Abstract
Dilated cardiomyopathy (DCM) is a severe disorder caused by medications or genetic mutations. D5 dopamine receptor (D5R) gene knockout (D5-/-) mice have cardiac hypertrophy and high blood pressure. To investigate the role and mechanism by which the D5R regulates cardiac function, we generated cardiac-specific human D5R F173L(hD5F173L-TG) and cardiac-specific human D5R wild-type (hD5WT-TG) transgenic mice, and H9c2 cells stably expressing hD5F173L and hD5WT. We found that cardiac-specific hD5F173L-TG mice, relative to hD5WT-TG mice, presented with DCM and increased cardiac expression of cardiac injury markers, NADPH oxidase activity, Nrf2 degradation, and activated ERK1/2/JNK pathway. H9c2-hD5F173L cells also had an increase in NADPH oxidase activity, Nrf2 degradation, and phospho-JNK (p-JNK) expression. A Nrf2 inhibitor also increased p-JNK expression in H9c2-hD5F173L cells but not in H9c2-hD5WT cells. We suggest that the D5R may play an important role in the preservation of normal heart function by inhibiting the production of reactive oxygen species, via inhibition of NADPH oxidase, Nrf2 degradation, and ERK1/2/JNK pathways.
Keywords: Dilated cardiomyopathy (DCM), Dopamine D5 receptor (D5R), Reactive oxygen species (ROS), Nrf2
1. Introduction
Dilated cardiomyopathy (DCM) is a severe disorder caused by ventricular dilatation and cardiac dysfunction in response to inflammatory, metabolic, and toxic effects of medications or genetic mutations [1], [2], [3], [4]. Despite the many causes of DCM, there are many similarities in the final structural and functional alterations, related to long-term stress and neurohormonal activation [5], that eventually lead to systolic heart failure. Increased left ventricular end-diastolic volume and decreased left ventricular ejection fraction are risk factors for cardiac morbidity and mortality in the general population [6]. The mechanisms that determine the progression of DCM are not completely understood, but 30–48% are related to genetic mutations [1], [2], [3], [4]; DCM is also closely associated with hypertension [3].
We have reported that the global disruption of the D5 receptor (D5R) gene (D5-/-), one of the two D1-like dopamine receptors, in mice causes high blood pressure [7], [8]. The D5-/- mice also have cardiac hypertrophy and increased heart weight [8]. Humans have single nucleotide polymorphisms in the D5R gene that are associated with diminished D5R function [9]. The mutant human D5R F173L is associated with decreased D5R function and transgenic (TG) mice globally expressing the human D5F173L (hD5F173L) have high blood pressure [10]. Global hD5F173L-TG mice present with cardiac hypertrophy at five months of age (unpublished data), presumably secondary to increased blood pressure, similar to that observed in D5-/- mice after two months of age [8].
The elevated blood pressure in D5-/- mice is related to increased oxidative stress, due in part to increased renal NADPH oxidase activity and decreased HO-1 activity [7], [11]. Renal NADPH oxidase activity is also increased in global hD5F173L-TG mice [10]. Therefore, we hypothesized that the D5R may directly regulate cardiac remodeling and function by inhibition of NADPH oxidase activity and stimulation of antioxidants, including HO-1. The D1R, unlike the D5R, has not been reported to affect HO-1 expression or function [12]. Nuclear factor E2-related factor 2 (Nrf2), which is an important positive regulator of anti-oxidant genes, has been reported to preserve the function of the D1R, the other D1-like receptor, in the presence of oxidative stress [13]. Therefore, we studied in vivo the role of NADPH oxidase and Nrf2 on pathways related to cardiac function in transgenic mice expressing human D5F173L or human D5WT only in the myocardium, cardiac specific hD5F173L-TG and hD5WT-TG mice, which endogenous D5R expression in heart was prevented. We determined further the role of Nrf2 and related pathways on oxidative stress in a rat cardiomyoblast cell line (H9c2) [14], heterologously expressing hD5F173L (H9c2-hD5F173L) or hD5WT (H9c2-hD5WT).
2. Methods
2.1. Materials
An immortalized rat heart cell line (H9c2 cell) was obtained from the Chinese Academy of Sciences for Type Culture Collection, Shanghai, China. Adult (2-month old) male C57Bl/6J mice were bought from Beijing HFK Bioscience Co, LTD. All animal-related studies were approved by the Institutional Animal Care and Use Committee of the Institute of Laboratory Animal Science, Peking Union Medical College, China, and the animals were handled according to the guiding principles published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. hD5WT and hD5F173L cell transfections
The full-length hD5WT or hD5F173L cDNA, subcloned into a pcDNA6/V5-His vector between the EcoRI and Xbal sites, was transfected into H9c2 cells [14], using LT1 transfection reagents. The successful transfection of hD5R cDNA was verified by immunoblotting for His/V5 expression and RT-PCR. Empty vector-transfected H9c2 cells served as negative controls.
2.3. Cell culture and drug treatment
H9c2-hD5F173L and H9c2-hD5WT cells were cultured in DMEM containing 10% fetal bovine serum (FBS), 1% penicillin, and 1% streptomycin in an incubator with a temperature set at 37 °C and 5% CO2 atmosphere. When the cells were 90–95% confluent, they were serum-starved for 2 h, and treated for 1 h in serum-free medium with phosphate-buffered saline (PBS), 1 μM ML385 (Nrf2 inhibitor)[15], or 10 μM SP600125 (JNK inhibitor)[16].
2.4. Cardiac-specific transgenic (TG) mice and drug administration
Cardiac-specific hD5F173-TG and hD5WT-TG mice were generated from C57Bl/6J mice, according to the following procedures. The full-length hD5WT or hD5F173L cDNA was subcloned into an expression plasmid under the α-myosin heavy chain (α-MHC) promoter. The TG mice were generated by the oocyte microinjection method [10]. The genotype of the TG mice was verified by polymerase chain reaction, using the primers 5′GGACCGCTACTGGGCCATCT and 5′GGGTCTTGAGAACCTTGGTC, and analysis of the sequence of the amplified 488 base-pair fragment of the hD5R gene.
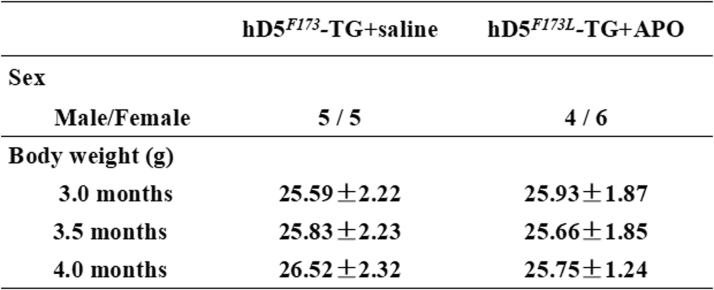
To determine the role of NADPH oxidase in the pathogenesis of DCM in hD5F173L-TG mice, we studied the effect of apocynin, a drug that inhibits NADPH oxidase activity by impeding the assembly of the p47phox and p67phox subunits into the NADPH oxidase complex at the plasma membrane [7], [17], [18], [19]. At 3 months of age, F0 generation of hD5F173L-TG and hD5WT-TG mice were injected intraperitoneally, daily for four weeks, with apocynin (1 mmol/kg/day, Sigma), or PBS (0.1–0.3 ml), as a control. Thereafter, echocardiographic examinations were performed weekly, as described below. At the end of the 4-week treatment, the mice were weighed and anesthetized (2.5% Avertin, 0.012 ml/g body weight) and the hearts were harvested, weighed, and subjected to studies, as described below.
2.5. Echocardiography
The mice, anesthetized by the intraperitoneal injection of 2.5% Avertin (as indicated above), were placed on a warm pad to keep the rectal temperature around 37 °C. The chests were shaved and covered with warm echocardiography gel. The heart rate, about 400–500 beats per minute, was monitored. The hearts were imaged with a 30 MHz linear transducer (VisualSonics Vevo 770), with the mouse at a shallow left-side position. Two-dimensional left ventricular (LV) echocardiograms were obtained by placing the transducer along the long-axis of the LV, and directed to the right side of the neck of the mouse. Then, the transducers were rotated 90° clockwise and the LV short-axis views were visualized. The images showing the positions and directions of the transducer for basic mouse echocardiography views are shown in Fig. 1A. Two D-guided LV M-modes at the papillary muscle level were recorded from either the short-axis and long-axis views. Doppler wave forms from other regions of the heart were recorded, as needed.
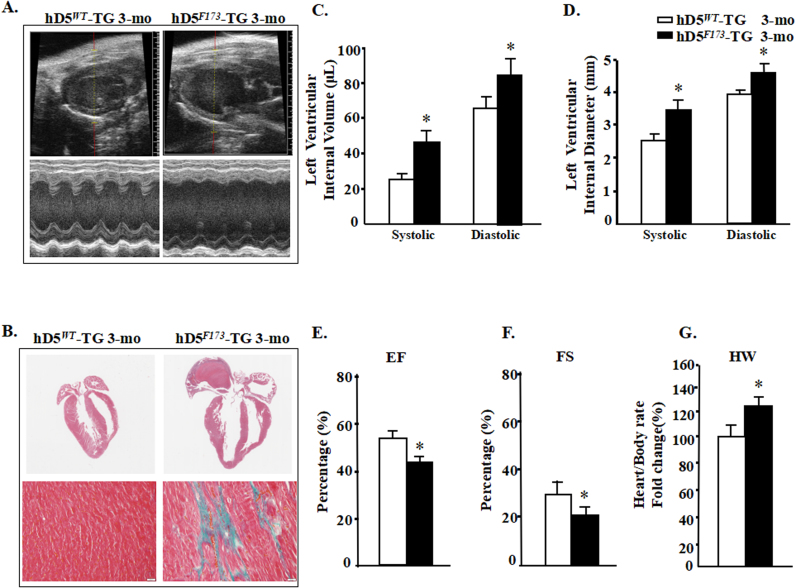
Fig. 1.
D5R functional deficiency produces dilated cardiomyopathy. A: Representative echocardiograms from cardiac-specific hD5WT-TG and hD5F173L-TG mice. B: Cardiac sections stained with H&E (top) and Masson's trichrome stain (bottom) from 3-month-old mice. Scale bar: 5 µm. C–G: Echocardiographic analysis of cardiac volume and function (n = 12 mice/group); *P < 0.05 vs. D5WT-TG. EF = ejection fraction, FS = fractional shortening, HW = heart weight.
2.6. NADPH oxidase activity
Cardiac membranes were prepared by a membrane and cytoplasmic protein extraction kit (Sangon Biotech, Shanghai). NADPH oxidase activity (light units per g protein per minute) of cardiac membranes was measured by NADPH-induced chemiluminescence with 5 μmol/L lucigenin and 100 μmol/L NADPH [7], [11]. The specificity of the NADPH-dependent O2- production was verified by treatment with the flavoprotein inhibitor diphenyleneiodonium (DPI).
