Abstract
Bile acids activate farnesoid X receptor (FXR) and G protein-coupled bile acid receptor-1 (Gpbar-1, aka TGR5) to regulate bile acid metabolism and glucose and insulin sensitivity. FXR and TGR5 are co-expressed in the enteroendocrine L cells but their roles in integrated regulation of metabolism are not completely understood. We reported recently that activation of FXR induces TGR5 to stimulate glucagon-like peptide-1 (GLP-1) secretion to improve insulin sensitivity and hepatic metabolism. In this study, we used the intestine-restricted FXR agonist fexaramine (FEX) to study the effect of activation of intestinal FXR on the gut microbiome, bile acid metabolism, and FXR and TGR5 signaling. The current study revealed that FEX markedly increased taurolithocholic acid (TLCA), increased fibroblast growth factor 15 (FGF15) and FGF21 and GLP-1 secretion, improved insulin and glucose tolerance, and promoted white adipose tissue browning in mice. Analysis of 16S ribosomal RNA sequences of the gut microbiome identified the FEX-induced and LCA-producing bacteria Acetatifactor and Bacteroides. Antibiotic treatment completely reversed the FEX-induced metabolic phenotypes and inhibited TLCA synthesis, adipose tissue browning, and liver bile acid synthesis gene expression, but further increased intestinal FXR target gene expression. FEX treatment effectively improved lipid profiles, increased GLP-1 secretion, improved glucose and insulin tolerance, and promoted adipose tissue browning, while antibiotic treatment reversed the beneficial metabolic effects of FEX in obese and diabetic mice. This study uncovered a novel mechanism in which activation of intestinal FXR shaped the gut microbiota to activate TGR5/GLP-1 signaling to improve hepatic glucose and insulin sensitivity and increase adipose tissue browning. The gut microbiota plays a critical role in bile acid metabolism and signaling to regulate metabolic homeostasis in health and disease.
Introduction
Bile acids are known to regulate lipid, glucose and energy homeostasis through activation of farnesoid X receptor (FXR) and G protein-coupled bile acid receptor-1 (Gpbar-1, aka Takeda G-protein-coupled receptor 5, TGR5) (1, 2). Bile acid synthesis in the liver generates two primary bile acids, cholic acid (CA) and chenodeoxycholic (CDCA) in humans (3). In mice, CDCA is converted to α- and β-muricholic acid (MCA). Bile acid are conjugated to the amino acids glycine and taurine for secretion into bile, and are released into the gastrointestinal system to aid in absorption of dietary nutrients. In the intestine, gut bacteria bile salt hydrolase (BSH) de-conjugates conjugated bile acids and bacterial 7α- and 7β-dehydroxylases convert the primary bile acids CA and CDCA to the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. Bacteria also can isomerize the 7α-hydroxyl group in CDCA to the 7β-hydroxyl group in ursodeoxycholic acid (UDCA), which is soluble and non-toxic. Enterohepatic circulation of bile acids from the intestine to the liver activates FXR, which represses bile acid synthesis through inhibition of Cyp7a1 and Cyp8b1 expression. In the intestine, FXR induces fibroblast growth factor 15 (FGF15), which activates hepatic FGF receptor 4 signaling in the liver to further inhibit Cyp7a1 transcription (4). However, under physiological concentrations, taurocholic acid (TCA) may not be able to activate FXR in mouse liver (EC50= ~0.6 mM), while TCDCA (EC50 = 17 μM) is the most potent endogenous FXR agonist and T-β-MCA (IC50= 40 μM) is an FXR antagonist.
Activation of FXR is thought to be beneficial in improving insulin and glucose sensitivity in diabetes and non-alcoholic fatty liver disease (NAFLD) (1, 2). Paradoxically, deficiency or antagonism of intestinal FXR was shown to improve obesity, insulin resistance and NAFLD (5, 6), while activation of intestinal FXR by intestine-restricted fexaramine (FEX) also improves metabolic disorders in mice (7). Thus, the role and mechanism of intestinal FXR in the regulation of lipid and glucose metabolism and NAFLD is controversial, not completely understood and requires further study.
TGR5 is a Gαs-protein-coupled receptor activated by the secondary bile acids TLCA (EC50 = 0.3 μM) and DCA (EC50 = 1 μM). TGR5 is widely expressed in the epithelial cells of the gastrointestinal system, including intestine, gallbladder, hepatic sinusoidal endothelial cells and Kupffer cells, but is not expressed in hepatocytes (8–10). In the gastrointestinal tract, activation of TGR5 by bile acids and synthetic agonists protects intestinal barrier function, reduces inflammation, and stimulates gallbladder refilling and GLP-1 secretion from enteroendocrine L cells (11). GLP-1 stimulates insulin synthesis, increases postprandial insulin secretion from pancreatic β cells and improves insulin resistance (12). GLP-1 secretion is stimulated by nutrients in the intestinal lumen, such as carbohydrates, fats and proteins, and glucose-induced GLP-1 secretion is enhanced by bile acids (13). Activation of TGR5 increases intracellular cAMP to stimulate cAMP-dependent protein kinase A, which activates cAMP response element binding protein and induces thyroid hormone deiodinase 2 (Dio2) and other browning factors, stimulating energy metabolism in white adipose tissue and alleviating obesity and hepatic steatosis in diet-induced obese mice (14, 15). Recently, TGR5 was found to have a key role in bile acid synthesis and fasting-induced hepatic steatosis in mice (16) and activation of FXR induces TGR5 expression and GLP-1 secretion, improving insulin and glucose sensitivity and symptoms of metabolic disorder (17).
The gut-to-liver axis plays a critical role in the regulation of bile acid metabolism, bile acid pool size and enterohepatic circulation of bile acids. Bile acids shape the gut microbiome to regulate host metabolism (18). In this study, the effects of the intestine-restricted FXR agonist FEX on hepatic bile acid metabolism and the gut microbiota were examined, with the aim of unveiling the mechanism of TGR5/GLP-1 signaling in improving glucose and lipid metabolism. The data suggest that FEX treatment induced gut bacteria that produce LCA to activate TGR5/GLP-1 signaling, thereby improving insulin sensitivity and promoting adipose tissue browning. Thus, activation of TGR5/GLP-1 signaling through modulation of the gut microbiota may have therapeutic potential for treating NAFLD, diabetes, and obesity.
Materials and Methods
MICE
Male wild-type C57BL/6J and leptin receptor-deficient diabetic mice Leprdb/db (db/db) were purchased from the Jackson Laboratory (Bar Harbor, ME). Fxr−/− mice (19) and Tgr5−/− mice (20) are on the C57BL/6J genetic background and were maintained in an AAALAC-certified animal facility at Northeast Ohio Medical University (NEOMED). The Institutional Animal Care and Use Committee of NEOMED approved all animal protocols. Mice were 12–16 weeks old at the time of study and were maintained on a standard chow diet and water ad libitum and housed in a room with a 12 h light (6 a.m. to 6 p.m.) and 12 h dark (6 p.m. to 6 a.m.) cycle.
