Abstract
Loss of photoreceptors and other retinal cells is a common endpoint in retinal degenerate (RD) diseases that cause blindness. Retinal transplantation is a potential therapy to replace damaged retinal cells and improve vision. In this study, we examined the development of human fetal retinal sheets with or without their retinal pigment epithelium (RPE) transplanted to immunodeficient retinal degenerate rho S334ter-3 rats. Sheets were dissected from fetal human eyes (11–15.7 weeks gestation) and then transplanted to the subretinal space of 24–31 d old RD nude rats. Every month post surgery, eyes were imaged by high-resolution spectral-domain optical coherence tomography (SD-OCT). SD-OCT showed that transplants were placed into the subretinal space and developed laminated areas or rosettes, with clear development of plexiform layers first seen in OCT at 3 months post surgery. Several months later, as could be expected by the much slower development of human cells compared to rat cells, transplant photoreceptors developed inner and later outer segments. Retinal sections were analyzed by immunohistochemistry for human and retinal markers and confirmed the formation of several retinal subtypes within the retinal layers. Transplant cells extended processes and a lot of the cells could also be seen migrating into the host retina. At 5.8 – 8.6 months post surgery, selected rats were exposed to light flashes and recorded for visual responses in superior colliculus, (visual center in midbrain). Four of seven rats with transplants showed responses to flashes of light in a limited area of superior colliculus. No response with the same dim light intensity was found in age-matched RD controls (non-surgery or sham surgery). In summary, our data showed that human fetal retinal sheets transplanted to the severely disturbed subretinal space of RD nude rats develop mature photoreceptors and other retinal cells, integrate with the host and induce vision improvement.
Keywords: Retinal restoration, retinal development, stem cells, immunodeficient rat, electrophysiology, retinal transplantation, optical coherence tomography, superior colliculus recording
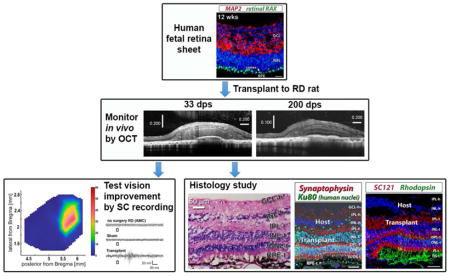
Graphical Abstract
1. Introduction
Age-related macular degeneration (de Jong, 2006; Hanus et al., 2016), Stargardt’s disease (Walia and Fishman, 2009), and retinitis pigmentosa (Hartong et al., 2006) are common RD diseases, which often result in irreversible blindness. Many treatments currently in use and in development, such as gene therapy (Bennett et al., 1996; Liu et al., 2011), and trophic factors (Lavail, 2005) show effect only in early disease stages when the host photoreceptors are damaged but still alive and can be rescued. Rescue treatments are unsuccessful when photoreceptors are irreversibly degenerated. Since retinal ganglion cells can survive after severe photoreceptor loss (Medeiros and Curcio, 2001), the most effective treatment presently would likely be to replace the photoreceptors and other retinal cells.
Extensive studies have shown that up to now, fetal donor tissue has been a very effective choice for transplantation in the central nervous system (Brundin et al., 1988; Lindvall and Bjorklund, 2011). Further studies indicated that this also applies to retinal transplantation for replacing cells of a degenerating retina (Seiler and Aramant, 1998; Ghosh et al., 1999). Fetal retina can be transplanted as dissociated cells (Huang et al., 2014), aggregates (Juliusson et al., 1993; Ivert et al., 1998; Gouras and Tanabe, 2003) and sheets (Silverman et al., 1992; Seiler and Aramant, 1998; Radtke et al., 2008; Li et al., 2009).
Later studies to transplant dissociated photoreceptor precursor cells did not report any convincing result of vision improvement (MacLaren et al., 2006; West et al., 2008; Pearson et al., 2010; West et al., 2010). Suspecting this may be due to the low numbers of photoreceptor precursors that integrated into the retina (0.2% of the injected cells; less than 1000 cells), Pearson et al optimized the procedure to increase the number of integrated cells to an average of 4.25% (17,000) of 400,000 injected cells. They reported that transplanted NRL-GFP mouse rod precursors formed synaptic connections with the host retina and improved visual function. At 4–6 weeks post transplantation (relatively short time), visual function was measured by whole-cell recording, electroretinograms, optical imaging, optomotor response and watermaze with improvements in some transplanted mice (Pearson et al., 2012).
Transplantation of dissociated cells has an advantage as the surgery is simpler than transplantation of aggregates and sheets. However, dissociated cells cannot form a laminated organization and have a limited survival long term (Juliusson et al., 1993; Mansergh et al., 2010). Transplants of cell aggregates can form rosettes but not parallel laminated layers (Juliusson et al., 1993; Ivert et al., 1998; Gouras and Tanabe, 2003). Previous work had shown that a degenerated retinal area replaced by a fetal sheet with or without its RPE, formed laminated transplants in rodents (Seiler and Aramant, 1998; Aramant et al., 1999; Aramant and Seiler, 2002b). In several rat RD models, rat fetal–derived retinal sheet transplants restored visual responses in an area of the superior colliculus corresponding to the transplant location in the retina (Woch et al., 2001; Sagdullaev et al., 2003; Thomas et al., 2004; Seiler et al., 2010; Yang et al., 2010; Seiler et al., 2017).
Pre-clinically, it was important to know if sheets of human fetal retina together with its RPE could be transplanted to the subretinal space. A study that used nude immunodeficient rats with normal vision showed that retinal sheet transplants with RPE could develop laminated structures after long survival times (Aramant and Seiler, 2002b), which led to the first clinical co-transplant trials (Radtke et al., 2002). In the following phase II clinical trial it was demonstrated that sheet transplants of human fetal retina with its RPE improved visual acuity in 7 of 10 patients after one year (Radtke et al., 2008). However, visual function improvement following retinal sheet transplantation had not been tested in a model of retinal degeneration. As the ultimate goal is to transplant in human patients suffering from retinal degeneration, the capability of human retinal sheet transplants to integrate into the remaining host architecture and the extent to which visual function is restored, needs to be tested in an animal model. Human tissue develops and matures at a slower rate compared to rat tissue, so the required time for experimental analysis needs to be extended. The current study aims to address these questions.
Immunosuppression is commonly applied when transplanting a xenograft such as human tissue to rats. However, immunosuppression gives unreliable results (Anderson et al., 2011), is labor-intensive and may cause pain and damage to the animals. For example, the immunosuppressant cyclosporine A can have nephrotoxic effects (Thliveris et al., 1991; Cibulskyte et al., 2005), and reduces visual responses in RCS rats (Cooper et al., 2016). Therefore, we developed a double mutant rat with a Foxn1−/− and rho S334ter−/− line-3 rhodopsin mutation (Seiler et al., 2014) which is both immunodeficient and retinal degenerate. This rat model eliminates the need for immunosuppression when transplanting xenografts and also eliminates a slow chronic immune rejection of allografts (Seiler et al., 2017).
The present study evaluated the visual function of this RD nude rat model after transplantation of human fetal retinal sheets, using extracellular recordings in the superior colliculus (SC). Transplant development and interaction between donor and host were studied using OCT and immunohistochemistry. Our data showed that transplantation of human fetal retinal sheets could improve visual function in this rat model of advanced stage of retinal degeneration.
2. Material and Methods
2.1. Animals
For all experimental procedures, animals were treated in accordance with NIH guidelines for the care and use of laboratory animals, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, under a protocol approved by the Institutional Animal Care and Use Committee of UC Irvine. Animals were group housed in cage racks with individually filtered air. All rats were maintained on a 12 hour light/dark cycle (lights on 6:30am – 6:30pm) at an ambient temperature of 21.5 ± 0.8 °C and a relative humidity of 50%. NIH nude rats (NTac:NIH-Foxn1rnu) were bred in-house from founder breeders purchased from Taconic (Taconic Bioscience, Inc., Hudson, NY). SD-Foxn1 Tg(S334ter)3Lav (RD nude rat) transplant recipients were generated by crossing SD-Tg(S334ter)3Lav rat and NTac:NIH-Whn rats (Seiler et al., 2014).
2.2. Donor Tissue Isolation and Transplantation Procedures
Human fetal eyes (11–15.7 weeks post-conception) were obtained from hSCRO approved sources after overnight shipping in hibernate E medium with B27 supplements (Life Technologies, now Thermo Fisher Scientific, Waltham, MA, USA). Donor human fetal retina was isolated in hibernate E medium and treated as previously described (Aramant and Seiler, 2002b). Briefly, donor eyes were incubated in 10% dispase for 30–40 min at 37°C to enable the RPE and retina to be dissected free from surrounding tissues. The donor tissue was taken from different areas of the fetal retina excluding the macula since the RPE is easily detached in this area. Prior to transplantation, a small piece of donor tissue (on average 0.7 mm × 1.2 mm) was prepared and inserted into a custom-made transplantation instrument (Aramant and Seiler, 2002b). Some transplanted pieces included the attached RPE while others were retina alone. Information on the donor tissue and the transplanted rats is summarized in Table 1. Retinal sheet transplantation was performed as previously described (Aramant and Seiler, 2002a; Yang et al., 2010). Recipient rats (P26–31, either sex) were anesthetized with Ketamine (40–55 mg/kg)/Xylazine (6.0 – 7.5 mg/kg). A ~1 mm incision was made posterior to the pars plana and followed by local mechanical retinal detachment. To prevent eyelid swelling, rats received a subcutaneous injection of Ketoprofen (4mg/kg) (Zoetis Inc., Kalamazoo, MI) prior to anesthesia. The eyes were also frequently treated with dexamethasone eye drops (Bausch & Lomb Inc., Tampa) tetracaine (Bausch & Lomb Inc., Tampa FL) and Atropine eye drops (Akorn Pharmaceuticals, Lake Forest, IL). Donor retinal transplant tissue was delivered to the subretinal space of the left eye using a custom implantation instrument. The incision was closed with 10–0 sutures. Sham surgery consisted in injection of media only. For recovery, rats were given a subcutaneous injection of Ringer saline solution and were then placed in a Thermocare incubator to recover. The surgery eye was treated with Betadyne and triple antibiotic ointment (neomycin/bacitracin/polymyxin). For pain management, the animals also received analgesic Buprenex (0.03 mg/kg) (Reckitt Benckiser Pharmaceuticals Inc., Richmond VA).
