Abstract
Perturbations in WNT signaling are associated with congenital eye disorders, including familial exudative vitreoretinopathy and Norrie disease. More recently, activation of the WNT pathway has also been shown to be associated with age-related macular degeneration (AMD). In this study, we identified that in choroidal neovascular membranes from AMD patients, β-catenin is activated specifically in the vascular endothelium, suggesting that WNT promotes pathologic angiogenesis by directly affecting vascular endothelial cells. WNT7B has been shown to be important during eye development for regression of the fetal hyaloid vasculature. However, it has not yet been established whether WNT7A and/or WNT7B are involved in neovascular AMD pathogenesis. Here, we show that WNT7A and WNT7B increase the proliferation of human dermal microvascular endothelial cells in a dose-dependent manner. Both WNT7A and WNT7B also stimulated vascular sprouting from mouse choroidal explants in vitro. To evaluate in vivo relevance, we generated mice systemically deficient in Wnt7a and/or Wnt7b. Genetic deletion of both Wnt7a and Wnt7b decreased the severity of laser injury-induced choroidal neovascularization (CNV), while individual deletion of either Wnt7a or Wnt7b did not have a significant effect on CNV, suggesting that WNT7A and WNT7B have redundant pro-angiogenic roles in vivo. Cumulatively, these findings identify specific WNT isoforms that may play a pathologic role in CNV as observed in patients with neovascular AMD. Although the source of increased WNT7A and/or WNT7B in CNV requires further investigation, WNT signaling may be a potential target for therapeutic intervention if these results are demonstrated to be relevant in human disease.
Keywords: WNT7A, WNT7B, β-catenin, age-related macular degeneration, choroidal neovascularization, angiogenesis
Wingless-related integration site (WNT) signaling is highly conserved and important for regulating developmental processes including cell proliferation, differentiation, and tissue patterning (Logan and Nusse, 2004). In the absence of WNT ligands, kinases held in complex by Axin and adenomatous polyposis coli protein phosphorylate cytoplasmic β-catenin, leading to its degradation by the proteasome (Logan and Nusse, 2004). When WNT binds the Frizzled (FZD)-low-density lipoprotein receptor-related protein 5/6 (LRP5/6) receptor complex in the plasma membrane, the degradation complex is disrupted, stabilizing β-catenin and allowing for its translocation to the nucleus where it upregulates T-cell factor/lymphoid enhancer-binding factor-driven genes (Logan and Nusse, 2004). WNT ligands have also been shown to mediate cytoskeletal changes and intracellular calcium levels via non-canonical pathways that do not involve β-catenin (Komiya and Habas, 2008). WNT signaling is crucial for normal development, as mice deficient in different WNT ligands can display early developmental failure or abnormalities in individual organ systems (Logan and Nusse, 2004). In the eye, WNT signaling has been shown to be important in the specification of various cell fates, maintenance of retinal progenitor cells, vascularization of the retina, regression of the hyaloid vasculature, and development of the cornea and lens (Lobov et al., 2005; Fuhrmann, 2008; Lad et al., 2009; Fujimura, 2016; Drenser, 2016).
Clinically, dysregulation of the WNT pathway can cause manifestations in patients including tetra-amelia, tooth and bone defects, colorectal cancer, colon cancer, as well as defects in the eye (Logan and Nusse, 2004). Familial exudative vitreoretinopathy (FEVR) and Norrie disease (ND) are inherited disorders with overlapping ocular phenotypes, both of which have been associated with aberrations in WNT signaling (Nikopoulos et al., 2010b). Patients with FEVR typically present with peripheral retinal avascularity but may also manifest secondary complications including retinal tears, aberrant neovascularization, exudation, and hemorrhage (Poulter et al., 2010). FEVR has been reportedly linked to several WNT-related genes: NDP (encodes Norrin), TSPAN12 (tetraspanin-12), CTNNB1 (β-catenin), FZD4, and LRP5 (Chen et al., 1993; Robitaille et al., 2002; Toomes et al., 2004; Jiao et al., 2004; Nikopoulos et al., 2010a; Poulter et al., 2010; Panagiotou et al., 2017). Mouse models with genetic manipulations in some of these associated genes recapitulate the human FEVR phenotype (Xu et al., 2004; Ye et al., 2009; Wang et al., 2012). On the other hand, patients with ND present with congenital blindness and bilateral retinal detachment, but can also include pseudoglioma, leukocoria, microphthalmia, retrolental fibrovascular tissue, cataracts, hemorrhages, retinal folding, subretinal exudates, mental retardation, and deafness (Chen et al., 1993; Nikopoulos et al., 2010b). In contrast to the genetically heterogenous disease FEVR, ND is considered to be caused only by mutations in NDP (Berger et al., 1992; Chen et al., 1993).