2.7. Intracellular ROS and mitochondrial ROS measurement
Intracellular ROS were analyzed using hydro-dichlorofluorescein diacetate (H2DCF-DA) (Life technologies) as probes. Post-treatment, H9c2 cells or heart homogenate were collected and incubated with 100 mM Mito-tracker Red FM/ H2DCF-DA (dissolved in DMSO) for 30 min at 37 °C. Cells were then washed three times with PBS and the intracellular accumulation of fluorescent DCF-DA was measured by fluorescence microplate (Carl Zeiss MicroImaging, NY).
For mitochondrial ROS, isolated mitochondria were loaded with MitoSOX red (5 μM, for 10 min) according to the manufacturer's instruction. Sequential 2-D confocal images were taken at 405 nm excitation, and emission was collected at > 560 nm. To calculate the rate of MitoSOX signal change (dF/dt), fluorescent signals during a 5-min period before [20].
2.8. Mitochondrial isolation and Enzyme activity
Heart mitochondria were isolated from the rest of the homogenatesas previously described [21]. In brief: Whole heart were placed in ice cold isolation medium (250 mM Sucrose, 10 mM Tris, 0.5 mM EDTA pH 7.4 at 4 °C) immediately after killing the mice, and transferred to the Dounce glass tissue grinder to homogenise. An aliquot of the homogenates was immediately snap frozen and stored at − 70 °C.
The mitochondrial membrane potential was measured by JC-1 staining (T3168, Life technologies) After incubation with JC-1 dye, fluorescence at 485 nm was measured by a fluorometer (BioTek, Winooski, VT) according to the manufacturer's instruction [22].
2.9. Histopathology
After the mice were euthanized with an overdose of pentobarbital, The anesthetized mice was exposured to the heart and cut at auricle. The heart was perfused with HTK solution 4 °C via the tip of heart at a constant perfusion pressure of 80 ± 2 mmHg using a peristaltic pump (Gilson Minipuls 4, Middleton, WI, USA). [K+] concentration in HTK solution is 9 mmol/L, low potassium can avoid the injury of high concentration of potassium on myocardium and coronary artery endothelium and make cardiac arrest during diastolic phase [23]. Then the heart was perfused in 10% formalin, and harvest. The whole heart was fixed in 10% formalin. The fixed hearts was embedded in paraffin, and then cut serially from the apex to the base. The sections were stained with hematoxylin-eosin and Masson's trichrome for histopathological analyses. Images of transverse sections were captured digitally, and the cardiomyocyte cross-sectional areas were measured using a Scion imaging system (Scion, Frederick, MD) [24], [25]. The outlines of at least 200 cardiomyocytes in each section were traced and the data were averaged. To assess the degree of fibrosis using Masson's trichrome stain, the images from at least 10 fields for each heart were analyzed, as described previously [25], [26].
2.10. Immunoblotting
Mouse cardiac plasma membranes and homogenates and H9c2 cell lysates were prepared for immunoblotting, as previously reported [7], [10], [11]. The samples were immunoblotted with well characterized anti-p40phox (1:500, Epitomics), anti-p47phox (1:1000, Epitomics), anti-Nrf2 (1:1000, Abcam), anti-P84 (1:500, Abcam), anti-phospho ERK1/2 (p-ERK1/2), anti-phospho JNK (p-JNK), anti-phospho P38 (p-P38), anti-ERK1/2, and anti-JNK, anti-P38 (1:1000, Cell Signaling Technology) antibodies. Uniformity of protein loading and membrane transfer was determined by immunoblotting for GAPDH.
2.11. Quantitative real-time PCR
Total RNA was purified using the RNeasy RNA Extraction Mini kit (Qiagen, Valencia, CA). RNA samples were converted into first strand cDNA using an RT2 First Strand kit, following the manufacturer's protocol (Qiagen). Quantitative gene expression was analyzed by real-time PCR, performed on an ABI Prism 7900 HT (Applied Biosystems, Foster City, CA). The assay used gene specific primers (Qiagen) and SYBR Green real-time PCR detection method (Qiagen) and was performed as described in the manufacturer's manual. Primers used were as Supplementary Table 1. Data were analyzed using the ΔΔCt method [27].
2.12. Co-immunoprecipitation
Co-immunoprecipitation was performed using an immunoprecipitation kit. Equal amounts of whole cell lysates (500 μg protein) were mixed with mouse anti-Nrf2 antibody, non-immune mouse serum (negative control), or mouse anti-ubiquitin antibody (positive control), specificities of which have been reported [28]. Protein A/G agarose beads were added and incubated overnight at 4 °C. The bound proteins were eluted using 30 μL of Laemmli buffer. The samples were subjected to immunoblotting and probed for ubiquitin, using a rabbit anti-ubiquitin antibody. Reverse co-immunoprecipitation was performed using the same method; cell lysates were mixed with mouse anti-ubiquitin antibody, non-immune mouse serum (negative control), or mouse anti-Nrf2 (positive control) and the bound proteins were subjected to immunoblotting and probed for Nrf2, using a rabbit anti-Nrf2 antibody, specificity of which has been reported [28].
2.13. Statistical analysis
The data are expressed as mean±SEM. Significant differences between and among groups were determined by one-way or two-way factorial ANOVA, Holm-Sidak test, for groups > 2 and the Student's t-test for groups = 2. Overall survival was analyzed with the Kaplan-Meier method and the log-rank test. P < 0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS 22.0 statistical software (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. DCM in cardiac-specific hD5F173L-TG and hD5WT-TG mice
We generated cardiac-specific hD5F173L-TG and hD5WT-TG mice expressing the mutant human D5R, hD5F173L, and wild-type human D5R, hD5WT, under the control of the α-MHC promoter. Three-month-old hD5F173L-TG mice had DCM (Fig. 1A) with advanced heart failure (Fig. 1A–G), associated with increased myocardial fibrosis observed by Masson's trichrome staining (Fig. 1B). LV M-mode allows the assessment of LV systolic function. Systolic and diastolic LV internal volume (LVIVs and LVIVd, respectively) and systolic and diastolic LV internal diameter (LVIDs and LVIDd, respectively) were measured from the M-mode images at the level of the papillary muscles. LV ejection fraction (EF) and LV fractional shortening (FS) were used to evaluate LV global systolic function [29]. Cardiac-specific hD5F173L-TG mice, relative to cardiac-specific hD5WT-TG mice, had increased LVIVs (44.97 ± 14.54 vs. 24.66 ± 5.34 μL) and LVIVd (83.99 ± 18.42 vs. 64.83 ± 9.90 μL) (P < 0.01) (Fig. 1C) and enlarged LVIDs (3.26 ± 0.42 vs. 2.58 ± 0.23 mm) and LIVDd (4.28 ± 0.39 vs. 3.86 ± 0.25 mm) (P < 0.05) (Fig. 1D), as well as impaired EF (42.01 ± 8.73% vs. 53.18 ± 4.84%) (Fig. 1E) and FS (21.23 ± 5.15% vs. 29.95 ± 3.52%, P < 0.05) (Fig. 1F). The hearts/body rate were significantly higher in hD5F173L-TG than hD5WT-TG mice (Fig. 1G). These results show that cardiac-specific D5R deficiency results in DCM, indicating that the D5R may have a cardioprotective effect.
3.2. Dopamine D5 receptor deficiency facilitates NOX assembly at the plasma membrane and increases NADPH oxidase activity and ROS generation
The impaired D5R-negative regulation of NADPH oxidase activity contributes to the pathogenesis of hypertension in global D5−/− mice [7], [11]. Cardiac NADPH oxidase activity (Fig. 2A) and ROS generation (Fig. 2B) were significantly increased in 3-month-old cardiac-specific hD5F173L-TG, relative to cardiac-specific hD5WT-TG mice. Notably, the reduction in D5R activity was associated with increased translocation of NADPH oxidase regulatory subunits p40phox and p47phox to the plasma membrane (Fig. 2C–E).
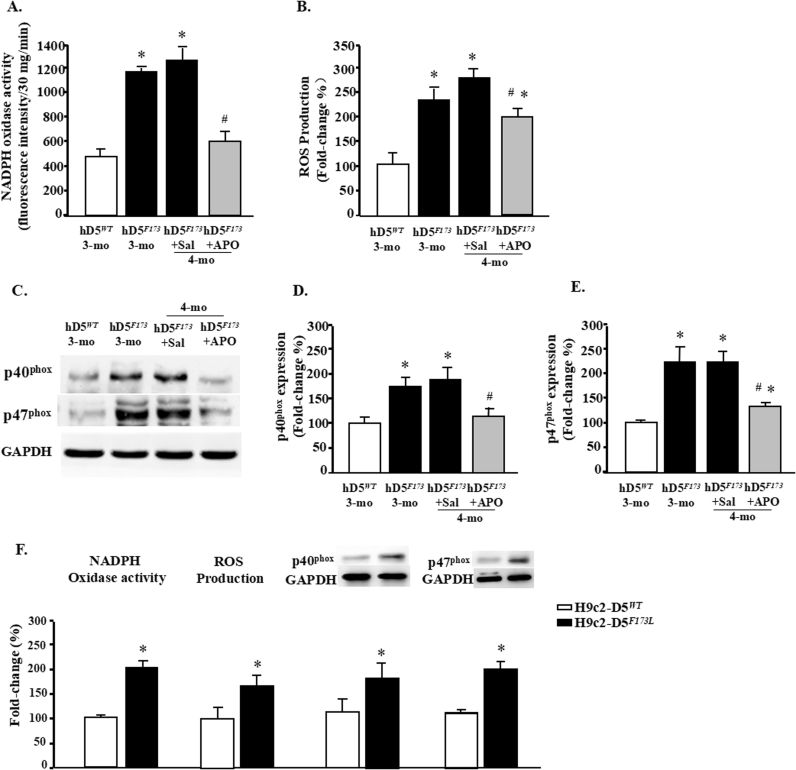
Fig. 2.
D5R functional deficiency increases myocardial oxidative stress. Plasma membrane proteins were extracted from hearts of 3-month-old cardiac-specific human D5WT-TG (hD5WT) and hD5F173L-TG mice (hD5F173) and 4-month-old cardiac-specific hD5F173 mice treated with saline (hD5F173 + Sal) or apocynin (hD5F173 + APO, 1 mmol/kg/day) for 4 weeks, starting at 3 months of age (n = 12 mice/group, regardless of age). A and F: NADPH oxidase activity was quantified by lucigenin chemiluminescence assay. *P < 0.05 vs. hD5WT mice; #P < 0.05 vs. hD5F173L + Sal. B and F: ROS production was quantified by H2DCF-DA. *P < 0.05 vs. hD5WT mice; #P < 0.05 vs. hD5F173L + Sal. C–E: p40phox and p47phox expression was semiquantified by western blot. *P < 0.05 vs. hD5WT-TG mice; #P < 0.05 vs. hD5F173L + Sal. F: NADPH oxidase activity, ROS production, and p40phox and p47phox protein expression were quantified in H9c2-D5WT and H9c2-D5F173L cells. *P < 0.05 vs. H9c2-D5WT cells (n = 8/group).