GUT MICROBIOTA ANALYSIS
Bacterial isolation and creation of the 16S amplicons
Cecal contents (100 mg) were used for bacterial DNA extraction with an E.Z.N.A isolation kit (Omega Biotek, Norcross, GA) according to provided instructions. Extracted DNA concentration was measured using a Nanodrop and samples were diluted to 10 ng/μl. DNA was then mixed with V4/V4 primers (515F: TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCCG CGGTAA and 806R: GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVG GGTWTCTAAT) and the Invitrogen Platinum SuperFi enzyme kit (ThermoFisher Scientific, Waltham, MA) for PCR amplification (98°C, 2 min; 98°C, 10 s, 56.5°C, 20 s; 72°C, 15 s for 20 cycles; 72°C, 5 min). Products were run on a 1X agarose gel, verifying a 350-bp product.
Sequencing and analysis
PCR products were sent to the Pennsylvania State University Genomics Core Facility for Illumina Miseq sequencing (150 × 150 paired end). Sequence files were analyzed using the Mothur software pipeline (21). Reads were trimmed at 320 bp and aligned to the SILVA database. Chimaeras were removed using UChime (22) and reads were classified at a 75% cutoff using the ribosomal database project’s (RDP) training set. The summary file was used for further taxonomic analysis. Further sequence analysis was done by converting the trimmed fasta file into a distance file and aligning it to a phylogenic tree. This tree file was compared to an operational taxonomic unit (OTU) file for Generalized Unifrac (GUnifrac) analysis (23). Significantly different genera were discovered using a Students t-test and the normalized values were converted into Z-scores ( ) for visualization. Sequencing data have been submitted to NCBI (Accession #PRJNA413375).
FXR AGONIST TREATMENT AND GLP-1 SECRETION ASSAY
Wild-type C57BL/6J, Fxr−/−, Tgr5−/− and db/db mice were treated by oral gavage with the intestine-specific FXR agonist fexaramine (7) (FEX, 50 mg/Kg, BioVision, Inc., Milpitas, CA), once a day for 7 to 9 days. FEX is highly insoluble and was first dissolved in DMSO and then diluted to 0.2% DMSO with PBS (14). For the GLP-1 secretion assay, mice (wild-type, Fxr−/−, Tgr5−/− and db/db) were treated with FEX by oral gavage once a day for 6 days. On the 7th day, the mice were fasted for 10 h and gavaged with the dipeptidyl peptidase 4 (DPP4) inhibitor, sitagliptin, (3 mg/kg) and liquid diet (Ensure Plus, 10 ml/kg, 1.5 calories/ml: 15% protein, 57% carbohydrate and 28% fat) to stimulate GLP-1 secretion as described (24). Blood samples were collected immediately (time 0), at 15 min, 30 min, and 60 min after liquid diet gavage. Serum GLP-1 levels were assayed using a GLP-1 (7-37) ELISA kit (Millipore, Billerica, MA).
BILE ACID ANALYSIS
For bile acid quantitation in serum, ileum, colon, and gallbladder, d5-TCA at 1 μM was used as an internal standard. Samples were prepared by acetonitrile precipitation and the supernatants were further diluted with 0.1% formic acid. The bile acid concentrations were determined by an UPLC/Synapt G2-Si QTOFMS system (Waters Corp., Milford, MA) with an ESI source. Chromatographic separation was archived on an Acquity BEH C18 column (100 mm × 2.1 mm i.d., 1.7 μm, Waters Corp.). The mobile phase consisted of a mixture of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient elution was applied. Column temperature was maintained at 45°C, and the flow rate was 0.4 mL/min. MS detection was operated in negative mode. A mass range of m/z 50 to 850 was acquired. All bile acid standards were purchased from Sigma-Aldrich (St. Louis, MO) or Toronto Research Chemicals (Toronto, Ontario, Canada).
ANTIBIOTIC TREATMENT
C57BL/6J wild-type mice (n=20) and db/db mice were provided a mixture of antibiotics (ABX) containing ampicillin (1 g/L; Sigma Aldrich), vancomycin (500 mg/L; Abbott Labs, Chicago, IL), neomycin sulfate (1 g/L; Pharmacia/Upjohn), and metronidazole (1 g/L; Sidmak Labs, Gujarat, India) in drinking water for 30 days. Antibiotic water was replaced every other day. During the last week mice were treated with FEX (50 mg/Kg, n=10) or vehicle (0.2% DMSO in PBS, n=10) by oral gavage for 7 –10 days. On the 8th day, mice (n=5) were used for GLP-1 secretion or oral glucose tolerance test. Mice were fasted 6 h and were euthanized.
STATISTICAL ANALYSIS
All experimental data are presented as mean ± standard error. Statistical analysis was performed by Student’s t-test for analysis of two variants. P < 0.05 was considered statistically significant.
SUPPLEMENTAL METHODS
Oral glucose and insulin tolerance tests; immunoblot analysis; bile acid pool size; quantitative real-time PCR assay (qPCR).
Results
The intestine-restricted FXR agonist fexaramine stimulated GLP-1 secretion and hepatic insulin sensitivity
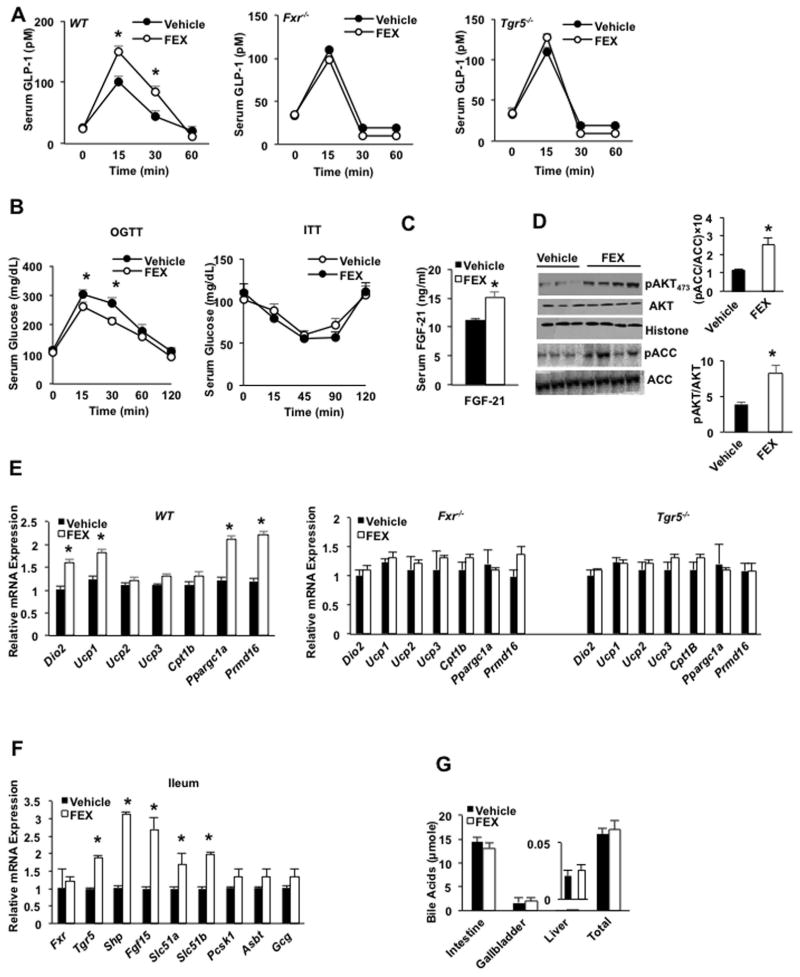