Table 1.
Overview of experimental rats
| Rat ID | Donor age | Transplant with RPE (Y: yes. N: no) | Days post-surgery (dps) when euthanized | Age(d) when euthanized | Transplant organization: (L: laminated R: rosettes) | Response to light by SC recording (Y: yes. N: no. N/A: not recorded) |
|---|---|---|---|---|---|---|
| 1 | 15 wk 5 day |
N | 55 | 81 | R | N/A |
| 2 | 11 wk 5 days |
Y | 68 | 95 | R | N/A |
| 3 | 15 wk 5 day |
N | 70 | 96 | R | N/A |
| 4 | 11 wk 3 day |
N | 99 | 130 | L | N/A |
| 5 | 11 wk 1 day |
Y | 175 | 201 | R | Y |
| 6 | 11 wk 1 day |
N | 189 | 215 | R | Y |
| 7 | 11 wk 3 day |
Y | 201 | 232 | L | N |
| 8 | 11 wk 5 days |
Y | 202 | 228 | L * | N/A |
| 9 | 11 wk 3 day |
Y | 207 | 238 | L | Y |
| 10 | 11 wk 5 days |
Y | 207 | 234 | L | N/A |
| 11 | 11 wk 3 day |
Y | 209 | 240 | R | N |
| 12 | 12 wk | Y | 242 | 272 | L | N |
| 13 | 11 wk 5 day |
N | 257 | 284 | R | Y |
Transplant almost completely laminated; rat died before it could be recorded
2.3. SD-OCT Imaging
As described previously (Seiler et al., 2017), rats were anesthetized with ketamine/xylazine, the eyes were dilated with atropine, and SD-OCT images of the retina were obtained using a Bioptigen Envisu R2200 Spectral Domain Ophthalmic Imaging System (Bioptigen, Research Triangle Park, NC). Transplanted rats were imaged every 1–2 months from 2 weeks after surgery and with the last scan as close to the terminal experiment (SC recording) time as possible.
2.4. Histology and Immunofluorescence
Most rats were perfusion-fixed with cold 4% paraformaldehyde in 0.1 M Na-phosphate buffer. The eye cups were processed and embedded in Tissue-Tek® O.C.T. compound and frozen using isopentane on dry ice. Serial 10μm cryostat sections were cut and stored at −20°C. Every fifth slide was stained using hematoxylin and eosin (H&E). For antigen retrieval during immunofluorescence experiments, cryostat sections were incubated for 30 minutes at 70 °C in HistoVT One (Nacalai Inc., San Diego, CA) Sections were blocked for at least 30 min in 10% donkey serum. Primaries were left on sections overnight at 4 °C and on the next day, sections were incubated for at least 30 min at room temperature in secondary antibodies (Jackson Immunoresearch). A list of primary and secondary antibodies is presented in Table 2. Sections were coverslipped using Vectashield mounting media (Vector Labs) with 5 μg/mL DAPI. Fluorescent images were captured either on an Olympus BXH10 microscope, a Nikon FXA microscope or a Zeiss LSM700 confocal microscope. After taking confocal stacks of 5–8 micron thickness at 20x, 40× and 63× (selected images), Zen 2012 software was used to extract 3D confocal images in separate color channels that were later combined in Adobe Photoshop CS6 software. Volocity (×64) software (Perkin-Elmer, www.cellularimaging.com) was used to obtain higher magnification 3D opacity rendered images.
Table 2.
List of primary antibodies
| Antibodies | species | specific for | dilution | supplier |
|---|---|---|---|---|
| Bassoon | Mouse | Ribbon synapses | 1:600 | Stressgen (San Diego, CA) |
| Calretinin | Goat | Ganglion and amacrine cells | 1:1000 | Chemicon (Temecula, CA) |
| Ch×10 | sheep | Retinal progenitor cells | 1:200 | Thermo Fisher (Huntington Beach, CA) |
| Cone Transducin | Rabbit | Cone outer segments | 1:200 | Cytosignal (Irvine, CA) |
| CRALBP | Rabbit | Cellular retinaldehyde binding protein; RPE and Müller cells | 1:1000 | Dr. Saari, Univ. of WA (Saari et al., 1984) |
| GFAP | Mouse | Reactive Müller cells | 1:100 | Cell Signaling (Danvers, MA) |
| Iba-1 | Rabbit | Microglia | 1:100 | Biocare Medical (Pacheco, CA) |
| Ku-80 | Rabbit | Human nuclei | 1:400 | Abcam (Eugene, OR) |
| MAP2 | Mouse | Microtubule-associated protein 2 | 1:100 | Millipore/Chemicon (Temecula, CA) |
| MAP2 | Rabbit | Microtubule-associated protein 2 | 1:500 | Chemicon |
| NeuN | Mouse | Neuronal nuclei | 1:400 | Chemicon |
| PKC α | Rabbit | Rod bipolar cells | 1:200 | Oxford Biomedical (Riviera, FL) |
| PKC α | Mouse | Rod bipolar cells | 1:50 | Amersham (Little Chalfont, U.K.) |
| OTX2 | Rabbit | Orthodenticle Homeobox 2 | 1:1000 | Chemicon |
| Recoverin | Rabbit | Photoreceptors, cone bipolar cells | 1:500 | Dr. Dizhoor, Univ. PA (Dizhoor et al., 1991);; Millipore/Chemicon |
| Red Green Opsin | Rabbit | Red and Green cones | 1:2000 | Chemicon |
| Ret. RAX | Rabbit | Retina And Anterior Neural Fold Homeobox Protein | 1:200 | Santa Cruz Biotechnology, Dallas, Texas |
| Rhodopsin (rho1D4) | Mouse | Rods | 1:100 | Dr. Molday, Univ. of British Columbia (Molday and MacKenzie, 1983) |
| Rhodopsin | Rabbit | Rods | 1:2000 | Dr. Plantner, Cleveland, OH (Plantner t al., 1982) |
| Secretagogin | Rabbit | Cone bipolar cells | 1:200 | Cell Signaling |
| α-Synuclein | Rabbit | Rodent amacrine cells and IPL; OLM (general) | 1:100 | Cell Signaling |
| Synaptophysin | mouse | Membrane protein of synaptic vesicles | 1:500 | Sigma |
| Synaptophysin | Goat | Membrane protein of synaptic vesicles | 1:100 | Novus Biologicals (Littleton, CO) |
| SC-121 | Mouse | Cytoplasm of human cells | 1:2K | Stem Cell Inc. (Newark, CA) |
| Vimentin | mouse | Intermediate filament present in most cells | 1:500 | ICN |
| Secondary Antibodies | ||||
| Conjugate | species | specific for | dilution | supplier |
| Alexa Fluor 488 | Donkey | Rabbit IgG (H+L) | 1:400 | Jacson Immuno Research (West Grove, PA) |
| Rhodamine X | Donkey | Rabbit IgG (H+L) | 1:400 | Jacson Immuno Research |
| Rhodamine X | Donkey | Mouse IgG (H+L) | 1:400 | Jacson Immuno Research |
| Alexa Fluor 647 | Donkey | Goat IgG (H+L) | 1:400 | Jacson Immuno Research |
2.5. Superior colliculus (SC) electrophysiology
Visual responses from the SC were recorded as previously described (Seiler et al., 2017). In brief, responses from selected transplanted RD nude rats were recorded between 6.6 – 9.5 months of age (5.8 – 8.6 months post surgery) and compared with responses from age-matched non-transplanted RD nude and sham rats. Multi-unit electrophysiological recording was performed from 50–60 locations on the SC surface approximately 200–400μm apart using a tungsten microelectrode (0.5 MΩ impedance; MicroProbe, Inc, Carlsbad, CA). At each location, light stimuli (20 ms duration) were delivered approximately 10-times at 10-seconds intervals at an intensity of 0.58 to −6.13 log cd/m2. To detect the response threshold, when responses were found, the intensity of the light stimuli was reduced until there was no response. Electrophysiological responses to the strongest light stimuli (stimulus level 0.58 log cd/m2, corresponding to 3.8 lux) were quantified and formed into a map over the area of the SC using a custom MATLAB program (Mathworks, Natick, MA). All spikes occurring 30 ms to 210 ms after the onset of the photic stimulus were counted.
2.8. Statistical Analysis
For all statistical analyses, the significance level was calculated in Graphpad Prism software (Graphpad Software LLC, La Jolla, CA) using t-tests (paired and unpaired), Level of significance was set at 0.05.