For the in vitro study, H9c2 cells were stably transfected with hD5RF173L (H9c2-hD5F173L) or hD5RWT (H9c2-hD5WT). NADPH oxidase activity, ROS production, and basal protein expressions of p40phox and p47phox were higher in H9c2-hD5F173L than H9c2-hD5WT cells (Fig. 2F), consistent with the in vivo study.
Apocynin has been used as an efficient NADPH oxidase inhibitor by impairing the translocation of the NADPH-oxidase complex cytosolic components, p47phox and p40phox, to the plasma membrane [7], [18], [19], [20], which may be a therapeutic target for the treatment of advanced heart failure [30]. Therefore, we determined if inhibition of NADPH oxidase activity prevents the progression of DCM in cardiac-specific hD5F173L-TG mice by treatment with apocynin (1 mmol/kg/day). Cardiac-specific hD5F173L-TG mice (3-month-old), treated with saline or apocynin, had similar body weights throughout the experimental period (Supplementary Tables 1 and 2). The intraperitoneal injection of apocynin that was started at 3 months of age (pretreatment) and continued for four weeks until 4 months of age decreased cardiac NADPH oxidase activity to the 3-month-old hD5WT-TG level (Fig. 2A) and the translocation of p47phox and p40phox to the plasma membrane (P < 0.05) (Figs. 2C–E), compared with the saline-treated group. ROS production was decreased by apocynin treatment (Fig. 2B), which was associated with a reduction in NADPH oxidase activity (Fig. 2A).
3.3. Cardiac-specific dopamine D5 receptor mutation induces a mitochondria-independent increase in reactive oxygen species levels in heart
Within cardiomyocytes, ROS are generated in different compartments by different enzymes. In addition to the NADPH oxidases, mitochondria are one of the most important cellular source of ROS [31]. To gain insight into the source and the potential mechanism of D5R mutation-induced generation of ROS in human cells, we analyzed the mitochondrial ROS and function of this process.
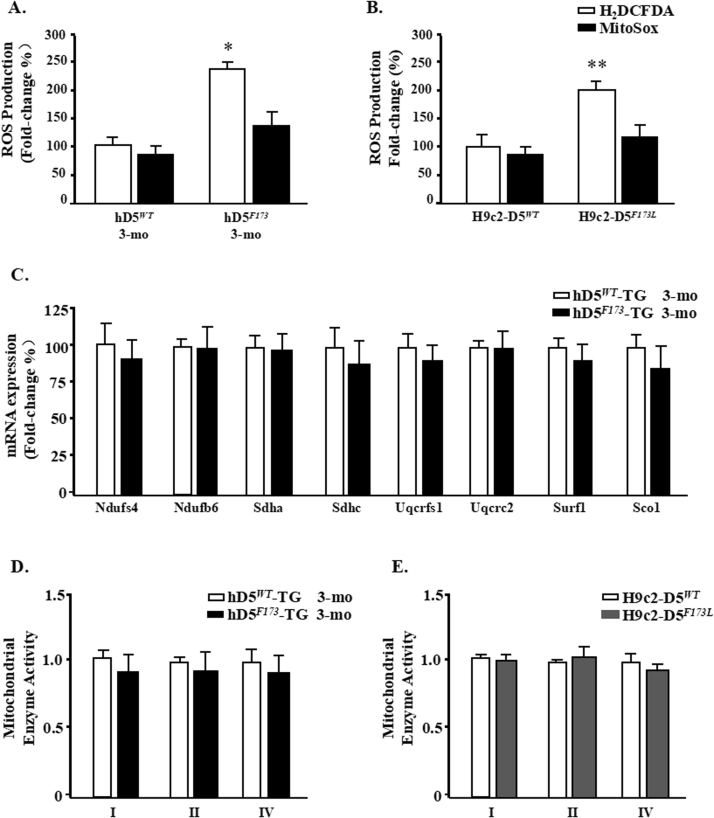
ROS levels of Mitochondrial were measured by using the mitochondrial-specific probe MitoSox. Compared with significant increase in intracellular ROS levels, a little bit higher in mitochondrial superoxide was observed, which was not significant either in cardic specific D5RF173L-TG mice or in H9c2-hD5F173L cell (P > 0.05) (Fig. 3A and B). The role of mitochondrial function was also detected in cardic specific D5RWT-TG and D5RF173L-TG mice (3 months). The mRNA expression of complex I genes(Ndufs4 and Ndufb6) remained unaltered. The mRNA levels of several genes representative of the ETC complexes II–IV also remained unchanged (Fig. 3C). Complex activities measured in isolated mitochondrial complexes (complex I–IV) showed slight decresed in cardic specific D5RF173L-TG mice compared to D5RWT-TG mice (3 months) (P > 0.05). A parallel decrease in complexes activity was observed in H9c2-D5F173 cells (P > 0.05) (Fig. 3D and E). These data shows that cardic specific D5R mutantion transfection did not affect mitochondria function and mitochondrial ROS production. Although mitochondria are the crucial source of ROS response in cardiomyocytes of D5RF173L-TG mice, but cardic specific D5R mutantion transfection increased ROS production may mainly come from NADPH oxidase.
Fig. 3.
Mitochondrial ROS and assessment in cardiac-specific hD5F173-TG mice. Temporal analysis of ROS levels in (A) D5RWT-TG and D5RF173L-TG mice (3 months) or in (B) H9c2-hD5F173L and H9c2-hD5WT cells. ROS levels were measured at the indicated time points by incubating with H2DCF-DA (total intracellular ROS) or MitoSox fluorescent probes (mitochondrial ROS). *p < 0.05 vs. D5RWT-TG mice (n = 7), **p < 0.05 vs. H9c2-hD5WT cells (n = 5). C: Fold changes of sub-mitochondrial particles gene expression in D5RF173L-TG mice relative to D5RWT-TG mice (n = 7). Fold changes of Complex I, II and IV enzyme activities for (D) in vivo study (n = 7) and (E) in vitro study (n = 5).
3.4. Apocynin ameliorates the cardiac enlargement and heart injury in cardiac-specific D5R deficiency-induced DCM
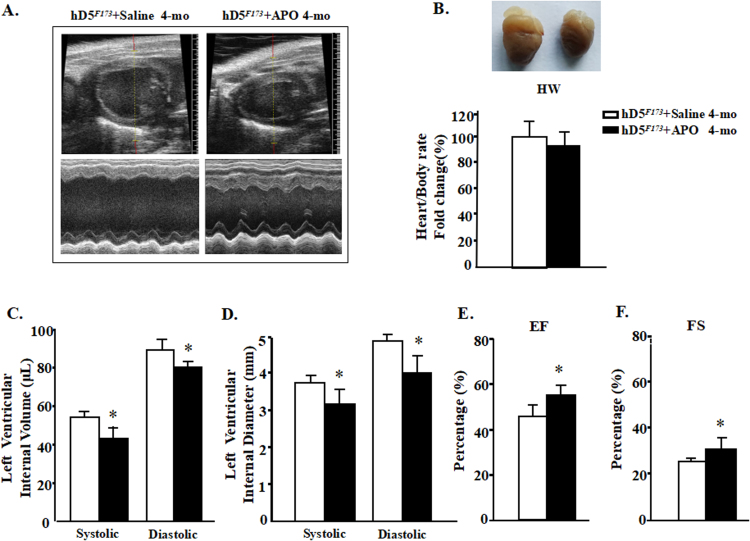
Apocynin had a favorable effect on cardiac performance (Fig. 4A) but had a minimal effect on heart/body rate in cardiac-specific hD5F173L-TG mice (4-month-old) (Fig. 4B). The administration of apocynin for 4 weeks in 3-month old hD5F173L-TG mice improved all of the measures (LVIV, LVID, EF, and FS) of cardiac performance (LVIVs: 41.97 ± 10.21 vs. 52.66 ± 6.11 μL; LVIVd: 80.98 ± 9.42 vs. 89.24 ± 12.90 μL P < 0.05; LVIDs: 3.11 ± 0.45 vs. 3.76 ± 0.13 mm; LVIDd: 4.28 ± 0.38 vs. 4.66 ± 0.23 mm P < 0.01) (Fig. 4C and D). Pump failure (% EF: 40.15 ± 5.62% vs. 31.23 ± 4.44%; % FS: 25.38 ± 5.04% vs. 17.05 ± 6.23%, P < 0.05) was also alleviated by apocynin (Fig. 4E and F). These results demonstrate that inhibition of NADPH oxidase significantly delays the progression of DCM caused by the cardiac-specific deficiency of D5R function. However, the apocynin-induced decrease in ROS production was not as large as the decrease in NADPH oxidase activity (Fig. 2A and B), which indicates that there is some counter-regulation of the apocynin-induced reduction in NADPH oxidase activity.
Fig. 4.
Apocynin prevents the cardiac enlargement and improves cardiac function in cardiac cardiac-specific hD5F173-TG mice (hD5F173L). A: Representative echocardiograms of 4-month-old cardiac-specific hD5F173L-TG mice treated with saline (hD5F173L + Saline) or apocynin (APO, 1 mmol/kg/day) (hD5F173L + APO) for 4 weeks, starting at 3 months of age (n = 10 mice/group). B: Heart size and weight. C–F: Echocardiographic analysis of cardiac volume and function. (n = 10 mice/group); *P < 0.05 vs. hD5F173 + Saline. EF = ejection fraction, FS = fractional shortening.
