Waters Technologies Corporation (Milford, MA).
2.3. Preparation of Stock Solutions and QC Materials
Hypogylcin A-trifluoroacetic acid (HGA-TFA) and methylenecyclopropylglycine-trifluoroacetic acid (MCPG-TFA) salts were dissolved in HPLC-grade water to concentrations of 10.0 mg/mL of HGA and 1.00 mg/mL of MCPG. Eight calibrators were made in HPLC-grade water from stock solutions. HGA ranged from 1.00 – 100. ng/mL and MCPG ranged from 5.00 – 150. ng/mL. The HGA internal standard (15N13C2-HGA-TFA) was diluted in HPLC-grade water to be 1.00 mg/mL of 15N13C2-HGA, and the MCPG internal standard (13C3-MCPG-TFA) was diluted in deionized water to be 10.0 mg/mL of 13C3-MCPG. A stock solution of both internal standards at 200. ng/mL was prepared from the stock solutions in HPLC-grade water. Quality controls (QCs) at high, medium, and low concentrations were prepared in pooled plasma from Tennessee Blood Services (Memphis, TN). The concentrations of the high, medium, and low HGA QCs were 35.0 ng/mL, 8.00 ng/mL, and 3.00 ng/mL in pooled plasma, respectively. The concentrations of the high, medium, and low MCPG QCs were 80.0 ng/mL, 35.0 ng/mL, and 8.00 ng/mL in pooled plasma, respectively. Plasma was purchased commercially from Tennessee Blood Services (Memphis, TN) and did not meet the definition of human subjects as specified in 45 CFR 46.102 (f).
2.4. Sample Preparation
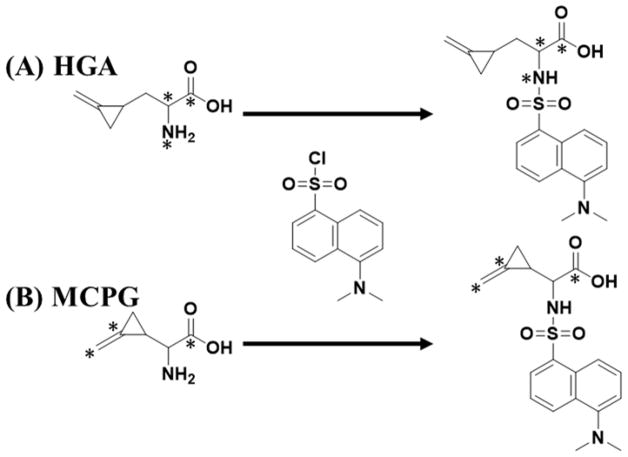
A 10 μL aliquot of the internal standard solution was added to each sample well to be analyzed in a deep well plate (Figure S1). Matrix blank (50 μL of pooled plasma) was then added to calibrator and matrix blank wells; QCs and samples were added to the respective wells. Calibrators, denoted as S1–S8 (50 μL) were added to their respective wells; water (50 μL) was added to all QC, matrix blank, and sample wells. Samples within the plate were transferred to a protein precipitation plate containing 200 μL of acetonitrile, and shaken for 30 seconds at 1,000 rpm at 25 °C. The calibrators, QCs, and samples were pulled through a vacuum and dried under nitrogen for 30 minutes at 60 °C. The analytes were derivatized, using previously reported methods for amino acid analysis [13, 21, 22]. To the dried wells, 20 μL of 10X PBS buffer (pH 11.0) and 50 μL of dansyl chloride (1 mg/mL) were added and shaken at 1,000 rpm at 60 °C for 10 minutes. Dansylation was carried out in order to improve separation by reversed-phase chromatography, and improve ionization efficiency (Scheme 1) [13, 21]. After derivatization, 430 μL of water was added to all wells. The samples were extracted using a 60 mg Oasis HLB SPE 96-well plate: conditioned with 800 μL of methanol, equilibrated with 800 μL of 98:2 water to acetonitrile, loaded with 500 μL of samples, washed with 800 μL 98:2 water to acetonitrile, and eluted with 800 μL 70:30 water to acetonitrile. The eluted samples were then dried under nitrogen at 60 °C for one hour. Formic acid (50 μL of 0.1% in water) was added to each well to reconstitute the sample volume, and samples were transferred to a PCR plate. The PCR plate was placed in a cooled (5 °C) autosampler, and a 4.0 μL injection of the samples was made onto a HPLC-MS/MS system for quantitative analysis.
