Graphical abstract
Keywords: Bacillus spp., Infant foods, Enterotoxigenic genes, Molecular detection
Abstract
A 205 samples representing eight different infant foods with various based materials were collected and analyzed for their microbiological properties. The contamination rate by aerobic spore formers was achieved 100% in milk based infant food with fruit, vegetables, honey, rice and infant milk powder. While, it was detected in 95, 60 and 65% of the infant food with wheat milk based, ready to use (infant food with fruit) and ready to use (infant food with vegetables), respectively. Biochemical Identification and API 50 CHB used to identify the obtained isolates and revealed that B. subtilis was the most frequently occurring Bacillus spp. Followed by B. licheniformis and B. circulans. While B. cereus was detected in 10.20% of the total isolates. Moreover, B. cereus was confirmed in 21.2% of milk based fruit, vegetables (15.7%), honey (17.2%), rice (14.1%) and wheat (12%) and vanished in the infant milk powder samples. Although, B. cereus noted in lower percentage but this strain is considered as the more harmful one in lower numbers. For that, the following part is focused on B. cereus. Forty five isolates obtained from B. Cereus contaminating samples were screened for prevalence of 3 important virulent enterotoxigenic genes using PCR technique. The CYTK gene had the highest presence which detected in 43 isolates (95.5%), followed by NHEC gene detected in 32 isolates. However, the HBLA gene was detected in just 5 isolates. So, many processes should be applied for controlling of pathogens to preserve infant lives.
1. Introduction
Feeding infant with powdered formula and ready to eat infant food issued from birth through first 2 years to feed millions of infants over the world. This kind of nutrition represents a rich source of nutrients and contains ingredients from various origins. So, it is considered an excellent medium to support bacterial growth and carrying potential risk of exposure to foodborne pathogens. Infants and babies are more susceptible to infection by such pathogens because of their less well-developed immune system and lack of competing intestinal flora [1]. Low moisture content of dried infant foods and even ready to eat baby’s acts as inhibitory factor with respect to any bacterial spores or vegetative that have survived drying or processing. These microorganisms cannot grow and play any direct role in their spoilage.Their occurrence in the products is a great significance served as an index of hygienic standards maintained during production, processing and handling [2]. But, reconstituted infant foods are considered to be a food class of high risk due to the infants susceptibility to enteric bacterial pathogens in order to mortality [3]. Epidemiological evidence suggests that the majority of outbreaks worldwide due to B. cereus have been associated with concentrations (105 cfu/g in implicated foods). Infants and babies are more susceptible to infection by such pathogens because of their less well-developed immune system and lack of competing intestinal flora [1].
The microflora of dried milk powders depends on the number and type of bacteria in the raw milk or milk by-product, preheating temperature, operation conditions, evaporator and/or dryer and plant hygiene [4]. B. cereus contamination of infant milk formulas was well documented in previous studies [5,6]. B. cereus was among the primary microbes associated with baby food contamination as reported by FAO/WHO Expert Consultations [7]. In spite of that B. cereus was classified as category C or of low risk, its prevalence in infant food was sufficiently high to cause food borne infection outbreaks [8,9]. B. cereus spores are resistant to many processes as low and high temperatures, desiccation, disinfectant agents, ionization, radiation and ultraviolet light [10,11]. B. cereus has been reported to produce 5 enterotoxins and 1 emetic toxin. From them, heamolysine BL (HBL) and non heamolytic enterotoxin (NHE) consists of 3 different exo-proteins while the other toxins, ENTFM, CYTK and BCE consist a single protein [12].
Therefore, this work aimed to investigate the incidence of Bacillus spp. and its enterotoxigenic virulence factors in infant formula and ready to use baby food collected from Cairo and Giza markets and pharmacies under Egyptian condition of production, storage and distribution.
2. Material and methods
2.1. Samples collection
A total of 205 samples were obtained from commercially available markets in Egypt. They were infant foods, infant milk powder and ready to use (infant food) such as infant food with honey and milk based, infant food with rice and milk based, infant food with wheat and milk based, ready to use infant food with fruit, ready to use infant food with vegetables (20 samples of each), infant food with fruit and milk based (45 samples), infant food with vegetables and milk based (15 samples) and infant milk powder (45 samples). These samples were randomly purchased from different pharmacies and supermarkets at Cairo and Giza. Sterile techniques were applied during samples collection, packaging and microbiological analysis. All samples were delivered directly to the laboratory according to American Public Health Association [13] protocols. Each sample was subjected for many analyses as total bacteria, total aerobic spore formers, total psychrotrophic spore formers and total Bacillus cereus group counts.
2.2. Preparation of sample
All samples were homogenated as standard methods reported by ICMSF [14]. In brief, 225 ml of sterile peptone water buffer were added to 25 g of the sample and thoroughly mixed using sterile homogenizer for 1–1.5 min, and then the tenfold serial dilution were prepared.
2.3. Total bacterial counts
The total bacterial count was carried out using plate count agar medium (Oxoid) using pour plate method with incubation at 37 °C for 48 h [15].
2.4. Total spore former counts
Spore formers were determined after heating the sample (10−1) in a water bath at 80–85 °C for 10 min and cooled rapidly [16], then plated on plate count agar medium using pour plate method and incubated at 37 °C for 48 h for total aerobic spore formers or 7 °C for 10 days for psychrotrophic spore formers. The numbers of colonies per countable plates was noted and expressed as CFU/g [17].
2.5. Determination of B. cereus group count
From each previously prepared dilution after pasteurization, 0.1 ml was spread onto the surface of Polymyxin Pyruvate Egg yolk Mannitol Bromothymol Blue Agar medium (PEMBA) by bent glass rod. The plates were incubated at 37 °C for 24 h as mentioned by Harrigan [18].
2.6. Isolation and identification of Bacillus spp
Colonies (3–5) were randomly picked from each plate of total aerobic spore forming count. Each colony of Bacillus spp. was isolated, purified, characterized and identified. A single representative colony was removed and inoculated into 10 ml tryptone soya broth. Each broth culture was incubated at 37 °C for 24 h and used to perform the various biochemical tests and identified according to Varadaraj [81] and API 50 CHB (Biomerieux, France).
2.7. Detection of haemolysin activity
Seventy eight obtained B. cereus isolates were tested for haemolysin activity on sheep blood agar plates (5%) by plating a loopful of 24 h old culture of the isolates. The plates were then incubated at 30 °C for 24 h and checked for hemolysis surrounding the growth [19].