3.5. Dopamine D5 receptor deficiency impairs Nrf2 antioxidant activity
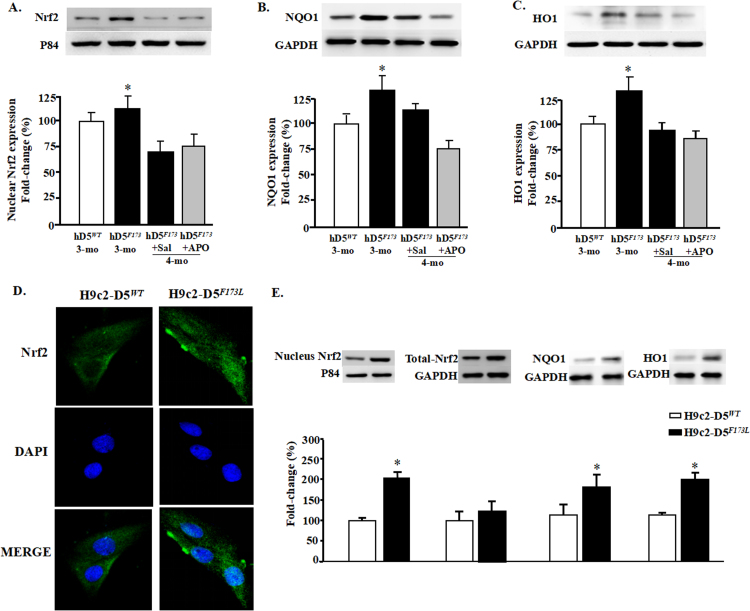
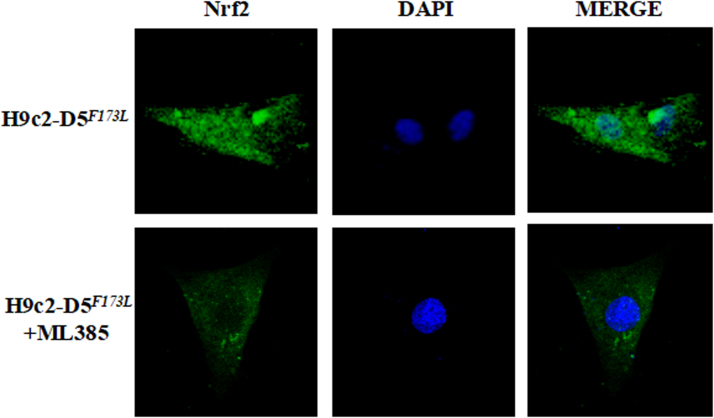
Activation of Nrf2 and its target genes plays an important role in protecting organs, including the heart, against pathological cardiac remodeling by suppressing oxidative stress [32], [33]. To determine if Nrf2-induced antioxidant function is affected by D5R deficiency-induced DCM, we studied the expression (Fig. 5) and ubiquitination (Fig. 6) of Nrf2 in the nucleus and cytoplasm. Cardiac-specific D5R deficiency slightly increased nuclear Nrf2 expression at 3 months of age (+ 12 ± 4%, hD5F713L-TG mice (3-month-old) vs. hDWT-TG) but significantly decreased it at 4 month of age (− 28 ± 2%, hD5F173L-TG + Sal (4-month-old) vs. hDWT-TG) (Fig. 5A); apocynin did not affect Nrf2 translocation into the nucleus in hD5F173L-TG + Apo at 4 months of age. The nuclear expression of NAD(P)H quinone 1 dehydrogenase (NQO1), which, prevents the reduction in one electron of quinones and heme oxygenase 1 (HO1), which removes toxic heme and produces antioxidants, such as biliverdin carbon monoxide [33]. NQO1 and HO-1, downstream targets of Nrf2 [29], [33], also increased at 3 months of age and decreased toward the 3 month old level at 4 months of age (hD5F713L-TG (3-month-old) vs. hD5F173L-TG + Sal (4-month-old)). Similar to the Nrf2 results (Fig. 5A), apocynin had no effect of NQO1 and HO1 expression in 4-month-old hD5F173L-TG mice (D5F173L-TG + Sal vs. hD5F173L-TG + Apo) (Figs. 5B and C). ROS stimulate the translocation of Nrf2 from the cytoplasm into the nucleus [34]. Therefore, it is possible that the increased nuclear Nrf2 expression at 3 months of age is a compensation for the increase in ROS production in hD5F173L-TG mice. Immunofluorescence microscopy of H9C2 cells showed that Nrf2 was located mainly in the cytoplasm of H9c2-hD5WT cells, while nuclear expression of Nrf2 was increased in the nucleus in H9c2-hD5F173Lcells (cyan color, Fig. 5D). Immunoblotting also showed that nuclear, but not total Nrf2, as well as cytoplasmic NQO1 and HO1 expressions were significantly increased in H9c2-hD5F173L compared with H9c2-hD5WTcells, which were consistent with the in vivo study in 3-month-old but not 4-month-old mice (Fig. 5E). However, total Nrf2 expression was not different between two cells, which further confirmed the hypothesis that cardiac-specific functional deficiency of D5R affects the translocation of Nrf2 in nucleus but not its total expression.
Fig. 5.
D5R functional deficiency impairs nuclear Nrf2 translocation and downstream pathway. A: Nuclear proteins were extracted from the hearts of 3-month-old cardiac-specific human D5WT-TG (hD5WT) and hD5F173L-TG (hD5F173) mice and 4-month-old cardiac-specific hD5F173 mice treated with saline (hD5F173 + Sal) or apocynin (hD5F173 + APO, 1 mmol/kg/day) for 4 weeks, starting at 3 months of age (n = 12 mice/group). Nrf2 and P84 (nucleus marker) were semiquantified by western blot. B: and C: NQO1 and HO1 expression was semiquantified by western blot. *P < 0.05 vs. hD5WT mice (3-month-old). D: Nrf2 location in H9c2-D5WT and H9c2-D5F173L cells was determined by immunofluorescence. Green: Nrf2 Blue: DAPI. E: Protein expressions of Nrf2, NQO1, and HO1 were quantified by western blotting in H9c2 cells. *P < 0.05 vs. H9c2-D5WT cells (n = 8 /group).
Fig. 6.
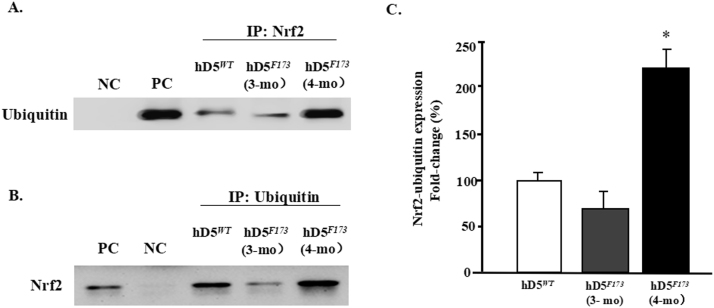
Nrf2 ubiquitination in hD5F173Lmice. A: Total heart lysates from cardiac-specific hD5WT- and hD5F173L-TG mice (3- and 4-month-old) were immunoprecipitated with anti-Nrf2 antibody and immunoblotted with anti-ubiquitin antibody. B: Total heart lysates from cardiac-specific D5WT- and hD5F173L-TG mice (3- and 4-month-old) were immunoprecipitated with anti-ubiquitin antibody and immunoblotted with anti-Nrf2 antibody. C: Data are expressed as mean ± S.E. n = 4mice/group.*P < 0.05 vs. others, one-way ANOVA, Holm-Sidak test, NC = negative control, PC = positive control.
3.6. Dopamine D5 receptor deficiency increases Nrf2 degradation via the ubiquitin-proteasome pathway
Nrf2 degradation is regulated by Nrf2 ubiquitination [28], [34]. Therefore, we determined if cardiac D5R could increase the stability of Nrf2 protein, by reducing its ubiquitination via the proteosome pathway. We immunoprecipitated Nrf2 from heart homogenates of hD5WT-TG and hD5F173L-TG mice at 3- and 4 months of age and immunoblotted the immunoprecipitates with anti-ubiquitin antibody. We corroborated the results by reversing the order of immunoprecipitation and immunoblotting, i.e., immunoprecipitation with ubiquitin and immunoblotting with Nrf2 antibody (Fig. 6A and B) The amount of ubiquitinated Nrf2 was slightly, but not significantly, decreased in hD5F173L-TG mice at 3 months of age but was significantly increased in hD5F173L-TG mice at 4 months of age, suggesting that the decrease in the nuclear Nrf2 expression in hD5F173L-TG mice at 4 months of age may be the result of increased Nrf2 degradation of ubiquitinated Nrf2 in the nucleus.
3.7. Dopamine D5 receptor deficiency induces cardiomyocyte dysfunction
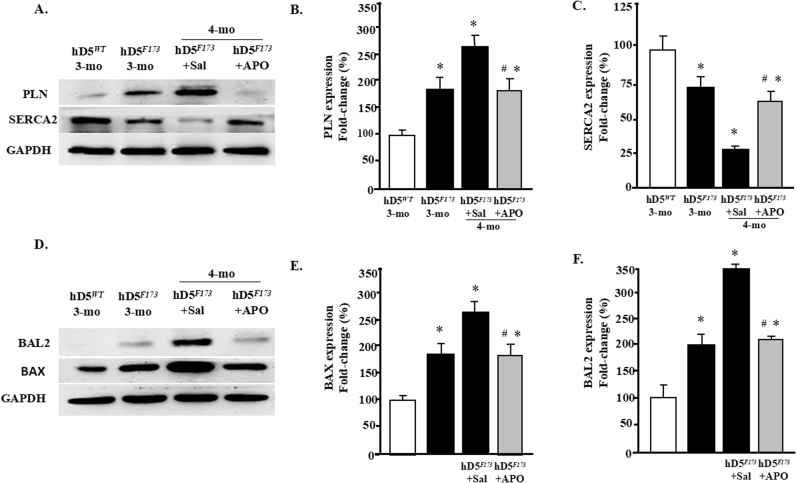
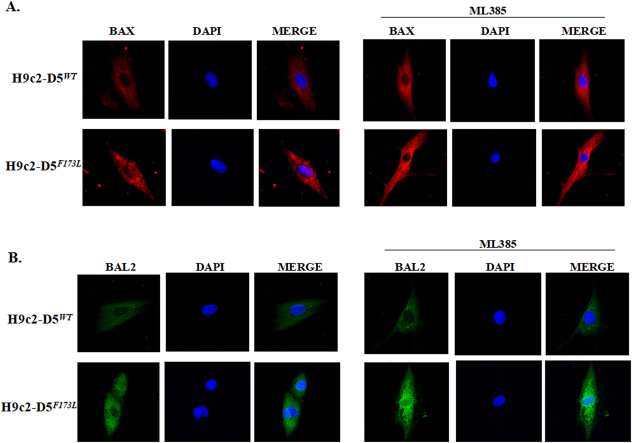
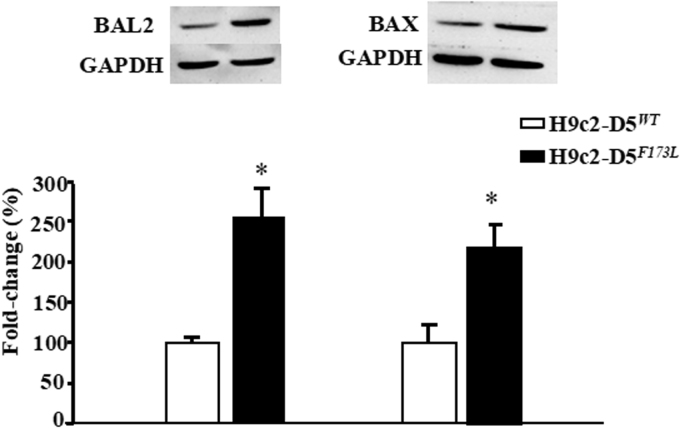
Disrupted cardiomyocyte Ca2+ homeostasis is recognized as a major contributor to the systolic and diastolic dysfunction phenotype. In our study, the expression of cardiac phospholamban (PLN), which regulates cardiac sarcoplasmic reticulum Ca2+ATPase (SERCA), was increased in cardiac-specific hD5F173L-TG mice at 3 months of age and increased further at 4 months of age (hD5F173L-TG + Sal) (Fig. 7A). PLN was decreased by apocynin treatment in 4-month-old mice to the 3-month old hD5F173L-TG level (Fig. 7A). By contrast, SERCA expression was decreased in cardiac-specific hD5F173L-TG mice at 3 months of age and was even more decreased at 4 months of age (hD5F173L-TG + Sal mice). Apocynin increased SERCA expression in the 4-month-old hD5F173L-TG level to the 3-month-old hD5F173L-TG level (Fig. 7C). We also measured the expression of apoptosis and myocardial injury markers BAX (BCL2 associated X, apoptosis regulator) and BAL2 (B aggressive lymphoma protein 2) and found that their expressions were increased in hD5F173L-TG mice at 3 months of age and increased further at 4 months of age in hD5F173L-TG (hD5F173L-TG + Sal mice) (Fig. 7E and F). As with the PLN results, apocynin treatment decreased the BAX and BAL2 in the 4-month-old hD5F173L-TG + Apo mice to the same level as that found in 3-month-old D5F173L-TG mice (Fig. 7D–F). These results indicate that D5R deficiency-induced ROS overproduction accelerates cardiomyocyte disruption of Ca2+ homeostasis and promotes apoptosis, in vivo. In vitro studies also showed that H9c2-hD5F173L cells have increased BAL2 and BAX expression compared with H9c2-hD5WTcells (Supplementary Fig. 2). Treatment with the Nrf2 inhibitor ML385, which decreased Nrf2 expression in the cytoplasm and nucleus (Supplementary Fig. 3), increased further the already increased expression of BAX and BAL2 in H9c2-hD5F173L, relative to H9c2-hD5WTcells (Fig. 8A and B), which would aggravate the cardiomyocyte apoptosis. These data confirm that Nrf2 plays an important role in the D5R deficiency-induced cardiomyocyte dysfunction.
