Abstract
Ketamine’s mechanism of action was assessed using gamma power from magnetoencephalography (MEG) as a proxy measure for homeostatic balance in 35 unmedicated subjects with major depressive disorder (MDD) and 25 healthy controls enrolled in a double-blind, placebo-controlled, randomized cross-over trial of 0.5 mg/kg ketamine. MDD subjects showed significant improvements in depressive symptoms, and healthy control subjects exhibited modest but significant increases in depressive symptoms for up to one day after ketamine administration. Both groups showed increased resting gamma power following ketamine. In MDD subjects, gamma power was not associated with the magnitude of the antidepressant effect. However, baseline gamma power was found to moderate the relationship between post-ketamine gamma power and antidepressant response; specifically, higher post-ketamine gamma power was associated with better response in MDD subjects with lower baseline gamma, with an inverted relationship in MDD subjects with higher baseline gamma. This relationship was observed in multiple regions involved in networks hypothesized to be involved in the pathophysiology of MDD. This finding suggests biological subtypes based on the direction of homeostatic dysregulation and has important implications for inferring ketamine’s mechanism of action from studies of healthy controls alone.
Introduction
Over half of patients with major depressive disorder (MDD) fail to respond to first-line treatments (1), and residual symptoms are common. In addition, the neurobiological basis for MDD and the mechanism of action of existing antidepressant drugs are only partially understood. Controlled studies of subjects with treatment-resistant MDD and bipolar depression have shown that the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine has rapid antidepressant and anti-suicidal effects (2–8). Convergent evidence supports the conclusion that enhanced α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activity, concomitant with increased synaptic potentiation, is critical to ketamine’s mechanism of action and may be crucial to developing similar rapid-acting antidepressants (9–12).
Both animal (13–16) and human (17, 18) studies indicate that acute, sub-anesthetic ketamine infusion is associated with robust increases in gamma power. Multiple synaptic mechanisms play a role in regulating gamma oscillations, including AMPA receptor-mediated depolarization and gamma aminobutyric acid (GABA)A receptor-mediated inhibition. Ketamine may influence both of these systems, both by silencing GABAergic inhibitory synapses and by increasing glutamate release, thereby activating AMPA receptors (19). The decreased activity in GABAergic interneurons and the disinhibition of excitatory pyramidal neurons (20) presumably provides the mechanism for increased gamma oscillations (21). The antidepressant mechanism is likely more complex, however, given that blockade of NMDA receptors on interneurons and the subsequent disinhibition of pyramidal neurons does not consistently produce an antidepressant effect (22). Notably, acute administration of the ketamine metabolite (2R,6R)-hydroxynorketamine (HNK) also increases gamma oscillations, despite the fact that the metabolite does not inhibit NMDA receptors at concentrations that increase gamma power (23). Because AMPA receptor blockade can attenuate HNK-induced gamma oscillations, enhanced AMPA receptor activity is likely to be the mechanism by which HNK increases gamma power, although the mechanism of increased AMPA receptor activity is not known (24). Importantly, gamma oscillations have been associated with cognition (25), attention (26), the hemodynamic (BOLD) response (27, 28), and functional connectivity (29), and are extremely sensitive to metabolic stress due to their influence on neuronal oxygen consumption (30). Taken together, these findings underscore the close relationship between gamma oscillations and inhibition/excitation balance (31, 32).
Recent evidence suggests that disruptions in synaptic homeostasis may underlie MDD (33). In this context, homeostasis refers to the stable functioning of neuronal networks in response to changes in excitatory input, alterations in neuronal excitability, and metabolic stressors, ensuring that an appropriate inhibition/excitation balance is maintained. Neurons respond to disruptions in function by altering their synaptic strength, a process known as homeostatic plasticity (34). For example, to maintain homeostasis, mice with GABA receptor γ2 subunit deficits (a model for anxious depression) manifest reduced function of both NMDA and AMPA receptors (35). Notably, ketamine administration reverses both the glutamatergic adaptations to the intrinsic GABA γ2 deficits and the abnormal behavioral phenotype (19). Chronic stress in rodents also downregulates AMPA and NMDA receptor function, and similar genetic and environmental mechanisms may lead to depressive symptoms in humans (36). Disruptions in inhibition/excitation balance and receptor function may lead to neuronal atrophy, synaptic loss, altered network level connectivity, and altered volume of brain structures involved in emotional processing (33), all of which have been associated with MDD (37, 38). These findings support the importance of homeostatic control of mood circuits and form the basis of a synaptic hypothesis of MDD and antidepressant response (33).
In addition to electrophysiological effects, preclinical studies have shown that ketamine rapidly induces synaptogenesis and reverses synaptic deficits caused by chronic stress, thus restoring network connectivity (33), while clinical studies support enhanced plasticity in response to ketamine (39). However, the hypotheses of homeostatic dysregulation in MDD patients and restoration of homeostasis by ketamine have not yet been demonstrated. The present study is part of a larger Ketamine Mechanism of Action (Ket-MOA) Study designed to identify clinical and neurobiological correlates of ketamine treatment in unmedicated inpatients with treatment-resistant MDD. This report focuses on the effects of ketamine on mood and resting gamma power (as a surrogate marker of inhibition/excitation balance) measured at baseline, at six to nine hours post-ketamine/post-placebo infusion, and at 11–13 days post-infusion. We hypothesized that sustained increases in gamma power would be associated with ketamine administration, commensurate with the degree of improvement in depressive symptoms.
Materials and Methods
Subjects
Eligible participants included men and women, ages 18 to 65 years. Subjects with MDD had been diagnosed with recurrent MDD without psychotic features using the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID)-Patient Version (40). Subjects were required to have a score ≥20 on the Montgomery-Åsberg Depression Rating Scale (MADRS) at screening and before each infusion. Subjects had to have not responded to at least one adequate antidepressant trial during their current episode, as assessed using the Antidepressant Treatment History Form (41), and the current episode had to have lasted at least four weeks. Subjects were free from psychotropic medications in the two weeks before randomization (five weeks for fluoxetine, three weeks for aripiprazole). A patient sample size of 34 individuals was necessary to have 80% power to detect an antidepressant effect of ketamine (d=0.5) with p<0.05, two-tailed.