2.8. Detection of virulence genes using PCR technique
Genomic DNA for 45 B. cereus isolates was extracted by lysozyme (20 mg/ml) and proteinase K (1 mg/ml) buffer. Total genomic DNA was purified using isopropanol buffer as described by Barakat et al. [20]. Amplification reaction (PCR) of the enterotoxin genes was carried out using extracted DNA and the primerssets as presented in Table 1. The PCR amplification included initial denaturation at 95 °C for 5 min, followed by 30 cycles of 95 °C for 1 min, 58 °C for 1 min, 72 °C for 1 min, and a final elongation step at 72 °C for 10 min, with a final hold at 4 °C [21]. PCR products were analyzed in 1.5% (wv−1) TAE agarose gel and all PCR experiments were performed twice for each strain [22].
Table 1.
The primer sequences and amplicon sizes used in PCR analysis.
| Gene | Primer sequences (50–30) F: forward; R: reverse |
Amplicon size (bp) | Reference |
|---|---|---|---|
| hblA | F-5׳ AAGCAATGGAATACAATGGG 3׳ R-5׳ AGAATCTAAATCATGCCACTGC 3׳ |
1154 | Minnaard et al. [23] |
| nheC | F-5׳ CGGTAGTGATTGCTGGG 3׳ R-5׳ CAGCATTCGTACTTGCCAA 3׳ |
581 | [24]) |
| cytK | F-5׳ CGACGTCACAAGTTGTAACA 3׳ R-5׳ CGTGTGTAAATACCCCAGTT 3׳ |
565 | [25]) |
3. Results and discussion
3.1. Incidence and distribution of total bacterial counts
One of the most important parameter of baby food products is their microbiological quality, to evaluate the safety of different infant formula products. Many detection experiments as total bacterial numbers, total spore formers, total pathogens and total bacillus spp. were used to evaluate the infant food microbiological quality based on standard international regulations. These parameters were as total bacterial numbers, total spore formers, total pathogens, total bacillus spp.…..etc. The results in Table 2 revealed that bacterial counts were detected in almost all the variety of tested infant formula and ready to use infant foods, with mean total viable counts varied over the range of 1.896–3.453 log10 cfu/g (1.896 log10 cfu/g for ready to use infant food with fruit and 3.453 log10 cfu/g for infant food with fruit milk based), these results are agreed with those obtained by Shadlia-Matug et al. [26] who reported that, total bacterial counts of infant food with banana ranged from <1.0 to 3.54 log10 cfu/g.
Table 2.
Total bacteria, total aerobic spore forming, total psychrotrophic spore forming, and total Bacillus cereus group counts in tested samples.
| Type of tested samples | Number of tested samples |
Bacterial groups* | Positive samples | Counts (log10cfu/g) |
||
|---|---|---|---|---|---|---|
| No % | Min. | Max. | Mean. | |||
| infant food with fruit milk based | 45 | 1 | 45(100) | 2.593 | 4.161 | 3.453 |
| 2 | 45(100) | 2.159 | 3.807 | 3.167 | ||
| 3 | 27(60) | 0 | 2.608 | 0.987 | ||
| 4 | 28(62.2) | 0 | 2.820 | 1.497 | ||
| infant food with vegetables milk based | 15 | 1 | 15(100) | 2.441 | 4.275 | 3.365 |
| 2 | 15(100) | 1.441 | 3.945 | 2.840 | ||
| 3 | 6(40) | 0 | 1.852 | 0.583 | ||
| 4 | 4(26.6) | 0 | 2.800 | 0.652 | ||
| infant food with honey milk based | 20 | 1 | 20(100) | 1.759 | 3.849 | 3.070 |
| 2 | 20(100) | 1.492 | 3.621 | 2.840 | ||
| 3 | 1(5) | 0 | 2.075 | 0.104 | ||
| 4 | 6(30) | 0 | 2.651 | 0.679 | ||
| infant food with rice milk based | 20 | 1 | 20(100) | 1.259 | 3.761 | 2.923 |
| 2 | 20(100) | 1.159 | 3.495 | 2.619 | ||
| 3 | 2(10) | 0 | 1.100 | 0.101 | ||
| 4 | 3(15) | 0 | 2.593 | 0.358 | ||
| infant food with wheat milk based | 20 | 1 | 20(100) | 1.833 | 3.819 | 2.856 |
| 2 | 19(95) | 0 | 3.394 | 2.410 | ||
| 3 | 2(10) | 0 | 1.100 | 0.082 | ||
| 4 | 4(20) | 0 | 2.360 | 0.451 | ||
| infant milk powder | 45 | 1 | 45(100) | 1.651 | 3.416 | 2.417 |
| 2 | 45(100) | 1.159 | 3.030 | 2.129 | ||
| 3 | -ve | -ve | -ve | -ve | ||
| 4 | -ve | -ve | -ve | -ve | ||
| ready to use (infant food with fruit) | 20 | 1 | 18(90) | 0 | 2.388 | 1.896 |
| 2 | 12(60) | 0 | 2.160 | 1.208 | ||
| 3 | -ve | -ve | -ve | -ve | ||
| 4 | -ve | -ve | -ve | -ve | ||
| ready to use (infant food with vegetables) | 20 | 1 | 20(100) | 1.360 | 2.463 | 1.982 |
| 2 | 13(65) | 0 | 2.229 | 1.199 | ||
| 3 | -ve | -ve | -ve | -ve | ||
| 4 | -ve | -ve | -ve | -ve | ||
Where, 1; Total bacterial counts, 2; Total aerobic spore forming counts, 3; Total psychrotrophic spore forming counts, 4; Total Bacillus cereus group counts.
Most of the examined samples were within the acceptable limit and met the standard limit (104) as recommended by FDA [27,28].The high aerobic count indicated neglected sanitary measures during manufacturing process, handling, packing and using the low quality milk or ingredients in the production [4]. Yacoub et al. [29] mentioned that, the total bacterial counts ranged between log 2.4 and 3.2 cfu/g of skim milk powder. According to the limits proposed by APHA, ES and USDA for dried milks, APC must not exceed 5 × 104, so, all the examined samples were within the acceptable limits.