Fig. 7.
D5R deficiency induces cardiomyocyte dysfunction A–C: Total proteins were extracted from hearts of 3-month-old cardiac-specific human D5WT-TG (hD5WT) and hD5F173L-TG (hD5F173) and 4-month-old cardiac-specific hD5F173 mice treated with saline (hD5F173L + Sal) or apocynin (hD5F173L + APO) for 4 weeks, starting at 3 months of age (n = 12 mice/group). PLN and SERCA2 expression was semiquantified by western blot. D–F: BAL2 and BAX expression was semiquantified by western blot. *P < 0.05 vs. hD5WT mice; #P < 0.05 vs. hD5F173L + Sal.
Fig. 8.
D5R deficiency induces cardiomyocyte injury that is aggravated by Nrf2 inhibition. A: and B: H9c2-D5WT and H9c2-D5F173L cells were treated with ML385 (1 μM), an inhibitor of Nrf2, for 12 h. Cytosol BAX (B) and BAL2 (C) expressions are increased by ML385 treatment. Red: BAX; Blue: DAPI; and Green: BAL2.
3.8. JNK/ERK pathway is activated by ROS in D5R deficiency-induced DCM
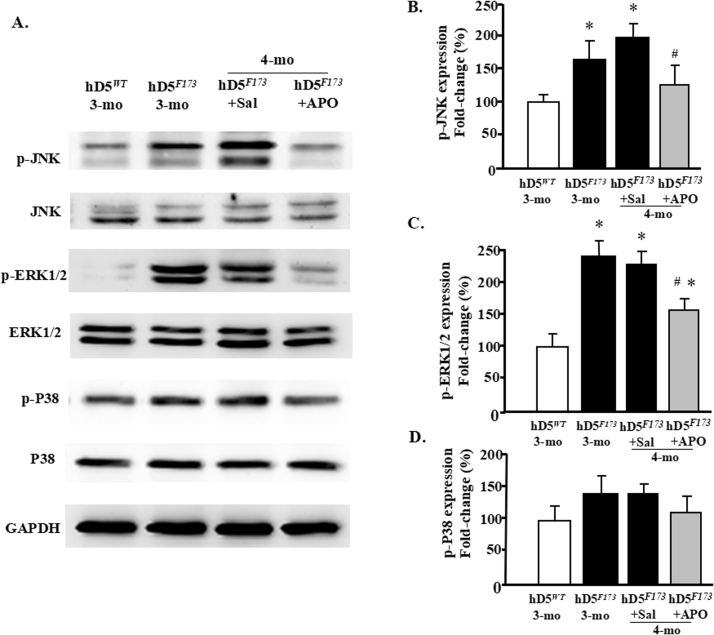
ROS induction of the MAPK signaling pathway may take part in the progression of DCM [35]. Therefore, we next determined if ERK, JNK, or p38 is involved in the D5R functional deficiency-induced DCM in hD5F173L-TG mice. We found that basal cardiac phospho-JNK (p-JNK) and phospho-ERK1/2 (p-ERK1/2) protein expressions but not their total protein expressions were higher in hD5F173L-TG than hD5WT-TG mice both at 3 months (hD5F173) and 4 months (hD5F173 + Sal) of age (Fig. 9A–C). By contrast, phospho- or total-P38 was not affected by the hD5F173 mutation (Fig. 9Aand D). ROS increased the phosphorylation and presumably the activity of these proteins because the increased p-JNK and p-ERK1/2 protein expressions in both (hD5F173L) 3-month- and (hD5F173 + Sal) 4-month-old mice were decreased by apocynin treatment in (Fig. 9A–C). These results indicate that ROS participate in the activation of JNK and ERK pathways in D5R functional deficiency-induced DCM.
Fig. 9.
D5R functional deficiency upregulates p-JNK and p-ERK1/2. Proteins were extracted from hearts of 3-month-old cardiac-specific hD5WT-TG (hD5WT) and hD5F173L-TG (hD5F173) and 4-month-old cardiac-specific hD5F173 mice treated with saline (hD5F173L + Sal) or apocynin (hD5F173L + APO) for 4 weeks, starting at 3 months of age (n = 12 mice/group). Protein expression was semiquantified by western blot using anti-p-JNK and anti-p-ERK1/2. A: and B: p-JNK, A: and C: p-ERK1/2, (D) p-P38 (n = 10 mice/group). The data were normalized with total ERK1/2, total JNK, and total P38; *P < 0.05 vs. hD5WT mice; #P < 0.05 vs. hD5F173L + Sal.
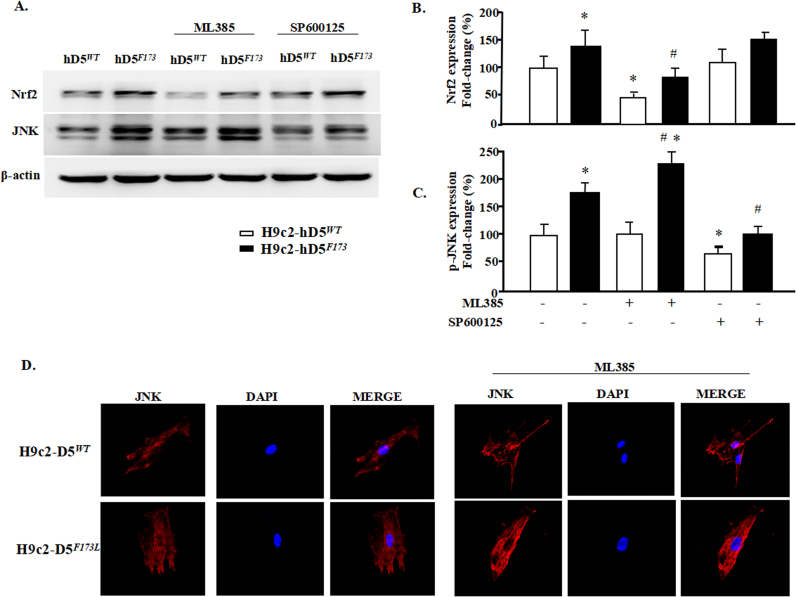
To determine further whether ERK and JNK are downstream effects of Nrf2 and ROS, we treated H9c2-hD5F173L cells with Nrf2 (ML385) [16] and JNK (SP600125) [17] inhibitors. The Nrf2 inhibitor, as expected, decreased Nrf2 expression in H9c2-D5WT cells and also decreased the slight increase in Nrf2 expression in H9c2-hD5F173L cells but not to the same level as that found in H9c2-D5WT cells (Fig. 10A and B). The Nrf2 inhibitor had no effect on p-JNK expression in H9c2-D5WT cells but increased the expression of total JNK in H9c2-hD5F173L cells. (Fig. 10A and C). By contrast, inhibition of JNK was unable to alter total Nrf2 expression (Fig. 10B and C) in either H9c2-D5WT or H9c2-hD5F173L cells. These results demonstrated that increased ROS production stimulates the ERK/JNK pathway, in part via a decrease in Nrf2 expression, which participates in the D5R functional deficiency-induced DCM.
Fig. 10.
JNK pathway is downstream of Nrf2. H9c2-D5WT and H9c2-D5F173L cells were treated with ML385 (1 μM), an inhibitor of Nrf2, or SP600125 (10 μM), an inhibitor of JNK, for 12 h. JNK inhibition does not affect Nrf2 expression (A and C). However, Nrf2 inhibition (A, B, D) increases further the D5R functional deficiency-induced increase in JNK in H9c2-D5F173L cells. *P < 0.05 vs. untreated H9c2-D5WT cells. #P < 0.05 vs. untreated H9c2-D5F173L cells. n = 8/group.