Healthy control subjects consisted of males and females, 18–65 years old with no Axis I disorder as determined by SCID-NP, and no family history of Axis I disorders in first degree relatives. Healthy control subjects were free of medications affecting neuronal function or cerebral blood flow or metabolism. Subjects in both groups were in good physical health as determined by medical history, physical exam, blood labs, electrocardiogram, chest x-ray, urinalysis, and toxicology. The study was approved by the National Institutes of Health (NIH) Combined Neuroscience Institutional Review Board. All subjects provided written informed consent before entry into the study (NCT00088699).
Data Collection
Ketamine and placebo infusions were administered two weeks apart using a double-blind, placebo-controlled, crossover design, with infusion order randomized. Subjects were rated 60 minutes prior to each infusion and at 40, 80, 120, and 230 minutes as well as at 1, 2, 3, 7, 10, and 11 days after the infusion.
The MADRS was the primary outcome measure. Additional secondary outcome measures included the 17-item Hamilton Depression Rating Scale (HAM-D17) (42), the reduced Hamilton-Bech designed to probe rapid changes in depressive symptoms (43), the Snaith-Hamilton Pleasure Scale (SHAPS) (44), and the Temporal Experience of Pleasure Scale (TEPS) (45). Additional secondary outcome measures administered only through Day 3 included the Hamilton Anxiety Rating Scale (HAM-A) (46), Beck Depression Inventory (BDI) (47), PTSD Checklist Civilian Version (PCL-C) (48), Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF) (49), Clinical Global Impression-Severity Scale (CGI-S) (50), and Scale for Suicide Ideation-5 items (SSI5) (51). Additional scales intended for use in an exploratory analysis of symptom clusters included the Profile of Mood States (POMS) (52), and a seven-item visual analog scale (VAS) (53). The Clinician-Administered Dissociative States Scale (CADSS) was used as the primary assessment of side effects (54), and the Brief Psychiatric Rating Scale-positive symptoms (BPRS) (55) and YMRS (56) were also administered.
Resting state magnetoencephalography (MEG) recordings were obtained at baseline (two to four days before the first infusion), the day of the infusion (KET-Day0 and Placebo-Day0, approximately six to nine hours post-infusion), and 11–13 days post-infusion (KET-Day12 and Placebo-Day12). Up to two 250-second resting state recordings per time point were analyzed; in general, one recording occurred at the beginning of the session, and the second was acquired approximately 30 minutes to one hour later after a series of tasks. For the resting state recordings, subjects were instructed to relax with their eyes closed and remain still. All data were acquired on a 275-channel CTF system (Coquitlam, BC) at 1200Hz. Background environmental magnetic noise was attenuated by synthetic third gradient balancing. T1 weighted MRI scans were acquired on a 3T GE scanner for co-registration. MEG, rather than EEG, recordings were obtained because MEG is associated with enhanced spatial specificity due to the lack of distortion of neuronal magnetic fields by the skull or scalp (57).
Clinical Data Analysis
IBM SPSS 23.0.0.3 (Armonk, NY) was used to analyze clinical outcome measures. Participants were included in the clinical analyses as long as at least one pre- and one post-infusion measure were available for at least one phase. All time points were analyzed, and the −60 minute time point was used as the baseline. Linear mixed models with restricted maximum likelihood estimation were estimated for each outcome measure. Time and drug factors were within-subjects repeated measures, and the interaction was included in the model along with the phase-specific baseline score as a main effect only. A fixed intercept was included. Post-hoc tests with Bonferroni adjustment were used to compare ketamine and placebo at individual time points if the main effect of drug or drug by time interaction was significant. For primary outcome measures, significance was evaluated at p<.05, two-tailed.
Magnetoencephalography Data Analysis
MEG data were processed using CTF software (http://www.ctf.com), MNE-python (58), Analysis of Functional NeuroImages (AFNI) (59), and routines developed in house. This work used the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov). Each MEG dataset was filtered using a high pass filter of 2 Hz and visually inspected to identify and mark time periods with significant muscular, ocular, or movement artifacts. Up to 10 segments of 15-second duration outside marked artifacts were identified in an automated fashion. Datasets were discarded if at least five 15-second artifact-free segments could not be defined. All further described imaging analyses and quality control measures were carried out on the clean epochs.
Data were localized to source space on a 5mm grid using synthetic aperture magnetometry (SAM (60)), and a multisphere head model was calculated from co-registered MRI scans. MRI and MEG images were coregistered using MRI-visible fiducial markers placed on the head at the time of MRI scanning. Beamformer weights were calculated using a bandpass frequency of 2–100 Hz, and power was normalized by the projected noise floor of the virtual sensor. The resulting images represented root-mean-square (RMS) power in the gamma band (30–50Hz). All images were warped to Talairach space using AFNI and masked to remove non-brain matter and cerebellum. The final gamma band images were then normalized by the square root of the sum of squared images for six canonical bands between 2–100Hz (delta, theta, alpha, beta, gamma, and high gamma). From this point forward, “gamma power” refers to the normalized RMS gamma power.
Images were analyzed using a linear mixed model implemented in the AFNI routine 3dLME (61). If more than one usable recording existed for a given subject and session, both were included and coded as having occurred before or after tasks. Session (baseline, Placebo-Day0, Placebo-Day12, KET-Day0, KET-Day12), diagnosis, and the pre-post task factor were included in the model, along with the interaction between session and diagnosis. Gender and age were initially included as main effects and removed if non-significant. Post-hoc tests were performed within the 3dLME routine to assess individual contrasts. Images are shown at a voxel uncorrected threshold of p<0.01 only if the FDR corrected p-value was less than 0.05, with the voxel uncorrected threshold lowered to p<0.001 as necessary to better distinguish clusters. Additional descriptive analyses were performed on regions of interest (ROIs), as described in the Supplementary Methods. Note that because these clusters were defined from the functional images, effect size estimates could not be made from extracted values.
As a secondary analysis, an additional mixed model was carried out using 3dLME using only the KET-Day0 and Placebo-Day0 recordings, with an additional covariate of absolute change in MADRS, centered within group and session, to determine if regional gamma power was associated with response to ketamine. Contrasts were performed to assess the relationship between gamma power and change in MADRS score within each diagnosis and session, as well as any differences between diagnostic groups with regard to the relationship between gamma power and change in MADRS score. =. In order to increase sensitivity, additional mixed models were performed in SPSS using the ROIs defined from the primary analysis of KET-Day0 vs. Placebo-Day0 recordings (See supplementary Methods). The mean gamma power in each ROI during the KET-Day0 session was examined, with the pre-post factor as a repeated measure and diagnoses as a factor. The absolute change in MADRS response at t=+40 minutes was entered as a covariate, and the diagnosis by MADRS interaction was modeled. As before, age and gender were included as main effects and retained only if significant. These models were repeated using the change in CADSS at t=+40 minutes.