The effects of the intestine-restricted FXR agonist fexaramine (FEX) on glucose-stimulated GLP-1 secretion were examined. Oral gavage of FEX stimulated GLP-1 secretion in wild-type C57BL/6J mice, but not in Tgr5−/− or Fxr−/− mice, indicating both FXR and TGR5 are involved in bile acid-stimulated GLP-1 secretion (Fig. 1A). FEX treatment significantly improved oral glucose tolerance but not insulin tolerance (Fig. 1B), and significantly increased serum FGF21 levels (Fig. 1C). Immunoblot analysis of liver extracts demonstrated that FEX treatment increased the ratios of phosphorylated AKT to total AKT and phosphorylated acetyl CoA-carboxylase (ACC) to total ACC, indicating increased hepatic insulin sensitivity (Fig. 1D). ACC is a key enzyme in lipogenesis and phosphorylation inactivates ACC enzyme activity. H&E and UCP-1 antibody staining show beiging of epididymal WAT (eWAT) in FEX-treated mice compared to vehicle control mice (Suppl. Fig. S1). FEX treatment increased mRNA expression of the adipose tissue browning factors Dio2, uncoupling protein-1 (Ucp-1), peroxisome proliferator-activated receptor-γ co-activator-1α (Ppargc1α), and PR domain containing 16 (Prmd16) in eWAT of wild-type mice (Fig. 1E). Prdm16 transcription factor induces brown-like adipocytes (beige) in WAT and is required for brown adipose identity and function (25, 26). FEX treatment did not induce browning factors in Fxr−/− and Tgr5−/− mice (Fig. 1E). FEX treatment significantly induced ileum FXR target genes, including Tgr5, Shp, Fgf15, organic solute transporter 1α (Ostα) and Ostβ mRNA levels (Fig. 1F). In contrast to reducing the bile acid pool size as seen with the FXR-selective agonist obeticholic acid and the FXR and TGR5 dual agonist INT-767 (17), FEX treatment did not significantly alter bile acid content in the intestine, gallbladder or liver, or total bile acid pool size (Fig. 1G). Overall, these data suggest that FEX activation of both FXR and TGR5 stimulated glucose-dependent GLP-1 secretion from intestine L cells to promote adipose tissue browning and increase glucose tolerance and hepatic insulin sensitivity.
Fig. 1.
The intestine-restricted FXR agonist FEX stimulated GLP-1 secretion, hepatic insulin sensitivity and adipose tissue browning in mice. Wild-type C57BL/6J mice were gavaged with FEX (50 mg/Kg, n=10), or vehicle (0.2% DMSO in PBS, n=10) for 7 days. For oral glucose tolerance testing, the mice were fasted for 6 h and gavaged with glucose (2 g/Kg). (A) Effect of FEX on GLP-1 secretion in wild-type, Fxr−/− and Tgr5−/− mice. (B) Oral glucose tolerance test (left) and insulin tolerance test (right) of wild-type mice treated with FEX. (C) ELISA assay of serum FGF21 levels in FEX-treated wild-type mice. (D) Effect of FEX treatment on phosphorylation of liver AKT and acetyl-CoA carboxylase. Total and phosphorylated AKT and ACC were assayed by immunoblot analysis of liver extracts of vehicle-treated (n=3) and FEX-treated (n=4) mice. (E) Real-time PCR analysis of the effect of FEX treatment on mRNA expression of browning factors in inguinal white adipose tissue (iWAT) of wild-type, Fxr−/− and Tgr5−/− mice. (F) Real-time PCR analysis of the effect of FEX treatment on mRNA expression of FXR targets in mouse ileum. (G) Effect of FEX treatment on bile acid contents in intestine, gallbladder and liver and total bile acid pool size in wild-type mice. Results were expressed as mean ± standard error. An “*” indicates statistically significant difference between treated vs. vehicle control, p ≤ 0.05. Student’s t-test was used for statistical analysis.
Fexaramine did not affect liver metabolism
In wild-type mice, FEX treatment did not affect serum or hepatic cholesterol and triglyceride levels (Suppl. Fig. S2A). Expression of the bile acid synthesis genes Cyp7a1, Cyp8b1, Cyp27a1 and Cyp7b1 mRNA and their respective protein expression levels, and the FXR targets small heterodimer partner (Shp) and bile salt export pump mRNA levels were unchanged in liver (Suppl. Fig. S2B). Also unaltered were mRNA levels encoded by genes involved in fatty acid synthesis, fatty acid oxidation, triglyceride synthesis and hydrolysis, and lipoprotein metabolism. (Suppl. Fig. S2C). These data are consistent with FEX being an intestine-restricted FXR agonist, which is not absorbed and thus does not affect FXR target genes involved in bile acid synthesis and transport in the liver.
FEX treatment increased lithocholic acid content and bile acid hydrophobicity in gallbladder and intestinal bile
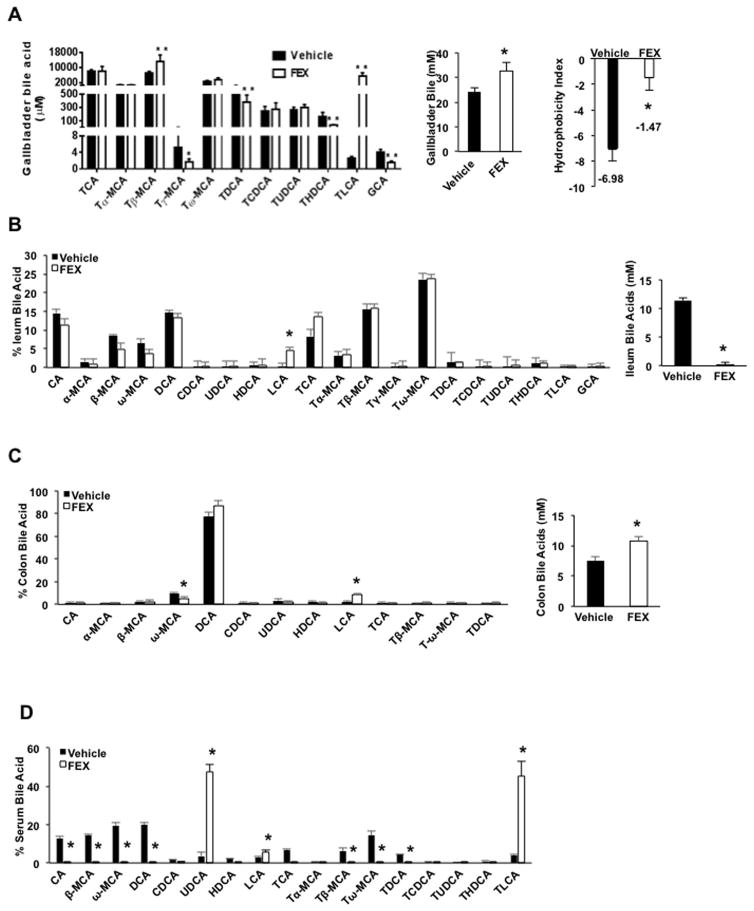
A previous study reported that FEX treatment increased LCA in mouse serum (7). Serum bile acid concentration is very low and does not contribute to the total bile acid pool. Bile acid composition in gallbladder bile best represents the bile acids synthesized in the liver and circulated in the pool. FEX significantly altered bile acid composition in the gallbladder by increasing TLCA concentration by ~1,000-fold, and significantly increasing T-βMCA, decreasing T-γ-MCA and TDCA, but not TCA concentration (Fig. 2A). However, when plotted as a percentage of bile acid in bile, TCA was reduced because drastic increasing of the % of TCA (Suppl. Fig. S2D). This change resulted in increased bile acid concentration and increased the hydrophobicity index of the bile (Fig. 2A). In the ileum, FEX treatment markedly reduced bile acid content and only LCA was significantly increased (Fig. 2B). In the colon, FEX treatment markedly increased total bile acid content, increased DCA content to 90% of total bile acids, and increased LCA but decreased ω-MCA (Fig. 2C). In serum, FEX treatment markedly increased UDCA and TLCA, which became the predominant bile acids (45% each) (Fig. 2D).
