Abstract
Background
Abnormal 12‑lead electrocardiogram (ECG) findings and proposing its ability for enhanced risk prediction, majority of the studies have been carried out with elderly populations with prior cardiovascular diseases. This study aims to denote the association of sudden cardiac death (SCD) and various abnormal ECG morphologies using middle-aged population without a known cardiac disease.
Methods
In total, 9511 middle-aged subjects (mean age 42 ± 8.2 years, 52% males) without a known cardiac disease were included in this study. Risk for SCD was assessed after 10 and 30-years of follow-up.
Results
Abnormal ECG was present in 16.3% (N = 1548) of subjects. The incidence of SCD was distinctly higher among those with any ECG abnormality in 10 and 30-year follow-ups (1.7/1000 years vs. 0.6/1000 years, P < 0.001; 3.4/1000 years vs. 1.9/1000 years, P < 0.001). At 10-year point, competing risk multivariate regression model showed HR of 1.62 (95% CI 1.0–2.6, P = 0.05) for SCD in subjects with abnormal ECG. QRS duration ≥ 110 ms, QRST-angle > 100°, left ventricular hypertrophy, and T-wave inversions were the most significant independent ECG risk markers for 10-year SCD prediction with up to 3-fold risk for SCD. Those with ECG abnormalities had a 1.3-fold risk (95% CI 1.07–1.57, P = 0.007) for SCD in 30-year follow-up, whereas QRST-angle > 100°, LVH, ER ≥ 0.1 mV and ≥0.2 mV were the strongest individual predictors. Subjects with multiple ECG abnormalities had up to 6.6-fold risk for SCD (P < 0.001).
Conclusion
Several ECG abnormalities are associated with the occurrence of early and late SCD events in the middle-age subjects without known history of cardiac disease.
Keywords: Sudden cardiac death, Risk prediction, Electrocardiogram, Follow-up studies
1. Introduction
The annual incidence of sudden cardiac death (SCD) in the United States is estimated to be as high as 450,000 cases (which accounts approximately 63% of all cardiac deaths) and the majority of SCD events occur in asymptomatic subjects considered to be at low- or intermediate risk for SCD [[1], [2], [3]]. Thus, improvements in risk stratification are urgently required and as SCD is primarily a result of electrical disturbance of the normal cardiac rhythm, 12‑lead electrocardiogram (ECG) is still an attractive non-invasive tool beyond clinical factors. In ideal circumstances, health care professionals would have simple tools for overall SCD risk evaluation combining genetic and demographic information to clinical data, such as12‑lead electrocardiogram (ECG) and echocardiography.
Risk prediction models for SCD and individual ECG abnormalities associated with SCD have been described earlier in numerous papers, but they have mainly been carried out in elderly populations and/or with patients with cardiovascular disease [[4], [5], [6], [7], [8], [9], [10]]. We aimed to clarify the prognostic significance of abnormal ECG findings in middle-aged subjects without known cardiac disease.
2. Methods
The Finnish Mobile Health Examination Survey, a large nationwide study was carried out in Finland between 1966 and 1972 [11]. As a part of this, The Coronary Heart Disease study (CHD study) was performed using 12 different geographical regions in Finland. Men and women aged 31 to 61 were invited to participate (N = 12,310, participation rate 89%). Age, body mass index (BMI), cholesterol, blood pressure, 12‑lead ECG as well as health questionnaire concerning current and prior health status (smoking, medications used, pain, chronic diseases etc.) were obtained as described earlier [12]. Overall, 10,904 ECGs were available for this study. Study was carried out following ethical guidelines and principals of the Declaration of Helsinki.
As this study focused on abnormal ECG findings in subjects without a known cardiac disease, exclusion was based on reported information and certain ECG findings. The exclusion criteria were identical to our previous study [12]. In brief, unreadable or missing ECGs, patients with atrial fibrillation, Wolf-Parkinsonson-White ECG pattern or pacemaker rhythm were excluded. Subject with a known cardiac disease (N = 895, information based on self-reported history of cardiac symptoms or medication, national registries using International Classification of Diseases (ICD) as well as the National Drug Reimbursement Registry maintained by the Finnish National Social Insurance Institution), symptoms of cardiac disease (N = 245) and those using cardiac medication (N = 253) were discounted from the analysis. The total of 9511 subjects (77.3% of the original population) were included in this study.