After the initial findings that gamma power in the MDD cohort did not correlate with MADRS response, we performed additional exploratory analyses using data previously defined ROIs. The purpose of these exploratory analyses was to determine if baseline gamma power moderated the relationship between post-ketamine gamma power and MADRS response. Seventeen subjects had both baseline and post-ketamine data; for subjects with two recordings in a session we used mean gamma power. Mixed models were performed on post-ketamine gamma power using baseline gamma power and change in MADRS score from t=−60 to t=+40 minutes as main effects, with the effect of interest being the interaction between baseline gamma power and MADRS response. tAs in previous analyses, age and gender were included as main effects and removed if non-significant. Identical models were then repeated using the MADRS percent change from t=−60 to t=+230 minutes, as this is a more common measure of antidepressant response.
Code Availability
All scripts and routines used for analyzing data are available from the corresponding author upon request.
Results
Subjects
Thirty-five unmedicated treatment-resistant subjects who met DSM-IV criteria for MDD and 26 healthy control subjects were included. One healthy control subject had a baseline MEG but was not randomized. Supplementary Figure S1 illustrates subject flow through the study, and demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic and clinical characteristics for the sample.
| MDD (N=35) | HC (N=26)a | |||
|---|---|---|---|---|
| N | % | N | % | |
| Female | 21 | 60.0 | 16 | 61.5 |
| Race (Caucasian) | 32 | 94.1 | 19 | 73.1 |
| History of Psychiatric Hospitalization | 19 | 54.3 | ||
| History of Suicide Attempt | 14 | 40.0 | ||
| History of Substance Abuse | 8 | 22.9 | ||
| Family History | N | % | ||
| - Alcohol Abuse (First Degree) | 13 | 37.1 | ||
| - Anxiety Disorder | 12 | 34.3 | ||
| - Mood Disorder | 25 | 71.4 | ||
| - Suicide Attempt | 6 | 17.7 | ||
| Mean | SD | Mean | SD | |
| Age | 35.5 | 9.6 | 33.9 | 10.4 |
| Age of Onset | 15.8 | 6.6 | ||
| Length of Current Episode (Months) | 41.4 | 68.0 | ||
| Mean number of failed trials (Lifetime, N=34)b | 6.4 | 3.4 | ||
| MADRS | 33.4 | 4.7 | 1.5 | 1.6 |
| HDRS | 21.6 | 4.3 | 1.6 | 1.4 |
| BDI | 29.0 | 7.6 | 0.2 | 0.6 |
| CADSS | 2.8 | 4.5 | 0.0 | 0.0 |
| BPRS Positive | 10.1 | 1.9 | 8.0 | 0.2 |
| HAMA | 22.6 | 6.3 | 1.4 | 1.3 |
| SHAPS | 39.5 | 3.9 | 18.5 | 4.3 |
| SSI (5 Items) | 1.6 | 1.8 | 0.0 | 0.0 |
| TEPS Consummatory | 23.4 | 4.6 | 39.6 | 5.2 |
| TEPS Anticipatory | 23 | 7.3 | 46.9 | 5.5 |
| PCLC | 42 | 12.3 | 17.6 | 1.1 |
| QLESQ | 30.8 | 7.2 | 64.1 | 5.2 |
Note that one healthy control subject was excluded prior to infusion and thus only baseline MEG recordings are included in the analysis. Mean values on rating scales are given for N=25.
All patients had failed to respond to at least one antidepressant trial in the current episode.
MDD subjects and healthy controls did not differ on the basis of age or gender composition (p>0.05).
MDD: major depressive disorder; HC: healthy control; MADRS: Montgomery Åsberg Depression Rating Scale; HAM-D: Hamilton Depression Rating Scale; BDI: Beck Depression Inventory; BPRS: Brief Psychiatric Rating Scale; CADSS: Clinician-Administered Dissociative States Scale; HAM-A: Hamilton Anxiety Rating Scale; SHAPS: Snaith-Hamilton Pleasure Scale; SSI: Scale for Suicide Ideation; TEPS: Temporal Experience of Pleasure Scale; PCLC: PTSD Checklist, Civilian Version; QLESQ: Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form.
Effects of Ketamine on Mood - MDD
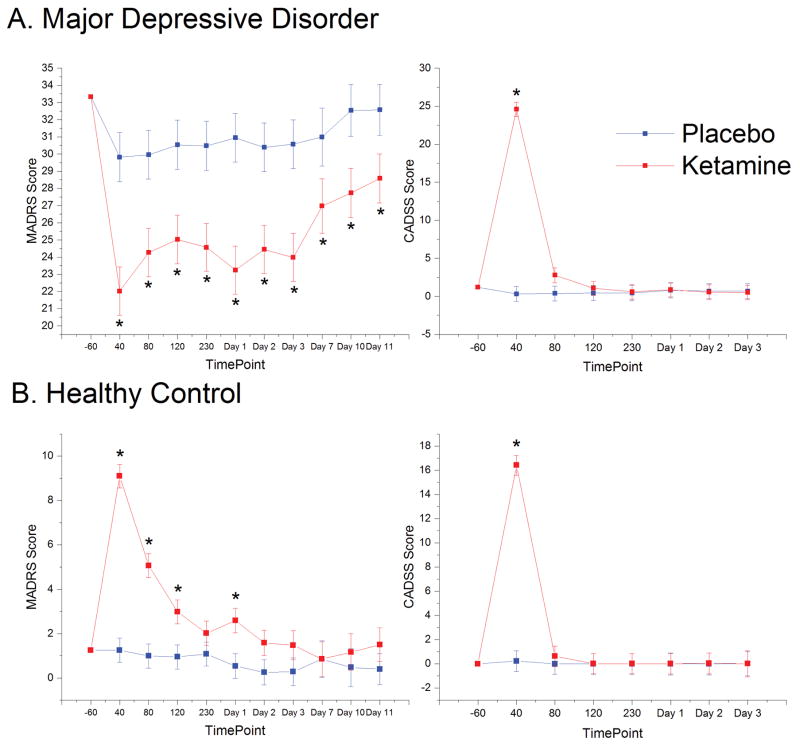
Antidepressant effects were assessed using the MADRS, and acute psychotomimetic effects were assessed via the CADSS. In MDD subjects, MADRS scores were significantly lower post-ketamine infusion compared to placebo (F1,77=84.5, p<.001; Supplementary Table S2, Fig 1A). The interaction with time was not significant (p=0.24), although placebo and ketamine scores approached convergence by the end of Day 11. CADSS scores showed significant effects of drug (F1,414=57.66, p<.001), time (F6,380=43.61, p<0.001), and a drug by time interaction (F6,380=45.59, <0.001; Fig 1A). Ketamine significantly improved symptoms across a wide variety of domains including anhedonia, anxiety, post-traumatic stress disorder (PTSD), suicidality, and quality of life (Supplementary Tables S2 and S3). Results from the independent ratings acquired by raters who were not present for the entire infusion (infusion-blinded ratings) as well as results exploring carryover effects appear in the Supplementary Materials (Supplementary Table S1).