3.2. Incidence and distribution of total aerobic spore forming bacteria
Spore forming bacteria are present in many food processing environments and may pose a threat to food safety and quality [30]. The mean total spore counts in the tested samples were reached to 2.410 log10 cfu/g (infant food with wheat milk based) and 3.167 log10 cfu/g (infant food with fruit milk based), from 1.199 log10 cfu/g to 1.208 log10 cfu/g for ready to use (infant food with vegetables) and ready to use (infant food with fruit), respectively (Table 2). As well as, it was detected in 19 from 20 (95%) samples of infant food with wheat and milk based, 13 from 20 (65%) samples of ready to use (infant food with vegetables) and 12 from 20 (60%) with ready to use (infant food with fruit), but it was detected in 100% of other tested samples. Other investigators recorded variable data concerning the incidence of aerobic spore former; Shadlia-Matug et al. [26] reported that 42.9% from infant foods with banana and 67% from infant foods with vegetables were positive for total aerobic spore counts. While, El-Gendi and Wahba [31] noted that, total spore groups were counted by 33.3, 60 and 83.3% in examined samples of milk-cereal based weaning food samples, infant milk powder and milk powder, respectively. In (2016), Sadiq et al. [32] reported that total mesophilic spore counts with average 4.82 × 103 were recorded in infant formula of milk powder. Recently, Yacoub et al. [29] reported that, the total aerobic spore-forming bacteria counts were ranged between <10 and log 2.85 cfu/g in the tested samples of skim milk powder.
3.3. Incidence and distribution of total psychrotrophic spore forming bacteria
The domination of psychrotrophic bacteria in the total microbial population is even more pronounced when milk is produced in poor hygiene conditions and/or contains increasing numbers of somatic cells. In the current study, this parameter was examined as a tool for infant food quality. Total psychrotrophic spore formers were detected in 60 and 40% of the total samples of milk based infant food with fruit and vegetables, respectively (Table 2). The counts were reached to 2.608 log10 cfu/g with average 0.987 log10 cfu/g in infant food with fruit milk based, followed by 1.852 log10 cfu/g with average 0.583 log10 cfu/g in infant food with vegetable milk based. Also, total psychrotrophic spore formers were observed in low percentage (5%) with average count 0.104 log10 cfu/g of infant food with honey milk based. While total psychrotrophic spore former counts was detected in 10% of milk based infant food with rice and wheat samples with average counts 0.101 and 0.082 log10 cfu/g, respectively as shown in Table 2. None of the surveyed infant milk powder, ready to use infant food with fruit and vegetables samples was contained detectable levels of total psychrotrophic spore former counts. In this target, Ahmed et al. [33] mentioned that, the mean total psychrotrophic spore former count was 2.9 × 102 cfu/g in infant’s milk formula (IMF) for babies. Also, Sadek et al. [34] reported that 46.6% from skim milk powder samples were positive for total psychrotrophic spore forming bacteria with counts ranged from 2 × 10 to 8 × 102 cfu/g and average 8.1 × 10 cfu/g. On the other hand, Yacoub et al. [29] noted that, Bacillus spp. were isolated from skim milk powder did not exhibit psychrotrophic growth at 7 °C. For that, the next part of the current study focused on detection of Bacillus spp. counts especially Bacillus cereus.
3.4. Identification and distribution of total aerobic spore forming counts
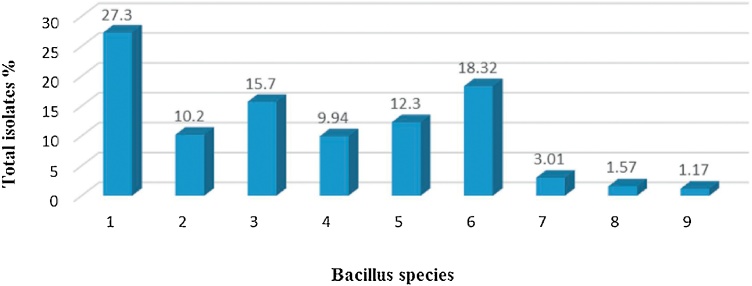
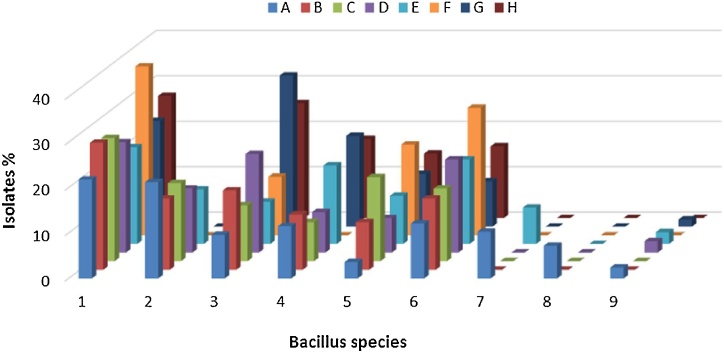
Isolates of aerobic spore formers were studied for morphological and biochemical properties. These tests were recommended for identification of Bacillus spp. according to Varadaraj [81] and API CHB50 biochemical tests. Data in Fig. 1, Fig. 2illustrated the frequency distribution of different Bacillus species isolated from the examined samples. The identification with classical methods based on morphological and biochemical criteria as well as API techniques showed that B. subtilis was the most frequently occurring Bacillus spp. with an over incidence of 27.3% of isolates followed by B. pumilus (18.32%), B. licheniformis (15.70%), B. circulans (12.30%) and B. cereus (10.20%). While, high frequency of B. cereus were observed in infant food with fruit milk based (21.2%). But, infant milk powder and ready to use infant food with fruit and vegetables were free of B. cereus. Moreover, isolates identified as B. firmus were recovered from infant food with fruit milk based and infant food with wheat milk based. Also, B. smithii was recovered only from infant food with fruit milk based. Of those most frequently isolates, B. cereus and B. subtilis are important for food hygiene because of their hydrolytic activities of food components and the ability of some strains to produce toxin or grew at refrigerator temperature [77]. Of the aerobic spore formers isolated from dried milk and cereal infant formulas, B. cereus, B. licheniformis, B. pumilus and B. coagulans have been occasionally implicated in either foodborne related illness or opportunist infections [78]. These results are nearly agreed with those obtained by Mostafa et al. [79] who reported that the most common Bacillus spp. detected in the commercial infant formulas was B. subtilis, with an over incidence (28%) followed by B. lichenifomis (20%) and B. cereus (14%). Moreover, [76] found that B. subtilis was the most commonly identified isolates from infant formula milks samples. Also, B. cereus, B. circulans and B. pumilus were isolated with percentage of 16.7, 13.3 and 20% in infant milk formulae, respectively [31]. B. smithii was isolated from diverse food products obtained from local dairies such as cocoa powder, milk powder and dessert products [80].