As highlighted above, it has been established that abnormal WNT signaling is pathogenic in some congenital retinal diseases. Yet, its role in age-related eye diseases remains poorly studied. Neovascular (wet) age-related macular degeneration (AMD) is a common blinding disease of the elderly in industrialized nations and is characterized by choroidal neovascularization (CNV). It has been shown that there is increased canonical WNT signaling in the maculae of AMD patients that may be secondary to decreased plasma concentrations of kallistatin and/or Dickkopf-related protein 1 (DKK1), both endogenous WNT inhibitors (Tuo et al., 2015; Qiu et al., 2017). Studies of laser injury-induced CNV in animals and genetic mouse models suggest that the mechanistic link between WNT/β-catenin activation and AMD may be due to upregulation of angiogenic and inflammatory factors including vascular endothelial growth factor (VEGF), MYC proto-oncogene, cyclin D1, intercellular adhesion molecule 1, and tumor necrosis factor (TNF) (Zhou et al., 2010; Hu et al., 2013; Tuo et al., 2015). The clinical utility of targeting WNT/β-catenin signaling for AMD has also been explored preclinically. Intravitreal administration of antibodies blocking LRP6 attenuates the formation of AMD-like retinal lesions and rescues retinal function in Ccl2−/−Cx3cr1−/−rd8 and Ccl2−/−Cx3cr1gfp/gfp mice (Tuo et al., 2015). Similarly, a separate group reported that intravitreal injection of anti-LRP6 antibodies reduced vascular leakage and CNV area in rats following laser injury and reduced retinal inflammation and vascular leakage in Vldlr−/− mice (Hu et al., 2013). These studies have further linked β-catenin activation with angiogenic and inflammatory factors, as anti-LRP6 antibodies attenuated expression of several angiogenic and inflammatory WNT target genes (Hu et al., 2013; Tuo et al., 2015).
While broad inhibition of LRP6 in the eye highlights the therapeutic potential of targeting WNT signaling for AMD, several factors that impair its translation to the clinic remain, including cell specificity of β-catenin activation and the specific roles of the many WNT isoforms in the AMD retina. Regarding cell specificity, previous studies reported LRP6 activation in the retinal ganglion cell layer in AMD patients (Tuo et al., 2015), but it remains undetermined how WNT/β-catenin signaling in ganglion cells contributes to photoreceptor death in AMD pathogenesis. It also remains unclear which WNT ligand is pathogenic in AMD, especially when considering that certain isoforms such as WNT5A have actually been shown to suppress β-catenin signaling in human retinal pigment epithelial cells (Kim et al., 2015). Although WNT3A has been shown to activate β-catenin, upregulate VEGF and TNF, and increase oxidative stress, its relevance has been limited in scope to in vitro studies with ARPE19 cells (Zhou et al., 2010). In the current work, we sought to elucidate the specific WNT isoform(s) whose dysregulation could contribute to pathogenesis in AMD patients.
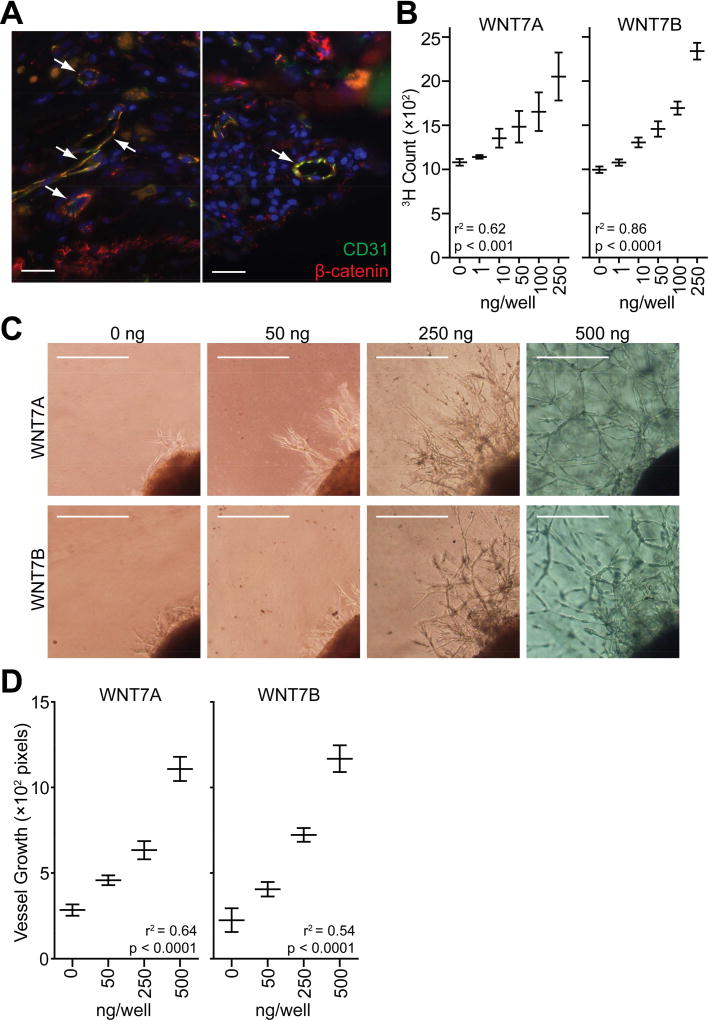
To clarify the precise tissue localization of β-catenin signaling in CNV seen in wet AMD, we analyzed choroidal neovascular membranes obtained from wet AMD patients. These membranes were surgically excised and preserved as part of usual patient treatment prior to the advent of anti-VEGF pharmacotherapy. Donors provided informed consent for these surgical procedures, and all human studies were performed in accordance with the Declaration of Helsinki and were approved by Washington University School of Medicine in St. Louis’s Human Research Protection Office. Tissue sections were prepared as described previously (Nakamura et al., 2015) and probed for active non-phosphorylated β-catenin (#8814, Cell Signaling Technologies) along with cluster of differentiation 31 (CD31, JC70A, Dako), a marker for endothelial cell junctions and CNV (Pennesi et al., 2012). Staining for activated β-catenin was prominent in endothelial cells surrounding blood vessels as indicated by the yellow overlay highlighting areas of co-localization with CD31 (white arrows in Fig. 1A). These data augment previous findings that WNT/β-catenin signaling is activated in retinal ganglion cells in AMD (Tuo et al., 2015) and demonstrate that this pathway is also activated in endothelial cells, suggesting that β-catenin may help promote pathologic angiogenesis.