Figure 1.
Effects of Ketamine on Mood. Graphs of the marginal means derived from the linear mixed models for the Montgomery-Åsberg Depression Rating Scale (MADRS) and Clinician Administered Dissociative States Scale (CADSS) time points significant at p<0.05 after Bonferroni correction are indicated with an asterisk. A) In major depressive disorder (MDD) subjects, MADRS scores demonstrated a significant main effect of drug (F1,559=140.70, p<.001; Supplementary Table S2). CADSS scores indicated significant main effects of drug (F1,414=57.66, p<.001), time (F6,380=43.61, p<0.001), and a drug by time interaction (F6,380=45.59, <0.001). CADSS scores peaked at 40 minutes post-ketamine (F1,389=326.7, p<.001); the effect of the drug was not significant at any other time point. B) In healthy controls, MADRS scores indicated significant main effects of drug (F1,328=61.87, p<0.001) and time (F9,314=14.31, p<0.001) and a significant drug by time interaction (F9,313=9.87, p<0.001). The increase in depressive symptoms was significant at 40, 80, and 120 minutes post-infusion and at Day 1. Seventeen of 24 healthy controls receiving ketamine (71%) showed an increase of at least five points on the MADRS at any time point, compared to only one of 23 healthy controls receiving placebo (4%). By Day 2, only one healthy control subject still scored above 5 on the MADRS. CADSS scores in healthy controls demonstrated significant main effects of drug (F1,274=26.21, p<0.001), time (F6,261=27.50, p<0.001), and a drug by time interaction (F6,259=26.01, p<0.001), with significant differences between ketamine and placebo observed only at the 40-minute time point.
Effects of Ketamine on Mood – Healthy Controls
Healthy control subjects exhibited an unexpected increase in depressive symptoms, with significant main effects of drug (F1,328=61.87, p<0.001), time (F9,314=14.31, p<0.001), and a drug by time interaction (F9,313=9.87, p<0.001). The increase in depressive symptoms was significant at 40, 80, and 120 minutes post-infusion and at Day 1; by Day 2, only one healthy control subject exhibited a MADRS score five points greater than at baseline. To understand which symptoms were driving the increase in MADRS score, we examined individual MADRS and HAM-D17 items (Supplementary Table S5 and Figure 2). Items showing the greatest increase in healthy controls (but decreases in MDD subjects) included inner tension, lassitude, inability to feel, psychic anxiety, and somatic anxiety. We also found significant increases in SHAPS, TEPS-Anticipatory, HAM-A, and BDI scores. Notably, every item on the SHAPS and TEPS (aside from those relating to food and appetite) showed increases in healthy controls and decreases in MDD subjects post-ketamine. These findings suggest that ketamine’s mood-lowering effect on healthy control subjects occurred primarily in the domains of anxiety, emotional blunting, and anhedonia.
Figure 2.
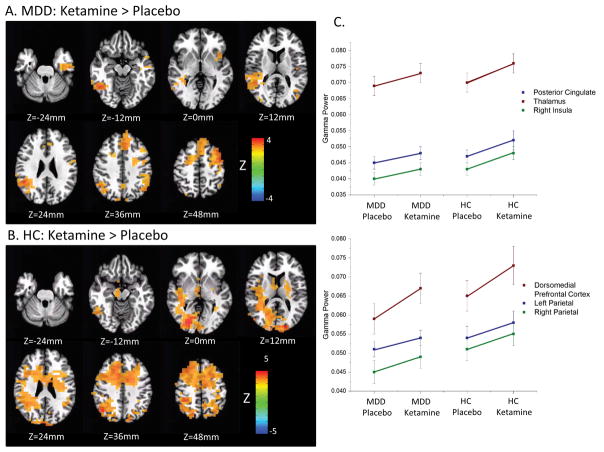
Images of Ketamine-Day0 vs. Placebo-Day0 contrasts derived from the mixed model are shown for A) patients with major depressive disorder (MDD). Images are thresholded at a voxel-level threshold of p<0.01, which corresponds to pFDR=0.049. The same images are shown for B) healthy controls, where in order to differentiate regions of peak change, images are thresholded at a more restrictive voxel-level threshold of p<0.001, which corresponds to pFDR=0.0014. Gamma power was robustly increased in regions of the central executive network (CEN; Figure 2a,b Z=36mm), including bilateral parietal cortex, dorsomedial prefrontal cortex, and dorsolateral prefrontal cortex (DLPFC) in both groups. The MDD group exhibited increases in the insula, which is involved in the salience network (SN; Figure 2a, Z=0mm). Healthy control subjects exhibited increases in gamma power in the posterior cingulate and thalamus (Figure 2b, Z=0mm to 12mm), regions related to the default mode network (DMN). MDD subjects exhibited increases under ketamine in the inferior temporal cortex extending into the parahippocampal cortex (Figure 2a, Z=−24mm to −12mm). C) The plots of estimated marginal means from mixed models performed on regions of interest (ROIs) defined on the Ketamine-Day0 vs. Placebo-Day0 contrast collapsed across groups. Coordinates are given in Supplementary Table S7. Because these are functionally defined ROIs, effect sizes cannot be interpreted, although the general trend of MDD subjects exhibiting increases in gamma power following ketamine infusion to a level commensurate with that of the healthy controls following placebo infusion can be observed. HC: healthy control.