Scheme 1.
Derivatization of (A) HGA and (B) MCPG with dansyl chloride. Asterisks indicate 13C and 15N label sites on the internal standards.
2.5. HPLC-MS/MS
The assay was performed on an Agilent 1260 series HPLC (Santa Clara, CA) coupled to a SCIEX 4000 triple quadrupole mass spectrometer (Framingham, MA). The HPLC column used was an Agilent Zorbax SB-C18 Rapid Resolution HT column, 2.1 × 50 mm with a 1.8 μm particle size (Santa Clara, CA). The column and autosampler temperatures were 60 °C and 5 °C, respectively. Mobile phase A was HPLC-grade water with 0.1% formic acid, and mobile phase B was HPLC-grade acetonitrile with 0.1% formic acid. Dansylated HGA and MCPG (dns-HGA and dns-MCPG) were eluted with a linear gradient at a flow rate of 500 μL/min. The 4.0 μL injection passed through an Agilent low dispersion in-line filter (2 μm frit) (Santa Clara, CA) and entered the column with 10% mobile phase B held for 0.10 minutes. From 0.10 minutes to 5.50 minutes, the % mobile phase B increased linearly to 70%. After 5.50 minutes, mobile phase B increased to 90% at 5.51 minutes and was held until 5.75 minutes. Mobile phase B was decreased to 10% at 5.76 minutes and held until 7.00 minutes. The injection needle was washed between samples for three seconds using a solution of 1:1 methanol to water.
Positive mode electrospray ionization was used for this method. Mass spectrometer source and acquisition parameters were as follows: collision gas, 7 psig; curtain gas, 10 psig; ion source gas 1, 60 psig; ion source gas 2, 60 psig; IS voltage, 4500 V; declustering potential, 45 V; entrance potential, 8 V; collision cell exit potential, 5 V. Multiple reaction monitoring (MRM) was used to acquire quantitative data for the following transitions: dns-HGA quantitation ion m/z 375.1 → 170.1, confirmation ion m/z 375.1 → 157.1; dns-15N13C2-HGA m/z 378.1 → 157.1; dns-MCPG quantitation ion m/z 361.1 → 157.1, confirmation ion m/z 361.1 → 170.1; dns-13C3-MCPG m/z 364.1 → 157.1. The collision energies were as follows: dns-HGA quantitation ion 27 V, confirmation ion 39 V; dns-15N13C2-HGA 39 V; dns-MCPG quantitation ion 39 V, confirmation ion 29 V; dns-13C3-MCPG 39 V.
2.6. Data Acquisition, Processing, and Reporting
Analyst v.1.6 software (SCIEX, Framingham, MA) was used for data acquisition and quantitative analysis. Precision and accuracy were evaluated by calculating percent relative standard deviation ( ) and percent relative error ( ), respectively. SD is the calculated standard deviation, Cavg is the average concentration observed, and Ce and Ct are experimental and theoretical concentrations, respectively. The percent recovery for SPE recovery was calculated by ( ), where Peak AreaPre and Peak AreaPost are the analyte peak areas when analyte was added before and after SPE, respectively. The percent recovery for stability studies was calculated by ( ), where Conc1 is the mean concentration from characterization (n=21) and Conc2 is the concentration measured after storage under each specified condition.
The peak area ratios of dns-HGA and dns-MCPG to dns-15N13C2-HGA and dns-13C3-MCPG were plotted as a function of the expected concentration of HGA and MCPG in each calibrator. Since the labeled internal standards are added prior to the dansyl chloride, the analyte response ratio in an unknown sample will be normalized even if the dansylation does not result in a 100% derivatization of the analytes. Concentrations of HGA and MCPG in plasma were reported with units of ng/mL. Three analysts contributed to this assay characterization with twenty-one analytical evaluations (i.e. calibration curves with QCs) over a six week period with no more than two analyses per day. The method’s characterization included statistical analyses using the the Centers for Disease Control and Prevention (CDC) Multi-Rule Quality Control System (MRQCS)[23]. A convenience set of 100 individual plasma specimens was purchased commercially from Tennessee Blood Services (Memphis, TN) and did not meet the definition of human subjects as specified in 45 CFR 46.102 (f).