2.9. Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
3. Results
3.1. Development and characterization of human fetal retinas
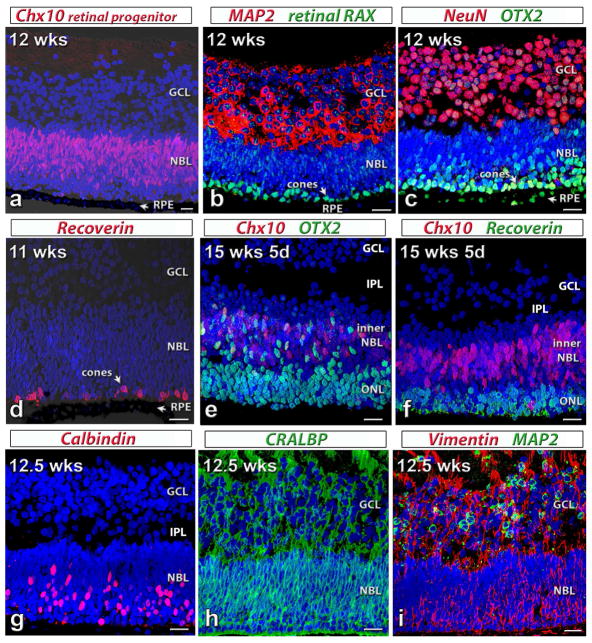
Donor tissue was isolated from five human fetuses at 11–15.7 weeks (wks) gestational age. As shown in figure 1, cells in the tissues were retinal progenitors, which were still developing. In a tissue at 12 weeks gestational age, strong Ch×10 (Visual System Homeobox 2 protein, VSX2) staining showed retinal progenitors in the whole neuroblastic layer (NBL; figure 1a). Developing ganglion cells were visible by strong MAP2 (Microtubule-associated protein 2) staining (cytoplasm of developing ganglion cells, figure 1b) and NeuN (Neuronal Nuclei) staining (figure 1c) in the ganglion cell layer. A layer of differentiating cone photoreceptors (based on their location close to the outer limiting membrane and the larger cell size), was strongly stained by retinal RAX (retinal Homeobox protein) (figure 1b) and OTX2 (Orthodenticle Homeobox 2) antibodies (figure 1c) in the outer neuroblastic layer (NBL). At this stage (12 weeks) there is no rhodopsin staining (data not shown) (Seiler and Aramant, 1994; O’Brien et al., 2003; Hoshino et al., 2017). RPE were also immunoreactive for OTX2 (figure 1c). At 11 wks, only 1 layer of developing photoreceptors was stained by recoverin in the outer NBL (figure 1d) but at 15.7 weeks, several layers of recoverin-positive photoreceptors were visible. Co-expression of OTX2 and Ch×10 were seen in the inner NBL of a retina at 15.7 wks. Other retinal cells, such as horizontal cells (Calbindin, figure 1g), RPE and Müller cells (CRALBP, cellular retinaldehyde binding protein, in figure 1h and Vimentin in figure 1i) were labeled in the human fetal retinal tissue.
Figure 1. Characterization of human fetal (HF) retina by immunohistochemistry.
Nuclei are stained by DAPI (blue).
(a)–(c): 12 weeks postconception. The retina contains a thick ganglion cell layer (GCL) and a neuroblastic layer (NBL). Arrow: retinal pigment epithelium (RPE). (a) Ch×10 (red, retinal progenitor cell marker) in neuroblastic layer (NBL).
(b) Cytoplasm of most cells in GCL: strongly label for MAP2 (microtubule-associated protein 2, red). Retinal RAX (retinal Homeobox protein, green): faint immunoreactivity in all nuclei; strong stain of nuclei of developing cones. (c) Intense NeuN (neuronal nuclei, red) stain of most ganglion cells. Strong OTX2 (Orthodenticle Homeobox 2, green) staining in RPE and developing photoreceptors.
(d) 11 weeks postconception. Recoverin (red): scattered differentiating cone photoreceptors in NBL close to RPE.
(e,f) 15 weeks 5 d postconception. The outer nuclear layer (ONL) has separated from the inner NBL. Retina has developed an inner plexiform layer (IPL). (e) Strong OTX2 (green) staining in developing photoreceptors and cells in inner NBL, some co-expressing Ch×10 (red). (f) Recoverin (green) staining of several layers of developing photoreceptors. Ch×10 (red) staining in inner NBL.
(g–i) 12.5 weeks postconception. (g) Calbindin (red) staining of developing horizontal cells and cones in outer NBL. (h) CRALBP (cellular retinaldehyde protein, green) immunoreactivity of developing Müller cells. (i) Strong Vimentin (red) staining throughout. MAP2 (green) staining in GCL.
Scale bars: 20 micron.
3.2. OCT results
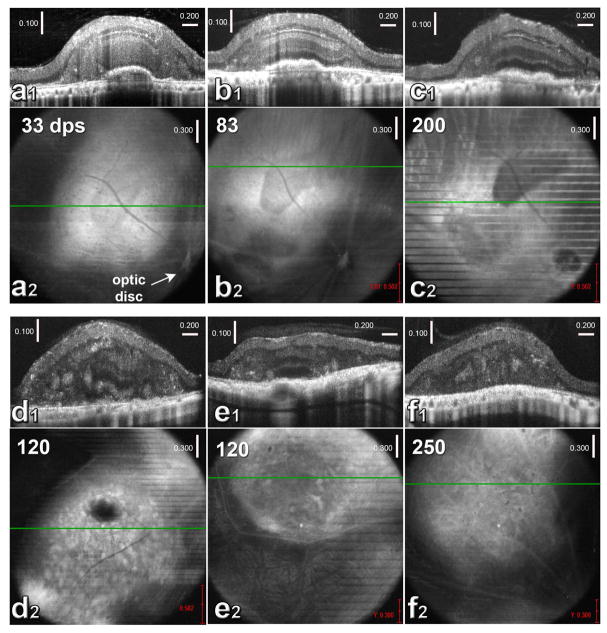
Thirteen of 22 rats were successfully transplanted. The transplants could be detected by OCT. As shown by OCT in figure 2, transplants of human fetal retinal sheets were immature at 1 month post-surgery (figure 2a1 and a2) with only IPL and outer neuroblastic layers recognizable. Around 3 months post-surgery, transplants developed into visible laminated retinal layers (figure 2b1 and b2). Transplants maintained the same structure until the end of experiment and with outer segments detectable in some cases (figure 2c1 and c2). Six of the transplants developed laminated layers (n= 6, figure 2a1–c1), and seven rosettes only (n=7, figure 2d1, d2, f1 and f2). Sometimes a mixture of both lamination and rosettes was seen (figure 2e1 and e2). Supplementary figure 1 shows OCT scans of rat #1 at 33, 83, and 200 dps.
Figure 2. OCT imaging of HF retinal transplants in vivo (a1, b1, c1, d1, e1, f1).
Cross-sectional B-scans. (a2, b2, c2, d2, e2, f2): corresponding fundus images (maximum projections). The green lines indicate the positions of the B-scans (above).
(a–c) show the same transplant (rat #8) at different times post-transplantation. Dark area in the fundus image indicates cografted RPE.
(a1) B-scan and (a2) fundus image at 33 day post-surgery (dps). At this time, only IPL and outer NBL of the transplant are recognizable in B-scan. (b1) B-scan and (b2) fundus image of the same eye at 83 dps. All retinal layers are visible in B-scan. (c1) B-scan and (c2) fundus image of the same eye at 200 dps. All retinal layers and outer segments are recognizable in B-scan. This fundus image is a narrower projection of the B-scans to show the RPE co-graft more clearly. Lines indicate rat breathing movements during scan.
(d1) B-scan and (d2) fundus image of the eye of transplanted rat #7 at 120 dps. B-scan shows partial laminated structure in transplant. Rosettes are visible in both images (hyper-reflective spots). Optic disc is outside the fundus image.
(e1) B-scan and (e2) fundus image of rat #9 at 120 dps. In B-scan, the center of the transplant appears laminated. Rosettes are recognizable too.
(f1) B-scan and (f2) fundus images of rat #13 at 250 dps. Rosettes are visible. Scales in a1, b1, c1, d1, e1, and f1: vertical: 100 μm; horizontal: 200 μm.
Scales in a2, b2, c2, d2, e2, and f2: 300 μm.
3.3 Histology
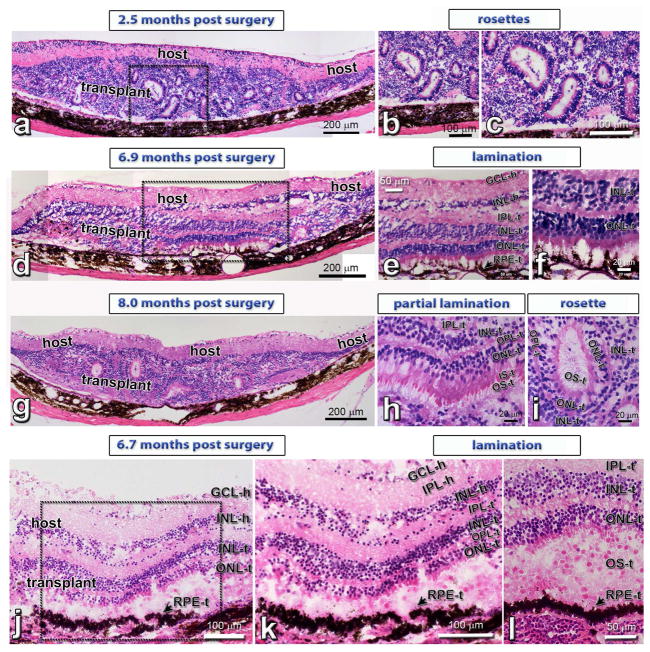
Hematoxylin-Eosin (H–E) staining confirmed the OCT results (figure 3). Transplants formed laminated layers (figure 3d–f and j–l), rosettes (figure 3a–c) and both (figure 3g–i).
Figure 3. Hematoxylin-Eosin (H–E) staining of HF retina transplants.
(a–c): HF retina + RPE transplant, 2.5 months post surgery (rat #2): Formation of photoreceptors in rosettes, due to the difficult surgery in the rat eye.
(d–f): HF retina + RPE transplant (#9), 6.9 months post surgery: lamination in transplant center with co-grafted RPE (see Fig. 7a.b). Same transplant as in Figure 2e).
(g–i): HF retinal transplant (#13), 8 months post surgery with partial lamination (h) and rosettes (i). Same transplant as in Figure 2f).
(j–l): Laminated HF retina + RPE transplant (#8; same as in Figure 2a–c), 6.7 months after surgery. This rat was found dead overnight, therefore there are some postmortem artefacts.