Fig. 1.
Distribution of different total Bacillus species from total isolates.
Where, A; infant food with fruit milk based, B; infant food with vegetables milk based, C; infant food with honey milk based, D; infant food with rice milk based, E; infant food with wheat milk based, F; infant milk powder, G; ready to use (infant food with fruit), H; ready to use (infant food with vegetables), 1; B. subtilis, 2; B. cereus, 3; B. licheniformis, 4; B. coagulans, 5; B. circulans, 6; B. pumilus, 7; B. firmus, 8; B. smithii, 9; None Bacillus.
Where, 1; B. subtilis, 2; B. cereus, 3; B. licheniformis, 4; B. coagulans, 5; B. circulans, 6; B.pumilus, 7; B. firmus, 8; B. smithii, 9; None Bacillus.
Fig. 2.
Distribution of different total Bacillus species in tested samples.
3.5. Incidence and distribution of total Bacillus cereus group counts
Bacillus cereus spores widely distribute in the nature. So, it is very important to investigate the presence of B. cereus in infant formula and possible pathogenicity of this microorganism in infant foods. According to FDA [35], the standard regulators stipulated that B. cereus must be less than and/or equal 100 cfu/g in infant formula. It is evident from the results in Table 2; the total B. cereus group counted on PEMBA medium was detected in the tested samples of milk based infant food with fruit and vegetable by 62.2 and 26.6%, respectively. It was reached to 2.820 log10 cfu/g with average 1.497 log10 cfu/g and 2.800 log10 cfu/g with average 0.652 log10 cfu/g, respectively. However, total B. cereus count was observed in low percentage; 15, 20 and 30% with average count 0.358, 0.451 and 0.679 log10 cfu/g in infant food with rice milk based, infant food with wheat milk based and infant food with honey milk based, respectively. On the contrary, B. cereus count was detected in the tested samples of infant milk powder, ready to use infant food with fruit and vegetables (Table 2 and Fig. 2). Generally, 45 out of 205 samples (21.9%) of the tested samples of infant foods were positive for total B. cereus counts. Other results were obtained by Dalea and Alexandra [36] who reported that B. cereus was detected in 6 out of 30 samples of powder milk samples for new bornes. Moreover, Kim et al. [37] showed that B. cereus was detected in infant and baby foods, including 23 of 100 (23.0%) cereal-based infant foods, 4 of 10 (40.0%) biscuits and 2 of 27 (7.4%) liquid infant foods. In another investigation, Asmaa et al. [38] commented that 10% of samples infant formula was contaminated with B. cereus with counts ranged from 4.0 × 101 to 2.1 × 102 and average 1.45 × 102. The high differences in percentage and range of B. cereus may be due to the different formulas, different types of samples and different tests. New nontoxic and high stable bioactive materials were developed for controlling of the pathogens [[39], [40], [41]]. In addition to food grade natural materials may be will the best chance to control of the pathogens [42].
3.6. Molecular investigation of Bacillus cereus and its toxins
3.6.1. Prevalence of hemolytic BL complex HBLA gene in B. cereus isolates
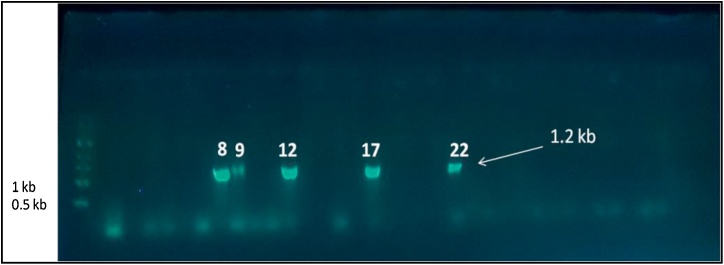
The prevalence of toxigenic strains of B. cereus has been extensively reported in different starchy foods such as vegetables, puddings, sauces, milk, dairy products, cereals, infant cereal formulas fried and cooked rice [[43], [44], [45], [46]]. Thus, it was important to evaluate the food safety of different baby food products widely manufacture, distributed and sold in Egypt. PCR technique has been recently applied for rapid detection and differentiation of enterotoxins related genes in B. cereus [47,25]. Results in Table 3 revealed that the B. cereus isolates carrying HBLA gene was detected in infant food with fruit milk based by 2 isolates, infant food with vegetables milk based (25%) and infant food with wheat milk based (50%). This gene was not detected in infant food with honey milk based and infant food with rice milk based (Fig. 3).
Table 3.
Prevalence of enterotoxin genes of 45 B. cereus isolates.
| NO. | Samples | No. of B. cereus isolates | Hemolytic BL complex hblA No. + (%) |
Non-hemolytic enterotoxin complex nheC No. + (%) |
cytotoxic gene cytK No. + (%) |
|---|---|---|---|---|---|
| 1 | infant food with fruit milk based | 28 | 2(7.1) | 21(75) | 27(96.4) |
| 2 | infant food with vegetables milk based | 4 | 1(25) | 3(75) | 3(75) |
| 3 | infant food with honey milk based | 6 | 0 | 5(83.3) | 6(100) |
| 4 | infant food with rice milk based | 3 | 0 | 0 | 3(100) |
| 5 | infant food with wheat milk based | 4 | 2(50) | 3(75) | 4(100) |
| Total | 45 | 5(11.1) | 32(71.1) | 43(95.5) | |
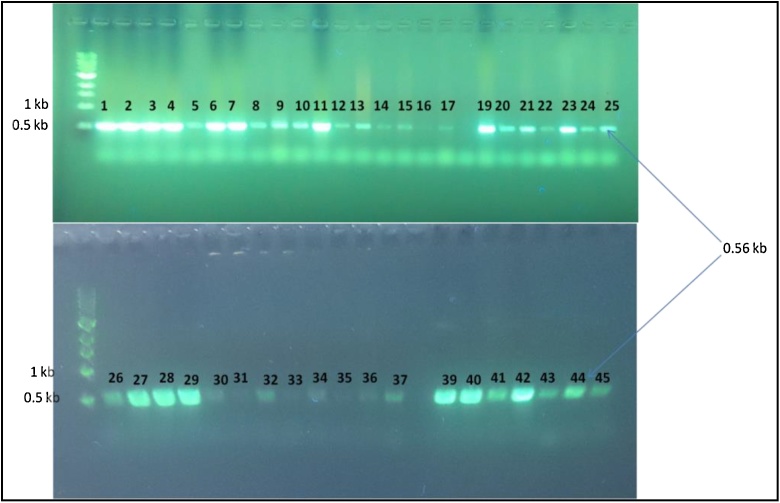
Fig. 3.