3. Results and Discussion
3.1. Sample Preparation
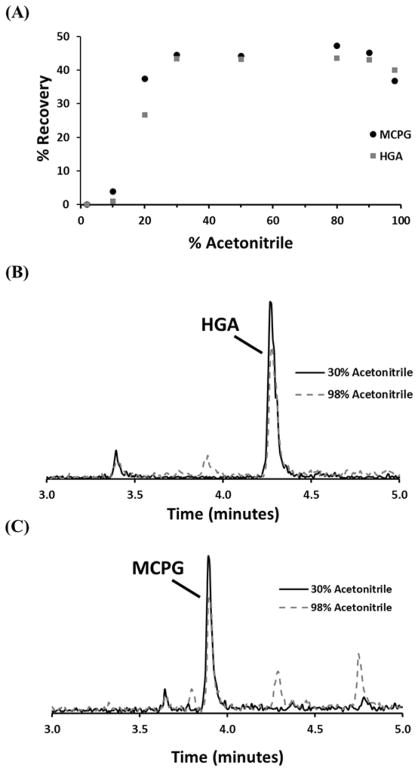
Previous method development for the quantification of HGA and MCPG in fruit extracts confirmed that a HLB SPE plate not only yielded sufficient recovery of the dansylated analytes but also removed fruit matrix interferences [21]. When switching from fruit extracts to a human plasma matrix, additional interferences were observed. To avoid unwanted interferences, SPE conditions were re-optimized to conserve the percent recoveries of HGA and MCPG, and to minimize the additional plasma matrix interferences in the eluent. The strength of the elution solvent was increased from 2 to 98% acetonitrile in water to determine the percent recovery (see Materials and Methods) of the ISTD mix spiked into blank pooled plasma (Figure 1A).. The optimized method used a 30% acetonitrile elution solvent and yielded lower background signal with fewer interfering peaks observed near the analytes of interest (Figure 1B and 1C).
Figure 1. Varying SPE elution strength.
Effects of varying the % acetonitrile in elution solvent on (A) recovery of HGA (gray squares) and MCPG (black circles), (B) extracted ion chromatograms of HGA with 30% (black solid) and 98% (gray dashed) acetonitrile elution, and (C) extracted ion chromatograms of MCPG with 30% (black solid) and 98% (gray dashed) acetonitrile elution.
3.2. Detection and Separation
Previous method development for HGA and MCPG in fruit extracts demonstrated that both dansylated analytes were well-retained on a C18 HPLC column [21]. In plasma, the elution gradient was modified to obtain greater separation of the dns-MCPG quantitation ion from a plasma interference peak with the same mass transition (m/z 361.1 → 157.1). The isobaric interference was observed in a 4 minute run with a 2.40 minute linear gradient and a resolution of only 1.52 (Figure S2). Increasing the linear gradient to 4.40 minutes with a total run time of 6 minutes increased the resolution from 1.52 to 2.44. Further, a final 5.50 minutes linear gradient with a total run time of 7 minutes was used for method characterization, and a resolution of 5.27 was observed. By designing an elution gradient with an excess in baseline resolution between dns-MCPG and the isobaric interference, the authors have ensured that future patient samples with potentially larger contributions to the isobaric interference will not affect analyte measurements. Using the 7 minute run, retention times of dns-HGA and dns-MCPG were 4.26 ± 0.03 minutes and 3.88 ± 0.03 minutes, respectively (n = 21).