Consistent with MDD patients, healthy controls demonstrated significant but acute increases in CADSS scores post-ketamine, with main effects for drug (F1,274=26.21, p<0.001), time (F6,261=27.50, p<0.001), and a drug by time interaction (F6,259=26.01, p<0.001). In post-hoc tests, only the 40-minute time point was significant (F1,261=187.54, p<0.001). Importantly, CADSS scores at 40 minutes post-infusion were only weakly correlated with MADRS scores (R=0.358, p=0.086), indicating that the increase in depressive symptoms extended beyond that associated with dissociative side effects.
Electrophysiology – MEG Results
Resting state MEG recordings were obtained at baseline, the day of the infusion, and 11–13 days post-infusion. Data were analyzed using linear mixed models, and post-hoc contrasts examining differences between diagnostic groups and sessions are reported.
Figure 2a shows the contrasts for Ketamine-Day0 versus Placebo-Day0 for the MDD group, and Figure 2b shows the same contrast in the healthy control group. Regions include those involved in the central executive (CEN), salience (SN), and default mode (DMN) networks (see figure legend). Figure 2c illustrates the estimated marginal means from mixed models of RMS gamma power extracted from selected regions of interest defined on the Ketamine-Day0 vs. Placebo-Day0 contrast across both groups. While unbiased effect sizes cannot be estimated from functional ROIs, they suggest that MDD subjects exhibited increases post-ketamine to levels commensurate with those seen in healthy control subjects following placebo infusion. Additional contrasts are described in the Supplementary Materials.
To examine the relationship between gamma power and MADRS response, we used absolute change in MADRS score from the t=−60 to the t=+40 minute time point as a covariate in a mixed model including both ketamine and placebo recordings. Although the +230-minute time point was closer to the MEG recording, there was no difference between placebo and ketamine MADRS scores in the healthy control group at this time point. We observed no significant relationship between MADRS response and gamma power in either group in the ketamine session. Similar mixed models carried out on ROIs derived from the data presented in Figure 2 produced equivalent results, with no relationship between gamma power and either MADRS or CADSS observed in either group.
Electrophysiology – Exploratory Analysis of Gamma Power and Antidepressant Response
We hypothesized that heterogeneity within the MDD sample might have contributed to the lack of association between antidepressant response and gamma power; in particular, we hypothesized that if response to ketamine depended on homeostatic regulation, as has been suggested previously (33), baseline gamma power might modulate the interaction. To investigate this, we used the regions of interest where we observed significant increases in gamma power post-ketamine in the combined depressed and healthy groups (See Supplementary Table S7)..
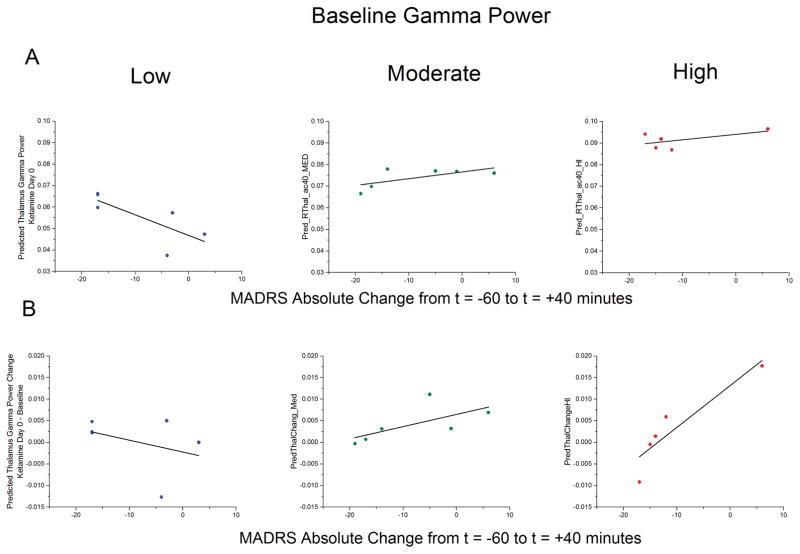
The first exploratory model examined gamma power at the Day0 post-ketamine time point using baseline gamma power and absolute change in MADRS score from the −60 to the +40-minute time points as covariates; the effect of interest was the interaction between baseline gamma power and MADRS response. As expected, there were significant main effects for baseline gamma power in all ROIs=. Additionally, after controlling for baseline gamma power, we observed a main effect of MADRS response, as well as a significant interaction between baseline gamma power and MADRS response in 8 of our 11 regions (See supplementary table S7). The interaction between baseline gamma power and MADRS response was most notable in the thalamus (F1,12=47.46, p<0.001) and the right insula (F1,13=22.24, p<0.001). We repeated these models using percent change in MADRS score from the −60 to +230-minute time point, as this is a more common measure of antidepressant response. The moderating effect of baseline gamma power on MADRS response was evident only at a trend level for the dorsomedial prefrontal cortex and right parietal cortex (See supplementary table S7). Figure 3a illustrates the difference in the relationship between gamma power post-ketamine and MADRS response as baseline gamma power increases; it should be noted that subgroup stratification was performed for visualization purposes only, and that baseline gamma power was a continuous variable in the statistical model. Figure 4b plots the change in gamma power between the post-ketamine and baseline sessions versus MADRS response for patients divided by the magnitude of their baseline gamma power.
Figure 3.
A) Exploratory results in major depressive disorder (MDD) patients from a mixed model examining post-infusion gamma power in the right thalamus with baseline gamma power and change in Montgomery Åsberg Depression Rating Scale (MADRS) score as a covariate. Significant main effects were noted for both baseline gamma power (F1,12=224.8, p<0.001) and MADRS response (F1,12=32.6, p<0.001), as was a significant interaction between baseline gamma power and MADRS response (F1,12=47.46, p<0.001). The predicted values are plotted versus change in MADRS score from t=−60 to t=+40 following ketamine infusion for three groups of patients stratified by baseline gamma power to visualize the interaction. Note that subgroup stratification was performed here for visualization purposes only; baseline gamma power was a continuous variable in the statistical model. B) The same data from A (above), except with change in gamma power from baseline to ketamine sessions plotted along the y-axis; note that the statistical effects are the same.