Agarose gel image for the PCR product of hblA gene in B. cereus isolates compared with marker (1 kb); Numbers on image related to positive detected isolates only.
On this respect, Hansen and Hendriksen [48] showed that 52% of B. cereus strains isolated from foods had HBLA genes. Nearly, the same trend of the HBLA gene distribution with different percentage were obtained from B. cereus isolated from baby food with rice milk based, wheat milk based and honey milk based with percentages of 19.35,12 and 5.55%, respectively. And it was not detected in baby food with banana milk based [49]. Moreover, Dhuha and Habeeb [50] reported that, HBLA gene was detected from B. cereus isolated from milk, dairy products and infant food in the percentages of 9.09, 20 and 20%, respectively. Variation in the obtained results is accepted based on various factors such as season, type of sample, collection condition, it was closely related with qualitative and quantitative results.
3.6.2. Prevalence of non-hemolytic enterotoxin complex NHEC gene
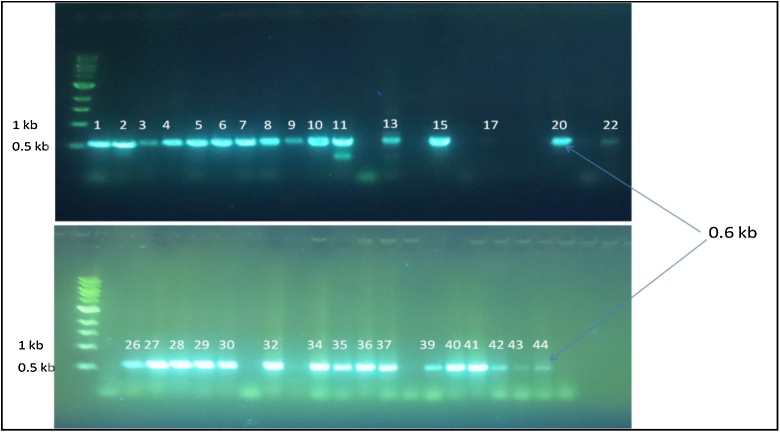
The presence of enterotoxigenic NHEC virulence gene seems to be widely distributed among B. cereus even in different isolates which found in 75, 75, 83.3 and 75% of B. cereus isolated from infant food with fruit milk based, infant food with vegetables milk based, infant food with honey milk based and infant food with wheat milk based, respectively (Table 3 and Fig. 4). This gene was not detected in infant food with rice milk based. The results were approximately similar with obtained by Guinebretiere et al. [24], who found 63 and 36% of food borne B. cereus isolates lack one or two of the NHE and HBL genes, respectively by PCR amplification. Also, Al-Khatib et al. [51] found the NHE genes in most of the strain of B. cereus were higher than HBL genes. Other study, Dhuha & Habeeb [50] reported that, NHEC gene was found in 80% of B. cereus isolated from infant food.
Fig. 4.
Agarose gel image for the PCR product of nheC gene in B. cereus isolates compared with marker (1 kb); Numbers on image related to positive detected isolates only.
3.6.3. Prevalence of cytotoxic CYTK gene
It is obvious that from Table 3 and Fig. 5 the most of the strains CYTK gene was in higher percent than HBLA and NHEC genes. This gene was detected in 95.5% of B. cereus isolates studied in this work. Also, the results showed CYTK gene was detected in 96.4, 75, 100, 100 and 100% of B. cereus strains isolated from milk based infant food with fruit, vegetables, honey, rice and wheat, respectively. The cytotoxic gene CYTK of B. cereus (a clinical isolate) was the only causer of severe food poisoning outbreak that killed people. They also reported that CYTK toxin had necrotic and hemolytic action. In addition to, it was completely different from other B. cereus enterotoxins [52]. Many authors commented that both HBLC and CYTK genes were detected with varying percentages ranged from 20 to 77% of screened isolates [53,54,66,55].
Fig. 5.
Agarose gel image for the PCR product of cytK gene in B. cereus isolates compared with marker (1 kb); Numbers on image related to positive detected isolates only.
In this study, results in Table 3 illustrated the HBLA, NHEC and CYTK genes were detected by 11.1, 71.1 and 95.5% of the examined samples, respectively. Samples of milk based infant food with fruit, vegetables and wheat were carried the 3 genes. Furthermore, 3 isolates from infant food with rice milk based had only CYTK gene. In this direction, Granum [56] found that, NHE gene was present in 100% of B. cereus isolates, while the HBL gene was present in 50% of B. cereus isolates only. Samapundo et al. [57] isolated 324 B. cereus strains from food products in Belgium. They showed 52.5% of strains had all HBLA, HBLB, HBLC, NHEA, NHEB, and NHEC enterotoxigenic genes.
3.6.4. Hemolytic activity of the B. cereus isolates
B. cereus produces a large number of potentially virulent factors including hemolysin [58,59]. The ability of B. cereus isolates to produce hemolysis of 7% horse erythrocytes on blood agar was examined. The results in Table 4 revealed that out of 78 isolates, 76 isolates (97.4%) showed hemolytic activity. These results are not far from those obtained by Wong et al. [60] who noted that 98% of B. cereus isolated from fermented milks and milk powders lysed ertherocytes were observed hemolytic activity. Also, Nour et al. [19] and Sadek et al. [61] recorded that 89.74 and 88.4% of B. cereus isolated from some dairy products showed hemolytic activity. Moreover, Sameer et al. [55] reported that 100% of B. cereus strains isolated from infant milk formula showed stronger hemolytic activity with lower frequency of harboring HBL and CYTK.
Table 4.