Scale bars are 200 μm in (a, d, g), 100 μm in (b, c, j, k), 50 μm in (e, l) and 20 μm (f, h, i). GCL = ganglion cell layer, IPL = inner plexiform layer, INL = inner nuclear layer, ONL = outer nuclear layer; RPE = retinal pigment epithelium; -h = host, -t = transplant
3.3.1. Photoreceptors
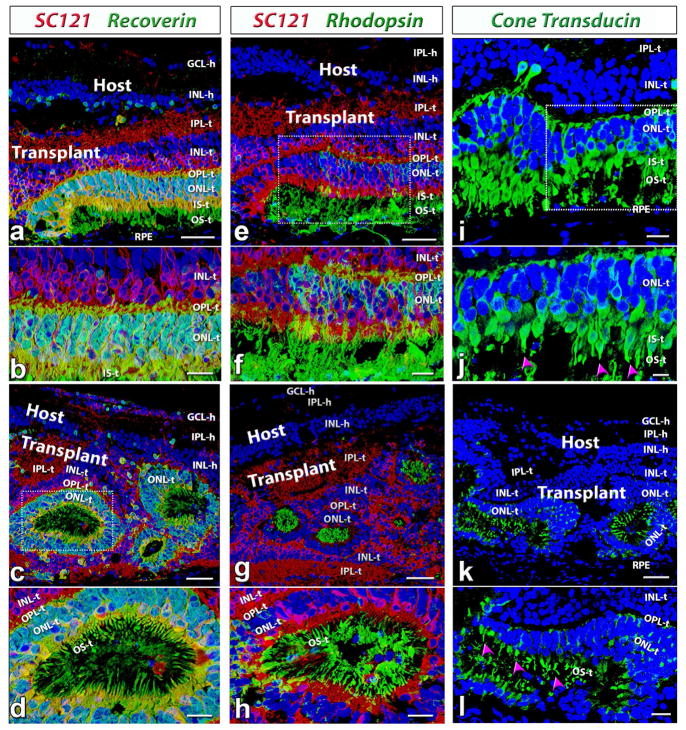
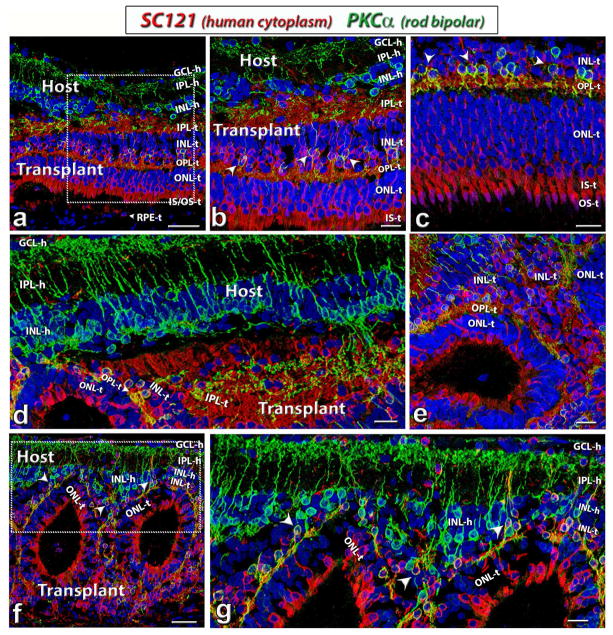
After transplantation into the subretinal space, the fetal retinal sheets developed and matured into laminated structures recognizable as IPL, INL, OPL and ONL layers. As shown in figure 4, matured photoreceptors with outer segments could be found in the transplants at 6 months post-surgery. Recoverin staining showed clear laminated photoreceptor layers in some transplants (n=6/13, figure 4a and b). Although most transplants formed rosette structures (spherical arrangement of retinal layers, with photoreceptors in the center, n=7/13), lamination within rosettes was still apparent (figure 4c and d). Rhodopsin (specific for rods, figures 4e–h) and cone transducin (specific for cones, figure 4i–l) staining confirmed the finding and showed that the rod and cone outer segments faced correctly to the RPE in laminated transplants and faced the rosette center in rosetted transplants. There was no rhodopsin or cone transducin staining in the host (data not shown; see Figs. 1, 2, 8 of (Seiler et al., 2017) indicating a complete loss of photoreceptor in the host at the time tested (5.8 – 8.6 months post surgery).
Figure 4. Photoreceptor development in HF retina transplants.
(a–d): Recoverin (photoreceptors and cone bipolar cells, green), SC121 (human cytoplasm, red). (e–h) Rhodopsin (rods, green), SC121 (red). (i–l): cone transducin (cones, green). Nuclei are stained blue (DAPI).
(a,b,e,f,i,j) Laminated transplant (rat # 9) at 207 dps (same transplant as in Figure 2e; 3d). (a,b) Strong recoverin (green) labeling of photoreceptors and cone bipolar cells within the HFE transplant (red). Note that there is much fewer recoverin label in the host retina. (e,f) Transplant-specific rods. Strong staining of rod outer segments in transplant. There is no rhodopsin staining in host. (i, j) Transplant-specific cones in the outer nuclear layer of the transplant are correctly oriented with putative outer segments facing the RPE (purple arrow heads in (j).
(c,d,g,h,k,l): Many transplants however formed a rosetted morphology (rat #13; same transplant as in Figure 2f; 3g). Lamination within the rosettes of the transplant, however, was still apparent. Distinct outer nuclear, outer plexiform and inner nuclear layers surrounded the rosetted photoreceptors (c,d). Putative outer segments of rods (g,h) and cones (k, l; purple arrow heads) formed within the rosettes.
Scale bars are 50 μm in (a, c, e, g, k), 20 μm in (b, d, f, h, i, l) and 10 μm (j). GCL = ganglion cell layer, IPL = inner plexiform layer, INL = inner nuclear layer, ONL = outer nuclear layer; IS = inner segments, OS = outer segments, RPE = retinal pigment epithelium; -h = host, -t = transplant
3.3.2. Bipolar cells
To study the rod and cone bipolar cells, PKCα and secretagogin antibodies were used, respectively. As shown in figure 5, strong PKCα-positive rod bipolar cells were found in the host retina. Co-localization of PKCα and SC121 (human cytoplasmic marker) in transplants demonstrated that some donor progenitor cells developed into mature rod bipolar cells after the transplantation. In some areas, host rod bipolar cells extended processes into transplants (figure 5d). Even in some transplants that only formed rosettes, the rosettes were very close to host rod bipolar cells and integrated with the host (figure 5f,g).
Figure 5. Rod bipolar cells (PKCa) development in HF retinal transplants.
Combination of label for PKCα (protein kinase C α; rod bipolar cells, green), SC121 (human cytoplasm, red) and DAPI (cell nuclei, blue).
(a–c): Well-laminated transplants in rat # 9 (a, b) and #12 (c).
(d–g): Rosetted transplants in rat #7 (d, e) and #11 (f, g). Arrowheads point out co-localization of SC121 and PKCα, indicating donor rod bipolar cells. Donor and host tissues are well-integrated and in some areas, host rod bipolar cells extend processes into transplant (d).
Scale bars are 50 μm in (a, c, f) and 20 μm in (b, d, e, g). GCL = ganglion cell layer, IPL = inner plexiform layer, INL = inner nuclear layer, OPL = outer plexiform layer, ONL = outer nuclear layer; IS = inner segments, OS = outer segments, RPE = retinal pigment epithelium; -h = host, -t = transplant
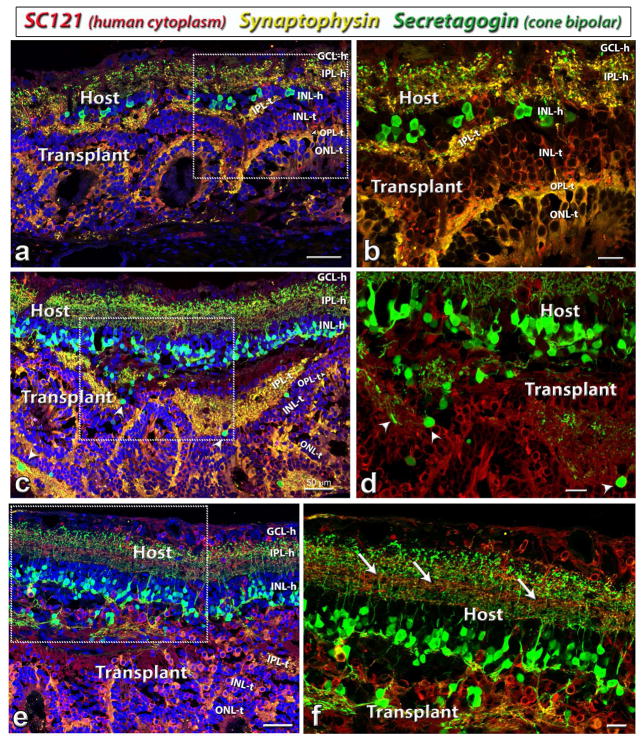
Similarly, after the transplantation, some cells in the transplant developed into secretagogin-positive cone bipolar cells (figure 6). Compared with rod bipolar cells, there were much less cone bipolar cells in the transplant than in the host (figure 6c and d). The transplant processes in the host IPL intermingled well with host cone bipolar cell processes (figure 6e,f). Synaptophysin, marker for membrane protein of synaptic vesicles, also showed strong staining in both host and donor, indicating the good integration of host and donor tissues (figures 6, 7).
Figure 6. Cone bipolar cells (Secretagogin) development in HF retinal transplants.
Combination of label for Secretagogin (Cone bipolar cells, green), Synaptophysin (membrane protein of synaptic vesicles, goat antibody; yellow-gold), SC121 (human cytoplasm, red) and DAPI (cell nuclei, blue). Secretagogin labeling of cone bipolar cells in rat #9 (a, b) rat #13 (c, d,) and rat #6 (e, f). Most secretagogin-positive cone bipolar cells are found in the host but some are found in transplants (co-localization of secretagogin and SC121, arrow heads in (c, d). Many SC121 positive human cells have migrated into the host. Arrows in (f) point to SC121 labeled processes in host IPL. (b, d, f) are enlargement of the boxed area in (a, c, e) respectively.