Fig. 2.
FEX treatment altered bile acid composition in wild-type mice. Wild-type C57BL/6J mice were orally gavaged with the FXR agonist FEX (50 mg/Kg, n=8), or vehicle (0.2% DMSO in PBS, n=7) as indicated for 9 days. Mice were fasted for 6 h and killed. (A) FEX altered bile acid concentration in gallbladder bile. Bile acids were extracted from 2 μl gallbladder bile from FEX-treated and vehicle control mice. Hydrophobicity index of gallbladder bile was calculated by multiplying the hydrophobicity index of individual bile acids by the concentration (mM) of the individual bile acids in the gallbladder. (B) Ileum bile acid composition. (C) Colon bile acid composition. (D) Serum bile acid composition. Hydrophobicity index used: TCA= 0, T7α-MCA= −0.84, Tβ-MCA= −0.78, THDCA= −0.37, Tγ-MCA and Tω-MCA = −0.33, TUDCA= −0.27, TCDCA= 0.46, TDCA= 0.59, TLCA= 1. Results were expressed as mean ± standard error. Results were expressed as mean ± standard error. An “*” indicates statistically significant difference (p ≤ 0.05) treated vs. vehicle control. An “**” indicates statistically significant difference between treated vs. vehicle control, p ≤ 0.01. Student’s t-test was used for statistical analysis.
FEX increased lithocholic acid synthesis and altered bile acid composition
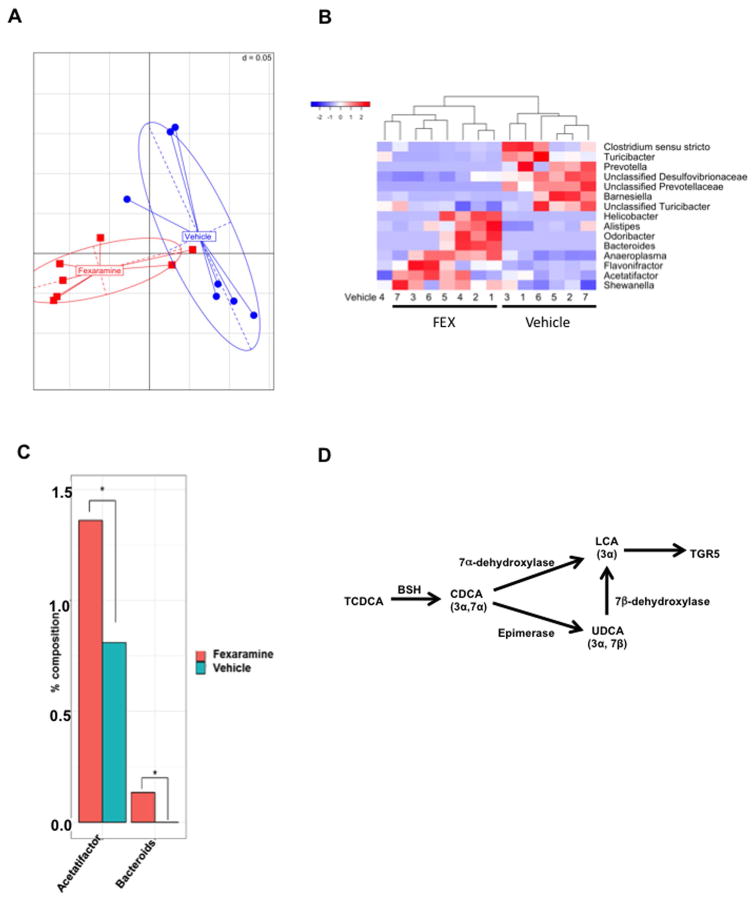
To investigate the mechanism of FEX induction of LCA, 16S ribosomal RNA sequencing analysis was performed on the gut microbiome from FEX-treated mice. Generalized unifrac analysis shows a clear separation of the gut microbiome in FEX-treated mice vs. vehicle control mice (Fig. 3A). A heatmap displaying the Z score distribution of significantly different genera revealed that FEX increased the abundance of Helicobacter, Alistipes, Bacteroides, Anaeroplasma, Flavonifractor, Acetalifactor and Shewanella (Fig. 3B). Figure 3B also illustrates that FEX reduced the abundance of Clostridium sensu stricto, Turicibacter, Prevolella, unclassified Desulfovibrionaceae, unclassified Prevotellaceae, Barnesiella, and unclassified Turicibacter compared to vehicle control mice. Acetatifactor was recently identified as a bile acid-induced anaerobic bacteria most closely related to Clostridium cluster XIV (27). A quantitative increase of Acetatifactor and Bacteroides was noted in FEX-treated mice compared to vehicle-treated control mice (Fig. 3C). Bacteroides are the predominant bacteria in the gut capable of converting TCDCA to TLCA by 7α-dehydroxylase activity, and UDCA to LCA by 7β-dehydroxylase activity (28, 29). BSH activity in Clostridium de-conjugates TCDCA to CDCA, which is converted to LCA by bacteria 7α-dehydroxylase. CDCA can be isomerized to UDCA, which can be converted to LCA by 7β-dehydroxylase activity in these bacteria (Fig. 3D).
Fig. 3.
Gut microbiota analysis. Gut microbes were extracted according the described methods after FEX treatment. FEX significantly altered the composition of the gut microbiota. (A) Generalized unifrac analysis of the whole gut microbiota population between vehicle and FEX-treated mice. Generalized unifrac combines weighted and unweighted unifrac analysis to incorporate both abundant and rare taxonomies, respectively. Significance was obtained via the R package Adonis, which is a permutational multivariate analysis of variance that is designed for distance matrices. (B) Effect of FEX on the genera of the gut microbiome. Z-scores are used to illustrate significantly different genera (p ≤ 0.05) between vehicle and FEX-treated mice. Samples were allowed to cluster under the R package heatmap.2’s hclust function. The dendrogram shows related samples. (C) Two genera, Acetatifactor and Bacteroides, were shown to increase after FEX treatment. (D) Gut bacteria induce LCA production to stimulate TGR5 signaling. Bile salt hydrolase de-conjugates TCDCA to CDCA, which is converted to LCA by bacterial 7α-dehydroxylase. CDCA can be epimerized to UDCA, which is converted to LCA by bacterial 7β-dehydroxylase. LCA activates TGR5 in the intestine. An “*” indicates statistically significant difference (p ≤ 0.05) between treated vs. vehicle control.