Fig. 1.
WNT7A and WNT7B promote vascular proliferation in vitro. (A) Tissue sections prepared from choroidal neovascular membranes excised from patients with wet AMD were probed with antibodies specific for active non-phosphorylated β-catenin (red) and CD31 (green) by immunohistochemistry. Areas of co-localized β-catenin and CD31 staining are indicated in yellow. White arrows indicate blood vessels. DAPI (blue) was used to counterstain cell nuclei. Scale bars: 25 µm. (B) HMVEC proliferation was stimulated by recombinant human WNT7A and WNT7B (1-way ANOVA with post hoc test for linear trend). Graphs indicate the mean ± SEM of 3 independent experiments. (C–D) WNT7A and WNT7B promote vascular sprouting from mouse choroidal explants (1-way ANOVA with post hoc test for linear trend). Representative images are shown in (C; scale bars: 75 µm). Quantifications shown in (D) represent the mean ± SEM vessel growth measured in 4–14 explants.
We adopted a more targeted approach and hypothesized that WNT7A or WNT7B signaling might contribute to pathogenic CNV in wet AMD. Our speculation was guided by previous knowledge that these two WNT ligands are important for developmental angiogenesis of the central nervous system and eye (Lobov et al., 2005; Stenman et al., 2008; Daneman et al., 2009). Therefore, we initially tested whether WNT7A or WNT7B can directly promote vascular proliferation and growth. We evaluated the effect of human recombinant WNT7A (3008-WN, R&D) and WNT7B (ab152805, Abcam) on proliferation of human dermal microvascular endothelial cells (HMVECs). HMVECs (3 × 103 cells) from Lonza were cultured overnight in 96-well round-bottomed plates in EGM2V media as described previously (Nakamura et al., 2015). On the next day, WNT7A or WNT7B was added to the culture media in concentrations ranging from 0–250 ng/well. Cells were then incubated with 1 µCi/ml [3H]thymidine (PerkinElmer) for 18 hours, and proliferation was measured by radioactive thymidine incorporation using a TopCount Scintillation Counter (PerkinElmer). We found that both WNT7A and WNT7B stimulated HMVEC proliferation in a dose-dependent manner (Fig. 1B). We tested for a trend between HMVEC proliferation and WNT ligand concentration using 1-way ANOVA with a post test for linear trend. HMVEC proliferation was positively correlated with both WNT7A and WNT7B concentration with p-values < 0.001 and < 0.0001, respectively (Fig. 1B). We next investigated the effect of human recombinant WNT7A and WNT7B protein on an ex vivo model of microvascular angiogenesis (Shao et al., 2013). Briefly, we excised portions of the sclerochoroidal complex from mouse eyes and embedded them in Cultrex reduced growth factor basement membrane extract (Trevigen) in 24-well tissue culture plates. We added human endothelial serum free media (0.5 ml/well, Gibco) containing 2% FBS, 200 µg/ml endothelial cell growth supplement (BD Biosciences), 1% penicillin/streptomycin, and either WNT7A or WNT7B in concentrations ranging from 0–500 ng/well. We used human recombinant protein because human WNT7A and WNT7B are 99% identical to mouse proteins based on amino acid sequence (UniProt IDs: human WNT7A O00755; human WNT7B P56706; mouse WNT7A P24383; mouse WNT7B P28047). Media were replaced every 2 days. After 6 days, the explants were imaged by phase-contrast microscopy, and the mean distances of vascular sprouts were quantified using ImageJ (National Institutes of Health). In line with the HMVEC proliferation assay, we observed a dose-dependent increase in the sprouting of microvessels from the explanted tissue in response to exogenous WNT7A or WNT7B (Fig. 1C–D). This positive correlation between choroidal sprouting and WNT concentration was statistically significant as assessed by 1-way ANOVA with a post test for linear trend (p-values < 0.0001 for both WNT7A and WNT7B). Together, these data indicate that WNT7A and WNT7B directly promote vascular growth and proliferation in vitro.
