Abstract
The present study was conducted to evaluate the ameliorative role of pumpkin seed oil (PSO) against potential adverse effects of bisphenol-A (BPA) in male mice. BPA was administered to the mice orally at a dose of 50 mg/kg body weight once a day for 28 successive days. While, PSO was administered to the mice orally at 1 mL/kg b w either before, with or after treatment of BPA, once a day for 28 successive days. The studied parameters were DNA damage evaluation using comet assay in liver and testes cells and micronucleus test in bone marrow; and histopathological examination of liver and testes tissues. Results revealed that BPA induced DNA damage in tested cells and marked histopathological alterations in liver and testes. In contrast, PSO treatments alleviated DNA damage and improved the histopathological alterations in liver and testes tissues. Furthermore, administration of mice with the PSO before BPA treatment was the best regimen in the alleviation of the adverse effects of BPA, followed by administration of PSO after then with treatment of BPA. It can be concluded that PSO may has a protective role against BPA genotoxicity and histopathological alterations in male mice.
Keywords: Pumpkin seed oil, Bisphenol-A, Genotoxicity, Micronucleus test, Comet assay, Histopathology
1. Introduction
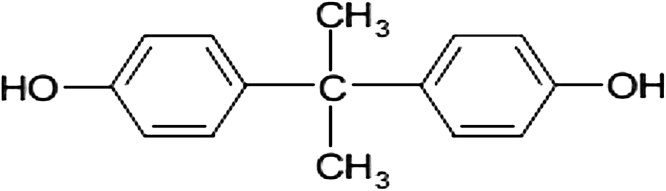
Among the various endocrine disrupting chemicals (EDCs), bisphenol-A (BPA) is the most vital synthetic compound with annual production greater than 3.8 million tons [1]. BPA chemical name is 4,4'-dihydroxy-2,2-diphenyl propane, its formula is (CH3)2C (C6H4OH)2 (or C15H16O2), its CAS No. is 80-05-7, and its chemical structure is shown in (Fig. 1). It is a synthetic monomer used in production of polycarbonate plastics, epoxy resins, food packaging, dental sealant, water pipes, toys, fire retardant, plastic bottles, paints, pesticides and lacquers for food cans [2,3].
Fig. 1.
Chemical structure of bisphenol-A (BPA).
Biomonitoring studies supported the widespread exposure to BPA [4,5]; and the potential human threat from usage of these products awing to the ability of BPA to leach out of plastics into the food and beverages they contain [6]. BPA causes hepatotoxic, mutagenic, reproductive and carcinogenic effects [[7], [8], [9], [10]]. Several studies confirmed the genotoxicity of BPA [11,12]. In addition, growing evidence indicates that the oxidative stress could be BPA mechanism to cause genotoxicity [9,13]. Moreover, Moustafa and Ahmed [14] data speculated that BPA long-term exposure depicted genomic damage, alterations in liver enzymes, lipid panel, antioxidant enzymes and reproductive hormones.
Natural antioxidants play a main role to diminish the oxidative stress by scavenging the free radicals and by further mechanisms that can prevent a variety of diseases [15]. The prevention of BPA-induced toxicity has been shown by using of antioxidants such as vitamin A [16] vitamin C [17], pomegranate juice [18] on experimental animals. In addition, many studies confirmed the modulator role of natural products against BPA toxicity. Morgan et al. [19] reported that the pretreatment with Cinnamon aqueous extract before BPA administration ameliorated the BPA-induced biochemical and pathological adverse effects in kidney, brain and testes. In the meantime, Aloe vera extract overcame the BPA damaging effects on the rat’s reproductive system and protects testes against BPA-induced toxicity [20].
In this study, pumpkin seed oil (PSO) was used as a protective agent. Pumpkin (Cucurbita pepo) is cultivated for human utilization and as functional food or medicine [21]. It is rich in various antioxidants and valuable nutritional components such as essential fatty acids, amino acids, phytosterols, β-carotenes and selenium [22]. Furthermore, it contains phenolic compounds as tyrosol, vanillic acid, vanillin, ferulic acid, and luteolin [23]. In addition, it shown to contain high levels of tocopherol that interpret its antioxidant activity and consequently may be able to reduce lipid peroxidation [24].
Therefore, the objective of this work was to assess the role of PSO in alleviating the adverse effects of BPA on DNA integrity in both somatic and germ cell and histopathological changes in male mice.
2. Materials and methods
2.1. Chemicals
Bisphenol-A (BPA) (≥ 99%) was purchased from Sigma-Aldrich Company (St. Louis, MO, USA). Pumpkin seed commercial oil was purchased from EL Captin Company (Al Obour City, Cairo, Egypt). All other chemicals were of analytical grade and purchased from standard commercial suppliers.
2.2. Dose preparation
BPA was dissolved in absolute ethyl alcohol (95%) and diluted with corn oil [1:20 alcohol: corn oil (vehicle)] to obtain a final concentration of BPA (50 mg/kg b.w.) and was always freshly prepared before use.
2.3. Experimental design
Seventy male swiss albino mice weighing 26 ± 5 g (10–12 week old) were purchased from the Theodor Bilharz Research Institute, Giza, Egypt. Animals were randomly divided into seven groups (n = 10), according to approximately equal mean body weight and were housed in individual cages with a 12-h light cycle at 23 ± 0.5 °C in 55 ± 5% relative humidity. Food and water were given ad libitum. The animals were cared for according to the published NIH guideline.