4. Discussion
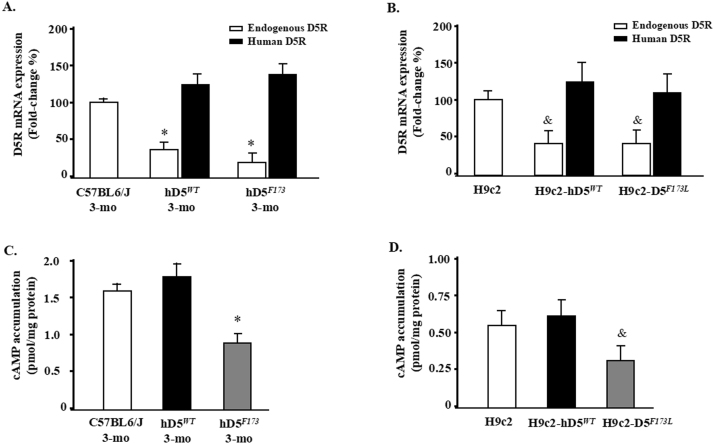
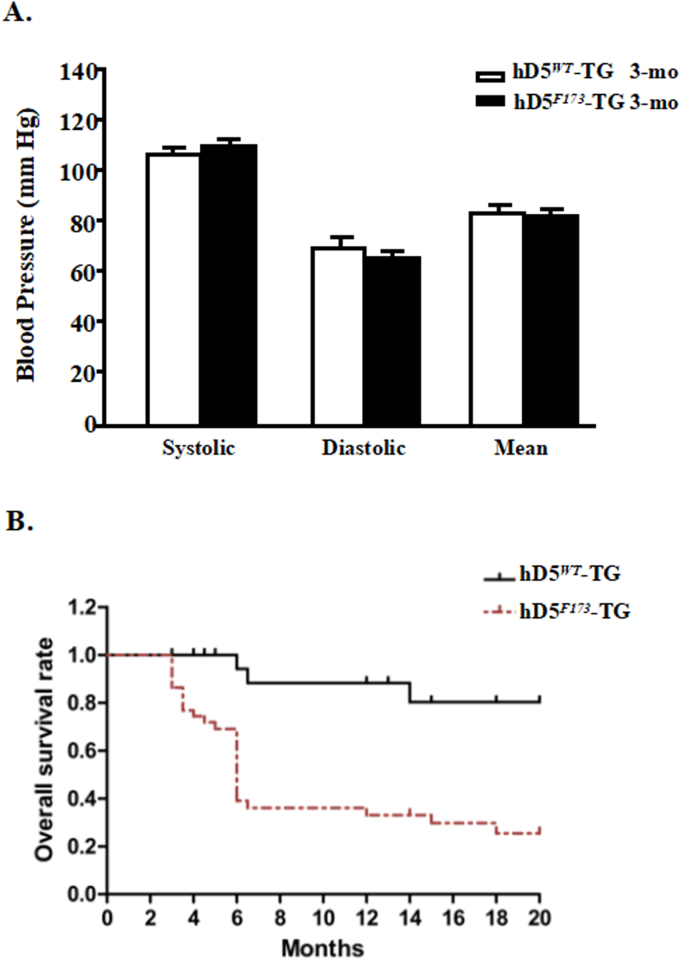
Cardiovascular diseases are closely linked to hypertension. The risk of DCM, characterized by ventricular dilatation and impaired systolic and diastolic function, is doubled when the blood pressure exceeds 160/95 mmHg [36]. A recent estimate indicated that the risk for cardiovascular disease for each 10 mm Hg increment in systolic blood pressure from baseline is 25% for women and 15% for men in the USA [37]. Genome-wide linkage studies have revealed that the loci of the human D5R (hD5R), 4p15.1–16.1, and its pseudo genes, 1q21.1 2p11.1-p11.2, are linked to essential hypertension [9], [38], [39]. Moreover, hD5F173L has diminished cAMP activity and D5R function, which transgenosis in mice signifiantly inhibit endogenous D5R expression (Supplementary Fig. 1). We have found that global hD5F173L-TG mice have high blood pressure and cardiac hypertrophy [10]. In our current study, the blood pressures of cardiac-specific hD5F173L-TG mice were normal and not significantly different from those observed in cardiac-specific hD5WT-TG mice at 3 months of age (Supplementary Fig. 4A) and 4 months of age (not shown). Therefore, the DCM in cardiac-specific hD5F173L-TG mice is a direct cardiac effect of the mutant human D5R gene rather than a compensatory dilatation due to pressure overload. We now highlight the observation that D5R is necessary for the normal expression of Nrf2 and inhibition of NADPH oxidase activity and that their dysfunctions stimulate ROS over-production and myocardial remodeling via ERK1/2/JNK pathway (Fig. 11).
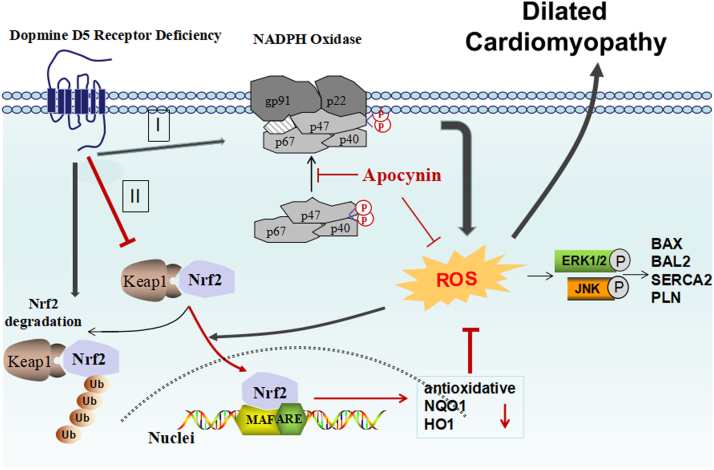
Fig. 11.
D5R mutation induces dilated cardiomyopathy that is related to oxidative stress. D5R deficiency causes an increase in NADPH oxidase activity (I), and prevents the translocation of Nrf2 into the nucleus (II), resulting in a failure of the transcription of a number of antioxidative genes via masculoaponeurotic fibrosarcoma (MAF) and antioxidant response elements (ARE). Antioxidant proteins include NAD(P)H quinone 1 dehydrogenase (NQO1) and heme oxygenase 1 (HO1). Oxidative stress accelerates the signaling of the ERK1/2 and JNK pathway, impairing the balance between apoptosis (BCL2 associated X, apoptosis regulator [BAX]) and anti-apoptosis (B aggressive lymphoma protein 2 (BAL2, PARP14 gene). Phospholamban (PLN) is also stimulated which inhibits cardiac muscle sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA, encoded by ATP2A2), which in turn causes cardiac remodeling and finally, dilated cardiomyopathy. D5R deficiency also allows Kelch-like ECH Associated Protein 1 (Keap1) to target Nrf2 in the cytoplasm for ubiquitination and degradation in the proteasome.
Oxidative stress has been identified as one of the key contributing factors in the pathogenesis of heart disease, including myocardial hypertrophy, myocardial remodeling, DCM, and heart failure [30], [40], [41], [42]. We have reported that global D5-/- mice have increased ROS production, associated with increased expression and activity of NADPH oxidase and phospholipase D in the kidney [7], [10], [11]. We have also reported that renal and brain NADPH oxidase activity is increased in global hD5F173L-TG mice [7], [10], [11]. NADPH oxidases are the major sources of ROS in many cells, including cardiac, vascular endothelial, and renal epithelial cells [7], [10], [11], [17], [18], [19], [30], [41], [42], [43], [44], [45]. NADPH oxidases, depending on the particular isoform, are multi-subunit enzymatic complexes consisting of membrane subunits gp91phox and p22phox and cytosolic subunits p47phox, p67phox, and p40phox [40], [41], [42], [43], [44]. In our current study, we found that membrane p40phox and p47phox levels were markedly increased in the heart of cardiac-specific hD5F173L-TG mice that had DCM. Apocynin decreases ROS production and inhibits NADPH oxidase activity by blocking the translocation of cytosolic subunits p47phox and p40phox to the plasma membrane [7], [17], [18], [19]. However, the apocynin-mediated reduction of cardiac ROS production was smaller than the reduction of NADPH oxidase activity which could be taken to indicate that factors other than oxidative stress are important in the pathogenesis of DCM in cardiac-specific hD5F173L-TG mice.
In addition to the NADPH oxidases, mitochondria are one of the most important cellular source of ROS in cardiomyocytes [31]. Human studies on heart failure as well as animal models on cardiovascular diseases suggest that mitochondrial bioenergetics deficiency may contribute to the development and progression of the disease [46], [47]. we found that mitochondria function and mitochondrial ROS was similar in D5RF173L-TG mice and D5RWT-TG mice. H9c2-hD5F173L cells also have normal mitochondria function, which was similar with H9c2-hD5WT cells. These data indicate that cardic specific D5R mutantion transfection increased ROS production may mainly come from NADPH oxidase but not from mitochondria, and the role of NADPH oxidase in the regulation of DCM may extend beyond ROS, which is unlikely. A better possibility is that ROS can stimulate the activity of antioxidant proteins that could serve as a negative feedback, Nrf2 for example [32], [33].
Several clinical studies have shown that the antioxidant effect of Nrf2 is a critical regulator for maintaining the structural and functional integrity of the heart in states of oxidative stress [32], [33]. We have reported that the dopamine D2 receptor is necessary for normal Nrf2 activity in the kidney [28]. Renal-selective silencing of Drd2 in the mouse increases ROS production which is partly mediated by increased Nrf2 degradation [28]. The D5R also inhibits ROS production, not only by inhibiting NADPH oxidase activity, but also by increasing HO-1 expression and activity [7], [11], [48]]. In our current study, cardiac-specific hD5F173L-TG mice had increased Nrf2 expression at 3 months of age but decreased Nrf2 expression at 4 months of age because of increased ubiquitination and subsequent degradation. We suggest that the increased cardiac Nrf2 expression at 3 months of age may be a mechanism to counteract the state of oxidative stress but eventually fails. This could also explain why Nrf2 expression was increased in H9c2-hD5F173L cells and its inhibition increased p-JNK expressions, a marker of tissue injury. The finding that D5R deficiency prevents the nuclear translocation of Nrf2, impairing the upregulation of the expression of antioxidant enzymes, e.g., HO1, highlights the important role of cardiac ROS and oxidative stress in myocardial dysfunction.
Increased generation of ROS in cardiomyocytes leads to increased necrosis and apoptosis, which induce cardiac remodeling and dysfunction [49]. Oxidative stress accelerates inflammation and apoptosis, which in turn cause cardiomyocyte hypertrophy and fibroblast proliferation, resulting in the cardiac remodeling [50]. In our current report, echocardiographic studies showed that the decrease in ROS production caused by apocynin was associated with a decrease in cardiac dilatation and an improvement in cardiac function; heart weight was also decreased. The apocynin-mediated beneficial effects in the myocardium, including the reduction in DCM, was associated with a decrease in apoptosis (BAX) and anti-apoptosis (BAL2) proteins, a decrease in PLN, but an increase in SERCA2 in hD5F173L-TG mice. H9c2-hD5F173L cells showed that the Nrf2 inhibitor [15] increased the expression of p-JNK, which plays a key role in tissue injury [51]. Our data indicate that Nrf2 and NADPH oxidase regulate ROS production involved in the promotion of hD5F173L-mediated cardiac dysfunction and DCM. DCM is a common cause of heart failure due to the progressive depression of myocardial contractile function and ventricular dilatation [1], [2], [3], [4], [5]. We found that more than 60% of the cardiac-specific hD5F173L-TG mice did not survive after 6 months of age (Supplementary data 4B). The etiology of the early death of cardiac-specific hD5F173L-TG mice may be due to heart failure.