To evaluate the in vivo relevance of WNT7A and WNT7B in neovascularization, we next obtained mice lacking expression of Wnt7a and/or Wnt7b. All experimental protocols involving animals conformed to the ARRIVE guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and protocols were approved by the Animal Studies Committee of the IACUC of Washington University School of Medicine in St. Louis. Wnt7a+/− mice were purchased from The Jackson Laboratory to be used for generation of Wnt7a−/− mice because complete deletion of Wnt7a causes mouse infertility (Dunlap et al., 2011). Homozygous mutant Wnt7b mice do not survive gestation (Parr et al., 2001), precluding the generation of germline Wnt7b−/− mice. Therefore, tamoxifen inducible Wnt7bf/fCreERtam mice were generated by breeding Wnt7bf/f mice, which were generously provided by Dr. Fanxin Long of Washington University School of Medicine in St. Louis, with CreERtam mice from The Jackson Laboratory. In addition, we generated Wnt7a−/−Wnt7bf/fCreERtam by breeding Wnt7a+/− Wnt7bf/fCreERtam with Wnt7a+/−Wnt7bf/f mice. Systemic Cre recombinase expression was induced as follows: tamoxifen (T5648, Sigma) was administered daily by intraperitoneal injections for 5 days (100 mg/kg bodyweight, 1% w/v in sunflower oil). This regimen was repeated after mice were rested for 2 days (i.e. 10 total tamoxifen injections were administered). All mice bred and used for these experiments were screened for rd1 and rd8 mutations using published protocols to assure that only mice with a wild type background at those alleles were used for the experiments (Giménez and Montoliu, 2001; Mattapallil et al., 2012).
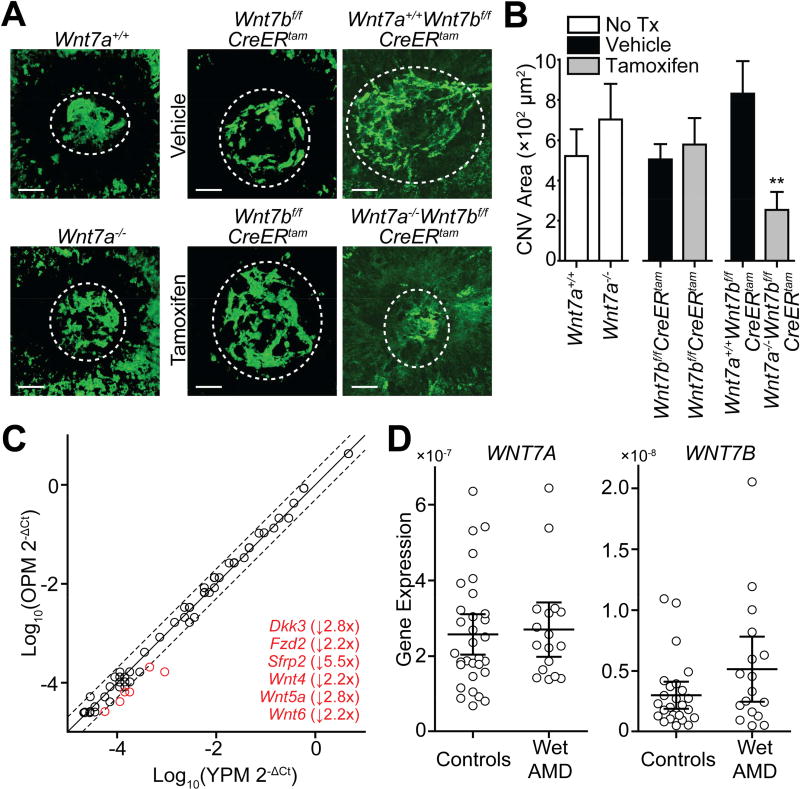
To determine whether these genetic manipulations affected pathologic angiogenesis in vivo, we measured CNV area in a laser injury-induced mouse model of neovascularization. This method involves rupturing Bruch’s membrane with an Argon laser to induce CNV as described previously (Apte et al., 2006; Kelly et al., 2007; Dace and Apte, 2008; Lambert et al., 2013; Sene et al., 2013). All mice used in this study were female and between 8–16 weeks of age. Briefly, mice were anesthetized by intraperitoneal injection of ketamine hydrochloride (100 mg/kg bodyweight) and xylazine (13.4 mg/kg bodyweight). Then, pupils were dilated with 1% tropicamide eye drops. Using an argon green laser (Phoenix Laboratory), 3–4 laser burns were placed around the optic nerve (0.1 second, 100 µm, 150–200 mW). One week after injury, mice were anesthetized and perfused with 100 µl of 50 mg/ml FITC-labeled dextran (MW 2,000,000) via the femoral vein. Eyes were enucleated and fixed immediately in 4% paraformaldehyde for 1 hour. Eyes were then washed with PBS and the choroid was flat mounted onto a glass slide. Z-stack images of CNV spots were acquired using an Olympus FV1000 confocal microscope and processed in ImageJ to create pseudo volumetric 2D images. Pixel intensity was quantified using MetaMorph Software (Molecular Devices). In mice with tamoxifen-inducible Cre recombinase expression, we performed laser injury immediately after the 5th tamoxifen injection, and mice were sacrificed 1 week later after the 10th tamoxifen injection. We tested for significant differences in CNV area using the Mann-Whitney U test. Mice deficient in either Wnt7a or Wnt7b exhibited no significant differences in the area of CNV, but mice lacking both Wnt7a and Wnt7b exhibited a 3.3-fold decrease in CNV area that was statistically significant (Fig. 2A–B). These data indicate that WNT7A and WNT7B have redundant roles promoting pathologic angiogenesis in vivo.