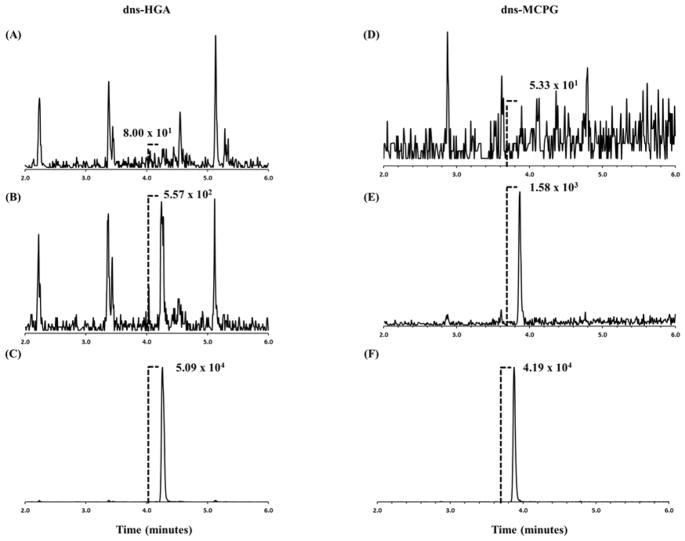
The lowest reportable limit (LRL) for HGA and MCPG was chosen based on which concentration was consistently observed at a signal-to-noise greater than three. The LRL was 1.00 ng/mL for HGA and 5.00 ng/mL for MCPG (Figure 2). Using the Taylor method, the theoretical limit of detection (LOD) was calculated to be 0.330 ng/mL for HGA and 0.697 ng/mL for MCPG [24]. The highest reportable limit (HRL) for the method was 100. ng/mL for HGA and 150. ng/mL for MCPG. In previous cases of SPM in horses, concentrations of HGA reached 8.5 × 103 ng/mL in blood [11]. Although this previously reported value is above the HRL of this assay, samples can be diluted up to 100X to give reportable results within the characterized range (Table 1). In order to ensure accuracy of dilutions, blank pooled plasma samples were enriched (n = 4) to a final concentration 500. ng/mL, 1.00 × 103 ng/mL, 5.00 × 103 ng/mL, and 1.00 × 104 ng/mL of HGA and MCPG and diluted to the reportable range. The %RSDs and %REs for the diluted plasma samples were ≤ 5.2% and ≤ 7.9%, respectively. Therefore, the precision and accuracy for diluted samples above the method HRL are consistent with the characterized method and within the guidelines for bioanalytical method validation [25].
Figure 2. Extracted ion chromatogram peak heights of spiked human plasma.
Chromatograms of dns-HGA (left) at concentrations of (A) 0.00 ng/mL or unspiked, (B) 1.00 ng/mL, and (C) 150. ng/mL. Chromatograms of dns-MCPG (right) at concentrations of (D) 0.00 ng/mL or unspiked, (E) 5.00 ng/mL, and (F) 100. ng/mL. The MS/MS quantitation transitions shown dns-HGA and dns-MCPG are m/z 375.1→170.1 and m/z 361.1→157.1, respectively.
Table 1.
Analyte dilution accuracy and precision in human plasma (n = 4).
| Analyte | Theoretical Concentration (ng/mL) | Experimental Concentration (ng/mL) | %RE* | %RSD* |
|---|---|---|---|---|
| HGA | 5.00 × 102 | 5.06 × 102 | 1.2 | 4.6 |
| 1.00 × 103 | 1.01 × 103 | 0.80 | 4.6 | |
| 5.00 × 103 | 4.60 × 103 | 7.9 | 1.9 | |
| 1.00 × 104 | 9.97 × 103 | 0.35 | 4.6 | |
|
| ||||
| MCPG | 5.00 × 102 | 5.26 × 102 | 5.2 | 4.2 |
| 1.00 × 103 | 1.04 × 103 | 4.4 | 3.7 | |
| 5.00 × 103 | 4.77 × 103 | 4.7 | 5.2 | |
| 1.00 × 104 | 1.03 × 104 | 2.7 | 4.7 | |
Percent relative error, ( ); Percent relative standard deviation, ( )
3.3. Precision, Accuracy, and Linearity
Three analysts participated in a six week QC characterization and method validation with no more than two analytical evaluations per day and employed the statistical analysis supported by the CDC MRQCS [23]. The R2 values for HGA and MCPG were 0.999 ± 0.001 and 0.998 ± 0.001, respectively (n=21). The %RE for calibrators and QCs were ≤ 7.5% for HGA and MCPG, and the %RSDs were ≤ 17% (Table 2). The assay accuracy and precision follow the guidelines in the FDA’s guidance for bioanalytical method validation and supports its application in the analysis of clinical samples [25].
Table 2.
Interday precision (%RSD) and accuracy (%RE) during characterization of QCs and calibrators (n = 21).