Hemolytic activity of the isolated B. cereus.
| Type of infant food samples | No. of isolates | Positive isolates |
|
|---|---|---|---|
| No. | % | ||
| Fruit milk based | 35 | 34 | 97.1 |
| Vegetables milk based | 9 | 9 | 100 |
| Honey milk based | 14 | 14 | 100 |
| Rice milk based | 11 | 11 | 100 |
| Wheat milk based | 9 | 8 | 88.8 |
| Total | 78 | 76 | 97.4 |
4. Conclusion
Based on the major foods for infants and young children are industrial synthesis foods, it is important to ensure about food safety application. The presence of B. cereus in the infant foods could be potentially alarming for the infant’s health. Unfortunately, found pathogenic strains of B. cereus in the collected infant food samples. For that, many processes should be applied for controlling of pathogens to preserve infant lives.
Conflict of interest statement
Authors declare that this work has not been published previously and there are no conflicts of interest.
Acknowledgement
We are thankful to National Research Centre, Cairo, Egypt for supporting this work.
References
- 1.Townsend S., Forsythe S.J. The neonatal intestinal microbial flora, immunity, and infections. In: Farber J.M., Forsythe S.J., editors. Enterobacter sakazakii. ASM Press; Washington DC: 2008. Chapter 3. [Google Scholar]
- 2.Yadav J.S., Grover S., Batish V.K. B.V. Gupta, Metropolitan Book Co Pvt. Ltd.; Delhi, India: 1993. Microbiology of Dried Milks: a Comprehensive Dairy Microbiology; pp. 315–349. pp,xiv + 764 pp. [Google Scholar]
- 3.Rowan N.J. Infant Milk Formulae. 4thed. University of Strathclyde; Glasgow, Scotland: 2006. Studies on the growth, survival, interaction, and detection of potentially pathogenic Listeria and Bacillus spp. Ph.D. thesis. [Google Scholar]
- 4.Deeb A.M., Al Hawary I.I., Aman I.M., Shahine D.M. Bacteriological investigation on milk powder in the Egyptian market with emphasis on its safety. Bangladesh J. Vet. Med. 2010;4:424–433. [Google Scholar]
- 5.Haughton P., Garvey M., Rowan N.J. Emergence of Bacillus cereus as a dominant organism in Irish retailed powdered infant formulae (Pif) when reconstituted and stored under abuse conditions. J. Food Saf. 2010;30(4):814–831. [Google Scholar]
- 6.Di Pinto A., Bonerba E., Bozzo G., Ceci E., Terio V., Tantillo G. Occurence of potentially enterotoxigenic Bacillus cereus in infant milk powder. Eur. Food Res. Technol. 2013;237:275–279. [Google Scholar]
- 7.Wang M., Cao B., Gao Q., Sun Y., Liu P., Feng L. Detection of Enterobacter sakazakii and other pathogens associated with infant formula powder by use of a DNA microarray. J. Clin. Microb. 2009;47:3178–3184. doi: 10.1128/JCM.00366-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Animal Plant and Fisheries Quarantine and Inspection Agency . 2013. Livestock Processing Standards and Ingredient Specifications No. 2012-118. Republic of Korea. [Google Scholar]
- 9.Lakshmi S.G., Jayanthi N., Saravanan M., Ratna M.S. Safety assesment of Bacillus clausii UBBC07, a spore forming probiotic. Tox. Rep. 2017;4(2017):62–71. doi: 10.1016/j.toxrep.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pirttijarvi T.S., Andersson M.A., Salkinoja-Salonen M.S. Properties of Bacillus cereus and other Bacilli contaminating biomaterial-based industrial processes. Internat. J. Food Microb. 2000;60(2-3):231–239. doi: 10.1016/s0168-1605(00)00313-5. [DOI] [PubMed] [Google Scholar]
- 11.Darwesh O.M., Sultan Y.Y., Seif M.M., Marrez D.A. Bio-evaluation of crustacean and fungal nano-chitosan for applying as food ingredient. Tox. Rep. 2018;5:348–356. doi: 10.1016/j.toxrep.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen B.M., Hoiby P.E., Jensen G.B., Hendrksen N.B. The Bacillus cereus BCET enterotoxin sequence eappraised. FEMS Microb. Lett. 2003;223:21–24. doi: 10.1016/S0378-1097(03)00249-0. [DOI] [PubMed] [Google Scholar]
- 13.APHA (American Public Health Association) 16th ed. 1992. Standard Methods for Examination of Dairy Products. Washington, DC. [Google Scholar]
- 14.ICMSF (International Commission on Microbiological Specification for Foods) Blackie Acad. Profess.; London.xv: 1998. Microorganisms in Food, Microbial Ecology of Food Commodities. 615 p.ISBN 0751404306. [Google Scholar]
- 15.Darwesh O.M., Moawad H., Abd El-Rahim W.M., Barakat O.S., Sedik M.Z. Bioremediation of textile Reactive Blue (RB) azo dye residues in wastewater using experimental prototype bioreactor. Res. J. Pharm. Biol. Chem. Sci. 2014;5(4):1203–1219. [Google Scholar]
- 16.Meer R.R., Baker J., Bodyfelt F.W., Griffiths M.W. PsychrotrophicBacillus spp. in fluid milk products: a review. J. Food Prot. 1991;54:969–979. doi: 10.4315/0362-028X-54.12.969. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Khalek A., El-Sherbini M. Prevalence of enterotoxigenic Bacillus cereus in raw and pasteurized milk. 4th Sci. Congr. Proc. 1996;44:157–161. [Google Scholar]
- 18.Harrigan W.F. 3rded. Academic Press; London: 1998. Laboratory Methods in Food Microbiology. 532 Pages. [Google Scholar]
- 19.Nour M.A., Tohami M., Kahter A.A. Identification and characterization of Bacillus cereus group isolated from different sources. Egypt. J. Dairy Sci. 2002;30:1–10. [Google Scholar]
- 20.Barakat K.M., Hassan S.W., Darwesh O.M. Biosurfactant production by haloalkaliphilic Bacillus strains isolated from Red Sea, Egypt. Egypt. J Aqua. Res. 2017;43(2017):205–211. [Google Scholar]
- 21.Kheiralla Z.H., Hewedy M.A., Mohammed H.R., Darwesh O.M. Isolation of pigment producing actinomycetes from rhizosphere soil and application it in textiles dyeing. Res. J. Pharm. Biol. Chem. Sci. 2016;7(5):2128–2136. [Google Scholar]