Antibiotics reversed the metabolic effects of FEX
Because FEX treatment altered the gut microbiota and increased LCA-producing bacteria, we then treated mice with antibiotics to test whether antibiotics would reduce TLCA and prevent the FEX-induced metabolic phenotype changes. Mice were given a mixture of antibiotics (ABX) containing ampicillin (1 g/L), vancomycin (500 mg/L), neomycin sulfate (1 g/L), and metronidazole (1 g/L) in drinking water for four weeks, followed by oral gavage of FEX during the last week of antibiotic treatment (Suppl. Fig. S4A). ABX did not affect serum AST and ALT levels (Suppl. Fig. S4B), but significantly reduced weight (Suppl. Fig. S4C).
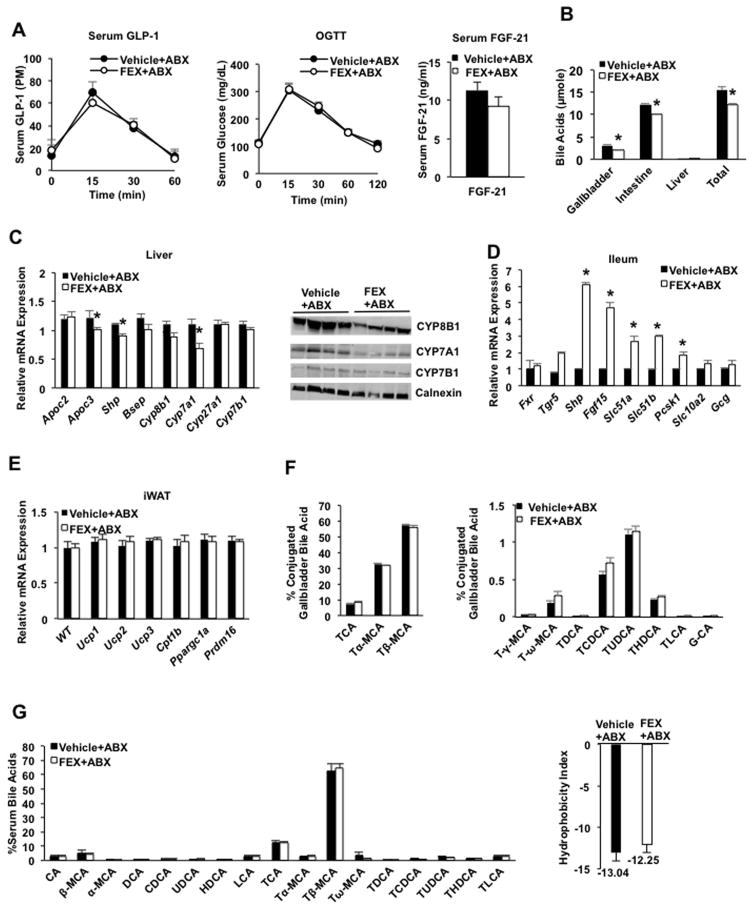
Antibiotic treatment completely prevented FEX-induced GLP-1 secretion (Fig. 4A, left), did not improve glucose tolerance (Fig. 4A, middle), and did not significantly change serum FGF21 levels (Fig. 4A, right). Interestingly, FEX treatment significantly reduced the bile acid pool size in mice treated with antibiotics compared to vehicle control (Fig. 4B), contrasted with a lack of effect in mice without antibiotic treatment (see Fig. 2E). Surprisingly, FEX treatment reduced mRNA expression levels of the FXR targets Cyp7a1, Cyp7b1 and Shp in the liver of antibiotic-treated mice (Fig. 4C), while expression of these genes was unaffected without antibiotic treatment. This may be due to increased Fgf15 production (Fig. 4D). Consistent with changes in mRNA levels, immunoblot analysis showed a decrease in CYP7A1 and CYP8B1 protein in FEX-treated mouse liver (Fig. 4C, right panel). In the ileum of antibiotic-treated mice, FEX induced mRNA encoding Tgr5, Shp, Ostα and Ostβ, and Prohormone convertase 1/3 (Pc1/3), which processes preproglucagon to GLP-1 (Fig. 4D). Interestingly, induction of intestinal FXR target genes by FEX was doubled in antibiotic-treated mice compared to mice without antibiotic treatment (see Fig. 1F). FEX also did not induce expression of white adipose tissue browning factors in inguinal white adipose tissue (iWAT) tissues of antibiotic-treated mice (Fig. 4E).
Fig. 4.
Antibiotics prevented FEX-induced metabolic phenotypes in wild-type mice. Wild-type C57BL/6J mice were given a mixture of antibiotics (ABX) in drinking water, containing ampicillin (1 g/L), vancomycin (500 mg/L), neomycin sulfate (g/L), and metronidazole (1 g/L) for 30 days. In the last week of antibiotic treatment, mice were orally gavaged with FEX (50 mg/Kg, n=10 each group), or vehicle (0.2% DMSO in PBS, n=10 each group) for 7 days. (A) FEX did not affect GLP-1 secretion in ABX-treated mice (left). Serum GLP-1 levels were assayed over 60 min in FEX + antibiotic (FEX+ABX) and vehicle + antibiotic (Vehicle+ABX) treated-mice. FEX did not stimulate oral glucose tolerance in ABX-treated mice (middle). FEX did not increase serum FGF21 levels in ABX-treated mice (right). (B) FEX reduced gallbladder and liver bile acid content, resulting in a significantly reduced bile acid pool.. (C) FEX treatment reduced FXR target gene mRNA expression (left panel) and CYP7A1 and CYP8B1 protein expression (right panel) in the liver of ABX-treated mice. (D) Effect of FEX treatment on mRNA expression levels of FXR targets in the ileum of mice treated with ABX. (E) Effect of FEX treatment on mRNA expression levels of browning factors in eWAT of ABX-treated mice. (F). FEX did not alter bile acid composition in gallbladder bile of ABX-treated mice. (G). FEX did not alter bile acid composition in serum of ABX-treated mice. Results in all panels were expressed as mean ± standard error. An “*” indicates statistically significant difference determined by Student’s t-test (p ≤ 0.05), FEX+ABX-treated vs. vehicle + ABX-treated wild-type mice.
Antibiotic treatment reduced bile acid hydrophobicity, completely abolished LCA and TLCA, and increased Tα-MCA and Tβ-MCA, which became the predominant bile acids (>90% total bile acids) in mouse gallbladder bile (Fig. 4F, compared to vehicle control, Fig. 2A). FEX treatment did not alter bile acid composition or the bile acid hydrophobicity index in antibiotic-treated mice (Fig. 4F). Antibiotics completely altered serum bile acid composition, abolished UDCA and TLCA, and increased Tβ-MCA, which became the predominant bile acid in the serum (Fig. 4G, compared to Fig. 2D). FEX did not alter serum bile acid composition in antibiotic-treated mice (Fig. 4G). These data support the finding that the metabolic effects of FEX depend on gut microbiota composition.