| HGA | MCPG | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Expected (ng/mL) | Measured (ng/mL) | %RSD* | %RE* | Expected (ng/mL) | Measured (ng/mL) | %RSD* | %RE* |
| 1.00 | 1.01 ± 0.15 | 15 | 0.60 | 5.00 | 5.12 ± 0.51 | 9.9 | 2.3 |
| 2.00 | 1.99 ± 0.20 | 10. | −0.71 | 7.50 | 7.68 ± 0.44 | 5.7 | 2.4 |
| 5.00 | 4.88 ± 0.41 | 8.5 | −2.3 | 10.0 | 9.69 ± 0.85 | 8.8 | −3.1 |
| 7.50 | 7.54 ± 0.35 | 4.6 | 0.58 | 20.0 | 20.0 ± 1.1 | 5.4 | −0.17 |
| 10.0 | 10.1 ± 0.9 | 8.6 | 1.4 | 50.0 | 49.7 ± 2.6 | 5.3 | −0.62 |
| 20.0 | 20.0 ± 1.3 | 6.5 | 0.12 | 75.0 | 73.3 ± 3.7 | 5.1 | −2.2 |
| 50.0 | 50.6 ± 2.4 | 4.8 | 1.3 | 100. | 100. ± 4 | 3.7 | 0.34 |
| 100. | 99.4 ± 2.5 | 2.5 | −0.60 | 150. | 152 ± 5 | 3.5 | 1.2 |
| 35.0 (QH) | 37.6 ± 3.2 | 8.4 | 7.3 | 80.0 (QH) | 82.9 ± 7.1 | 8.6 | 3.6 |
| 8.00 (QM) | 7.72 ± 1.35 | 17 | −3.5 | 35.0 (QM) | 34.4 ± 5.3 | 16 | −1.8 |
| 3.00 (QL) | 3.06 ± 0.32 | 10. | 2.1 | 8.00 (QL) | 8.15 ± 0.70 | 8.6 | 1.9 |
Percent relative error ( ); Percent relative standard deviation ( )
3.4. Plasma Matrix Effects
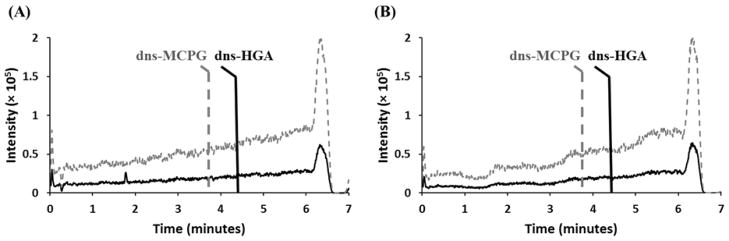
Plasma matrix effects were evaluated using a post-column infusion experiment [26]. A 200. ng/mL neat solution of HGA and MCPG was derivatized and infused directly into the mass spectrometer source while 4.0 μL of processed pooled plasma was injected from the HPLC autosampler. At the expected retention times of 4.26 ± 0.03 minutes for dns-HGA and 3.88 ± 0.03 minutes for dns-MCPG, there were no characteristic markers of suppression or enhancement observed (Figure 3). Therefore, this experiment demonstrates the applicability of the optimized elution gradient for the analysis of dns-HGA and dns-MCPG in plasma specimens.
Figure 3. Evaluation of plasma matrix effects.
(A) A 4.0 μL injection of processed pooled plasma in 0.1% formic acid in water was injected onto the C18 column. During injection, a solution of dns-HGA (top, gray, dashed) and dns-MCPG (bottom, black, solid) was infused post-column. No significant plasma matrix effects were observed at the expected retention times of 4.28 ± 0.03 minutes for dns-HGA and 3.88 ± 0.03 minutes for dns-MCPG. (B) For comparison, the same experiment was performed, with 4.0 μL of solvent blank (0.1% formic acid) injected onto the column during the infusion.
3.5. Stability of QC Materials
To assess the stability of QC materials, the calculated concentrations after various storage conditions were compared to the average calculated concentrations from characterization to determine a percent recovery (see Materials and Methods). Each QC was stored at 4 °C and 22 °C (n = 3 for each QC) for 24 hours in order to evaluate the stability after leaving materials overnight to thaw in a refrigerator or on a laboratory benchtop. At both temperatures, the recovery was greater than 86% for both analytes, which suggests that both HGA and MCPG are stable at 4 °C and 22 °C for at least 24 hours (Table 3). At 60 °C, the viscosity of the plasma QCs increased significantly; calibrators in water were used instead to assess compound stability at the highest temperature. Three calibrators (S2, S5, and S8) were stored at 60 °C for four hours (n = 3 of each calibrator) to establish stability during derivatization and nitrogen evaporation during sample preparation. After storage at 60 °C for four hours, both analytes achieved greater than 92% recovery (Table 3). Both compounds were evaluated after ten QC freeze-thaw cycles in order to demonstrate stability within storage vials containing ten aliquots of QC material at 500 μL volumes. (Table 3). Although the recovery was not within 20% of all characterized values, each of the QCs were within three standard deviations of their characterized means and therefore representative of the characterized precision from 22 analytical runs.