Fig. 2.
WNT7A and WNT7B promote laser injury-induced CNV in mice but appear to function independent of monocytes in wet AMD. (A–B) Double knockout mice lacking systemic Wnt7a and Wnt7b expression demonstrated significantly decreased area of laser injury-induced CNV (Mann-Whitney U tests). Representative images are shown in (A; scale bars: 50 µm). Quantifications are provided in (B); each bar represents the mean ± SEM CNV area in 9–29 eyes. (C) Aged mouse peritoneal macrophages exhibit dysregulation of 6 WNT-related genes (>2.0-fold change cutoff as indicated by dashed lines). Thioglycollate-elicited peritoneal macrophages from old (17 months old, n=3) and young (1.5 months old, n=3) female mice were pooled, and we averaged 3 measurements per gene for each age group. Each open circle represents a single gene in the array. An inset list of downregulated genes (in red) is provided. (D) Expression of WNT7A and WNT7B is unchanged in PBMCs isolated from wet AMD patients as compared to healthy controls (Mann-Whitney U tests). Each open circle represents the mean of 2–3 PCR reactions performed for each case or control. Lines indicate the means with 95% confidence intervals for each group. (**, p<0.01; OPM, old peritoneal macrophages; Tx, treatment; YPM, young peritoneal macrophages).
The data presented above implicate that WNT7A and/or WNT7B activate β-catenin in endothelial cells in AMD to thereby promote pathogenic angiogenesis. Based on previous work from our lab showing that aging macrophages undergo a constellation of pro-angiogenic changes, we hypothesized that up-regulation of the WNT/β-catenin pathway in the choroidal neovascular membranes of wet AMD patients is due to aberrant WNT7A and/or WNT7B secretion from aged macrophages (Kelly et al., 2007; Sene et al., 2013; Nakamura et al., 2015; Lin et al., 2018). Thus, we profiled by PCR array the expression of 84 WNT-related genes in thioglycollate-elicited peritoneal macrophages harvested from old (17 months old, n=3) and young (1.5 months old, n=3) female mice. After harvesting, macrophages were cultured in RPMI-1640 media with 10% FBS and 1% penicillin/streptomycin. The next day, total RNA was extracted from macrophages using RNeasy mini kits (Qiagen), and RNA was reverse-transcribed to cDNA using a high capacity cDNA reverse transcription kit (Thermo Fisher Scientific). We used the Mouse WNT Signaling Pathway array (PAMM-043Z, Qiagen) and followed the manufacturer’s recommended protocol. Seventeen genes in the array were detected with Ct > 35, indicating little, if any, mRNA expression. Of the 67 remaining genes, only 6 displayed a >2.0-fold change in transcript expression (Fig. 2C), including: 1 gene related to non-canonical WNT pathways (Wnt5a); 1 member of the WNT receptor complex (Fzd2); 2 genes related to canonical WNT signaling (Wnt4 and Wnt6); and 2 inhibitors of WNT signaling (Dkk3 and Sfrp2). These decreases in gene expression were either consistent (Dkk3, Sfrp2, and Wnt5a), inconsistent (Wnt4 and Wnt6), or of unknown apparent relevance (Fzd2) with respect to activation of β-catenin observed in wet AMD choroidal neovascular membranes.