Discussion
In this study, and consistent with prior work (2–6), we report a robust, rapid, and relatively sustained antidepressant response to a single dose of ketamine in MDD patients. Unexpectedly, we also observed significant increases in depressive symptoms in healthy control subjects lasting up to 24 hours post-infusion, primarily in the domains of anxiety and anhedonia. Importantly, increased MADRS scores only weakly correlated with CADSS scores, indicating that depressive symptoms were not entirely accounted for by increased dissociative symptoms. We also observed increased gamma power following ketamine infusion compared to placebo infusion in widespread cortical and subcortical areas in both healthy control and MDD groups six to nine hours post-infusion.. Contrary to our a priori hypothesis, we did not observe a simple relationship between gamma power post-ketamine infusion and antidepressant response. Our post-hoc exploratory models, however, revealed a more complex interaction. In MDD subjects, these models showed that baseline gamma power moderated the relationship between change in gamma power post-ketamine and antidepressant response in multiple regions; subjects with higher baseline gamma power who experienced larger increases in gamma power showed a worse antidepressant response, while subjects with lower baseline gamma power who also experienced larger increases in gamma power showed a better antidepressant response. Taken together, these data suggest that alterations to homeostatic balance may be a crucial piece of ketamine’s mechanism of action and, potentially, the pathophysiology of MDD, at least in a treatment-resistant population.
Although ketamine infusion in psychiatrically healthy control subjects has been used extensively as a model for schizophrenia (62), only one study specifically assessed depressive symptoms, reporting a small but significant increase in HAM-D17 scores in eight subjects (63). Acute increases in BPRS-negative (dysphoric) and anxiety symptoms in healthy controls have also been reported (64), a measure that was acutely reduced in our MDD subjects. Multiple studies have also reported increased anhedonia following ketamine infusion in healthy volunteers (65–67). While sad mood was not among the most robust symptom domains affected by ketamine infusion in healthy controls in the present study, it is important to note that diagnosing a major depressive episode requires the presence of either sad mood or anhedonia, not both. The short duration of depressive symptoms in response to ketamine in healthy controls may indicate a rapid adaptation to the actions of ketamine; this would be analogous to resiliency in response to stressors, a process known to be altered in individuals with MDD (36). However, because gamma power was still elevated at the six- to nine-hour post-ketamine time point compared to placebo, this indicates that synaptic homeostasis was not yet fully restored.
Notably, the extant literature investigating ketamine’s influence on resting gamma oscillations has primarily examined acute response. Studies have reported acute increases in gamma power in widespread frontal, temporal, and parietal areas (17, 68), as well as thalamic gamma oscillations (17). Acute increases in gamma power in cortical areas associated with visual and motor tasks have also been reported (18). Although few studies have investigated time points occurring hours or days post-infusion, gamma band synaptic potentiation has been observed six to seven hours post-infusion, although only the motor cortex was examined (39). The only fMRI study measuring the non-acute effects of ketamine found reduced resting state functional connectivity 24 hours post-infusion between the medial prefrontal cortex and the posterior cingulate (69), regions where we observed increased gamma power six to nine hours post-infusion. Because gamma synchrony typically correlates positively with functional connectivity, taken together, these results may indicate that—after a relatively acute phase of increased gamma synchrony and functional connectivity—there may be a rebound phase as homeostasis is restored. Longitudinal studies at multiple time points following ketamine infusion are needed to confirm this hypothesis. Consistent with this notion, an MRS study in healthy control subjects showed an increase in the glutamine/glutamate ratio 24 hours following ketamine infusion that was partly driven by decreases in glutamate (70); notably, no increase in glutamate was found one hour post-infusion, and other spectroscopy studies in healthy control subjects failed to find differences in glutamate immediately following ketamine infusion (63).
The finding that ketamine induced depressive symptoms in healthy controls, particularly in the domains of anxiety and anhedonia, has important implications. It suggests that ketamine’s effects in healthy control subjects may represent a potential model for dysphoria. In addition, while many studies have investigated ketamine infusion in healthy controls in an effort to discern its antidepressant mechanism of action, our findings indicate that one cannot presume that biological findings in healthy subjects will accurately represent the biology of the antidepressant response.
Within our MDD subject group, we found that baseline gamma power moderated the relationship between increased gamma power post-ketamine and antidepressant response in multiple regions, consistent with the notion that resting gamma oscillations may be a proxy measure of inhibition/excitation balance and homeostasis, although these exploratory results should be considered preliminary. As a potential explanation for our findings, we would hypothesize that subjects with low baseline gamma power may be in a state where ketamine-induced increases in gamma power (via decreased interneuron and/or increased AMPA receptor activity) re-establish optimal homeostatic balance; in contrast, subjects with high baseline gamma power may experience a further disruption of the homeostatic balance and thus no antidepressant effect (although it should be noted that thalamic gamma power was nominally although not significantly reduced in MDD subjects compared to healthy controls (Figure S5)). Notably, however, gamma power dysfunction has not been identified as a biomarker for MDD, consistent with the idea that both increases and decreases in gamma power beyond a homeostatic ideal may be pathological. Additionally, it may be difficult to discern an ideal value for gamma power, as there is likely high inter-subject variability, analogous to the high inter-subject variability of the peak alpha frequency (71). In the present study, gamma power also depended on age and/or gender (see Supplementary Results). Further research is needed to determine whether clinical characteristics or secondary biomarkers can differentiate subjects above versus below their ideal point of inhibition/excitation balance. Substantial evidence for biologically-based MDD subpopulations exists, as recently demonstrated in a large sample of resting state fMRI images collected from over 1,100 patients with MDD (72). Given the extant correlations between resting state fMRI connectivity, glucose utilization (73–76), and gamma synchrony (27), these fMRI-based subgroups may potentially reflect altered glutamatergic function as well as inhibition/excitation balance. MRS studies in depression have been inconsistent, with both increases and reductions observed in prefrontal glutamate concentrations relative to controls; this may also be attributable to biological subtypes of MDD that may be poorly distinguished on the basis of clinical features alone (reviewed in (77)).