Table 3.
Stability of QCs and calibrators during ten freeze-thaw cycles and storage at temperatures of 4, 22, and 60 °C for up to 24 hours (n=3).
| HGA (%Recovery)* | MCPG (%Recovery)* | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| QH | QM | QL | QH | QM | QL | |
| 4 °C | 101 | 89.6 | 96.5 | 107 | 90.8 | 97.3 |
| 22 °C | 94.2 | 102 | 86.1 | 106 | 104 | 98.8 |
| Freeze Thaw | 105 | 128 | 122 | 109 | 134 | 121 |
|
| ||||||
| S8 | S5 | S2 | S8 | S5 | S2 | |
|
| ||||||
| 60 °C | 92.6 | 95.6 | 99.9 | 95.4 | 98.4 | 105 |
%Recovery, ( )
3.6. Ruggedness
Column temperature, injection volume, and mobile phase flow rate were varied to evaluate the analytical ruggedness of the assay. Each parameter was tested at a lower and higher value than the optimized parameters used. The column temperature was varied to 55 °C and 65 °C, the injection volume was changed to 3.0 μL and 5.0 μL, and the liquid chromatography flow rate was modified to 450 μL/min and 550 μL/min. All results fell within the characterized limits of two standard deviations from the mean. Three material lots from the HPLC column, SPE plate, and protein precipitation plate were evaluated during characterization and were not found to affect the calculated QC concentrations.
3.7. Application of Method
This method was applied to a commercial convenience set consisting of 100 individual plasma samples that served as a reference range for HGA and MCPG expected in a commercially available, unexposed population. No concentrations above the method LRL were detected for HGA or MCPG. In order to apply the method in a laboratory exercise to simulate a public health exposure response, a blinded convenience set was designed and analyzed (Table 4). Ten samples were prepared and blinded for analysis, wherein eight were enriched with varying levels of HGA and MCPG, while two remained blank. Samples quantified above the method HRL were diluted 50X and re-prepared for analysis. Experimental concentrations were compared to the theoretical concentration, and %RE was calculated. All samples were within 16% of their theoretical value. No false negatives nor false positives were observed in the blinded exercise (i.e. a 100% identification rate). As such, this assay is well-designed for the investigation of illness outbreaks where soapberry toxins are suspected as the causative agents.
Table 4.
Application of assay to a blinded exercise using a convenience sample set (n=10).
| HGA | MCPG | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Blinded Sample | Expected (ng/mL) | Actual (ng/mL) | %RE* | Expected (ng/mL) | Actual (ng/mL) | %RE* |
| 1 | 5.00 × 102 | 5.80 × 102 | 16 | 5.00 × 102 | 5.80 × 102 | 14 |
| 2 | 3.00 × 100 | 3.40 × 100 | 13 | 8.00 × 100 | 8.02 × 100 | 0.25 |
| 3 | 3.50 × 101 | 3.82 × 101 | 9.1 | 8.00 × 101 | 7.80 × 101 | 2.5 |
| 4 | 5.00 × 101 | 4.78 × 101 | 4.4 | 1.00 × 102 | 9.81 × 101 | 1.9 |
| 5 | Blank | Not Detected | N/A | Blank | Not Detected | N/A |
| 6 | 5.00 × 100 | 5.29 × 100 | 5.8 | 1.00 × 101 | 1.00 × 101 | 0.0 |
| 7 | 1.00 × 101 | 1.16 × 101 | 16 | 5.00 × 101 | 5.39 × 101 | 7.8 |
| 8 | Blank | Not Detected | N/A | Blank | Not Detected | N/A |
| 9 | 1.00 × 103 | 1.10 × 103 | 10. | 1.00 × 103 | 1.13 × 103 | 13 |
| 10 | 5.00 × 103 | 4.87 × 103 | 2.6 | 5.00 × 103 | 4.90 × 103 | 2.0 |
Percent relative error ( ); N/A = Not applicable
4. Conclusion
Reports of illnesses associated with the ingestion of soapberry fruits have commonly been linked to HGA. More recently, the lower mass analogue of HGA, MCPG, has gained interest due to its link to acute toxic encephalopathy. Although methods for HGA in blood products have been reported previously, the assay reported herein is the first to additionally quantify the lower mass analogue MCPG in blood products for simultaneous quantification of both HGA and MCPG. The assay reports levels of HGA from 1.00 to 100. and MCPG from 5.00 to 150. ng/mL in human blood plasma. Furthermore, blood samples detected above the assay’s highest reportable limit can be reported up to 10,000 ng/mL within a 7.9% relative error using dilution. A blinded laboratory exercise resulted in calculated concentrations within a 16% relative error of target theoretical values and a 100% identification rate. In the future, this quantitative assay can be applied to blood specimens from Jamaican Vomiting Sickness, acute toxic encephalopathy, and other illness-outbreaks linked to soapberry fruit exposures. With the addition of this assay to current clinical and agricultural methods for soapberry toxins, for the first time, laboratories can assist with public health investigations whether specimens are urine, fruit, or blood.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the Centers for Disease Control and Prevention, the Oak Ridge Institute for Science and Education, and the Battelle Memorial Institute.