Scale bars are 50 μm in (a, c, e) and 20 μm in (b, d, f). GCL = ganglion cell layer, IPL = inner plexiform layer, INL = inner nuclear layer, OPL = outer plexiform layer, ONL = outer nuclear layer; -h = host, -t = transplant
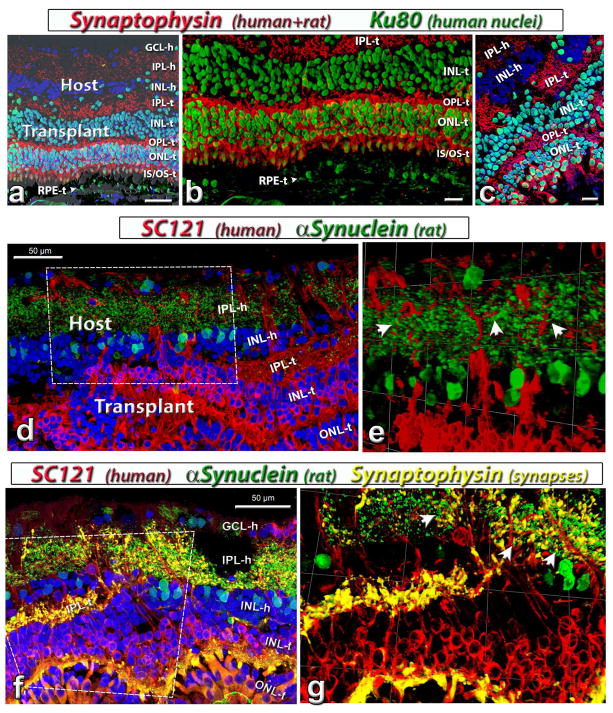
Figure 7. Synaptic markers and connectivity in host and transplant.
(a, b, c) Combination of label for synaptophysin (synaptic vesicles, mouse antibody; red), Ku80 (human nuclei, green) with DAPI (nuclei, blue).
(a, b) Laminated transplant in rat #9. Synaptophysin staining of plexiform layers and cone inner segments. The image in (a) is in combination with brightfield and shows donor RPE (RPE-t) in interaction with transplant outer segments. Some transplant cells migrated into the host. (b) is enlargement of (a) without DAPI. (c) Rosetted transplant in rat #11.
(d, e) Combination of SC121 (human cytoplasm, red) and α-Synuclein (rodent amacrine cells and IPL, green) in rat #9. (e) is a tilted image created in Volocity (www.cellularimaging.com) of the boxed area in (d), showing host IPL and transplant interface. Arrow heads point to the possible direct contact of host and transplant processes.
(f, g) Combination of SC121 (human cytoplasm, red), synaptophysin (goat antibody, yellow-gold), α-Synuclein (rodent amacrine cells and IPL, green) and DAPI (nuclei, blue) in rat #13. Strong synaptophysin staining of transplant inner plexiform (IPL-t) and outer plexiform layer (OPL-t). Note the extension of SC121-positive processes into the host IPL (IPL-h). (g) is a tilted image created in Volocity (www.cellularimaging.com) of the boxed area in (f). Arrows point to possible synaptic contacts of host and transplant. Scale bars are 50 μm in (a, d, f) and 20 μm in (b, c, g). 1 unit in (e) equals 40.05 μm, and 60.06 μm in (g). GCL = ganglion cell layer, IPL = inner plexiform layer, INL = inner nuclear layer, OPL = outer plexiform layer, ONL = outer nuclear layer; -h = host, -t = transplant
3.3.3. Human donor markers
Donor cellular processes and cells immunoreactive for SC121 (marker for cytoplasm of human cells) were found in the host retina (figures 4 and 5). To clarify whether donor cells migrate into the host retina, Ku80 (human nuclei marker) was used (figures 7a–c, 8c,d). As shown in figures 7a, and 8c, human retinal sheet transplants could form laminated structures, and some Ku80-positive (human nuclei marker) cells did migrate into host retina. H–E staining showed RPE in contact with transplant photoreceptors (figures 3d, e and f). The cells were identified as RPE based on their location in the RPE layer and their pigmentation. In the same location on other sections nearby, these cells were Ku80-positive (figures 7a, b) indicating they originated from human RPE of the donor. This was confirmed by CRALBP and Ku80 staining of adjacent sections (supplementary figure 2).
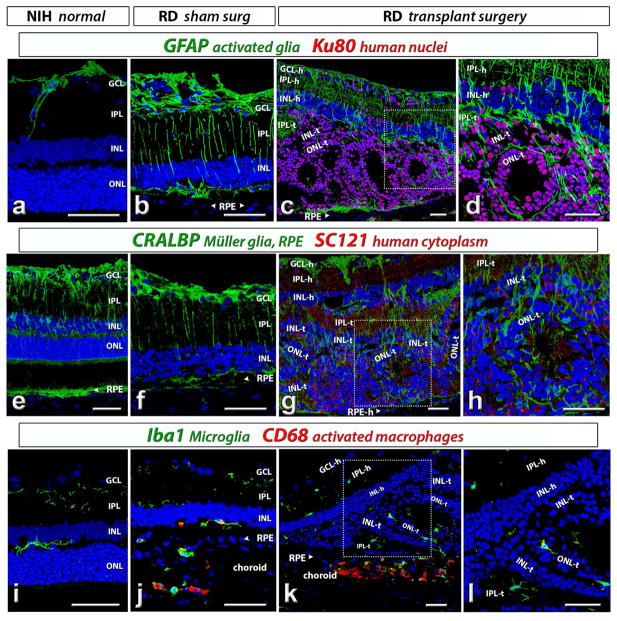
Figure 8. Analysis of glia and microglia cells in host and transplant.
(a–d) GFAP (glial fibrillary acidic protein, green). (a) In an NIH rat with normal retina, GFAP is only expressed in astrocytes on retinal surface and along blood vessels. (b) In a sham surgery RD rat, GFAP is also strongly expressed in Müller cells, in addition to astrocytes. (c,d) GFAP (green) and Ku80 (human nuclei, red) expression in a transplanted RD rat (#6). There is more GFAP staining in the retinal degenerate host than in transplant. Some donor cells have migrated into host retina.
(e–h) CRALBP (cellular retinaldehyde binding protein, green). (e) CRALBP in Müller cells and RPE of a NIH rat with normal retina. (f) CRALBP expression in Müller cells and RPE of a sham surgery RD rat. (g,h) CRALBP and SC121 (human cytoplasm, red) staining in Müller cells and RPE of a transplanted RD rat (#13). Donor processes (SC121 +) extend into the host INL and IPL. The Müller cells in the transplant rosettes are radially oriented.
(i–l) Iba1 (microglia, green) in combination with (j–l) CD68 (activated macrophages, red). (i) Microglia in IPL and OPL of a NIH rat with normal retina. (j) microglia (green) and activated macrophages (red) staining in a sham surgery RD rat. Microglia are mainly observed in IPL and subretinal space; while activated macrophages are mainly found in the choroid. (k,l) Iba1 (green) and CD68 (red) staining in a transplanted RD rat (#13). Iba1 + microglia are seen in both host and donor and CD68 + cells were mostly restricted to the choroid and not found in the transplant. (d), (h) and (l) were enlargement of the boxed area in (c), (g) and (k) respectively. Scale bars: 50 μm. GCL = ganglion cell layer, IPL = inner plexiform layer, INL = inner nuclear layer, ONL = outer nuclear layer; -h = host, -t = transplant
3.3.4. Transplant-host connectivity and synaptic markers
To examine synaptic connectivity between donor and host cells, a combination of SC121 and α-synuclein (marker for rodent amacrine cells and IPL) showed colocalization of both labels in the host IPL (figure 7d,e). The addition of goat anti-synaptophysin (with a higher affinity for human) showed the SC121 (human), α-Synuclein (rat) and Synaptophysin stained processes were seen in close contact to each other, suggesting that donor cells made synaptic connections with the host (figure 7f,g). The synaptophysin marker showed clear IPL and OPL in the donor tissue but only IPL in the host retina (figure 6 and 7a–c, f).
3.3.5. Glia and microglia
Several glial markers (GFAP, CRALBP) and microglia markers (Iba1 and CD68) were used to study glial and inflammatory responses to donor tissues in the host. In NIH nude rats with normal retina, GFAP + astrocytes were found on the retinal surface (optic fiber layer) and along blood vessels only (figure 8a). More GFAP + glia were found in in Müller cells of RD rats without surgery (data not shown) and sham RD rats (figure 8b) and in the host retina of transplanted RD rats (figure 8c and d) likely due to retinal degeneration and injury caused by surgery. However, there was less GFAP-reactive glia in the donor tissue than in the host retina (figure 8c and d). The CRALBP antibody labeled Müller cells and RPE. The intensity of CRALBP staining in NIH nude rats with normal retina (figure 8e), sham RD rats (figure 8f) and transplanted RD rats (figure 8g, h; supplementary figure 2) was similar. Müller cells were oriented radially in rosettes. Only few Iba1 stained microglia cells were found in IPL and OPL in the normal retina of NIH nude rats, which have no T cells. Iba1 staining appeared to be similar in sham RD rats (figure 8j) and transplanted RD rats (figure 8k,l), indicating that there was no increase in microglia. CD68 + activated macrophages were found in sham RD rats (figure 8j) and transplanted rats (figure 8k and l) but mostly restricted to choroid. These data suggested that the donor tissue did not induce an inflammatory response after transplantation to this immunodeficient rat model.
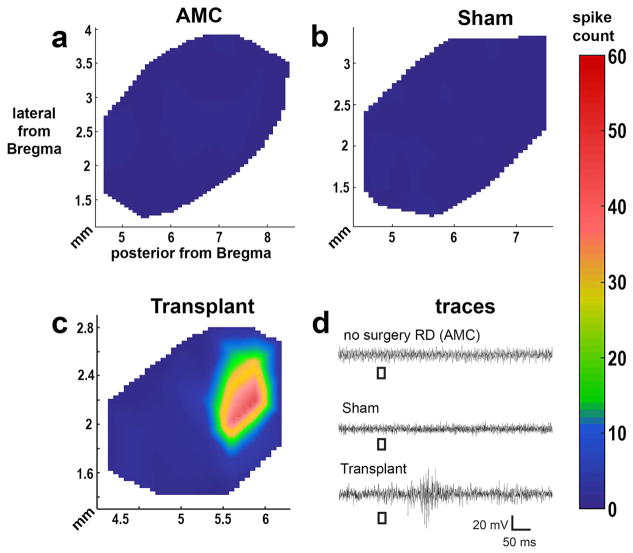
3.4. Visual function improvement evaluated by superior colliculus (SC) electrophysiological recording
To access the visual improvement after transplantation, selected rats were analyzed by electrophysiological recording in the SC at 5.8 – 8.6 months after transplantation. Four of seven transplanted rats selected for SC electrophysiological recording showed responses to flashes of light (0.58 log cd/m2) in a limited area of SC after transplantation. In some rats, dimmer light (0.1 log cd/m2) could still evoke responses (figure 9, Table 3). In contrast, there was no response to flashes of light (0.58 log cd/m2) in age-matched sham surgery rats (n=9, 3.3 – 8.9 MPS, age 4.2 – 9.9 months) or age matched control non-surgery rats (n=12, age 4.5 – 9 months, table 3).