Animals were orally administered BPA (50 mg/kg b.w.) and/or PSO (1 mL/kg b.w.) before either with or after BPA administration for 28 successive days as follows: group 1 (control); group 2 (vehicle); group 3 (PSO); group 4 (BPA); group 5 (PSO before BPA); group 6 (PSO with BPA) and group 7 (PSO after BPA). Clinical signs were examined twice a day, and body weight was measured once a week during the experimental period.
2.4. Oil analysis
2.4.1. Fatty acids analysis
The fatty acids profile were determined as fatty acid methyl esters by Thermo scientific TRACE 1310 gas chromatograph attached with ISQ LT single quadruple mass spectrometer (GC-MS). The methyl esters were prepared using the method prescribed by AOAC [25].
2.4.2. Total phenolic content
Total phenolic content (TPC) was determined using the Folin–Ciocalteau’s reagent according to the method reported by Lin and Tang [26] at 760 nm with a spectrophotometer (UV-1601; Shimadzu, Tokyo, Japan) and the quantification was done on the basis of the standard curve of gallic acid concentration ranging between 10–80 mg/mL (r2 = 0.99).
2.4.3. Determination of total tocopherol (Vitamin E)
Sample preparation was carried out based on a method described by Gimeno et al. [27]. High Performance Liquid Chromatography (HPLC) system (1100 series, Agilent Technologies) was used for the quantification of vitamin E.
2.4.4. Measurement of antioxidant activity
The ability of PSO at 200 u L to scavenge 2.9 mL of 1, 1'-diphenyl 1-2-picrylhydrazyl (DPPH) free radical was estimated by the method of Singh et al. [28].
2.5. Relative organs weights
At the termination of the experiments, internal organs such as liver and testes were dissected out, trimmed of excess fat and weighted before genetic and histopathological studies took place. The organs weight was presented as relative organ weight as follows:
2.6. Evaluation of DNA damage
2.6.1. Comet assay in cells of liver and testes
The comet assay was performed in liver and testes cells according to Bandyopadhyaya et al. [29]. Briefly, 50 μL of cell suspension was mixed with 100 μL of 1% low melting point (LMP) agarose and added to fully frosted slides coated with 80 μL of 1% normal melting point (NMP) agarose. The cells were then incubated in a lysis solution (2.5 mol L−1 NaCl, 100 mmol L−1 EDTA, 10 mmol L−1 Tris−HCL, 1% Triton X-100, pH 10) at 4 °C for at least 2 h, at which the slides were placed into an alkaline solution (300 mmol L−1 NaOH, 1 mmol L−1 EDTA, pH 13) at 4 °C for 20 min so as to allow DNA unwinding, and electrophoresed at 25 V (300 mA) for 20 min. Finally, the slides were neutralized in a 400 mmol L−1 Tris buffer (pH 7.5) for 15 min and stained with ethidium bromide (5 μg mL−1). Images of 50 randomly selected nuclei per experimental group were captured using a fluorescence microscope (Eclipse 800, Nikon, Tokyo, Japan) and analyzed with image analysis software (Comet Assay IV, Perceptive Instruments, Suffolk, UK). Scored parameters included tail length, DNA percentage in tail and Olive tail moment (OTM). Tail length is the maximum distance that the damaged DNA migrates from the centre of the cell nucleus. DNA Percentage in tail is the DNA content that migrates from the nucleus into the comet tail. OTM is the product of the tail length and percentage DNA, which gives a more integrated measurement of overall DNA damage in the cell.
2.6.2. Bone marrow micronucleus assay
The micronucleus test was carried out in mice femoral bone marrow cells according to Chauhan et al. [30]. Numbers of normochromatic, polychromatic erythrocytes and micronuclei were evaluated in control and treated groups. For micronuclei evaluation, 2000 polychromatic erythrocytes were scored per animal. Both normochromatic erythrocytes (NCE) and polychromatic erythrocytes (PCE) were scored in 500 erythrocytes for determination of the PCE: NCE ratio according to the OECD No. 474 guideline of mammalian erythrocyte micronucleus test for chemicals testing [31].
2.7. Histopathological examination
Liver and testes from each sacrificed mouse were dissected out and trimmed of excess fat tissues. Tissues were fixed in 10% buffered formalin and processed for paraffin sectioning by dehydration in different concentrations of alcohol, cleared with xylol and embedded in paraffin blocks. Sections of about 5 μm thickness were stained with Harris haematoxylin and eosin (H&E) for histological study [32].
2.8. Statistical analyses
Statistical analyses were performed with SPSS 16 software. Experimental data were analyzed using one-way analysis of variance (ANOVA). Duncan's multiple range test was used to determine the significant differences between means. All values were expressed as mean ± SD and the significance level was set at P ≤ 0.05.
3. Results and discussion
3.1. Pumpkin seed oil analyses
The fatty acids composition, total phenolic content, vitamin E concentration and antioxidant activity of PSO are summarized in (Table 1). The results revealed that the main fatty acids content in PSO are palmitic (17.53 ± 2.20), stearic (7.13 ± 0.40), oleic (14.80 ± 0.87) and linoleic (58.23 ± 2.31). Besides, some traces of linolenic acid (0.87 ± 0.30) and myristic (0.43 ± 0.15) were found. Furthermore, PSO phenolic and vitamin E content was (24 ± 1.5) and (70.69 ± 1.39) respectively. In addition, PSO at 200 μL exhibited an antioxidant activity of 82.13 ± 0.57.