FEX induced bile acid sulfotransferase gene expression in the intestine
LCA is a potent pregnane X receptor (PXR) agonist, which induces CYP3A4 (or mouse Cyp3a11) to metabolize LCA and induce bile acid-specific sulfotransferase 2 (Sult2) family to conjugate sulfur to LCA for renal and fecal excretion (30, 31). The liver of FEX-treated mice appears normal despite high levels of LCA. Thus, the effect of FEX treatment on Sult expression in liver and intestine was examined. Interestingly, FEX strongly induced liver-specific and male-predominant Sult2a8 mRNA (32) and female-specific Sult2a1 mRNA in liver, ileum and colon of wild-type mice (Suppl. Fig. S3A), while FEX induced Sult2a2 mRNA in ileum and colon, and Sult2a3 mRNA in colon only (Suppl. Fig. S3A). FEX also induced Sult2a1, Sult2a2 and Sult2a8 mRNA in Fxr−/− and Tgr5−/− mice, indicating that FEX induction of Sults is independent of FXR and TGR5 (Suppl. Fig. S3B). Antibiotic treatment reversed FEX induction of Sult2a1, Sult2a2 and Sult2a3 in the ileum (Suppl. Fig. SC). These data suggest that in mice, the increase in LCA by FEX induces Sults to detoxify LCA, thus protecting mice from LCA toxicity.
FEX improved glucose tolerance in Leprdb/db mice
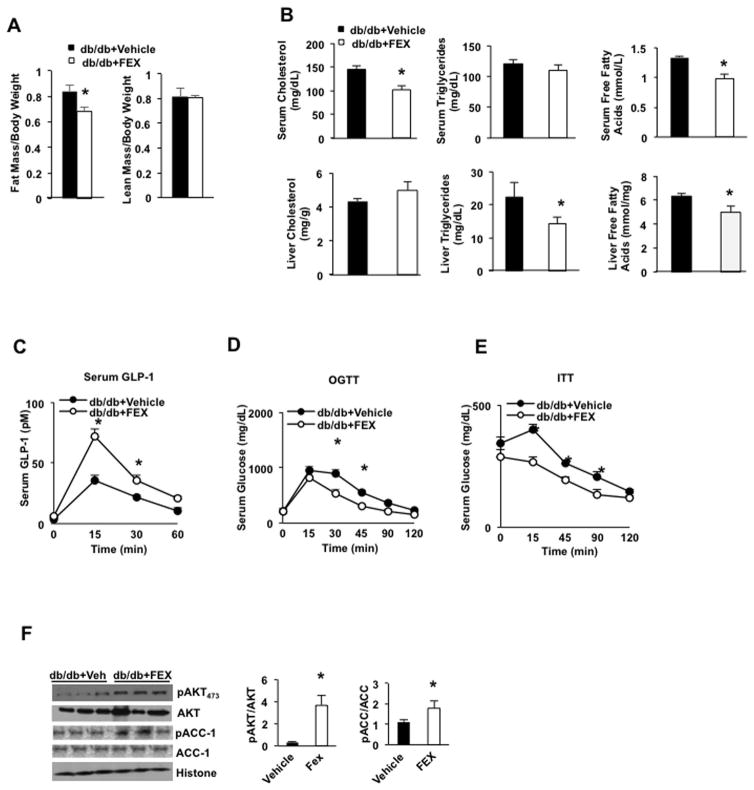
To test if activation of intestinal FXR improves insulin sensitivity and glucose metabolism, leptin receptor deficient and diabetic db/db mice were gavaged with FEX. FEX treatment significantly reduced the fat mass to body weight ratio, but did not change lean mass to body weight ratio in db/db mice compared to wild-type mice (Fig. 5A). FEX treatment significantly decreased serum cholesterol and free fatty acids, but not triglyceride levels, and decreased hepatic triglycerides and free fatty acids (Fig. 5B). FEX treatment stimulated serum GLP-1 levels by ~2-fold compared to vehicle-treated db/db mice (Fig. 5C). Oral glucose and insulin tolerance tests demonstrate that FEX treatment significantly improved glucose tolerance (Fig. 5D) and insulin tolerance (Fig. 5E) in db/db mice. FEX treatment also significantly increased the pAKT473/AKT and pACC-1/ACC-1 ratios in the liver, indicating improved insulin signaling (Fig. 5F).
Fig. 5.
FEX increases GLP-1 secretion and improves insulin sensitivity in db/db mice. db/db mice (n=6 in each group) were treated by oral gavage with FEX (30 mg/Kg) + sitagliptin (3 mg/Kg) or vehicle (0.2% DMSO) + sitagliptin (3 mg/Kg) for 9 days. (A) Effect of FEX treatment on fat mass and lean mass in db/db mice. (B) Effect of FEX treatment on serum lipid profiles in db/db mice. (C) GLP-1 secretion assay of FEX in db/db mice. (D) Oral glucose tolerance assay of effect of FEX in db/db mice. (E) Insulin tolerance test of effect of FEX in db/db mice. (F) Effect of FEX on phosphorylation of liver AKT473 and ACC-1 in db/db mice. Each lane represents one mouse (n=3 per group). An “*” indicates statistically significant difference between treated vs. vehicle control (p ≤ 0.05), determined by Student’s t-test.
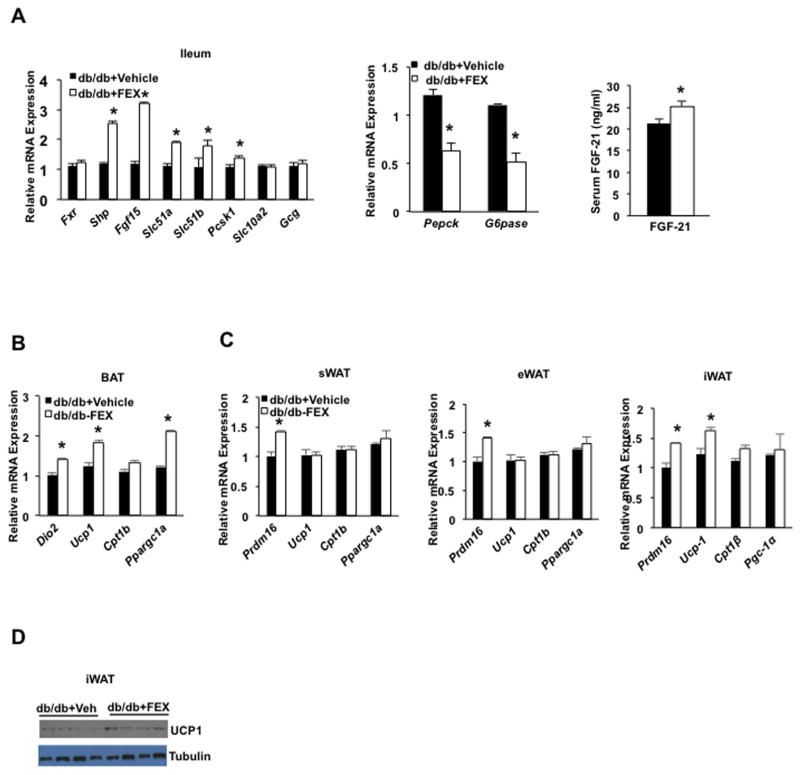
FEX treatment significantly increased liver FXR targets Shp, Fgf15, OSTα/β and Pc1/3 mRNAs, but not Asbt or Gcg mRNA expression in db/db mice compared to wild-type mice (Fig. 6A, left). Furthermore, FEX treatment suppressed Pepck and G6pase mRNA in db/db mice (Fig. 6A, middle). The db/db mice had a 2-fold higher serum FGF21 concentration compared to wild-type mice (see Fig. 1C), and FEX treatment did not significantly alter serum FGF21 levels in db/db mice (Fig. 6A, right). FEX treatment induced Dio2, Ucp1 and Ppargc-1α in brown adipose tissue (BAT) (Fig. 6B). FEX induced Pdrm16 in subcutaneous WAT and eWAT, and Pdrm16 and Ucp1 in iWAT (Fig. 6C). Immunoblot analysis show FEX induced UCP-1 protein in iWAT (Fig. 6D). H&E staining showed FEX treatment caused beiging of iWAT and eWAT (Suppl. Fig. S5A), but did not alter liver gene expression in db/db mice (Suppl. Fig. S5B). Thus, FEX treatment improved glucose and lipid metabolism, insulin sensitivity, and white adipose tissue browning (beiging) in db/db mice.