Figure 9. Visual improvement assessed by superior colliculus (SC) recording.
Representative spike count heatmaps of (a) non-surgery age matched control (AMC), (b) sham surgery, and (c) transplanted rat with response (#13). No response was found in the entire SC area of non-surgery AMC and sham rats. Strong responses were found in some areas of the SC in 4 of 7 recorded transplant rats (see Table 3). (d) Traces are representative from either the location with the strongest response in transplant rats or a random location in non-surgery AMC and sham rat. The scale bar applies to all traces and the color bar applies to all heatmaps.
Table 3.
Summary of SC data of human fetal retinal (HFR) transplants, and compared with that of rat fetal retinal transplants
| Rat ID | Donor age | Age at surgery (d) | Age at recording (d) | Time post-surgery (d) | % responsive/recorded area | Max spike count | Best response threshold (log cd/m2) | |
|---|---|---|---|---|---|---|---|---|
| HFR transplants | 5 | 11 wk 1 day | 26 | 199 | 173 | 8 | 24.2 | 0.36 |
| 6 | 11 wk 1 day | 26 | 229 | 203 | 10.20 | 19.0 | 0.36 | |
| 9 | 11 wk 3 day | 31 | 238 | 207 | 6.67 | 11.5 | 0.58 | |
| 13 | 11 wk 5 days | 27 | 284 | 257 | 31.43 | 51.7 | 0.10 | |
| Mean | 27.50 | 237.50 | 210.00 | 14.07 | 26.6 | 0.35 | ||
| SEM | 1.19 | 17.60 | 17.41 | 5.05 | 7.6 | 0.08 | ||
| rat * transplants | Mean | 191.38 | 159.13 | 19.75 | 50.05 | -0.13 | ||
| SEM | 22.27 | 24.19 | 6.23 | 10.93 | 0.40 |
summary of the data in previous work (Seiler et al., 2017)
4. Discussion
This is the first study evaluating the development of human fetal retinal sheets and their effect on visual function after transplantation to the RD nude rat model (rho S334ter-3 rat), using OCT, immunohistochemistry and extracellular recordings in the superior colliculus. After transplantation, human retinal sheets survive long-term in an environment of advanced retinal degeneration, mature and develop into different retinal cells, integrate with the host retina, and improve visual function. Previous work suggested that pre-differentiation of retinal stem cells to photoreceptors might be needed before they could integrate with the host retina (Canola et al., 2007). In the present study, human fetal retinas at 11–15.7 weeks gestation were used for transplantation. The cells were not mature although preliminary lamination had started to develop (figure 1). Thus, they had the plasticity to integrate with the host retina after transplantation, and were primed to differentiate into various retinal cell types, including photoreceptors.
The idea that fetal retinal sheets are the optimal transplants for cell replacement in advanced stage of retinal degenerate patients has been studied for many years. These sheets already contain a primordial circuitry and cells that are committed to differentiate into various retinal cells (Seiler and Aramant, 2012). Our first published study of transplanted fetal retinal sheets was to a rat light damage model (Seiler and Aramant, 1998). Using a custom-made instrument and procedure, the sheets were gently placed into the subretinal space and later developed laminated layers of different cell types. Later, the same procedure was used to transplant rat fetal retina together with its RPE to RCS rats (Aramant et al., 1999) and human fetal retina with its RPE to athymic nude rats (Aramant and Seiler, 2002b). Another group transplanted matrix-embedded human fetal neuroretina and RPE in a non-immunosuppressed minipig model of light-induced retinal degeneration (Li et al., 2009). The authors claimed that the transplants survived for up to 12 months, but rhodopsin-positive photoreceptor staining was not observed within the grafted tissue, which appeared degenerated. mfERG revealed some retinal functional improvement in regions both inside and outside of the grafted area. In contrast, our unique transplantation method generated laminated retinal sheets that were well integrated with the host retina and improved visual function in several rat degeneration models (Woch et al., 2001; Sagdullaev et al., 2003; Thomas et al., 2004; Yang et al., 2010; Seiler et al., 2017).
Later, in a phase II clinical trial, sheets of human fetal retina transplants with RPE improved visual acuity in 7 of 10 patients (Radtke et al., 2008). The aim of the current study was to learn more about the mechanism of visual improvement with the same type of human transplant using an immunodeficient RD rat model. However, subretinal transplantation in the small rat eye with a large lens is more difficult than in the larger human eye where the surgeon can see and control the placement. Therefore, many transplants developed rosettes.
Comparable to the development of fetal retinal sheet transplantation in other groups (Ghosh et al., 1998; Ghosh and Arner, 2002; Bragadottir and Narfstrom, 2003; Ghosh et al., 2004), the transplants in the present study developed well laminated areas although rosettes were also present. Most mature retinal cells, including rod and cone photoreceptors, but not ganglion cells, were found in the transplant. Donor processes labeled with the human cytoplasm marker SC121 were found in host retina, suggesting that the donor tissue fully integrated into the host. Recently, several groups found donor-host cytoplasmic exchange in the retina after transplantation of photoreceptor precursors, which required a re-evaluation of the mechanisms underlying rescue by photoreceptor transplantation (Pearson et al., 2016; Sanges et al., 2016; Santos-Ferreira et al., 2016; Ortin-Martinez et al., 2017). To test if the donor cells do migrate into the host, we used Ku80, a human nuclear marker, and found many Ku80-stained cells in the host retina. Although we cannot exclude the possibility of cytoplasmic exchange (Pearson et al., 2016; Singh et al., 2016; Ortin-Martinez et al., 2017), our data did provide the proof of concept that transplanted human fetal retina tissue could migrate and integrate with the severely degenerated host retina. Interestingly, our previous work showed that although rat fetal donor retina fully integrated with the same RD rat host, the donor tissue stayed in the subretinal space and did not migrate much into the host retina; but in the present study, the human donor cells migrated into the host retina to a large extent. These results suggest that human fetal retina, transplanted at an earlier developmental stage, may have a larger migration potential than the more mature transplanted rat fetal tissue. These results may shed some light on the mechanism of the increased retinal sensitivity not only in the transplant area but also in the adjacent area in the phase II clinical trial (Radtke et al., 2008). However, since the retinal transplants were often seen to enlarge in size, migrate into the adjacent area after the transplantation and improve vision, it cannot be excluded that some trophic effect might also contribute.
Although the outer limiting membrane of the Müller cells in a degenerating retina has been considered an obstacle for transplant integration (Hippert et al., 2016), this does not appear to be the case with human fetal retinal sheets. Staining for glial markers indicated activation of Müller glia in the RD retina due to the photoreceptor degeneration, but much less GFAP staining in the transplant. The number of microglia cells appeared to be unchanged after transplantation. This indicated the absence of an inflammatory response in the immunodeficient host retina to the human xenograft. Another interesting observation in this study: the vision improvements with human transplants were weaker than those found with rat transplants in the same RD rat model (Seiler et al., 2017). Two of the reasons might be (1) the different development time of rat versus human cells, since at the time of recording, the human photoreceptors had not yet matured to the same extent as the rat cells; (2) more difficulty in development and synaptic formation of xenotransplantation (human to rat) compared with allotransplantation (rat to rat). Our result is consistent with the report of another lab showing less vision improvement of hESC retina transplants compared with miPSC retina in NOG-rd1-2J mice (Iraha et al., 2018).
In the past decade, following the findings that stem cells, such as hESCs and iPSCs, can be differentiated into 3 D retinal organoids comparable to fetal retina (Osakada et al., 2009; La Torre et al., 2012; Mellough et al., 2012; Nakano et al., 2012; Zhong et al., 2014), stem cells have been considered a promising source of cell therapy for retinal diseases and attract more and more interests. Stem cell-derived RPE cells were successfully transplanted into the eyes of RD animal models and patients in phase I/II studies, and showed function improvement (Schwartz et al., 2015; Koss et al., 2016; Peng et al., 2016; Schwartz et al., 2016). After transplantation into the eyes of RD animal models, hESC/iPSC-derived photoreceptor precursors could develop into mature photoreceptors, integrate with the host, and improve visual function (Assawachananont et al., 2014; Shirai et al., 2016; Iraha et al., 2018). Because of the difference in animal model and methods for functional testing, it is hard to compare their data with our current results. Recently, we transplanted hESC-derived retinal organoid sheets to the same RD rat model and found qualitatively better vision improvement than with human fetal retinal transplants (McLelland et al., in press). It may be attributed to (1) fetal retinas were harvested at least one day before transplantation and shipped overnight in hibernate E medium on ice, while hESC-derived retinal organoids could be used right after being taken from the incubator; (2) hESC-derived retinal organoids may be more immature than fetal retina so that they have better potential to survive in the host. Two advantages of fetal retinal transplants were noticed: (1) they never grew into tumors, maybe because the cells were committed to differentiate into mature retinal cells; (2) they develop into laminated layers similar to normal retinal structure while stem cell-derived retinal cells formed only rosettes but not parallel laminated layers in all the studies so far (Iraha et al., 2018; McLelland et al., in press). More studies are needed to optimize stem cell transplants before they could be used in RD patients as a regular therapy.