- 22.Barakat K.M., Mattar M.Z., Sabae S.Z., Darwesh O.M., Hassan S.H. Production and characterization of bioactive pyocyanin pigment by marine Pseudomonas aeruginosa OSh1. Res. J. Pharm., Biolog. Chem. Sci. 2015;6(5):933–943. [Google Scholar]
- 23.Minnaard J., Delfederico L., Vasseur V. Virulence of Bacillus cereus: a multivariate analysis. Intern. J. Food Microb. 2007;116(2):197–206. doi: 10.1016/j.ijfoodmicro.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Guinebretiere M., Broussolle V., Nguyen C. Enterotoxigenic profiles of food poisoning and food borne Bacillus cereus strains. J. Clin. Microb. 2002;40(8):3053–3056. doi: 10.1128/JCM.40.8.3053-3056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngamwongsatit P., Buasri W., Pianariyanon P., Pulsrikarrrn C., Ohba M., Assavaning A., Panbangred W. Broad distribution of enterotoxin genes HBLCDA, NHEABC, CYTK and ENTFM among Bacillus thuringiensis and Bacillus cereus as shown by novel primers. Intern. J. Food Microb. 2008;121:352–356. doi: 10.1016/j.ijfoodmicro.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Shadlia-Matug M., Aidoo K.E., Candlish A.A., Elgerbi A.M. Evaluation of some antibiotics against pathogenic bacteria isolated from infant foods in North Africa. Open Food Sci. J. 2008;2:95–101. [Google Scholar]
- 27.Perial M., Abu-Salem H.H., Khalaf H.H. Nutritional and microbiological evaluation of some canned baby foods. Ann. Agric. Sci. Mosht. J. 1988;26:2605–2615. [Google Scholar]
- 28.Khalaf H., Marth E.H. Aerobic bacteria in commercially canned baby foods and other products. Ann. Agric. Sci. Mosht. J. 1994;21:760–766. [Google Scholar]
- 29.Yacoub S.S., Shamsia S.M., Awad S.A., Ziena H.M., Safwat N.M. Characterization of aerobic spore-forming bacteria isolated from raw milk, skim milk powder and UHT milk. Alexandria Sci. Exch. J. Int. Q. J. Sci. Agric. Environ. 2017;38(1):99–111. [Google Scholar]
- 30.Postollec F., Mathot A.G., Bernard M., Divanac’h M.L., Pavan S., Sohier D. Tracking spore-forming bacteria in food: from natural biodiversity to selection by processes. Intern. J. Food Microb. 2012;158(1):1–8. doi: 10.1016/j.ijfoodmicro.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 31.El-Gendi M.N., Wahba N.M. The importance of Geobacillus spp. As group of bacterial contaminates in the dairy industry. Ass. Veter. Med. J. 2013;139(59):86–92. [Google Scholar]
- 32.Sadiq F.A., Li Y., Liu T., Flint S., Zhang G., Yuan L., Pei Z., He G. The heat resistance and spoilage potential of aerobic mesophilic and thermophilic spore forming bacteria isolated from Chinese milk powders. Intern. J. Food Microb. 2016;238:193–201. doi: 10.1016/j.ijfoodmicro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed A.H., El-Prince E., Korashy E., Al-Gendy M.N. Microbiological evaluation of some infants powdered milk-based foods. Ass. Veter. Med. J. 2008;118(54):66–79. [Google Scholar]
- 34.Sadek Z.I., Refaat B.M., Abd El-Shakour E.H., Mehanna N.S., Hassan M.S. Potential sources of aerobic and anaerobic spore former bacteria in processed cheese. Res. J. Pharm. Biol. Chem. Sci. 2015;6(1):757–769. [Google Scholar]
- 35.FDA (Food and Drug Administration) vol. 61. 1996. pp. 36154–36219. (Food and Drug Administration. Microbiological Standards for Infant Formula Proposed in 1996). (132) [Google Scholar]
- 36.Dalea I., Alexandra L. Evaluation of hygienic quality of some powder milk samples for new borne and hazard evaluation through spores. Bull. Univer. Agric. Sci. Veter. Med. Cluj-Napoca. 2008;65(2):236–239. [Google Scholar]
- 37.Kim S.W., Oh Y.M., Lee J.Y., Imm I.G., Hwang D.H., Kang M.S. Microbial contamination of food products consumed by infants and babies in Korea. Lett. Appl. Microb. 2011;53:532–538. doi: 10.1111/j.1472-765X.2011.03142.x. [DOI] [PubMed] [Google Scholar]
- 38.Asmaa S.M., Alnakip E.A., Abd-El Aal S.F. Occurrence of Bacillus cereus in raw milk and some dairy products in Egypt. Japan. J Veter. Res. 2016;64(2):95–102. [Google Scholar]
- 39.Khalil A.M., Abdel-Monem R.A., Darwesh O.M., Hashim A.I., Nada A.A., Rabie S.T. Synthesis, Characterization, and Evaluation of Antimicrobial Activities of Chitosan and Carboxymethyl Chitosan Schiff-Base/Silver Nanoparticles. J. Chem. 2017;2017 11 pages. [Google Scholar]
- 40.Abdelhameed R.M., El-Sayed H.A., El-Shahat M., El-Sayed A.A., Darwesh O.M. Novel triazolothiadiazole and triazolothiadiazine derivatives containing pyridine moiety: design, synthesis, bactericidal and fungicidal activities. Curr. Bioact. Comp. 2018;14(2):169–179. [Google Scholar]
- 41.Emam H.E., Darwesh O.M., Abdelhameed R.M. In-growth metal organic framework/synthetic hybrids as antimicrobial fabrics and its toxicity. Coll. Surf. B: Biointerf. 2018;165:219–228. doi: 10.1016/j.colsurfb.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed A.A., Ali S.I., Darwesh O.M., El-Hallouty S.M., Sameeh M.Y. Chemical compositions, potential cytotoxic and antimicrobial activities of Nitraria retusamethanolic extract sub-fractions. Intern. J Toxicol. Pharmacol. Res. 2015;7(4):204–212. [Google Scholar]
- 43.Schneider K.R., Parish M.E., Goodrich R.M., Cookingham T. 2004. Preventing Foodborne Illness: Bacillus cereus and Bacillus anthracis. University of Florida, gainesville FL 32611.INstitute of Food and Agricultural Sciences, Extension. FSHNO04-05. [Google Scholar]