Fig. 6.
Effect of FEX in liver and adipose tissue gene mRNA expression in db/db mice (A) Effect of FEX on intestinal FXR target gene mRNA expression (left), hepatic gluconeogenic gene mRNA expression, serum FGF21. (B) Effect of FEX on brown adipose activation genes in brown adipose tissue (BAT). (C) Effect of FEX on adipose browning factors in subcutaneous WAT (sWAT), epididymal WAT (eWAT), and inguinal WAT (iWAT). (D) UCP-1 protein expression in the inguinal fat. An “*” indicates statistically significant difference between treated vs. vehicle control (p ≤ 0.05), determined by Student’s t-test.
Antibiotic treatment reversed metabolic benefit effects of FEX in db/db mice
To determine if antibiotic treatment would prevent FEX-induced metabolic benefits in db/db mice, we treated db/db mice with antibiotics for four weeks and in the last week gavaged FEX. FEX did not affect serum and liver cholesterol and triglyceride levels (Suppl. Fig. S6A) or serum FGF21 (Suppl. Fig. S6B). FEX had no effect on glucose-induced GLP-1 secretion (Suppl. Fig.S6C), glucose tolerance (Suppl. Fig. S6D) or insulin tolerance (Suppl. Fig. S6E). However, FEX still induced FXR target genes Shp and Fgf15, but not Ost or Pc1/3 in ABX treated db/db mice (Suppl. Fig. S6F). FEX did not affect adipose tissue browning factors in iWAT (Suppl. Fig. S6G), nor liver bile acid or lipogenesis gene mRNA expression (Suppl. Fig. S6D). Overall, antibiotic treatment completely reversed the beneficial metabolic effects of FEX in db/db mice.
Discussion
Activation of intestinal FXR signaling was shown to protect against NAFLD (7), but deficiency of intestinal FXR was also shown to prevent diet-induced obesity, diabetes and NAFLD (5, 6). Different mechanisms may be involved in the paradoxical effects of intestinal FXR signaling on obesity and diabetes. Results from this study demonstrate that the intestine-restricted FXR agonist FEX modulates the gut microbiota to induce Acetatifactor and Bacteroides, both of which have high BSH and 7α- and 7β-dehydroxylase activity to produce LCA from CDCA and UDCA. LCA then activates TGR5 signaling to stimulate GLP-1 secretion from L cells, resulting in promoting adipose tissue browning, and improving hepatic insulin signaling and glucose metabolism. Acetatifactor belongs to the Clostridium cluster XIV and is most closely related to the genera Anaerostipes, Blautia, Clostridum, and Ruminococcus (27). Acetatifactor grows under strictly anoxic conditions and produces acetate and butyrate. Clostridium and Bacteroides are Gram-positive anaerobic bacteria in the gut with high BSH activity for de-conjugation of TCDCA and TUDCA to CDCA and UDCA, which are converted to LCA by 7α-dehydroxylase and 7β-dehydroxylase, respectively (28, 29, 33).
FEX treatment improved lipid profiles, GLP-1 secretion, insulin and glucose tolerance and white adipose tissue browning in db/db mice, and antibiotics treatment completely reversed the beneficial metabolic effects of FEX in db/db mice. Interestingly, FEX induction of intestinal Shp and Fgf15 mRNA in antibiotic-treated mice was significantly higher in wild-type mice without antibiotic treatment but not in db/db mice without antibiotic treatment. This may be because gut microbiota blunts FEX efficacy and antibiotic treatment restored FEX efficacy to increase FGF15 and reduce Cyp7a1 and Cyp8b1 expression and bile acid pool size. FEX is an intestine-restricted FXR agonist and is not absorbed into the intestine or in blood circulation. FGF15, FGF21 and GLP-1 all have been shown to improve hepatic metabolism and adipose tissue energy metabolism (15, 34, 35). An interesting question arises regarding the mechanism by which FEX regulates hepatic metabolism, glucose sensitivity, and adipose browning. It has been reported that the effects of FGF21 in WAT browning is independent of UCP-1 (36). The current study demonstrates that antibiotic treatment abolished the FEX induction of GLP-1 and adipose tissue browning, while increased intestinal FGF15 production suggesting that FGF15 was not involved in WAT browning induced by FEX. Thus, TGR5/GLP-1 signaling may play a major role in FEX-induced adipocyte browning and improving hepatic glucose and insulin sensitivity in db/db mice.
Suppl. Fig. S7 illustrates that FXR and TGR5 are co-expressed in intestinal L cells (17). FEX activation of intestinal FXR shaped the gut microbiota to induce Acetatifactor and Bacteroides to convert TCDCA to LCA, which activates TGR5 to stimulate GLP-1 secretion from L cells, ultimately improving liver function and insulin and glucose tolerance. LCA also activates TGR5-cAMP signaling to induce WAT browning and energy metabolism to reduce weight. Free fatty acids released from adipose tissues activates PPARγ in adipose tissue and PPARα in hepatocytes to induce FGF21, which activates PGC-1α to stimulate energy metabolism and enhance insulin sensitivity in diabetes (34). The hepatic FXR/SHP pathway is not activated by FEX treatment. FGF21 antagonizes FGF15/FGFR4 signaling (37) and results in no effect on CYP7A1 expression and bile acid synthesis in FEX-treated mice.
Germ-free mice, antibiotic-treated mice, and Cyp8b1−/− mice all have increased bile acid pool size and Tβ-MCA, and are resistant to diet-induced hepatic steatosis (38, 39). In Intestine-specific Fxr-null mice, Gly-MCA antagonism of intestinal FXR activity and the antioxidant tempol all show beneficial effects in reducing weight and improving hepatic steatosis. However, the underlying mechanisms for improved metabolic phenotypes may be different in these mouse models. Intestinal FXR agonists or antagonists may shape the gut microbiota differently to regulate host metabolism and could explain the reported paradoxical effects of intestinal FXR in obesity and diabetes. Gly-MCA decreases the ratio of Firmicutes to Bacteroidetes and inhibits BSH activity, and the potent FXR agonist GW4064 reversed Gly-MCA-induced changes of metabolic phenotypes and the gut microbiome (40). Tempol alters the gut microbiome by reducing Lactobacillus and its BSH activity, leading to increase in Tβ-MCA (41).