5. Conclusions
This study demonstrates that transplanted human fetal retinal sheets continue to develop and differentiate into mature retinal cells in a severely degenerative environment and improve vision by direct connection with the host retinal circuitry. The findings provide significant insight for using fetal retinal sheet transplantation as a therapeutic strategy to restore vision after severe retinal degeneration. Ethically obtained fetal retinas can potentially be used to improve vision of RD patients while other methods such as stem cell transplants are still under development.
Supplementary Material
Video of selected OCT B-scans of transplant #8 at d33, d83 and d200 post transplantation.
Ku80 (human nuclei, red, in combination with brightfield; a,c,e) and CRALBP (cellular retinaldehyde protein, marker for RPE and Müller cells; green; b, d, f) staining of adjacent transplant sections. Nuclei are stained blue with DAPI. Maximum intensity projections (a, c, e) and 3D projections (b, d, f) of confocal stacks. Cone inner segments are autofluorescent in e) and f). (a, b, c, d) Laminated transplant in rat #9. Arrow indicates the vortex vein in choroid as a reference point. Section is 5 slides away from section depicted in Fig. 7a,b. (a,b) Overview of transplant. (c,d) enlargement. (e,f) Laminated transplant in rat #12. Scale = 50 μm.
Highlights.
Sheets of human fetal retina with its RPE were transplanted to severely retinal degenerate rats
In spite of the apparently unfavorable environment, transplants survived and differentiated
Transplants continued to develop into mature photoreceptors and other retinal cells
Transplants integrated with the degenerated host retina
In rats with long-term transplants, visual responses could be recorded in the midbrain
Acknowledgments
Supported by: CIRM TR4-06648; Lincy Foundation
The authors wish to thank Fengrong Yan, Lakshmi Patil (technical assistance); Alexander de Guzman (OCT), Navjot Kaur, Jacson Wan, Parth Patel, Mony Sary (cryostat cutting and staining); Robert Lin, Ph.D. (SC recording); Brian Cummings, Ph.D. (help and support); Hans S. Keirstead (HSK), Ph.D. (previously UC Irvine; now AIVITA Biomedical Inc., support). Supported by CIRM TR4-06648 (MJS), and NIH grant R01EY024045 (HSK). A grant of the Lincy foundation (HSK) provided support previously for obtaining fetal tissue for immunohistochemical analysis.
List of Abbreviations in Text and Figures
- DAPI
4,6-diamidinodiamidino-2-phenylindole
- AMC
age-matched control
- BM
Bruch’s membrane
- CRALBP
cellular retinaldehyde binding protein
- dps
days post-surgery
- DAB
diaminobenzidine
- GCL
ganglion cell layer
- GFAP
glial fibrillary acidic protein
- H&E
hematoxylin and eosin
- -h
host
- HFR
human fetal retina
- INL
inner nuclear layer
- IPL
inner plexiform layer
- IS
inner segments
- Iba-1
ionized calcium binding adaptor molecule 1
- MAP2
microtubule-associated protein 2
- MPS
months post-surgery
- NBL
neuroblastic layer
- NeuN
neuronal nuclei
- OLM
Outer limiting membrane
- OTX2
orthodenticle homeobox 2
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- OS
outer segments
- PBS
phosphate buffered saline
- PKC α
protein kinase C α retinal degenerate;
- RD
retinal degeneration
- RPE
retinal pigment epithelium
- SD-OCT
spectral-domain optical coherence tomography
- SC121
stem cell 121 antibody
- SC
superior colliculus
- -t
transplant
- wks
weeks
Footnotes
Commercial Relationships Disclosure: MJS and RBA have a proprietary interest in the implantation instruments and method.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AJ, Haus DL, Hooshmand MJ, Perez H, Sontag CJ, Cummings BJ. Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient? Regen Med. 2011;6:367–406. doi: 10.2217/rme.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ. Retinal transplantation--advantages of intact fetal sheets. Prog Retin Eye Res. 2002a;21:57–73. doi: 10.1016/s1350-9462(01)00020-9. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ. Transplanted sheets of human retina and retinal pigment epithelium develop normally in nude rats. Exp Eye Res. 2002b;75:115–125. doi: 10.1006/exer.2002.2001. [DOI] [PubMed] [Google Scholar]
- Aramant RB, Seiler MJ, Ball SL. Successful cotransplantation of intact sheets of fetal retina with retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1999;40:1557–1564. [PubMed] [Google Scholar]
- Assawachananont J, Mandai M, Okamoto S, Yamada C, Eiraku M, Yonemura S, Sasai Y, Takahashi M. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem cell reports. 2014;2:662–674. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, Maguire AM. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- Bragadottir R, Narfstrom K. Lens sparing pars plana vitrectomy and retinal transplantation in cats. Vet Ophthalmol. 2003;6:135–139. doi: 10.1046/j.1463-5224.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- Brundin P, Barbin G, Strecker RE, Isacson O, Prochiantz A, Bjorklund A. Survival and function of dissociated rat dopamine neurones grafted at different developmental stages or after being cultured in vitro. Brain Res. 1988;467:233–243. doi: 10.1016/0165-3806(88)90027-2. [DOI] [PubMed] [Google Scholar]
- Canola K, Angenieux B, Tekaya M, Quiambao A, Naash MI, Munier FL, Schorderet DF, Arsenijevic Y. Retinal stem cells transplanted into models of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate. Invest Ophthalmol Vis Sci. 2007;48:446–454. doi: 10.1167/iovs.06-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulskyte D, Kaalund H, Pedersen M, Horlyck A, Marcussen N, Hansen HE, Madsen M, Mortensen J. Chronic cyclosporine nephrotoxicity: a pig model. Transplant Proc. 2005;37:3298–3301. doi: 10.1016/j.transproceed.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Cooper AE, Cho JH, Menges S, Masood S, Xie J, Yang J, Klassen H. Immunosuppressive Treatment Can Alter Visual Performance in the Royal College of Surgeons Rat. J Ocul Pharmacol Ther. 2016;32:296–303. doi: 10.1089/jop.2015.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474–1485. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991;251:915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Arner K. Transplantation of full-thickness retina in the normal porcine eye: surgical and morphologic aspects. Retina. 2002;22:478–486. doi: 10.1097/00006982-200208000-00013. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Arner K, Ehinger B. Transplant of full-thickness embryonic rabbit retina using pars plana vitrectomy. Retina. 1998;18:136–142. doi: 10.1097/00006982-199818020-00007. [DOI] [PubMed] [Google Scholar]
- Ghosh F, Johansson K, Ehinger B. Long-term full-thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999;40:133–142. [PubMed] [Google Scholar]
- Ghosh F, Wong F, Johansson K, Bruun A, Petters RM. Transplantation of full-thickness retina in the rhodopsin transgenic pig. Retina. 2004;24:98–109. doi: 10.1097/00006982-200402000-00014. [DOI] [PubMed] [Google Scholar]
- Gouras P, Tanabe T. Survival and integration of neural retinal transplants in rd mice. Graefes Arch Clin Exp Ophthalmol. 2003;241:403–409. doi: 10.1007/s00417-003-0648-2. [DOI] [PubMed] [Google Scholar]
- Hanus J, Zhao F, Wang S. Current therapeutic developments in atrophic age-related macular degeneration. Br J Ophthalmol. 2016;100:122–127. doi: 10.1136/bjophthalmol-2015-306972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Hippert C, Graca AB, Pearson RA. Gliosis Can Impede Integration Following Photoreceptor Transplantation into the Diseased Retina. Adv Exp Med Biol. 2016;854:579–585. doi: 10.1007/978-3-319-17121-0_77. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Ratnapriya R, Brooks MJ, Chaitankar V, Wilken MS, Zhang C, Starostik MR, Gieser L, La Torre A, Nishio M, Bates O, Walton A, Bermingham-McDonogh O, Glass IA, Wong ROL, Swaroop A, Reh TA. Molecular Anatomy of the Developing Human Retina. Dev Cell. 2017;43:763–779. e764. doi: 10.1016/j.devcel.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Baranov P, Lai K, Zhang X, Ge J, Young MJ. Functional and morphological analysis of the subretinal injection of human retinal progenitor cells under Cyclosporin A treatment. Mol Vis. 2014;20:1271–1280. [PMC free article] [PubMed] [Google Scholar]
- Iraha S, Tu HY, Yamasaki S, Kagawa T, Goto M, Takahashi R, Watanabe T, Sugita S, Yonemura S, Sunagawa GA, Matsuyama T, Fujii M, Kuwahara A, Kishino A, Koide N, Eiraku M, Tanihara H, Takahashi M, Mandai M. Establishment of Immunodeficient Retinal Degeneration Model Mice and Functional Maturation of Human ESC-Derived Retinal Sheets after Transplantation. Stem cell reports. 2018;10:1059–1074. doi: 10.1016/j.stemcr.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivert L, Gouras P, Naeser P, Narfstrom K. Photoreceptor allografts in a feline model of retinal degeneration. Graefes Arch Clin Exp Ophthalmol. 1998;236:844–852. doi: 10.1007/s004170050169. [DOI] [PubMed] [Google Scholar]
- Juliusson B, Bergstrom A, van Veen T, Ehinger B. Cellular organization in retinal transplants using cell suspensions or fragments of embryonic retinal tissue. Cell Transplant. 1993;2:411–418. doi: 10.1177/096368979300200509. [DOI] [PubMed] [Google Scholar]
- Koss MJ, Falabella P, Stefanini FR, Pfister M, Thomas BB, Kashani AH, Brant R, Zhu D, Clegg DO, Hinton DR, Humayun MS. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: a feasibility and safety study in Yucatan minipigs. Graefes Arch Clin Exp Ophthalmol. 2016;254:1553–1565. doi: 10.1007/s00417-016-3386-y. [DOI] [PubMed] [Google Scholar]
- La Torre A, Lamba DA, Jayabalu A, Reh TA. Production and transplantation of retinal cells from human and mouse embryonic stem cells. Methods Mol Biol. 2012;884:229–246. doi: 10.1007/978-1-61779-848-1_16. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Survival factors for treatment of retinal degenerative disorders: preclinical gains and issues for translation into clinical studies. Retina. 2005;25:S25–S26. doi: 10.1097/00006982-200512001-00009. [DOI] [PubMed] [Google Scholar]