- 44.Pinto B., Chenoll E., Aznar R. Identification and typing of food borne Staphylococcus aureus by PCR-based techniques. Syst. Appl. Microb. 2005;37:4012–4019. doi: 10.1016/j.syapm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Shaheen R., Andersson M.A., Apetroaie C., Schulz A., Ehling-Schulz M., Ollilainen V.M., Salkinoja-Aalonen M.S. Potential of selected infant food formulas for production of Bacillus cereus emetic toxin, cereulide. Intern. J. Food Microb. 2006;107:287–294. doi: 10.1016/j.ijfoodmicro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Svensson B., Monthan A., Guinebretiere H.M., Nguyen C., Christiansson A. Toxin production potential and the detection of toxin genes among strains of Bacillus cereus group isolated along the dairy production chain. Intern. Dairy J. 2007;17:1201–1208. [Google Scholar]
- 47.Guinebretiere M., Fagerlund A., Granum P.E., Nguyen C. Rapid discrimination of CYTK-1 and CYTK-2 genes in Bacillus cereus strains by a novel PCR system. FEMS Microb. Lett. 2006;59(1):74–80. doi: 10.1111/j.1574-6968.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- 48.Hansen B.M., Hendriksen N.B. Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl. Environ. Microb. 2001;67:185–189. doi: 10.1128/AEM.67.1.185-189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rahimi E., Abdos F., Momtaz H., Baghbadorani Z.T., Jalali M. Bacillus cereus in infant foods: prevalence study and distribution of enterotoxigenic virulence factors in Isfahan Province, Iran. Transfus. Apher. Sci. 2013;292571:1–5. doi: 10.1155/2013/292571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhuha A.K., Habeeb S.N. In-vitro and in-vivo study on Bacillus cereus isolated from different foods samples. W. J Pharm. Res. 2015;4(11):87–102. [Google Scholar]
- 51.Al-Khatib M.S., Khyami-Horani H., Badran E., Shehabi A. Incidence and characterization of diarrheal enterotoxins of fecal Bacillus cereus isolates associated with diarrhea. Diagn. Microb. Infect. Dis. 2007;59:383–387. doi: 10.1016/j.diagmicrobio.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Lund T., Debuyser M.L., Granum A. Newcy toxin from B. cereus that may cause necrotic enteritis. Molec. Microb. 2000;38:254–261. doi: 10.1046/j.1365-2958.2000.02147.x. [DOI] [PubMed] [Google Scholar]
- 53.Chon J.W., Kim J.H., Lee S.J., Hyeon J.Y., Seo K.H. Toxin profile, antibiotic resistance, and phenotypic and molecular characterization of Bacillus cereus in sun silk. J. Food Microbiol. Saf. Hyg. 2012;32:217–222. doi: 10.1016/j.fm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Angela D.P., Elisabetta B., Giancarlo B., Edmondo B., Valentina C.T., Giuseppina T. Occurrence of potentially enterotoxigenic Bacillus cereus in infant milk powder. Eur. Food Res. Technol. 2013;237:275–279. [Google Scholar]
- 55.Sameer R.O., Hussein H.A., Ebrahim H.O., Manal K. Occurrence and characterization of toxigenic Bacillus cereus in food and infant feces. Asian Pac. J. Trop. Biomed. 2015;5(7):515–520. [Google Scholar]
- 56.Granum P.E. Bacillus cereus. In: Doyle M.P., Beuchat L.R., Montville T.J., editors. Food Microbiology. Fundamental and Frontiers. 2nd ed. ASM Press; Washington, D.C: 2001. pp. 373–381. [Google Scholar]
- 57.Samapundo S., Heyndrickx M., Xhaferi R., Devlieghere F. Incidence, diversity and toxin gene characteristics of Bacillus cereus group strains isolated from food products marketed in Belgium. Intern. J. Food Microb. 2011;150(1):34–41. doi: 10.1016/j.ijfoodmicro.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Brynestad S., Granum P.E. Clostridium perfringens and foodborne infections. Intern. J. Food Microb. 2002;74(3):195–202. doi: 10.1016/s0168-1605(01)00680-8. [DOI] [PubMed] [Google Scholar]
- 59.Schoeni J.L., Wong A.C. Bacillus cereus food poisoning and its toxins. J. Food Prot. 2005;68:636–648. doi: 10.4315/0362-028x-68.3.636. [DOI] [PubMed] [Google Scholar]
- 60.Wong H.C., Chen Y.L., Chen C.L. Growth, germination and toxigenic activity of Bacillus cereus in milk produces. J. Food Prot. 1988;1(9):707–710. doi: 10.4315/0362-028X-51.9.707. [DOI] [PubMed] [Google Scholar]
- 61.Sadek Z.I., Fathi F.A., Salem M.M. Incidance, survival and biocontrol of psycrotrophic Bacillus cereus and its toxin in milk and tallaga cheese. Pol. Food Nutr. Sci. 2006;56(4):419–425. [Google Scholar]
- 66.Arslan S., Eyi A., Küçüksari R. Toxigenic genes, spoilage potential and antimicrobial resistance of Bacillus cereus group strains from ice cream. Anaerob. 2014;25:42–46. doi: 10.1016/j.anaerobe.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Al-Timimi B.J. Antimicrobial resistance of bacteria isolated from powdered infant formulas (PIF) Med. J Bab. 2007;4(3):251–262. [Google Scholar]
- 77.Darwesh O.M., Moawad H., Barakat O.S., Abd El-Rahim W.M. Bioremediation of textile reactive blue azo dye residues using nanobiotechnology approaches. Res. J. Pharm. Biol. Chem. Sci. 2015;6(1):1202–1211. [Google Scholar]
- 78.Logan N.A. Bacillus species of medical and veterinary importance. J. Med. Microb. 1988;25:157–165. doi: 10.1099/00222615-25-3-157. [DOI] [PubMed] [Google Scholar]
- 79.Mostafa U.E., Filipiak M., Sekulska M.S. Evaluation of bacteriological quality in selected commercial infant formulas available in Poland and Egypt. J. Food Saf. 2002;22:197–208. [Google Scholar]
- 80.Stoeckel M., Lücking G., Ehling-Schulz M., Atamer Z., Hinrichs J. Bacterial spores isolated from ingredients, intermediate and final products obtained from dairies: thermal resistance in milk. Dairy Sci. Tech. 2016;96(4):569–577. [Google Scholar]
- 81.Varadaraj M.C. Methods for detection and enumeration of food born bacterial pathogenes. A critical evaluation. J. Food Sci. Technol. 1993;30:1–13. [Google Scholar]