Downregulation of Dkk3 and Sfrp2 may be of particular interest for future study as decreased plasma levels of kallistatin and DKK1, additional endogenous WNT inhibitors, have already been reported to be associated with AMD (Tuo et al., 2015; Qiu et al., 2017). However, the array did not identify any changes in Wnt7a or Wnt7b expression (both genes were included in the array). We next compared expression of WNT7A and WNT7B in peripheral blood mononuclear cells (PBMCs) taken from wet AMD patients to expression in healthy PBMCs as described previously (Sene et al., 2013). Briefly, wet AMD patients and healthy controls donated blood in a case-control study design. Donors provided informed consent and were classified as having either ‘no AMD’ or ‘wet AMD’ according to previously-established criteria with wet AMD patients having CNV in at least 1 eye (Ferris et al., 2005). Cases and controls were relatively equally split between male and female participants (WNT7A: Chi-square p-value = 0.56, df = 1; WNT7B: Chi-square p-value = 0.49, df = 1). PBMCs were isolated from blood by density gradient centrifugation, total RNA was extracted from cells and reverse-transcribed to cDNA, and gene expression was assessed using a Taqman probe-based gene expression assay. Ct numbers for WNT7A and WNT7B were normalized to expression of 18S rRNA, and statistical significance was assessed using the Mann-Whitney U test. Expression of WNT7A and WNT7B was unchanged in wet AMD PBMCs (p-values = 0.67 and 0.21, respectively, Fig. 2D). Although WNT7A and WNT7B appear to have pro-angiogenic roles, these results suggest that the source of these WNT ligands is neither macrophages nor PBMCs.
In this work, we have demonstrated that β-catenin signaling is activated in endothelial cells within choroidal neovascular membranes of wet AMD patients and that this activation appears to be independent of signaling from circulating monocytes. We also show that WNT7A and WNT7B both promote endothelial cell proliferation in vitro and increase vascular sprouting from murine choroidal explants. We also provide evidence that WNT7A and WNT7B have redundant roles in vivo as mice lacking both Wnt7a and Wnt7b exhibit decreased laser-induced CNV while deficiencies in either Wnt7a or Wnt7b alone do not affect CNV area.
The data presented in this work shed further light on the role of β-catenin signaling in the pathobiology of wet AMD. It has been shown previously that upregulation of the WNT/β-catenin pathway is associated with AMD (Hu et al., 2013; Tuo et al., 2015; Qiu et al., 2017). The mechanism linking β-catenin activation with AMD has been suggested to be due to increased expression of angiogenic and inflammatory factors, namely TNF and VEGF (Vallée et al., 2017). It has been postulated that VEGF’s upregulation could be due to accumulation of cytosolic lactate produced by β-catenin-mediated activation of aerobic glycolysis (Vallée et al., 2017).
We have for the first time identified that WNT7A and WNT7B could link WNT signaling to pathologic neovascularization as seen in wet AMD. In doing so, we have identified a pathway that could be pharmacologically targeted within the eye, potentially limiting some of the adverse effects of targeting WNT signaling systemically (Kahn, 2014). We have also demonstrated that β-catenin activation through WNT7A and/or WNT7B in wet AMD seems to occur independently from PBMC signaling although it remains possible that WNT7A and/or WNT7B could be upregulated in activated resident eye macrophages in an age- or disease-related process. It is also possible that expression of WNT7A and/or WNT7B are unchanged, but decreased levels of WNT inhibitors kallistatin and/or DKK1 in wet AMD (Tuo et al., 2015; Qiu et al., 2017) cause over-activation of β-catenin signaling.
More notably, these findings highlight a pathologic role for WNT7A and WNT7B that could be more broadly applicable. Cancer and cardiovascular disease are also disorders involving angiogenesis that have been associated with aberrant WNT/β-catenin activation (Zhan et al., 2017; Foulquier et al., 2018). It is possible that upregulation of WNT7A and/or WNT7B might also contribute to aberrant angiogenesis in cancers or in atherosclerotic plaques.
In conclusion, we have provided novel evidence for a pathologic role of WNT7A and WNT7B in CNV. These findings highlight novel therapeutic options, particularly for wet AMD in which local administration may limit potential adverse effects.
Highlights.
β-catenin is activated in choroidal neovascular membranes in wet AMD.
WNT7A and WNT7B promote vascular proliferation in in vitro and ex vivo models.
In vivo, WNT7A and WNT7B promote choroidal neovascularization.
Acknowledgments
This work was supported by NIH grants R01 EY019287 (RSA), P30 EY02687 (Vision Core Grant); the Starr Foundation (RSA); the Carl Marshall Reeves and Mildred Almen Reeves Foundation (RSA); the Bill and Emily Kuzma Family Gift for retinal research (RSA); a Physician8 Scientist Award and a Nelson Trust Award from Research to Prevent Blindness (RSA); the Jeffrey Fort Innovation Fund (RSA); the Glenn Foundation (RSA); the Macula Society Cox Research Award (RSA); and the Thome Foundation (RSA). Additional funding comes from an unrestricted grant to the Department of Ophthalmology & Visual Sciences of Washington University School of Medicine from Research to Prevent Blindness. Joseph B. Lin was supported by the Washington University in St. Louis Medical Scientist Training Program (NIH grant T32 GM07200) and the VitreoRetinal Surgery Foundation. Jonathan B. Lin was supported by NIH grants T32 GM07200, UL1 TR002345, and TL1 TR002344. None of these funding sources were involved in study design; in data collection, analysis, or interpretation; or in the preparation of the manuscript.