Table 1.
Chemical composition of main Fatty acids, phenolic compounds and antioxidants activity of pumpkin seed oil.
| Parameters | Value |
|---|---|
| Fatty acids (%) | |
| Myristic | 0.43 ± 0.15 |
| Palmitic | 17.53 ± 2.20 |
| Stearic | 7.13 ± 0.40 |
| Oleic | 14.80 ± 0.87 |
| Linoleic | 58.23 ± 2.31 |
| Linolenic | 0.87 ± 0.30 |
| Total Phenolic Content (mg gallic acid /100 g oil) | 24 ± 1.5 |
| Vitamin E Concentration (μg/mL) | 70.69 ± 1.39 |
| Percentage of antioxidant activity (200 u L) | 82.13 ± 0.57 |
3.2. Relative organs weights
According to the data in (Table 2), oral administration of BPA to male mice at a dose of 50 mg/kg body weight caused a significant loss (p ≤ 0.05) in the relative weights of tested organs as compared to control. In contrast, oral administration of PSO through the three regimens of treatment caused a significant increase (P ≤ 0.05) in the relative weights of liver and testes compared with the BPA treated male mice. Furthermore, administration of PSO before BPA treatment was the best regimen in the alleviation of the adverse effect of BPA on the relative organs weights
Table 2.
Means and standard deviations of relative organs weights of treated male mice with bisphenol-A (BPA) and/or pumpkin seed oil (PSO) for 28 consecutive days.
| Treatments | Relative organs weights (g/100g) |
|
|---|---|---|
| Liver | Testes | |
| Control | 3.38 ± 0.05a | 0.82±0.03ab |
| Vehicle | 3.39 ± 0.05a | 0.80±0.02ab |
| PSO(1 mL/kg b.w.) | 3.37 ± 0.07a | 0.83±0.03a |
| BPA(50 mg/kg b.w.) | 3.19 ± 0.05b | 0.64±0.02e |
| PSO before BPA | 3.37 ± 0.05a | 0.78±0.02cb |
| PSO with BPA | 3.34 ± 0.06a | 0.73±0.03d |
| PSO after BPA | 3.35 ± 0.08a | 0.76±0.02c |
Data are expressed as means ± SD. Mean values in the same column within each parameter bearing the same superscript do not differ significantly (P ≤ 0.05).
These results are in agreement with several studies, which demonstrated that embryo BPA exposure during developmental stages; infancy decreased the brain, kidney, and testes weights in male mice and rats [33,34]. In addition, Hashemi [35] reported that pretreatment with PSO increased the weight of testes in sodium valproate's treated rats.
3.3. DNA damage evaluation
3.3.1. Comet assay in liver and testes cells
The comet assay results of liver and testes are summarized in (Table 3). BPA induced significant increase (P ≤ 0.05) in the mean values of tail length, percentage of tail DNA and olive tail moment in liver and testes cells as compared to control. In contrast, administration of male mice with PSO at the three regimens with BPA diminished significantly (P ≤ 0.05) the increase in the mean values of comet parameters induced by BPA in liver and testes cells.
Table 3.
Comet assay parameters in liver and testes cells of treated male mice with bisphenol-A (BPA) and/or pumpkin seed oil (PSO) for 28 consecutive days.
| Treatments | Tail length (μm) |
Tail DNA (%) |
Olive tail moment (μm) |
|||
|---|---|---|---|---|---|---|
| Liver | testes | Liver | testes | Liver | testes | |
| Control | 7.66 ± 0.80e | 6.73 ± 0.46e | 13.53 ± 0.51c | 14.26 ± 0.20e | 1.03 ± 0.08d | 0.98 ± 0.11e |
| Vehicle | 8.56 ± 0.41de | 7.26 ± 0.30cde | 13.15 ± 0.22c | 15.00 ± 0.50de | 1.12 ± 0.04cd | 1.09 ± 0.08de |
| PSO(1 mL/kg b.w.) | 8.38 ± 0.37e | 7.20 ± 0.33de | 11.82 ± 0.71d | 14.70 ± 0.52de | 0.99 ± 0.05d | 1.05 ± 0.03de |
| BPA(50 mg/kg b.w.) | 17.30 ± 1.08a | 9.83 ± 0.15a | 19.93 ± 0.68a | 23.56 ± 0.45a | 3.63 ± 0.52a | 2.37 ± 0.05a |
| PSO before BPA | 9.66 ± 0.23cd | 7.50 ± 0.30cd | 13.57 ± 0.65c | 15.36 ± 0.56cd | 1.31 ± 0.03cd | 1.15 ± 0.03d |
| PSO with BPA | 11.80 ± 0.50b | 8.70 ± 0.20b | 15.36 ± 1.02b | 17.43 ± 0.31b | 1.85 ± 0.08b | 1.51 ± 0.05b |
| PSO after BPA | 10.00 ± 0.78c | 7.83 ± 0.40c | 14.86 ± 0.15b | 15.80 ± 0.26c | 1.48 ± 0.10bc | 1.26 ± 0.01c |
Data are expressed as means ± SD. Mean values in the same column within each parameter bearing the same superscript do not differ significantly (P≤ 0.05).