Increased gamma power in both healthy controls and MDD subjects was observed in regions relating to the SN, CEN, and DMN, a triad of networks hypothesized to be intimately involved in the pathophysiology of MDD (78–80). Many of these same regions also showed a relationship in MDD patients between MADRS response and gamma power after controlling for baseline gamma power, most notably thalamus and insula (an SN region). Interestingly, an fMRI study in healthy control subjects showed increased thalamo-cortical connectivity during acute ketamine infusion (82), and Dreisen and colleagues showed that the magnitude of the increase in global connectivity of the thalamus was negatively associated with negative symptoms experienced during the infusion (83). Although MEG is less sensitive to subcortical sources than cortical surface sources, it was used to observe cortico-thalamic gamma oscillations as early as 1991 (84). The beamforming technique used herein enables visualization of deep sources, and prior studies have demonstrated altered gamma power in the thalamus in response to fearful faces in individuals with MDD (85). Consistent with the idea that prolonged dysrhythmia or deviation from homeostasis can result in neuronal damage, studies have found decreased thalamic volumes in MDD (86), along with abnormally increased blood flow and metabolism (87) and DMN connectivity (88).
Our exploratory results, while tantalizing, should be treated with caution. Due to the assiduous quality control of our scans, the final sample size for exploratory MEG analysis used only 17 MDD subjects. In addition, these results alone do not provide a biomarker for response to ketamine. While subjects with lower baseline gamma who had the greatest increase in gamma power post-ketamine experienced the greatest antidepressant response, we cannot predict who is likely to experience the greatest gamma power increase in response to ketamine. In addition, there is no clear “ideal” value for raw gamma power post-ketamine, and it is unlikely that an ideal value would apply to all subjects, given that the range of resting gamma power in healthy subjects is relatively broad. We should also note that while, for consistency, we chose the measure of response to ketamine to match what we used in our analyses including the healthy controls (absolute change between MADRS score from t=−60 to +40 minutes), percent change at other time points is more commonly used as a metric of the antidepressant response. Although results no longer remained significant using percent change at later time points, the antidepressant response in our sample was maximal at 40 minutes, even in the depressed subjects, and the variance was significantly larger in the percent change at +230 and day 1. Finally, because these were post-hoc exploratory analyses, further hypothesis-driven investigations are required.
Nevertheless, these data have broad implications. First, we found that ketamine infusion in healthy control subjects robustly and rapidly induced depressive symptoms across multiple symptom domains, complicating the interpretation of studies examining the biological response to ketamine in healthy subjects as well as the applicability of those results to individuals with MDD. Second, our finding that gamma power was increased even six to nine hours post-ketamine infusion in both healthy controls and MDD subjects indicates that the influence of ketamine and its metabolites on synaptic plasticity persists outside of the acute infusion period. Third, and most importantly, our results potentially identify gamma power as a marker for synaptic homeostasis, which may enable the discovery of more accurate markers pointing to the degree and direction of divergence from ideal synaptic function. Building on this work, future treatments could be tailored to the degree and direction of dysregulation for each subject individually, potentially enabling a personalized approach to psychiatry.
Supplementary Material
Acknowledgments
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Footnotes
ClinicalTrials.gov Identifier: NCT00088699
Conflict of Interest
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; NCT00088699/04-M-0222/ZIA MH002857), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. Dr. Zarate is listed as a coinventor on a patent for the use of ketamine in major depression and suicidal ideation. Dr. Zarate is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Dr. Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders; he has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
References
- 1.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006 Jan;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 2.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006 Aug;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 3.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011 Feb 28;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013 Oct;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, et al. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry. 2016 Aug 01;173:816–826. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 6.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012 Jun 01;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010 Dec;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. 2015 Dec;45:3571–3580. doi: 10.1017/S0033291715001506. [DOI] [PubMed] [Google Scholar]
- 9.Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007 Dec;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- 10.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008 Feb 15;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010 Aug 20;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011 Jun 15;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008 Apr 15;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Anderson PM, Pinault D, O’Brien TJ, Jones NC. Chronic administration of antipsychotics attenuates ongoing and ketamine-induced increases in cortical gamma oscillations. Int J Neuropsychopharmacol. 2014 Nov;17:1895–1904. doi: 10.1017/S1461145714000959. [DOI] [PubMed] [Google Scholar]
- 15.Jones NC, Anderson P, Rind G, Sullivan C, van den Buuse M, O’Brien TJ. Effects of aberrant gamma frequency oscillations on prepulse inhibition. Int J Neuropsychopharmacol. 2014 Oct;17:1671–1681. doi: 10.1017/S1461145714000492. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Yoshida T, Katz DB, Lisman JE. NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. J Neurophysiol. 2012 Jun;107:3181–3189. doi: 10.1152/jn.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivolta D, Heidegger T, Scheller B, Sauer A, Schaum M, Birkner K, et al. Ketamine Dysregulates the Amplitude and Connectivity of High-Frequency Oscillations in Cortical-Subcortical Networks in Humans: Evidence From Resting-State Magnetoencephalography-Recordings. Schizophr Bull. 2015 Sep;41:1105–1114. doi: 10.1093/schbul/sbv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw AD, Saxena N, LEJ, Hall JE, Singh KD, Muthukumaraswamy SD. Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. Eur Neuropsychopharmacol. 2015 Aug;25:1136–1146. doi: 10.1016/j.euroneuro.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional Homeostatic Regulation of a Depression-Related Brain State by Gamma-Aminobutyric Acidergic Deficits and Ketamine Treatment. Biol Psychiatry. 2016 Sep 15;80:457–468. doi: 10.1016/j.biopsych.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007 Oct 24;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012 May;17:537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016 Jan;100:17–26. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDA receptor inhibition-independent antidepressant actions of a ketamine metabolite. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007 Jan;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 25.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 26.Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK. Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport. 2003 Apr 15;14:683–686. doi: 10.1097/00001756-200304150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Mulert C, Leicht G, Hepp P, Kirsch V, Karch S, Pogarell O, et al. Single-trial coupling of the gamma-band response and the corresponding BOLD signal. Neuroimage. 2010 Feb 01;49:2238–2247. doi: 10.1016/j.neuroimage.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 28.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005 Aug 05;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 29.Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008 Jul;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kann O, Hollnagel JO, Elzoheiry S, Schneider J. Energy and Potassium Ion Homeostasis during Gamma Oscillations. Front Mol Neurosci. 2016;9:47. doi: 10.3389/fnmol.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y, et al. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry. 2012 Jul 17;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered Excitatory-Inhibitory Balance in the NMDA-Hypofunction Model of Schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012 Oct 05;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008 Oct 31;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith KS, Rudolph U. Anxiety and depression: mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes. Neuropharmacology. 2012 Jan;62:54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015 May;38:279–294. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drevets WC. Neuroplasticity in Mood Disorders. Dialogues Clin Neurosci. 2004;6:199–216. doi: 10.31887/DCNS.2004.6.2/wdrevets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drevets WC, Gadde KM, Krishnan KR. Neuroimaging Studies of Mood Disorders. In: Charney DS, editor. Neurobiology of Mental Illness. 2. Oxford University Press; Oxford, UK: 2004. pp. 461–490. [Google Scholar]
- 39.Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012 Oct 1;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- 41.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62( Suppl 16):10–17. [PubMed] [Google Scholar]
- 42.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960 Feb;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bech P, Boyer P, Germain JM, Padmanabhan K, Haudiquet V, Pitrosky B, et al. HAM-D17 and HAM-D6 sensitivity to change in relation to desvenlafaxine dose and baseline depression severity in major depressive disorder. Pharmacopsychiatry. 2010 Nov;43:271–276. doi: 10.1055/s-0030-1263173. [DOI] [PubMed] [Google Scholar]
- 44.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995 Jul;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 45.Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J Res Personal. 2006 Dec;40:1086–1102. [Google Scholar]
- 46.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 47.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 48.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996 Aug;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 49.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 50.Guy W. Clinical Global Impressions. In: Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. National Institute of Mental Health; 1976. [Google Scholar]
- 51.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979 Apr;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 52.McNair DM, Lorr M, Droppleman LF. Educational and Testing Service. 1971. EITS Manual for the Profile of Mood States. [Google Scholar]
- 53.Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969 Oct;62:989–993. doi: 10.1177/003591576906201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998 Jan;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 55.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 56.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978 Nov;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 57.Lopes da Silva F. EEG and MEG: relevance to neuroscience. Neuron. 2013;80:1112–1128. doi: 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, et al. MEG and EEG data analysis with MNE-Python. Front Neurosci. 2013 Dec 26;7:267. doi: 10.3389/fnins.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996 Jun;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 60.Robinson SE, Vrba J. Biomag. Tohoku University Press; Sendai: 1999. Functional neuroimaging by synthetic aperture magnetometry (SAM) pp. 302–305. [Google Scholar]
- 61.Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed–effects modeling approach to FMRI group analysis. Neuroimage. 2013 Jun;73:176–190. doi: 10.1016/j.neuroimage.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perry EB, Jr, Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, et al. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 2007 Jun;192:253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 63.Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol. 2012 May;26:733–737. doi: 10.1177/0269881111405359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994 Mar;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 65.Stone JM, Erlandsson K, Arstad E, Squassante L, Teneggi V, Bressan RA, et al. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: a [(123)I]CNS-1261 SPET study. Psychopharmacology (Berl) 2008 Apr;197:401–408. doi: 10.1007/s00213-007-1047-x. [DOI] [PubMed] [Google Scholar]
- 66.Mason OJ, Morgan CJ, Stefanovic A, Curran HV. The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res. 2008 Aug;103:138–142. doi: 10.1016/j.schres.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 67.Pollak TA, De Simoni S, Barimani B, Zelaya FO, Stone JM, Mehta MA. Phenomenologically distinct psychotomimetic effects of ketamine are associated with cerebral blood flow changes in functionally relevant cerebral foci: a continuous arterial spin labelling study. Psychopharmacology (Berl) 2015 Dec;232:4515–4524. doi: 10.1007/s00213-015-4078-8. [DOI] [PubMed] [Google Scholar]
- 68.Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J Neurosci. 2015 Aug 19;35:11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7:e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li M, Demenescu LR, Colic L, Metzger CD, Heinze HJ, Steiner J, et al. Temporal Dynamics of Antidepressant Ketamine Effects on Glutamine Cycling Follow Regional Fingerprints of AMPA and NMDA Receptor Densities. Neuropsychopharmacology. 2017 May;42:1201–1209. doi: 10.1038/npp.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haegens S, Cousijn H, Wallis G, Harrison PJ, Nobre AC. Inter- and intra-individual variability in alpha peak frequency. Neuroimage. 2014 May 15;92:46–55. doi: 10.1016/j.neuroimage.2014.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017 Jan;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duncan NW, Wiebking C, Tiret B, Marjanska M, Hayes DJ, Lyttleton O, et al. Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. PLoS One. 2013;8:e60312. doi: 10.1371/journal.pone.0060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013 Jan 1;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nugent AC, Martinez A, D’Alfonso A, Zarate CA, Theodore WH. The relationship between glucose metabolism, resting-state fMRI BOLD signal, and GABA-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J Cereb Blood Flow Metab. 2015 Jan 7; doi: 10.1038/jcbfm.2014.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. Proc Natl Acad Sci U S A. 2013 Aug 13;110:13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niciu MJ, Ionescu DF, Richards EM, Zarate CA., Jr Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm (Vienna) 2014 Aug;121:907–924. doi: 10.1007/s00702-013-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011 Oct;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 79.Zheng H, Xu L, Xie F, Guo X, Zhang J, Yao L, et al. The Altered Triple Networks Interaction in Depression under Resting State Based on Graph Theory. Biomed Res Int. 2015;2015:386326. doi: 10.1155/2015/386326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pinault D, Deschenes M. Voltage-dependent 40-Hz oscillations in rat reticular thalamic neurons in vivo. Neuroscience. 1992 Nov;51:245–258. doi: 10.1016/0306-4522(92)90312-p. [DOI] [PubMed] [Google Scholar]
- 82.Hoflich A, Hahn A, Kublbock M, Kranz GS, Vanicek T, Windischberger C, et al. Ketamine-induced modulation of the thalamo-cortical network in healthy volunteers as a model for schizophrenia. Int J Neuropsychopharmacol. 2015 Apr;19:18. doi: 10.1093/ijnp/pyv040. pii:pyv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013 Jan 22;18:1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A. 1991 Dec 15;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007 Jan 15;34:839–847. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nugent AC, Davis RM, Zarate CA, Jr, Drevets WC. Reduced thalamic volumes in major depressive disorder. Psychiatry Res. 2013 Sep 30;213:179–185. doi: 10.1016/j.pscychresns.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992 Sep;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007 Sep 1;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.