- Li SY, Yin ZQ, Chen SJ, Chen LF, Liu Y. Rescue from light-induced retinal degeneration by human fetal retinal transplantation in minipigs. Curr Eye Res. 2009;34:523–535. doi: 10.1080/02713680902936148. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. Cell therapeutics in Parkinson’s disease. Neurotherapeutics. 2011;8:539–548. doi: 10.1007/s13311-011-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MM, Tuo J, Chan CC. Gene therapy for ocular diseases. Br J Ophthalmol. 2011;95:604–612. doi: 10.1136/bjo.2009.174912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Mansergh FC, Vawda R, Millington-Ward S, Kenna PF, Haas J, Gallagher C, Wilson JH, Humphries P, Ader M, Farrar GJ. Loss of photoreceptor potential from retinal progenitor cell cultures, despite improvements in survival. Exp Eye Res. 2010;91:500–512. doi: 10.1016/j.exer.2010.07.003. [DOI] [PubMed] [Google Scholar]
- McLelland BT, Lin B, Mathur A, Aramant RB, Thomas BB, Nistor G, Keirstead HS, Seiler MJ. Transplanted hESC-retina organoid sheets differentiate, integrate and improve visual function in retinal degenerate rats. Invest Ophthalmol Vis Sci. :59. doi: 10.1167/iovs.17-23646. in press. [DOI] [PMC free article] [PubMed]
- Medeiros NE, Curcio CA. Preservation of ganglion cell layer neurons in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:795–803. [PubMed] [Google Scholar]
- Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem cells (Dayton, Ohio) 2012;30:673–686. doi: 10.1002/stem.1037. [DOI] [PubMed] [Google Scholar]
- Molday RS, MacKenzie D. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Schulte D, Hendrickson AE. Expression of photoreceptor-associated molecules during human fetal eye development. Mol Vis. 2003;9:401–409. [PubMed] [Google Scholar]
- Ortin-Martinez A, Tsai EL, Nickerson PE, Bergeret M, Lu Y, Smiley S, Comanita L, Wallace VA. A Reinterpretation of Cell Transplantation: GFP Transfer From Donor to Host Photoreceptors. Stem cells (Dayton, Ohio) 2017;35:932–939. doi: 10.1002/stem.2552. [DOI] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4:811–824. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA, Luhmann UF, Benucci A, Sung CH, Bainbridge JW, Carandini M, Yau KW, Sowden JC, Ali RR. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, West EL, MacLaren RE, Duran Y, Bainbridge JW, Sowden JC, Ali RR. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19:487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Gonzalez-Cordero A, West EL, Ribeiro JR, Aghaizu N, Goh D, Sampson RD, Georgiadis A, Waldron PV, Duran Y, Naeem A, Kloc M, Cristante E, Kruczek K, Warre-Cornish K, Sowden JC, Smith AJ, Ali RR. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nature communications. 2016;7:13029. doi: 10.1038/ncomms13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CH, Chuang JH, Wang ML, Jhan YY, Chien KH, Chung YC, Hung KH, Chang CC, Lee CK, Tseng WL, Hwang DK, Hsu CH, Lin TC, Chiou SH, Chen SJ. Laminin modification subretinal bio-scaffold remodels retinal pigment epithelium-driven microenvironment in vitro and in vivo. Oncotarget. 2016;7:64631–64648. doi: 10.18632/oncotarget.11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantner JJ, Hara S, Kean EL. Improved, rapid radioimmunoassay for rhodopsin. Exp Eye Res. 1982;35:543–548. doi: 10.1016/s0014-4835(82)80068-7. [DOI] [PubMed] [Google Scholar]
- Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ. Vision Improvement in Retinal Degeneration Patients by Implantation of Retina Together with Retinal Pigment Epithelium. Am J Ophthalmol. 2008;146:172–182. doi: 10.1016/j.ajo.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Radtke ND, Seiler MJ, Aramant RB, Petry HM, Pidwell DJ. Transplantation of intact sheets of fetal neural retina with its retinal pigment epithelium in retinitis pigmentosa patients. Am J Ophthalmol. 2002;133:544–550. doi: 10.1016/s0002-9394(02)01322-3. [DOI] [PubMed] [Google Scholar]
- Saari JC, Bunt-Milam AH, Bredberg DL, Garwin GG. Properties and immunocytochemical localization of three retinoid-binding proteins from bovine retina. Vision Res. 1984;24:1595–1603. doi: 10.1016/0042-6989(84)90317-1. [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, Aramant RB, Seiler MJ, Woch G, McCall MA. Retinal transplantation-induced recovery of retinotectal visual function in a rodent model of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:1686–1695. doi: 10.1167/iovs.02-0615. [DOI] [PubMed] [Google Scholar]
- Sanges D, Simonte G, Di Vicino U, Romo N, Pinilla I, Nicolas M, Cosma MP. Reprogramming Muller glia via in vivo cell fusion regenerates murine photoreceptors. J Clin Invest. 2016;126:3104–3116. doi: 10.1172/JCI85193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T, Llonch S, Borsch O, Postel K, Haas J, Ader M. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nature communications. 2016;7:13028. doi: 10.1038/ncomms13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Tan G, Hosseini H, Nagiel A. Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Invest Ophthalmol Vis Sci. 2016;57:ORSFc1–9. doi: 10.1167/iovs.15-18681. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB. Photoreceptor and glial markers in human embryonic retina and in human embryonic retinal transplants to rat retina. Brain Res Dev Brain Res. 1994;80:81–95. doi: 10.1016/0165-3806(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB. Intact sheets of fetal retina transplanted to restore damaged rat retinas. Invest Ophthalmol Vis Sci. 1998;39:2121–2131. [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB. Cell replacement and visual restoration by retinal sheet transplants. Progress in retinal and eye research. 2012;31:661–687. doi: 10.1016/j.preteyeres.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB, Jones MK, Ferguson DL, Bryda EC, Keirstead HS. A new immunodeficient pigmented retinal degenerate rat strain to study transplantation of human cells without immunosuppression. Graefes Arch Clin Exp Ophthalmol. 2014;252:1079–1092. doi: 10.1007/s00417-014-2638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Aramant RB, Thomas BB, Peng Q, Sadda SR, Keirstead HS. Visual restoration and transplant connectivity in degenerate rats implanted with retinal progenitor sheets. Eur J Neurosci. 2010;31:508–520. doi: 10.1111/j.1460-9568.2010.07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MJ, Lin RE, McLelland BT, Mathur A, Lin B, Sigman J, De Guzman AT, Kitzes LM, Aramant RB, Thomas BB. Vision Recovery and Connectivity by Fetal Retinal Sheet Transplantation in an Immunodeficient Retinal Degenerate Rat Model - Sheet Transplants Recover Vision in RD Nude Rats. Investigative Ophthalmology & Visual Science. 2017;58:614–630. doi: 10.1167/iovs.15-19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, Assawachananont J, Kimura T, Saito K, Terasaki H, Eiraku M, Sasai Y, Takahashi M. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A. 2016;113:E81–90. doi: 10.1073/pnas.1512590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MS, Hughes SE, Valentino TL, Liu Y. Photoreceptor transplantation: anatomic, electrophysiologic, and behavioral evidence for the functional reconstruction of retinas lacking photoreceptors. Exp Neurol. 1992;115:87–94. doi: 10.1016/0014-4886(92)90227-h. [DOI] [PubMed] [Google Scholar]
- Singh MS, Balmer J, Barnard AR, Aslam SA, Moralli D, Green CM, Barnea-Cramer A, Duncan I, MacLaren RE. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nature communications. 2016;7:13537. doi: 10.1038/ncomms13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thliveris JA, Yatscoff RW, Lukowski MP, Copeland KR. Cyclosporine nephrotoxicity--experimental models. Clin Biochem. 1991;24:93–95. doi: 10.1016/0009-9120(91)90357-k. [DOI] [PubMed] [Google Scholar]
- Thomas BB, Seiler MJ, Sadda SR, Aramant RB. Superior colliculus responses to light - preserved by transplantation in a slow degeneration rat model. Exp Eye Res. 2004;79:29–39. doi: 10.1016/j.exer.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet. 2009;30:63–68. doi: 10.1080/13816810802695550. [DOI] [PubMed] [Google Scholar]
- West EL, Pearson RA, Barker SE, Luhmann UF, Maclaren RE, Barber AC, Duran Y, Smith AJ, Sowden JC, Ali RR. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem cells (Dayton, Ohio) 2010;28:1997–2007. doi: 10.1002/stem.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EL, Pearson RA, Tschernutter M, Sowden JC, MacLaren RE, Ali RR. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008;86:601–611. doi: 10.1016/j.exer.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woch G, Aramant RB, Seiler MJ, Sagdullaev BT, McCall MA. Retinal transplants restore visually evoked responses in rats with photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42:1669–1676. [PubMed] [Google Scholar]
- Yang PB, Seiler MJ, Aramant RB, Yan F, Mahoney MJ, Kitzes LM, Keirstead HS. Trophic factors GDNF and BDNF improve function of retinal sheet transplants. Exp Eye Res. 2010;91:727–738. doi: 10.1016/j.exer.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature Communications. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of selected OCT B-scans of transplant #8 at d33, d83 and d200 post transplantation.
Ku80 (human nuclei, red, in combination with brightfield; a,c,e) and CRALBP (cellular retinaldehyde protein, marker for RPE and Müller cells; green; b, d, f) staining of adjacent transplant sections. Nuclei are stained blue with DAPI. Maximum intensity projections (a, c, e) and 3D projections (b, d, f) of confocal stacks. Cone inner segments are autofluorescent in e) and f). (a, b, c, d) Laminated transplant in rat #9. Arrow indicates the vortex vein in choroid as a reference point. Section is 5 slides away from section depicted in Fig. 7a,b. (a,b) Overview of transplant. (c,d) enlargement. (e,f) Laminated transplant in rat #12. Scale = 50 μm.