3.3.2. Micronucleus test
Results in (Table 4) showed that BPA treatment increased (P ≤ 0.05) the mean values of MNPCEs significantly compared to control. In contrary, oral administration of PSO through the three regimens of treatment decreased significantly (P ≤ 0.05) the mean value of MNPCEs in bone marrow cells. In addition, cytotoxicity evaluation of bone marrow erythrocytes showed that BPA induced significant reduction (P ≤ 0.05) in the ratio of PCE/NCE as compared to control. Whereas, PSO through the three regimens of treatment has protection against BPA cytotoxicity. This protection was appeared in the elevation of the PCEs/NCEs ratio as compared with those of BPA treated group.
Table 4.
Frequencies of micronucleated polychromatic erythrocytes (MNPCEs) and polychromatics/normochromatics (PCEs/NCEs) ratio in bone marrow cells of bisphenol-A (BPA) and/or pumpkin seed oil (PSO) treated male mice for 28 consecutive days.
| Treatments | MNPCEs | PCEs | NCEs | PCEs/NCEs ratio |
|---|---|---|---|---|
| Control | 10.40 ± 2.96d | 1438.00 ± 32.95ab | 562.00 ± 32.95cd | 5.13 ± 0.43ab |
| Vehicle | 10.00 ± 4.00d | 1434.40 ± 57.36ab | 565.60 ± 57.36cd | 5.13 ± 0.75ab |
| PSO (1 mL/kg) | 6.80 ± 3.63d | 1478.80 ± 40.53a | 521.20 ± 40.53d | 5.70 ± 0.62a |
| BPA (50 mg/kg) | 66.40 ± 9.94a | 1149.60 ± 56.80d | 850.40 ± 56.80a | 2.71 ± 0.31d |
| PSO before BPA | 18.40 ± 4.33c | 1386.40 ± 37.77bc | 613.60 ± 37.77bc | 4.52 ± 0.40bc |
| PSO with BPA | 28.40 ± 7.26b | 1330.80 ± 60.82c | 669.20 ± 60.82b | 3.99 ± 0.58c |
| PSO after BPA | 20.80 ± 6.26bc | 1364.80 ± 59.64c | 635.20 ± 59.64b | 4.34 ± 0.61c |
Data are expressed as means ± SD. Mean values in the same column within each parameter bearing the same superscript do not differ significantly (P≤ 0.05).
Our results indicated that the BPA oral administration for 28 successive days showed genotoxic and cytotoxic activity in tested cells and PSO oral administration through the three different regimens of treatment with BPA significantly ameliorated the genotoxic and cytototoxic effects of BPA.
These results are in harmony with the findings of several studies which demonstrated that BPA induced DNA damage via comet assay and micronucleus test in blood lymphocytes, rat spermatocytes and Chinese hamster ovary (CHO) cells [12,13,36].
The mechanism of BPA genotoxicity might work throughout the incidence of oxidative stress and the depletion of antioxidant enzymes [9,37]. Beside to that, BPA is an endocrine disruptor structurally similar to estrogen, exerts a weak estrogen-like effect as well as an anti-androgenic effect [38]. It revealed that estrogen causes DNA damage by estrogen-derived oxidants [39]. In addition, ISO et al. [40] observed that BPA causes estrogen receptor dependent genotoxicity.
Concerning PSO treatments that alleviated the BPA induction of DNA damage in tested cells, our results are in agreement with those reported by Elfiky et al. [41]. They found that oral administration of PSO for ten consecutive days either before or after treatment of azathioprine was effective in the reduction of DNA fragmentation and MNPCEs frequencies, meanwhile, improved the PCEs/NCEs ratio. Therefore, it might suggest that the protecting effects of PSO against BPA genotoxicity could be due to their content of varied bioactive compounds, which were mentioned early.
Phenolics and polyphenols are fitting the degenerative diseases by inhibit the oxidative stress mechanisms [42]. The oil analysis in our study confirmed that PSO is rich in polyphenolics and vitamin E. Phenolic compounds have antioxidant, anti-mutagenic, anticarcinogenic and anti-inflammatory properties that might be beneficial in protecting the genome stability [43]. In the same manner, polyphenolics ameliorate cell injury and protect oxidant induced DNA from lesion by reducing the free radical mediated oxidative damage [44]. Meanwhile, vitamin E has an antioxidant role and can contribute to the cells protection against the free radicals deleterious property [45].