The authors acknowledge Scott Landvatter, Rich Tyburski, and Stephen Jones for their efforts towards the initial custom synthesis that led to the commercial availability of the native standards and isotopically-labeled standards that were imperative for this work.
Abbreviations
- ave
average concentration
- CDC
Centers for Disease Control and Prevention
- dns-Cl
dansyl chloride
- dns-HGA
dansyl-hypoglycin A
- dns-15N13C2-HGA
dansyl-15N13C2-hypoglycin A
- dns-MCPG
dansyl-methylenecyclopropylglycine
- dns-13C3-MCPG
dansyl-13C3-methylenecyclopropylglycine
- DI
deionized
- ESI
electrospray ionization
- FDA
U.S. Food and Drug Administration
- HGA
hypoglycin A
- HPLC–MS/MS
high-pressure liquid chromatography–tandem mass spectrometry
- HRL
highest reportable limit
- ISTD
isotopically labeled calibrator solution
- LOD
limit of detection
- LRL
lowest reportable limit
- MCPG
methylenecyclopropylglycine
- MRM
multiple-reaction monitoring
- MRQCS
multirule quality-control system
- PPE
personal protective equipment
- QC
quality control
- QH
quality control high
- QM
quality control medium
- QL
quality control low
- %RE
percent relative error
- %RSD
percent relative standard deviation
- SD
standard deviation
- SPE
solid-phase extraction
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Disclaimer
The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services or the U.S. Centers for Disease Control and Prevention. The use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Service or the U.S. Centers for Disease Control and Prevention. The authors declare no competing financial interest.
References
- 1.Blake OA, Bennink MR, Jackson JC. Ackee (Blighia sapida) hypoglycin A toxicity: Dose response assessment in laboratory rats. Food and chemical toxicology. 2006;44(2):207–213. doi: 10.1016/j.fct.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Melde KJ, Bartlett SK, Sherratt SA, Ghisla S. Metabolic consequences of methylenecyclopropylglycine poisoning in rats. Biochem J. 1991;274:395–400. doi: 10.1042/bj2740395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka KK, Johnson EAB. Jamaican Vomiting Sickness: Biochemical investigation of two cases. N Engl J Med. 1976;295:461–467. doi: 10.1056/NEJM197608262950901. [DOI] [PubMed] [Google Scholar]
- 4.Shrivastava A, et al. Association of acute toxic encephalopathy with litchi consumption in an outbreak in Muzaffarpur, India, 2014: a case-control study. The Lancet Global Health. 2017;5(4):e458–e466. doi: 10.1016/S2214-109X(17)30035-9. [DOI] [PubMed] [Google Scholar]
- 5.Valberg S, et al. Seasonal pasture myopathy/atypical myopathy in North America associated with ingestion of hypoglycin A within seeds of the box elder tree. Equine veterinary journal. 2013;45(4):419–426. doi: 10.1111/j.2042-3306.2012.00684.x. [DOI] [PubMed] [Google Scholar]
- 6.Gaillard Y, et al. Fatal intoxication due to ackee (Blighia sapida) in Suriname and French Guyana. GC–MS detection and quantification of hypoglycin-A. Forensic science international. 2011;206(1):e103–e107. doi: 10.1016/j.forsciint.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Joskow R, et al. Ackee fruit poisoning: an outbreak investigation in Haiti 2000–2001, and review of the literature. Clinical Toxicology. 2006;44(3):267–273. doi: 10.1080/15563650600584410. [DOI] [PubMed] [Google Scholar]
- 8.McTague JA, Forney R. Jamaican vomiting sickness in Toledo, Ohio. Annals of emergency medicine. 1994;23(5):1116–1118. doi: 10.1016/s0196-0644(94)70112-1. [DOI] [PubMed] [Google Scholar]
- 9.Sherratt H. Hypoglycin, the famous toxin of the unripe Jamaican ackee fruit. Trends in Pharmacological Sciences. 1986;7:186–191. [Google Scholar]