In the dilated failing heart, multiple signaling pathways are activated, including ERK1/2, JNK,P38a MAP kinases, and AKT [52]. There is increasing evidence that NADPH oxidase plays an important role in modulating ERK1/2 and AKT activity in the myocardium undergoing ischemia-reperfusion injury [35]. In this study, we found that the levels of p-ERK1/2 and p-JNK but not p-P38 were increased in the heart of cardiac-specific hD5F173L-TG mice. The increased expression of p40 and p47 was also accompanied by increased levels of p-ERK1/2 and p-JNK in H9c2-D5F173L-cells compared with H9c2-D5WT cells. The levels of p-ERK1/2 and p-JNK in the hearts of cardiac-specific hD5F173L-TG mice, which were increased than in hD5F173L-TG mice, were decreased by apocynin. The in vitro studies also demonstrated that ERK1/2 and JNK are the downstream of Nrf2 pathway. Therefore, p-ERK1/2 and p-JNK are involved in the D5R deficiency-induced DCM, which are up-regulated by NADPH oxidase and Nrf2-deficiency-stimulated ROS production.
Ca2+ plays a pivotal role in the normal cardiac function and pathogenesis of cardiac disease [53], [54]. Ca2+-handling abnormalities have been found in various forms of heart failure, including DCM [55], [56], [57]. Indeed, in our cardiac-specific hD5F173L TG mice, SERCA2 expression was decreased, relative to cardiac-specific hD5WT TG mice, indicating an abnormal transduction of SERCA2 with D5R dysfunction. By contrast, the levels of PLN, a negative regulator of SERCA [58], were increased in cardiac-specific hD5F173L TG mice, especially at 4 months of age, indicating that a dysfunction of SERCA2 may take part in the progression of D5R deficiency-induced DCM. Therefore, the D5R negatively regulates NADPH oxidase activity, probably by adjusting the concentration of intracellular Ca2+ ion. However, the detailed mechanism of the D5R-mediated regulation of SERCA2 remains to be clarified.
5. Conclusion
In summary, our result shows that the cardiac D5R is necessary for maintaining normal heart function. We demonstrated that the cardiac-specific decrease in D5R function, caused by the cardiac-specific expression of hD5F173L, results in DCM accompanied by increased ROS production come from cardiac NADPH oxidase and Nrf2 degradation but not mitochondria and stimulation of ERK1/2/JNK activities, accompanied by an increase in pro-apoptotic BAX and anti-apoptotic BAL2, an increase in PLN, and a decrease in SERCA2. Modulation of cardiac D5R could be a therapeutic approach in DCM.
Funding sources
These studies were supported, in part, by grants from Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-1-016), National Natural Science Foundation (China) (81600334, 81670387), Life science society consortium "Youth Talent Promotion Project"and National Institutes of Health (USA) (P01HL074940, PAJ).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.07.008.
Contributor Information
Qiang Wei, Email: weiqiang0430@cnilas.org.
Zhiwei Yang, Email: yangzhiwei@cnilas.pumc.edu.cn.
Appendix A. Supplementary material
Table S1.
Primer sequence for real-time PCR. The mRNA expression levels of mitochondrial genes were examined by Real-time PCR using the primer pairs with the sequences below. m: mouse; h: human.
Table S2.
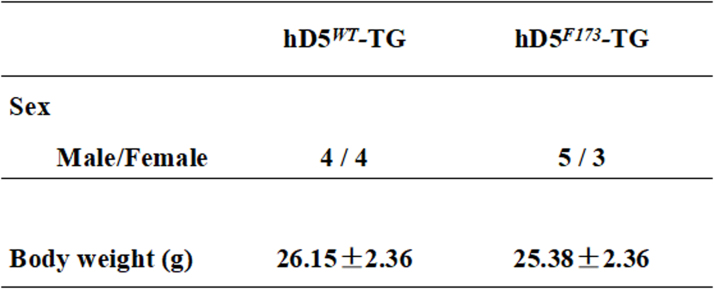
Body weights (g) of cardiac-specific hD5WT-TG and hD5F173L-TG mice at 3 months of age (n = 8/group).
Table S3.
Body weights (g) of cardiac-specific hD5F173L-TG mice treated with saline or apocynin. Cardiac-specific hD5F173L-TG mice were injected intraperitoneally with apocynin (1 mmol/kg/day) (hD5F173-TG + APO) or phosphate-buffered saline (0.1–0.3 ml) as control daily, for four weeks, starting at 3 months of age (hD5F173-TG + saline). (n = 10/group).
Fig. S1.
Endogenous D5R expression and cAMP accumulation in 3-month-old hD5F173L-TG and D5WT-TG mice and H9c2 cells. A: Fold changes of endogenous mouse D5R and human D5R mRNA expression in D5RF173L-TG mice relative to D5RWT-TG mice and in H9c2 cells. B: cAMP accumulation was determined in the presence of 1 mM 3-isobutyl-1-methylxanthine. *p < 0.05 vs. C57BL6/J mice (n = 7), &p < 0.05 vs. H9c2 cells (n = 5).
Fig. S2.
BAL2 and BAX protein expressions in H9c2 cells. BAL2 and BAX protein expressions were semiquantified by western blot in H9c2-D5WT and H9c2-D5F173L cells (n = 12/group, *P < 0.05 vs. H9c2-D5WT cells).
Fig. S3.
ML385 significant prevent Nrf2 expression. Nrf2 inhibitor, ML385, decreases Nrf2 expression in H9c2-D5WT and H9c2-D5F173L cells studied by immunofluorescence microscopy.
Fig. S4.
Arterial blood pressure(3-month-old) and survival curve in cardiac-specific hD5WT-TG and hD5F173L-TG mice. A: Systolic, diastolic, and mean arterial blood pressures were measured under pentobarbital anesthesia in cardiac-specific hD5WT and hD5F173L TG mice (n = 12/group). B: Kaplan-Meier survival curve of cardiac-specific hD5WT-TG(upper, n = 25) and hD5F173L-TG mice(lower, n = 20) was assessed by Mantel Cox log-rank test.
References
- 1.Hughes S.E., McKenna W.J. New insights into the pathology of inherited cardiomyopathy. Heart. 2005;91:257–264. doi: 10.1136/hrt.2004.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fatkin D., Graham R.M. Molecular mechanisms of inherited cardiomyopathies. Physiol. Rev. 2002;82:945–980. doi: 10.1152/physrev.00012.2002. [DOI] [PubMed] [Google Scholar]
- 3.Seidman J.G., Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 4.Jefferies J.L., Towbin J.A. Dilated cardiomyopathy. Lancet. 2010;375:752–762. doi: 10.1016/S0140-6736(09)62023-7. [DOI] [PubMed] [Google Scholar]
- 5.Houser S.R., Margulies K.B., Murphy A.M., Spinale F.G., Francis G.S., Prabhu S.D., Rockman H.A., Kass D.A., Molkentin J.D., Sussman M.A., Koch W.J., American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology Animal models of heart failure: a scientific statement from the American Heart Association. Circ. Res. 2012;111:131–150. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 6.Arsanjani R., Berman D.S., Gransar H., Cheng V.Y., Dunning A., Lin F.Y., Achenbach S., Al-Mallah M., Budoff M.J., Arsanjani T.Q., Chang H.J., Cademartiri F., Chinnaiyan K.M., Chow B.J., DeLago A., Hadamitzky M., Hausleiter J., Kaufmann P., LaBounty T.M., Leipsic J., Raff G., Shaw L.J., Villines T.C., Cury R.C., Feuchtner G., Kim Y.J., Min J.K., Investigators C. Left ventricular function and volume with coronary CT angiography improves risk stratification and identification of patients at risk for incident mortality: results from 7758 patients in the prospective multinational confirm observational cohort study. Radiology. 2014;273:70–77. doi: 10.1148/radiol.14122816. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z., Asico L.D., Yu P., Wang Z., Jones J.E., Escano C.S., Wang X., Quinn M.T., Sibley D.R., Romero G.G., Felder R.A., Jose P.A. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R96–R104. doi: 10.1152/ajpregu.00434.2005. [DOI] [PubMed] [Google Scholar]
- 8.Hollon T.R., Bek M.J., Lachowicz J.E., Ariano M.A., Mezey E., Ramachandran R., Wersinger S.R., Soares-da-Silva P., Liu Z.F., Grinberg A., Drago J., Young W.S., 3rd, Westphal H., Jose P.A., Sibley D.R. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J. Neurosci. 2002;22:10801–10810. doi: 10.1523/JNEUROSCI.22-24-10801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cravchik A., Gejman P.V. Functional analysis of the human D5 dopamine receptor missense and nonsense variants: differences in dopamine binding affinities. Pharmacogenetics. 1999;9:199–206. [PubMed] [Google Scholar]
- 10.Liu X., Wang W., Chen W., Jiang X., Zhang Y., Wang Z., Yang J., Jones J.E., Jose P.A., Yang Z. Regulation of blood pressure, oxidative stress and AT1R by high salt diet in mutant human dopamine D5 receptor transgenic mice. Hypertens. Res. 2015;38:394–399. doi: 10.1038/hr.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Q., Yang Y., Villar V.A., Asico L., Jones J.E., Yu P., Li H., Weinman E.J., Eisner G.M., Jose P.A. D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1. Hypertens. Res. 2013;36:684–690. doi: 10.1038/hr.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuevas S., Villar V.A., Jose P.A., Armando I. Renal dopamine receptors, oxidative stress, and hypertension. Int. J. Mol. Sci. 2013;27:17553–17572. doi: 10.3390/ijms140917553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banday A.A., Lokhandwala M.F. Transcription factor Nrf2 protects renal dopamine D1 receptor function during oxidative stress. Hypertension. 2013;62:512–517. doi: 10.1161/HYPERTENSIONAHA.113.01358. [DOI] [PubMed] [Google Scholar]
- 14.Cheung K.G., Cole L.K., Xiang B., Chen K., Ma X., Myal Y., Hatch G.M., Tong Q., Dolinsky V.W. sirtuin-3 (SIRT3) protein attenuates doxorubicin-induced oxidative stress and improves mitochondrial respiration in H9c2 cardiomyocytes. J. Biol. Chem. 2015;290:10981–10993. doi: 10.1074/jbc.M114.607960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh A., Venkannagari S., Oh K.H., Zhang Y.Q., Rohde J.M., Liu L., Nimmagadda S., Sudini K., Brimacombe K.R., Gajghate S., Ma J., Wang A., Xu X., Shahane S.A., Xia M., Woo J., Mensah G.A., Wang Z., Ferrer M., Gabrielson E., Li Z., Rastinejad F., Shen M., Boxer M.B., Biswal S. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem. Biol. 2016;11:3214–3225. doi: 10.1021/acschembio.6b00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q., Xiang Y., Chen Y., Tang Y., Zhang Y. Ginsenoside Rg1 protects cardiomyocytes against hypoxia/reoxygenation injury via activation of Nrf2/HO-1 signaling and inhibition of JNK. Cell. Physiol. Biochem. 2017;44:21–37. doi: 10.1159/000484578. [DOI] [PubMed] [Google Scholar]
- 17.Mora-Pale M., Weiwer M., Yu J., Linhardt R.J., Dordick J.S. Inhibition of human vascular NADPH oxidase by apocynin derived oligophenols. Bioorg. Med. Chem. 2009;17:5146–5152. doi: 10.1016/j.bmc.2009.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson D.K., Schillinger K.J., Kwait D.M., Hughes C.V., McNamara E.J., Ishmael F., O'Donnell R.W., Chang M.M., Hogg M.G., Dordick J.S., Santhanam L., Ziegler L.M., Holland J.A. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium. 2002;9:191–203. doi: 10.1080/10623320213638. [DOI] [PubMed] [Google Scholar]