3.4. Histopathological examination
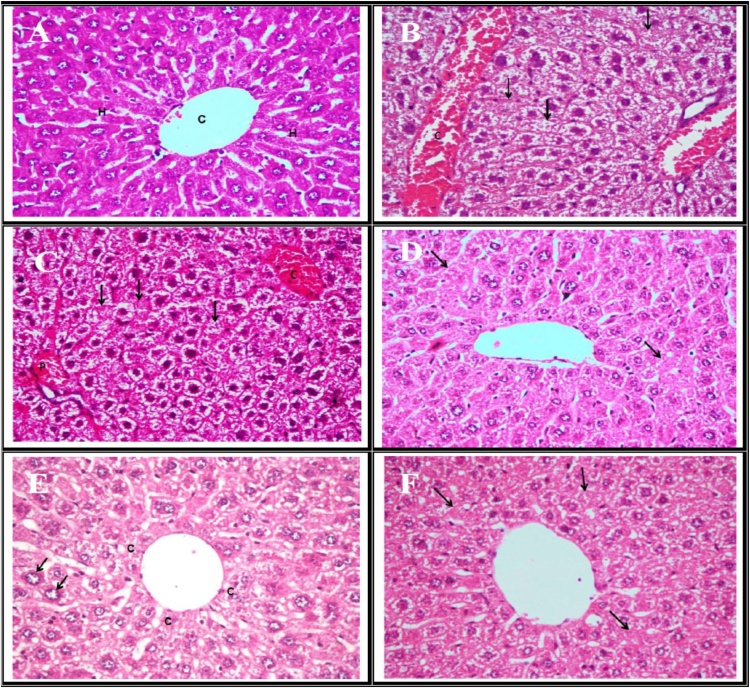
Regarding the examined liver sections of BPA's treated mice; it revealed marked congestion of the hepatic blood vessels as well as marked hepatocytes vacuolar degeneration with many necrotic cells which appeared either with pyknotic nuclei or without any nuclear structure (Fig. 2B and C). On the other hand, liver histopathological examination of PSO's treated mice for 28 successive days before BPA treatment showed good protection against BPA's hepatic cellular alteration. It revealed only mild vacuolar degeneration of the hepatocytes with some necrotic cells (Fig. 2D). In regards to liver of PSO and BPA co-treated mice, it showed centrilobular degeneration and necrosis of the hepatocytes with appearance of increased number of binucleated cells and karyomegaly of some cells (Fig. 2E). While, the hepatic tissue of PSO's treated mice for 28 successive days after BPA treatment showed moderated degree of granular degeneration and scattered necrotic hepatocytes (Fig. 2F).
Fig. 2.
(A) Liver of control mice showing normal appearance of the central vein (C) and hepatic parenchyml cells (H). (B) Liver of BPA treated mice showing congestion (C) of the hepatic blood vessels and marked vacuolar degeneration of the hepatocytes with many necrotic cells (arrow). (C) Liver of BPA treated mice showing congestion of the central vein (C) and portal blood vessels (P) with marked hepatocellular vacuolar degeneration and necrosis (arrow). (D) Liver of PSO treated mice before BPA treatment showing mild vacuolar degeneration of the hepatocytes with some necrotic cells (arrow). (E) Liver of PSO treated mice concurrently with BPA showing centrilobular degeneration (C) and necrosis of the hepatocytes, notice the karyomegaly of some cells (arrow). (F) Liver of PSO treated mice after BPA treatment showing granular degeneration and scattered necrotic hepatocytes (arrow). (H&E X400).
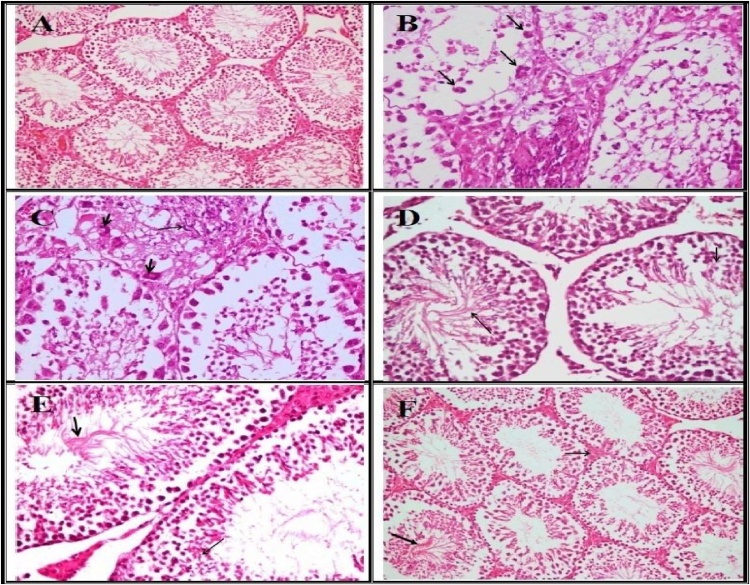
As for the examined testicular tissue of BPA treated mice, it showed an obvious defective spermatogenesis that characterized by severe necrosis and loss of the spermatogonial layers with multiple spermatid giant cells formation in most of the seminiferous tubules. Congestion of the interstitial blood vessel, destructed and abnormal spermatid formation with spermatid giant cells and loss of most of the spermatogonial cell layers were observed as well. Some seminiferous tubules showed destructed materials in the lumen with multiple spermatid giant cells formation were also observed (Fig. 3B and C). Testes of PSO's treated mice before BPA treatment showed marked protection of the spermatogonial cells layers with active spermatogenesis in the lumen of some seminiferous tubules (Fig. 3D). Whereas, testes of PSO's treated mice in concurrently with BPA showed necrosis of some spermatogonial cells with starting of active spermatogenesis in some other seminiferous tubules (Fig. 3E). In addition, testes of PSO's treated mice after BPA treatment showed degeneration and pyknosis of some spermatognial cells with active spermatogenesis in some other tubule and notice interstitial edema (Fig. 3F).