- 10.FDA. Guidance for FDA Staff: Compliance Policy Guide Sec. 550.050 Canned Ackee, Frozen Ackee, and Other Ackee Products-Hypoglycin A Toxin. US Department of Health and Human Services. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Regulatory Affairs; 2014. [Google Scholar]
- 11.Bochnia M, et al. Hypoglycin A content in blood and urine discriminates horses with atypical myopathy from clinically normal horses grazing on the same pasture. PloS one. 2015;10(9):e0136785. doi: 10.1371/journal.pone.0136785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boemer F, et al. Quantification of hypoglycin A in serum using aTRAQ® assay. Journal of Chromatography B. 2015;997:75–80. doi: 10.1016/j.jchromb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Carlier J, et al. A validated method for quantifying hypoglycin A in whole blood by UHPLC–HRMS/MS. Journal of Chromatography B. 2015;978:70–77. doi: 10.1016/j.jchromb.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 14.Karlíková R, et al. Equine atypical myopathy: A metabolic study. The Veterinary Journal. 2016;216:125–132. doi: 10.1016/j.tvjl.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Sander J, et al. Rapid diagnosis of hypoglycin A intoxication in atypical myopathy of horses. Journal of Veterinary Diagnostic Investigation. 2016;28(2):98–104. doi: 10.1177/1040638715624736. [DOI] [PubMed] [Google Scholar]
- 16.Sander J, et al. Quantification of hypoglycin A as butyl ester. Journal of Chromatography B. 2016;1029:169–173. doi: 10.1016/j.jchromb.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Sander J, et al. Quantification of methylenecyclopropyl compounds and acyl conjugates by UPLC-MS/MS in the study of the biochemical effects of the ingestion of canned ackee (Blighia sapida) and lychee (Litchi chinensis) Journal of Agricultural and Food Chemistry. 2017;65(12):2603–2608. doi: 10.1021/acs.jafc.7b00224. [DOI] [PubMed] [Google Scholar]
- 18.Votion DM. Analysing hypoglycin A, methylenecyclopropylacetic acid conjugates and acylcarnitines in blood to confirm the diagnosis and improve our understanding of atypical myopathy. Equine Veterinary Education. 2018;30(1):29–30. [Google Scholar]
- 19.Isenberg SL, et al. Quantification of metabolites for assessing human exposure to soapberry toxins hypoglycin A and methylenecyclopropylglycine. Chem Res Toxicol. 2015;28:1753–1759. doi: 10.1021/acs.chemrestox.5b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chase G, Jr, Landen W, Jr, Soliman A. Hypoglycin A content in the aril, seeds, and husks of ackee fruit at various stages of ripeness. Journal-Association of Official Analytical Chemists. 1989;73(2):318–319. [PubMed] [Google Scholar]
- 21.Isenberg SL, et al. Quantification of Toxins in Soapberry (Sapindaceae) Arils: Hypoglycin A and Methylenecyclopropylglycine. Journal of Agricultural and Food Chemistry. 2016;64(27):5607–5613. doi: 10.1021/acs.jafc.6b02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebane R, Oldekop M-L, Herodes K. Comparison of amino acid derivatization reagents for LC–ESI-MS analysis. Introducing a novel phosphazene-based derivatization reagent. Journal of Chromatography B. 2012;904:99–106. doi: 10.1016/j.jchromb.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 23.Caudill SP, Schleicher RL, Pirkle JL. Multi-Rule quality for the age-related eye disease study. Statist Med. 2008;27:4094–4106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 24.Taylor JK. Quality Assurance of Chemical Measurements. Boca Raton, FL: CRC Press; 1987. [Google Scholar]
- 25.FDA. Guidance for industry and reviewers: Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers. US Department of Health and Human Services.. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER); 2002. [Google Scholar]
- 26.Annesley TM. Ion suppression in mass spectrometry. Clinical chemistry. 2003;49(7):1041–1044. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.