Abbreviations
- AMD
age-related macular degeneration
- CD31
cluster of differentiation 31
- CNV
choroidal neovascularization
- DKK1
Dickkopf-related protein 1
- FEVR
familial exudative vitreoretinopathy
- FZD
Frizzled
- HMVECs
human dermal microvascular endothelial cells
- LRP
low density lipoprotein receptor-related protein
- ND
Norrie disease
- NDP
Norrin
- PBMCs
peripheral blood mononuclear cells
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- WNT
wingless-related integration site
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Declarations of interest: none.
References
- Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006;3:e310. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W, van de Pol D, Warburg M, Gal A, Bleeker-Wagemakers L, de Silva H, Meindl A, Meitinger T, Cremers F, Ropers HH. Mutations in the candidate gene for Norrie disease. Hum. Mol. Genet. 1992;1:461–465. doi: 10.1093/hmg/1.7.461. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Battinelli EM, Fielder A, Bundey S, Sims K, Breakefield XO, Craig IW. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat. Genet. 1993;5:180–183. doi: 10.1038/ng1093-180. [DOI] [PubMed] [Google Scholar]
- Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11:177–185. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenser KA. Wnt signaling pathway in retinal vascularization. Eye Brain. 2016;8:141–146. doi: 10.2147/EB.S94452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap KA, Filant J, Hayashi K, Rucker EB, Song G, Deng JM, Behringer RR, DeMayo FJ, Lydon J, Jeong J-W, Spencer TE. Postnatal Deletion of Wnt7a Inhibits Uterine Gland Morphogenesis and Compromises Adult Fertility in Mice. Biol. Reprod. 2011;85:386–396. doi: 10.1095/biolreprod.111.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris FL, Davis MD, Clemons TE, Lee L-Y, Chew EY, Lindblad AS, Milton RC, Bressler SB, Klein R Age-Related Eye Disease Study (AREDS) Research Group. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch. Ophthalmol. Chic. Ill 1960. 2005;123:1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulquier S, Daskalopoulos EP, Lluri G, Hermans KCM, Deb A, Blankesteijn WM. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2018;70:68–141. doi: 10.1124/pr.117.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S. Wnt signaling in eye organogenesis. Organogenesis. 2008;4:60–67. doi: 10.4161/org.4.2.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N. WNT/β-Catenin Signaling in Vertebrate Eye Development. Front. Cell Dev. Biol. 2016;4:138. doi: 10.3389/fcell.2016.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez E, Montoliu L. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdeb(rd1)) in FVB/N-derived transgenic mice. Lab. Anim. 2001;35:153–156. doi: 10.1258/0023677011911525. [DOI] [PubMed] [Google Scholar]
- Hu Y, Chen Y, Lin M, Lee K, Mott RA, Ma J. Pathogenic role of the Wnt signaling pathway activation in laser-induced choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2013;54:141–154. doi: 10.1167/iovs.12-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Ventruto V, Trese MT, Shastry BS, Hejtmancik JF. Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in LRP5. Am. J. Hum. Genet. 2004;75:878–884. doi: 10.1086/425080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J. Clin. Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-H, Park S, Chung H, Oh S. Wnt5a attenuates the pathogenic effects of the Wnt/β-catenin pathway in human retinal pigment epithelial cells via down-regulating β-catenin and Snail. BMB Rep. 2015;48:525–530. doi: 10.5483/BMBRep.2015.48.9.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad EM, Cheshier SH, Kalani MYS. Wnt-signaling in retinal development and disease. Stem Cells Dev. 2009;18:7–16. doi: 10.1089/scd.2008.0169. [DOI] [PubMed] [Google Scholar]
- Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez M-LA, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart JM, Rakic J-M, Noel A. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013;8:2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- Lin Jonathan B, Moolani HV, Sene A, Sidhu R, Kell P, Lin Joseph B, Dong Z, Ban N, Ory DS, Apte RS. Macrophage microRNA-150 promotes pathological angiogenesis as seen in age-related macular degeneration. JCI Insight. 2018;3 doi: 10.1172/jci.insight.120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Mattapallil MJ, Wawrousek EF, Chan C-C, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest. Ophthalmol. Vis. Sci. 2012;53:2921–2927. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Sene A, Santeford A, Gdoura A, Kubota S, Zapata N, Apte RS. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat. Commun. 2015;6:7847. doi: 10.1038/ncomms8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikopoulos K, Gilissen C, Hoischen A, van Nouhuys CE, Boonstra FN, Blokland EAW, Arts P, Wieskamp N, Strom TM, Ayuso C, Tilanus MAD, Bouwhuis S, Mukhopadhyay A, Scheffer H, Hoefsloot LH, Veltman JA, Cremers FPM, Collin RWJ. Next-generation sequencing of a 40 Mb linkage interval reveals TSPAN12 mutations in patients with familial exudative vitreoretinopathy. Am. J. Hum. Genet. 2010a;86:240–247. doi: 10.1016/j.ajhg.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikopoulos K, Venselaar H, Collin RWJ, Riveiro-Alvarez R, Boonstra FN, Hooymans JMM, Mukhopadhyay A, Shears D, van Bers M, de Wijs IJ, van Essen AJ, Sijmons RH, Tilanus MAD, van Nouhuys CE, Ayuso C, Hoefsloot LH, Cremers FPM. Overview of the mutation spectrum in familial exudative vitreoretinopathy and Norrie disease with identification of 21 novel variants in FZD4, LRP5, and NDP. Hum. Mutat. 2010b;31:656–666. doi: 10.1002/humu.21250. [DOI] [PubMed] [Google Scholar]
- Panagiotou ES, Sanjurjo Soriano C, Poulter JA, Lord EC, Dzulova D, Kondo H, Hiyoshi A, Chung BH-Y, Chu YW-Y, Lai CHY, Tafoya ME, Karjosukarso D, Collin RWJ, Topping J, Downey LM, Ali M, Inglehearn CF, Toomes C. Defects in the Cell Signaling Mediator β-Catenin Cause the Retinal Vascular Condition FEVR. Am. J. Hum. Genet. 2017;100:960–968. doi: 10.1016/j.ajhg.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, Cornish VA, Cybulsky MI, McMahon AP. Wnt7b regulates placental development in mice. Dev. Biol. 2001;237:324–332. doi: 10.1006/dbio.2001.0373. [DOI] [PubMed] [Google Scholar]
- Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol. Aspects Med. 2012;33:487–509. doi: 10.1016/j.mam.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter JA, Ali M, Gilmour DF, Rice A, Kondo H, Hayashi K, Mackey DA, Kearns LS, Ruddle JB, Craig JE, Pierce EA, Downey LM, Mohamed MD, Markham AF, Inglehearn CF, Toomes C. Mutations in TSPAN12 Cause Autosomal-Dominant Familial Exudative Vitreoretinopathy. Am. J. Hum. Genet. 2010;86:248–253. doi: 10.1016/j.ajhg.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Liu Zhen, Zhou Y, He J, Gong S, Bai X, Zeng Y, Liu Zuguo, Ma J-X. Decreased Circulating Levels of Dickkopf-1 in Patients with Exudative Age-related Macular Degeneration. Sci. Rep. 2017;7:1263. doi: 10.1038/s41598-017-01119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille J, MacDonald MLE, Kaykas A, Sheldahl LC, Zeisler J, Dubé M-P, Zhang L-H, Singaraja RR, Guernsey DL, Zheng B, Siebert LF, Hoskin-Mott A, Trese MT, Pimstone SN, Shastry BS, Moon RT, Hayden MR, Goldberg YP, Samuels ME. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat. Genet. 2002;32:326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- Sene A, Khan AA, Cox D, Nakamura REI, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Haqbi-Levi S, Chowers I, Edwards PA, Baldan A, Parks JS, Ory DS, Apte RS. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013;17:549–561. doi: 10.1016/j.cmet.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Friedlander M, Hurst CG, Cui Z, Pei DT, Evans LP, Juan AM, Tahiri H, Tahir H, Duhamel F, Chen J, Sapieha P, Chemtob S, Joyal J-S, Smith LEH. Choroid sprouting assay: an ex vivo model of microvascular angiogenesis. PloS One. 2013;8:e69552. doi: 10.1371/journal.pone.0069552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA, Craig JE, Jiang L, Yang Z, Trembath R, Woodruff G, Gregory-Evans CY, Gregory-Evans K, Parker MJ, Black GCM, Downey LM, Zhang K, Inglehearn CF. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am. J. Hum. Genet. 2004;74:721–730. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Wang Y, Cheng R, Li Y, Chen M, Qiu F, Qian H, Shen D, Penalva R, Xu H, Ma J-X, Chan C-C. Wnt signaling in age-related macular degeneration: human macular tissue and mouse model. J. Transl. Med. 2015;13:330. doi: 10.1186/s12967-015-0683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée A, Lecarpentier Y, Guillevin R, Vallée J-N. Aerobic Glycolysis Hypothesis Through WNT/Beta-Catenin Pathway in Exudative Age-Related Macular Degeneration. J. Mol. Neurosci. MN. 2017;62:368–379. doi: 10.1007/s12031-017-0947-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, Ma J. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2010;51:4371–4379. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]