Fig. 3.
(A) Testes of control mice showing active spermatogenesis within most of the seminiferous tubules. (B) Testes of BPA treated mice showing severe necrosis and loss of the spermatogonial layers with multiple spermatide giant cells formation (arrow) in most of the seminiferous tubules. (C) Testes of BPA treated mice showing congestion of the interstitial blood vessel, destructed and abnormal spermatid formation (thin arrow), spermatid giant cells (thick arrow) and loss of most of the spermatogonial cell layers. (D) Testes of PSO treated mice before BPA treatment showing marked protection of the spermatogonial cells layers with active spermatogenesis (arrow) in the lumen of some seminiferous tubules. (E) Testes of PSO treated mice concurrently with BPA showing necrosis of some spermatogonial cells (thin arrow) with starting of active spermatogenesis (arrow) in some other seminiferous tubules. (F) Testes of PSO treated mice after BPA treatment showing degeneration and pyknosis of some spermatognial cells with active spermatogenesis in some other tubule (arrow), notice interstitial edema (thin arrow). (H&E X400).
The evaluation of histopathological alterations in liver and testes of treated male mice are comparable to the genetic findings. These findings are in agreement with Korkmaz et al. [17] who noticed hepatic necrosis and congestion in the liver of BPA (25 mg/kg/day) treated male rats three times a week for 50 day. Moreover, Kalb et al. [11] showed that male mice exposure to BPA (3000 μg/kg b.w.) through breast milk in the breastfeeding period caused testicular degeneration and aplasia in a number of seminiferous tubules of their testes.
In addition to that, Sangai and Verma [46] found that BPA-caused changes in the activities of ATPase in liver of mice thereby causing a reduction in ATP produced in the cell which lead to necrosis. It has been reported that BPA produce ROS that caused brain, reproductive tract and kidney oxidative damage [47,48]. Furthermore, the great histopathological changes in testes tissues caused by BPA estrogenic activity [17].
On the other side, the protective role of PSO was confirmed by Ramah et al. [49] who found that treatment with PSO markedly alleviated lead acetate induced pathological changes in testes tissues of rats. Phenolic compounds, vitamins and zinc in pumpkin are attributed factors for its antioxidant activity by neutralizing free radical generation [50,51]. In addition, the presence of oleic acid in pumpkin diminishes the ability of the testes and epididymis to lipid peroxidation [52].
4. Conclusion
It can be concluded that BPA has probable genotoxicity and histopathological alterations in male mice. Accordingly, strict limitations on the use of this compound must be put especially in food contact materials. On the other hand, PSO can be useful as therapeutic agent in the alleviation of the adverse effects of BPA in human.
References
- 1.Michalowicz J. Bisphenol A-sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014;37:738–758. doi: 10.1016/j.etap.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Burridge E. Bisphenol A: product profile. Eur. Chem. News. 2003;20:17. [Google Scholar]
- 3.Fleisch A.F., Sheffield P.E., Chinn C., Edelstein B.L., Landrigan P.J. Bisphenol A and related compounds in dental materials. Pediatrics. 2010;126:760–768. doi: 10.1542/peds.2009-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calafat A.M., Ye X., Wong L.Y., Reidy J.A., Needham L.L. Exposure of the U.S. Population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu P., Kawamura K. Ubiquity of Bisphenol A in the atmosphere. Environ. Pollut. 2010;158:3138–3143. doi: 10.1016/j.envpol.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Berger A., Ziv-Gal A., Cudiamat J., Wang W., Zhou C., Flaws J.A. The effects of in utero bisphenol A exposure on the ovaries in multiplegenerations of mice. Reprod. Toxicol. 2016;60:39–52. doi: 10.1016/j.reprotox.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty L., Bromer J., Zhou Y., Aldad T., Taylor H. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: anepigenetic mechanism linking endocrine disruptors to breast cancer. Horm. Cancer. 2010;1(3):148–155. doi: 10.1007/s12672-010-0015-9. Horm. Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erler C., Novak J. Bisphenol A exposure: human risk and health policy. J. Pediatr. Nurs. 2010;25:400–407. doi: 10.1016/j.pedn.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Meeker J., Calafat A., Hauser R. Urinary bisphenol A concentration in relation to serum thyroid and reproductive hormones in men from an infertility clinic. Environ. Sci. Technol. 2010;44:1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeinab K., Hassan Z., Elobeid M., Virk P., Omer S., ElAmin M., Daghestani M., Ebtisam M., Al Olayan E. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid. Med. Cell. Longev. 2012 doi: 10.1155/2012/194829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalb A.C., Kalb A.L., Cardoso T.F., Fernandes C.G., Corcini C.D., Junior A.S.V., Martinez P.E. Maternal transfer of bisphenol A during nursing causes sperm impairment in male offspring. Arch. Environ. Contam. Toxicol. 2015;70(4):793–801. doi: 10.1007/s00244-015-0199-7. [DOI] [PubMed] [Google Scholar]