- 19.Vallejo S., Romacho T., Angulo J., Villalobos L.A., Cercas E., Leivas A., Bermejo E., Carraro R., Sanchez-Ferrer C.F., Peiro C. Visfatin impairs endothelium-dependent relaxation in rat and human mesenteric microvessels through nicotinamide phosphoribosyltransferase activity. PLoS One. 2011;6:e27299. doi: 10.1371/journal.pone.0027299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karamanlidis Georgios, Lee Chi Fung, Garcia-Menendez Lorena, Kolwicz Stephen C., Jr, Suthammarak Wichit, Gong Guohua, Sedensky Margaret M., Morgan Philip G., Wang Wang, Tian Rong. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013;18:239–250. doi: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehm E.A., Jones B.E., Radda G.K., Veech R.L., Clarke K. Increased uncoupling proteins and decreased efficiency in palmitate-perfused hyperthyroid rat heart. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H977–H983. doi: 10.1152/ajpheart.2001.280.3.H977. [DOI] [PubMed] [Google Scholar]
- 22.Zhuang Yan, Li Yi, Li Xuefeng, Xie Qing, Wu Min. Atg7 knockdown augments concanavalin A-induced acute hepatitis through an ROS-mediated p38/MAPK pathway. PLoS One. 2016;11:e0149754. doi: 10.1371/journal.pone.0149754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J., Wu Y., Tian X., Wang J., Dong M., Wang A., Ma S. Protective effect of HTK solution on postoperative pulmonary function in infants with CHD and PAH. Biosci. Rep. 2017;37:11–16. doi: 10.1042/BSR20170984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engberding N., Spiekermann S., Schaefer A., Heineke A., Wiencke A., Muller M., Fuchs M., Hilfiker-Kleiner D., Hornig B., Drexler H., Landmesser U. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation. 2004;110:2175–2179. doi: 10.1161/01.CIR.0000144303.24894.1C. [DOI] [PubMed] [Google Scholar]
- 25.Funayama A., Shishido T., Netsu S., Narumi T., Kadowaki S., Takahashi H., Miyamoto T., Watanabe T., Woo C.H., Abe J., Kuwahara K., Nakao K., Takeishi Y., Kubota I. Cardiac nuclear high mobility group box 1 prevents the development of cardiac hypertrophy and heart failure. Cardiovasc. Res. 2013;99:657–664. doi: 10.1093/cvr/cvt128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shishido T., Nozaki N., Yamaguchi S., Shibata Y., Nitobe J., Miyamoto T., Takahashi H., Arimoto T., Maeda K., Yamakawa M., Takeuchi O., Akira S., Takeishi Y., Kubota I. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation. 2003;108:2905–2910. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 27.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the ∆∆ CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Cuevas S., Yang Y., Konkalmatt P., Asico L.D., Feranil J., Jones J., Villar V.A., Armando I., Jose P.A. Role of nuclear factor erythroid 2-related factor 2 in the oxidative stress-dependent hypertension associated with the depletion of DJ-1. Hypertension. 2015;65:1251–1257. doi: 10.1161/HYPERTENSIONAHA.114.04525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsujita Y., Kato T., Sussman M.A. Evaluation of left ventricular function in cardiomyopathic mice by tissue Doppler and color M-mode Doppler echocardiography. Echocardiography. 2005;22:245–253. doi: 10.1111/j.0742-2822.2005.04014.x. [DOI] [PubMed] [Google Scholar]
- 30.Parajuli N., Patel V.B., Wang W., Basu R., Oudit G.Y. Loss of NOX2 (gp91phox) prevents oxidative stress and progression to advanced heart failure. Clin. Sci. 2014;127:331–340. doi: 10.1042/CS20130787. [DOI] [PubMed] [Google Scholar]
- 31.Boengler K., Kosiol M., Mayr M., Schulz R., Rohrbach S. Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopenia Muscle. 2017;8(3):349–369. doi: 10.1002/jcsm.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Z., Xu X., Hu X., Zhu G., Zhang P., van Deel E.D., French J.P., Fassett J.T., Oury T.D., Bache R.J., Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing Y., Niu T., Wang W., Li J., Li S., Janicki J.S., Ruiz S., Meyer C.J., Wang X.L., Tang D., Zhao Y., Cui T. Triterpenoid dihydro-CDDO-trifluoroethyl amide protects against maladaptive cardiac remodeling and dysfunction in mice: a critical role of Nrf2. PLoS One. 2012;7:e44899. doi: 10.1371/journal.pone.0044899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart D., Killeen E., Naquin R., Alam S., Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J. Biol. Chem. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- 35.Chen J.X., Zeng H., Tuo Q.H., Yu H., Meyrick B., Aschner J.L. NADPH oxidase modulates myocardial Akt, ERK1/2 activation, and angiogenesis after hypoxia-reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1664–H1674. doi: 10.1152/ajpheart.01138.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yusuf S., Pitt B. A lifetime of prevention: the case of heart failure. Circulation. 2002;106:2997–2998. doi: 10.1161/01.cir.0000046804.13847.5d. [DOI] [PubMed] [Google Scholar]
- 37.Wei Y.C., George N.I., Chang C.W., Hicks K.A. Assessing sex differences in the risk of cardiovascular disease and mortality per increment in systolic blood pressure: a systematic review and meta-analysis of follow-up studies in the United States. PLoS One. 2017;12:e0170218. doi: 10.1371/journal.pone.0170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allayee H., de Bruin T.W., Dominguez K. Michelle, Cheng L.S., Ipp E., Cantor R.M., Krass K.L., Keulen E.T., Aouizerat B.E., Lusis A.J., Rotter J.I. Genome scan for blood pressure in Dutch dyslipidemic families reveals linkage to a locus on chromosome 4p. Hypertension. 2001;38:773–778. doi: 10.1161/hy1001.092617. [DOI] [PubMed] [Google Scholar]
- 39.Rice T., Rankinen T., Province M.A., Chagnon Y.C., Perusse L., Borecki I.B., Bouchard C., Rao D.C. Genome-wide linkage analysis of systolic and diastolic blood pressure: the Quebec Family Study. Circulation. 2000;102:1956–1963. doi: 10.1161/01.cir.102.16.1956. [DOI] [PubMed] [Google Scholar]
- 40.Gorlach A., Brandes R.P., Nguyen K., Amidi M., Dehghani F., Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 41.Griendling K.K., Sorescu D., Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 42.Okamura D.M., Himmelfarb J. Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr. Nephrol. 2009;24:2309–2319. doi: 10.1007/s00467-009-1199-5. [DOI] [PubMed] [Google Scholar]
- 43.Jaimes E.A., Zhou M.S., Pearse D.D., Puzis L., Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am. J. Physiol. Ren. Physiol. 2008;294:F385–F392. doi: 10.1152/ajprenal.00302.2007. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan-Gunn M.J., Campbell-O'Sullivan S.P., Tisdale M.J., Lewandowski P.A. Decreased NADPH oxidase expression and antioxidant activity in cachectic skeletal muscle. J. Cachexia Sarcopenia Muscle. 2011;2:181–188. doi: 10.1007/s13539-011-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang M., Perino A., Ghigo A., Hirsch E., Shah A.M. NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid. Redox Signal. 2013;18:1024–1041. doi: 10.1089/ars.2012.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nickel A., Löffler J., Maack C. Myocardial energetics in heart failure. Basic Res. Cardiol. 2013;108(4):358–362. doi: 10.1007/s00395-013-0358-9. [DOI] [PubMed] [Google Scholar]
- 47.Neubauer S. The failing heart – an engine out of fuel. N. Engl. J. Med. 2007;356(11):1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z., Asico L.D., Yu P., Wang Z., Jones J.E., Bai R.K., Sibley D.R., Felder R.A., Jose P.A. D5 dopamine receptor regulation of phospholipase D. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H55–H61. doi: 10.1152/ajpheart.00627.2004. [DOI] [PubMed] [Google Scholar]
- 49.Gupta S., Das B., Sen S. Cardiac hypertrophy: mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2007;9:623–652. doi: 10.1089/ars.2007.1474. [DOI] [PubMed] [Google Scholar]
- 50.Haq S., Choukroun G., Lim H., Tymitz K.M., del Monte F., Gwathmey J., Grazette L., Michael A., Hajjar R., Force T., Molkentin J.D. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- 51.Li J., Shao Z.H., Xie J.T., Wang C.Z., Ramachandran S., Yin J.J., Aung H., Li C.Q., Qin G., Vanden Hoek T., Yuan C.S. The effects of ginsenoside Rb1 on JNK in oxidative injury in cardiomyocytes. Arch. Pharm. Res. 2012;35:1259–1267. doi: 10.1007/s12272-012-0717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isserlin R., Merico D., Alikhani-Koupaei R., Gramolini A., Bader G.D., Emili A. Pathway analysis of dilated cardiomyopathy using global proteomic profiling and enrichment maps. Proteomics. 2010;10:1316–1327. doi: 10.1002/pmic.200900412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeong E.M., Liu M., Sturdy M., Gao G., Varghese S.T., Sovari A.A., Dudley S.C., Jr. Metabolic stress, reactive oxygen species, and arrhythmia. J. Mol. Cell. Cardiol. 2012;52:454–463. doi: 10.1016/j.yjmcc.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toko H., Takahashi H., Kayama Y., Oka T., Minamino T., Okada S., Morimoto S., Zhan D.Y., Terasaki F., Anderson M.E., Inoue M., Yao A., Nagai R., Kitaura Y., Sasaguri T., Komuro I. Ca2+/calmodulin-dependent kinase IIdelta causes heart failure by accumulation of p53 in dilated cardiomyopathy. Circulation. 2010;122:891–899. doi: 10.1161/CIRCULATIONAHA.109.935296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beuckelmann D.J., Nabauer M., Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- 56.Lindner M., Brandt M.C., Sauer H., Hescheler J., Bohle T., Beuckelmann D.J. Calcium sparks in human ventricular cardiomyocytes from patients with terminal heart failure. Cell Calcium. 2002;31:175–182. doi: 10.1054/ceca.2002.0272. [DOI] [PubMed] [Google Scholar]
- 57.Louch W.E., Bito V., Heinzel F.R., Macianskiene R., Vanhaecke J., Flameng W., Mubagwa K., Sipido K.R. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc. Res. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 58.Stammers A.N., Susser S.E., Hamm N.C., Hlynsky M.W., Kimber D.E., Kehler D.S., Duhamel T.A. The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA) Can. J. Physiol. Pharmacol. 2015;93:843–854. doi: 10.1139/cjpp-2014-0463. [DOI] [PubMed] [Google Scholar]