In conclusion, this study uncovered a novel mechanism whereby activation of intestinal FXR altered bile acid metabolism by increasing LCA-producing bacteria in the gut. The elevated LCA levels activate intestinal TGR5, which then stimulates GLP-1 secretion from intestinal L cells to improve hepatic glucose and lipid metabolism and induces adipose tissue browning. Interestingly, a recent study reported that C57BL/6J mice reconstituted with Bacteroides acidifaciens had increased serum insulin and GLP-1, and decreased dipeptidyl peptidase-4 (42), suggesting that B. acidifaciens may prevent obesity and improve insulin sensitivity in mice, and supports the view that the gut microbiota can be reshaped, by stimulating TGR5/GLP-1 signaling to reduce weight and improve liver metabolism. The intestine FXR-gut microbiota-TGR5-GLP-1 axis plays a critical role in mediating intestinal bile acid receptor signaling to regulate liver metabolism and homeostasis. Activation of TGR5/GLP-1 signaling through modulation of the gut microbiota may have therapeutic potential for treating NAFLD, diabetes, and obesity.
Supplementary Material
Acknowledgments
Grant support: This study is supported by grants DK58379 and DK44442 from the National Institute of Diabetes Digestive and Kidney Diseases (JYLC), ES022186 from the National Institute of Environmental Health (ADP), and the National Cancer Institute Intramural Research Program (FJG).
Abbreviations list
- BSH
bile salt hydrolase
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CYP7A1
cholesterol 7α-hydroxylase
- CYP7B1
oxysterol 7α-hydroxylase
- CYP8B1
sterol 12α-hydroxylase
- DCA
deoxycholic acid
- LCA
lithocholic acid
- FGF15
fibroblast growth factor 15
- FGF21
fibroblast growth factor 21
- FXR
farnesoid X receptor
- FEX
fexaramine
- GPCR
G protein-coupled receptor
- GLP-1
glucagon-like peptide-1
- MCA
muricholic acid
- NAFLD
non-alcoholic fatty liver disease
- Gpbar-1
G protein-coupled bile acid receptor-1
- aka TGR5
Takeda G protein-coupled receptor 5
- Prmd16
PR domain containing 16
- TLCA
taurolithocholic acid
- Tα-MCA
tauro-α-muricholic acid
- Tβ-MCA
tauro-β-muricholic acid
- UDCA
ursodeoxycholic acid
- WAT
white adipose tissue
Footnotes
Disclosures: No potential conflicts of interest relevant to this article were reported.
Author Contributions: PP contributed to the design and performance of most experiments, data analysis, and preparation of the manuscript; SB contributed to breeding mice, JMF edited the manuscript; CX and KWK analyzed bile acid composition; FJG and AP contributed to data analysis and manuscript writing. JYLC contributed to concept design, hypothesis, data analysis and writing of the manuscript.
References
- 1.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile Acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 2.Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, Brocker CN, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Itadani H, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 9.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 10.Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 11.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 12.Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159–166. doi: 10.1210/endo.130.1.1309325. [DOI] [PubMed] [Google Scholar]
- 13.Parker HE, Wallis K, le Roux CW, Wong KY, Reimann F, Gribble FM. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol. 2012;165:414–423. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 16.Donepudi AC, Boehme S, Li F, Chiang JY. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology. 2017;65:813–827. doi: 10.1002/hep.28707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, Chiang JYL. Farnesoid X receptor induces Takeda G-protein receptor 5 Crosstalk to regulate Bile Acid Synthesis and Hepatic Metabolism. J Biol Chem. 2017;292:11055–11069. doi: 10.1074/jbc.M117.784322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 20.Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, Hoos L, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J. 2006;398:423–430. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Bittinger K, Charlson ES, Hoffmann C, Lewis J, Wu GD, Collman RG, et al. Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics. 2012;28:2106–2113. doi: 10.1093/bioinformatics/bts342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harach T, Pols TW, Nomura M, Maida A, Watanabe M, Auwerx J, Schoonjans K. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep. 2012;2:430. doi: 10.1038/srep00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, Kurokawa M, et al. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014;19:593–604. doi: 10.1016/j.cmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeiffer N, Desmarchelier C, Blaut M, Daniel H, Haller D, Clavel T. Acetatifactor muris gen. nov., sp. nov. a novel bacterium isolated from the intestine of an obese mouse. Arch Microbiol. 2012;194:901–907. doi: 10.1007/s00203-012-0822-1. [DOI] [PubMed] [Google Scholar]
- 28.Hirano S, Masuda N. Enhancement of the 7 alpha-dehydroxylase activity of a gram-positive intestinal anaerobe by Bacteroides and its significance in the 7-dehydroxylation of ursodeoxycholic acid. J Lipid Res. 1982;23:1152–1158. [PubMed] [Google Scholar]
- 29.Ishii M, Toda T, Ikarashi N, Kusunoki Y, Kon R, Ochiai W, Machida Y, et al. Gastrectomy increases the expression of hepatic cytochrome P450 3A by increasing lithocholic acid-producing enteric bacteria in mice. Biol Pharm Bull. 2014;37:298–305. doi: 10.1248/bpb.b13-00824. [DOI] [PubMed] [Google Scholar]
- 30.Echchgadda I, Song CS, Oh T, Ahmed M, De La Cruz IJ, Chatterjee B. The xenobiotic-sensing nuclear receptors pregnane X receptor, constitutive androstane receptor, and orphan nuclear receptor hepatocyte nuclear factor 4alpha in the regulation of human steroid-/bile acid-sulfotransferase. Mol Endocrinol. 2007;21:2099–2111. doi: 10.1210/me.2007-0002. [DOI] [PubMed] [Google Scholar]
- 31.Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng L, Yuen YL, Xu J, Liu X, Chan MY, Wang K, Fong WP, et al. Identification and characterization of a novel PPARalpha-regulated and 7alpha-hydroxyl bile acid-preferring cytosolic sulfotransferase mL-STL (Sult2a8) J Lipid Res. 2017;58:1114–1131. doi: 10.1194/jlr.M074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, Kharitonenkov A, et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veniant MM, Sivits G, Helmering J, Komorowski R, Lee J, Fan W, Moyer C, et al. Pharmacologic Effects of FGF21 Are Independent of the “Browning” of White Adipose Tissue. Cell Metab. 2015;21:731–738. doi: 10.1016/j.cmet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Gupte J, Gong Y, Weiszmann J, Zhang Y, Lee KJ, Richards WG, et al. Chronic Over-expression of Fibroblast Growth Factor 21 Increases Bile Acid Biosynthesis by Opposing FGF15/19 Action. EBioMedicine. 2017;15:173–183. doi: 10.1016/j.ebiom.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Hu X, Bonde Y, Eggertsen G, Rudling M. Muricholic bile acids are potent regulators of bile acid synthesis via a positive feedback mechanism. J Intern Med. 2014;275:27–38. doi: 10.1111/joim.12140. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Xie C, Nichols RG, Chan SH, Jiang C, Hao R, Smith PB, et al. Farnesoid X Receptor Signaling Shapes the Gut Microbiota and Controls Hepatic Lipid Metabolism. mSystems. 2016:1. doi: 10.1128/mSystems.00070-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017;10:104–116. doi: 10.1038/mi.2016.42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.