- 12.Xin L., Lin Y., Wang A., Zhu W., Liang Y., Su X., Hong C., Wan J., Wang Y., Tian H. Cytogenetic evaluation for the genotoxicity of bisphenol-A in Chinese hamster ovary cells. Environ. Toxicol. Pharmacol. 2015;40:524–529. doi: 10.1016/j.etap.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari D., Kamble J., Chilgunde S., Patil P., Maru G., Kawle D., Bhartiya U., Joseph L., Vanage G. Clastogenic and mutagenic effects of bisphenol A: an endocrine disruptor. Mutat. Res. 2012;743:83–90. doi: 10.1016/j.mrgentox.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Moustafa G.G., Ahmed A.M.A. Impact of prenatal and postnatal exposure to bisphenol A on female rats in a two generational study: genotoxic and immune-histochemical implications. Toxicol. Rep. 2016;3:685–695. doi: 10.1016/j.toxrep.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad S., Khan M.B., Hoda M.N., Bhatia K., Haque R., Fazili I.S., Jamal A., Khan J.S., Katare D.P. Neuroprotective effect of sesame seed oil in 6-hydroxydopamine induced neurotoxicity in mice model: cellular, biochemical and neurochemical evidence. Neurochem. Res. 2012;37(3):516–526. doi: 10.1007/s11064-011-0638-4. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa H., Koyama S., Matsuda M., Nakahashi K., Akazome Y., Mori T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004;315:119–124. doi: 10.1007/s00441-003-0806-1. [DOI] [PubMed] [Google Scholar]
- 17.Korkmaz A., Ahab M.A., Kolankaya D., Barlas N. Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem. Toxicol. 2010;48(10):2865–2871. doi: 10.1016/j.fct.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 18.El Ghazzawy I.F., Meleis A.E., Farghaly E.F., Solaiman A. Histological study of the possible protective effect of pomegranate juice on bisphenol-A induced changes of the caput epididymal epithelium and sperms of adult albino rats. Alexandria J. Med. 2011;47:125–137. [Google Scholar]
- 19.Morgan M.A., El-Balla S.S., El-Bialy E.B., EL-Bora B.N. Studies on the potential protective effect of cinnamon against bisphenol A- and octylphenol-induced oxidative stress in male albino rats. Toxicol. Rep. 2014;1:92–102. doi: 10.1016/j.toxrep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behmanesh M.A., Najafzadehvarzi H., Poormoosavi S.M. Protective effect of Aloe Vera extract against Bisphenol A-induced testicular toxicity in Wistar rats. Cell J. 2018;20(2):278–283. doi: 10.22074/cellj.2018.5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caili F., Huan S., Quanhong L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006;61:73–80. doi: 10.1007/s11130-006-0016-6. [DOI] [PubMed] [Google Scholar]
- 22.Procida G., Snatcher B., Cateni F., Zaccchigna M. Chemical composition and functional characterization of commercial pumpkin seed oil. J. Sci. Food Agric. 2012;93(5):1035–1041. doi: 10.1002/jsfa.5843. [DOI] [PubMed] [Google Scholar]
- 23.Andjelkovic M., Camp J.V., Trawka A., Verhe R. Phenolic compounds and some quality parameters of pumpkin seed oil. Eur. J. Lipid Sci. Technol. 2010;112:208–217. [Google Scholar]
- 24.Van Hoed V., Felkner B., Bavec F., Grobelnik S., Bavec M., Verhe R. Influences of processing on antioxidants content of pumpkin seed oil. 7th euro. Fed Lipid Congress Lipids Fats and Oils” from Knowledge to Application. 2009 [Google Scholar]
- 25.AOAC . 17th ed. 2000. Association of Official Analytical Chemists. Official Methods of Analysis. Gaithersburg, M.D. U.S.A. [Google Scholar]
- 26.Lin J.Y., Tang C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–147. [Google Scholar]
- 27.Gimeno E., Castellote A.I., Lamuela-Raventos R.M., Torre M.C., Lopez-Sabater M.C. Rapid determination of vitamin E in vegetable oils by reversed phase high-performance liquid chromatography. J. Chromatogr. A. 2000;881:251–254. doi: 10.1016/s0021-9673(00)00219-3. [DOI] [PubMed] [Google Scholar]
- 28.Singh R.P., Chidambara Murthy K.N., Jayaprakasha G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002;50(1):81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- 29.Bandyopadhyaya G., Sinha S., Chattopadhyay B.D., Chakraborty A. Protective role of curcumin against nicotine-induced genotoxicity on rat liver under restricted dietary protein. Eur. J. Pharmacol. 2008;588:151–157. doi: 10.1016/j.ejphar.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Chauhan L.K., Pant N., Gupta S.K., Srivastava S.P. Induction of chromosome aberrations, micronucleus formation and sperm abnormalities in mouse following carbofuran exposure. Mutat. Res. 2000;465:123–129. doi: 10.1016/s1383-5718(99)00219-3. [DOI] [PubMed] [Google Scholar]
- 31.OECD . OECD Publishing; Paris: 1997. Test No. 474: Mammalian Erythrocyte Micronucleus Test. [Google Scholar]
- 32.Delafield F. Oxford University Press; London: 1984. Haematoxylin and Eosin for General Staining. Staining of the Animal Tissues Practical and Theoretical. [Google Scholar]
- 33.Kabuto H., Amakawa M., Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74:2931–2940. doi: 10.1016/j.lfs.2003.07.060. [DOI] [PubMed] [Google Scholar]
- 34.Gamez J.M., Penalba R., Cardoso N., Ponzo O., Carbone S., Pandolfi M., Scacchi P., Reynoso R. Low dose of bisphenol A impairs the reproductive axis of prepuberal male rats. J. Physiol. Biochem. 2014;70:239–246. doi: 10.1007/s13105-013-0298-8. [DOI] [PubMed] [Google Scholar]
- 35.Hashemi J.M. Pumpkin seed oil and vitamin E improve reproductive function of male rats inflicted by testicular injury. World Appl. Sci. J. 2013;23(10):1351–1359. [Google Scholar]
- 36.Wu H.J., Liu C., Duan W.X., Xu S.C., He M.D., Chen C.H., Wang Y., Zhou Z., Yu Z.P., Zhang L., Chen Y. Melatonin ameliorates bisphenol A-induced DNA damage in the germ cells of adult male rats. Mutat. Res. 2013;752:57–67. doi: 10.1016/j.mrgentox.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Jiang F., Zhang Y., Dusting G.J. NADPH oxidase-mediated redox signaling: rolesin cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 38.Li Y., Duan F., Yang F., Zhou X., Pan H., Li Y., Li R. Pubertal exposure to bisphenol A affects the reproduction of male mice and sex ratio of offspring. J. Reprod. Contracept. 2015;26(1):14–21. [Google Scholar]
- 39.Mobley J.A., Brueggemeier R.W. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis. 2004;25:3–9. doi: 10.1093/carcin/bgg175. Carcinog. [DOI] [PubMed] [Google Scholar]
- 40.Iso T., Watanabe T., Iwamoto T., Shimamoto A., Furuichi Y. DNA damage caused by bisphenol A and estradiol through estrogenic activity. Biol. Pharm. Bull. 2006;29(2):206–209. doi: 10.1248/bpb.29.206. [DOI] [PubMed] [Google Scholar]
- 41.Elfiky S.A., Elelaimy I.A., Hassan A.M., Ibrahim H.M., Elsayad R.I. Protective effect of pumpkin seed oil against genotoxicity induced by azathioprine. J. Basic Appl. Zool. 2012;65:289–298. [Google Scholar]
- 42.Shahriar M., Hossain M.I., Bahar A.N.M., Akhter S., Haque M.A., Bhuiyan M.A. Preliminary phytochemical screening, in-vitro antioxidant and cytotoxic activity of five different extracts of moringa oleifera leaf. J. Appl. Pharm. Sci. 2012;2(5):65–68. [Google Scholar]
- 43.Xie C., Wang C., Wang X., Yang X. Two modified RNA extraction methods compatible with transcript profiling and gene expression analysis for cotton roots. Prep. Biochem. Biotechnol. 2013;43(5):500–511. doi: 10.1080/10826068.2012.759967. [DOI] [PubMed] [Google Scholar]
- 44.Urquiaga I., Leighton F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000;33:55–64. doi: 10.4067/s0716-97602000000200004. [DOI] [PubMed] [Google Scholar]
- 45.Santana A.T., Guelfi M., Medeiros H.C.D., Tavares M.A., Bizerra P.F.V., Mingatto F.E. Mechanisms involved in reproductive damage caused by gossypol in rats and protective effects of vitamin E. Biol. Res. 2015;48(1):43–51. doi: 10.1186/s40659-015-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sangai N.P., Verma R.J. Effect of quercetin on bisphenol A-caused alterations in succinate dehydrogenase and adenosine triphosphatase activities in liver and kidney of mice. Acta Pol. Pharm. 2012;69(6):1189–1194. [PubMed] [Google Scholar]
- 47.Aydogan M., Korkmaz A., Barlas N., Kolankaya D. The effect of vitamin C on bisphenol A, nonylphenol and octylphenol induced brain damages of male rats. Toxicology. 2008;249:35–39. doi: 10.1016/j.tox.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Korkmaz A., Aydoğan M., Kolankaya D., Barlas N. Vitamin C coadministration augments bisphenol A, nonylphenol, and octylphenol induced oxidative damage on kidney of rats. Environ. Toxicol. 2009;26(4):325–337. doi: 10.1002/tox.20556. [DOI] [PubMed] [Google Scholar]
- 49.Ramah A., EL-shwarby R.M., Nabila M.A., El-shewey E.A. The effect of lead toxicity on male albino rats reproduction with ameliorate by vitamin E and pumpkin seeds oil. Benha Vet. Med. J. 2015;28(1):43–52. [Google Scholar]
- 50.Amara S., Abdelmelek H., Garrel C., Guiraud P., Douki T., Ravanat J.L. Preventive effect of zinc against cadmiuminduced oxidative stress in the rat testis. J. Reprod. Dev. 2008;54(2):129–134. doi: 10.1262/jrd.18110. Reprod Develop. [DOI] [PubMed] [Google Scholar]
- 51.Morakinyo O.A., Achema P.U., Adegoke O.A. Effect of zingiber officinale (Ginger) on sodium arsenite induced reproductive toxicity in male rats. J. Biomed. Res. 2010;13(1):39–45. [Google Scholar]
- 52.Bourre J.M., Dumont O., Durand G. Dose-effect of dietary oleic acid: oleic acid is conditionally essential for some organs. Reprod. Nutr. Dev. 2004;44(4):371–380. doi: 10.1051/rnd:2004042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.