All participants had 12‑lead ECG recordings at baseline (paper speed 50 mm/s). Abnormal ECG findings were defined as: 1) QRS duration over 110 ms (interpreted from leads II or V5); 2) QRST angle over 100°; 3) QTc interval over 440 ms/460 ms (men/women); 4) left ventricular hypertrophy ([LVH] defined by Sokolow-Lyon criteria or Romhilt-Estes point score ≥ 5); 5) early repolarization (ER ≥ 0,1 mV and ER ≥ 0,2 mV) in inferior/lateral leads with descending or horizontal ST-segment; 6) T-wave inversions (≥1.0 mm deep in other leads than aVR, Minnesota codes 5.1 to 5.2).
The death certificate diagnoses, assigned by the physician responsible for the care at the time of death, was obtained from the Causes of Death Registry which is maintained by the Statistics of Finland. These certificates were manually studied by a committee of experienced cardiologists unaware of the data analysis. Events of SCD were defined as arrhythmic according to the Cardiac Arrhythmia Pilot Study, criteria being described earlier in detail [13,14]. The primary endpoint was SCD during a follow-up of 30 years and secondary endpoint was SCD in 10 years of follow-up.
Continuous variables are presented as means ± standard deviation (SD). We used the Fine and Gray competing risk model for assessment of adjusted and unadjusted hazard ratios (HR) and 95% confidence intervals (95% CI). Adjustments in multivariate model included age, gender, systolic blood pressure, smoking, body mass index (BMI), diabetes, blood cholesterol and smoking. In baseline, diabetes was screened with a one-hour glucose tolerance test and urine sample, if not diagnosed earlier. For further assessment of risk prediction value, we used the Integrated Discrimination Increment (IDI) analysis and C-statistics. The log-rank test was used in our Kaplan-Meier graphs. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS, version 24, IBM SPSS Statistics, Armonk, NY) and R Statistics (3.4.1, The R Foundation for Statistical Computing, Vienna, Austria). Two-sided P-values < 0.05 were considered significant.
3. Results
The demographic comparison of the groups is presented in Table 1. After exclusions, a total of 9511 subjects included in the analyses. A total of 1548 had at least one ECG abnormality present (test group). A total of 73 subjects suffered SCD in 10-year follow-up and 641 in 30-year follow-up. The incidence of SCD in the test group at 10-year point was 1.7/1000 years compared to 0.6/1000 years in the reference group. Incidences were 3.4/1000 years and 1.9/1000 years during the 30-year follow-up, respectively. The negative predictive value of normal ECG in 10-year follow-up was 99.4%.
Table 1.
Characteristics of subjects at baseline.
| Normal ECG |
Any ECG abnormality |
P value | |
|---|---|---|---|
| (N = 7963) | (N = 1548) | ||
| Males (%)a | 50.8 | 63.0 | <0.001 |
| Age (years)b | 42.8 ± 8.1 | 44.5 ± 8.6 | <0.001 |
| Current smoker (%)c | 33.9 | 38.2 | 0.001 |
| Diabetes (%)c | 1.3 | 1.9 | 0.369 |
| Cholesterol (mmol/l)c | 6.46 ± 1.3 | 6.49 ± 1.3 | 0.695 |
| BMI (kg/m2)c | 25.8 ± 3.7 | 25.2 ± 3.6 | 0.001 |
| Systolic blood pressure (mmHg)c | 135 ± 19 | 144 ± 23 | <0.001 |
Adjusted for age.
Adjusted for gender.
Adjusted for age and gender.
3.1. 10-year analysis
The competing risk regression model (adjusted and unadjusted) for 10-year events is presented in Table 2. In the 10-year analysis the univariate yielded a 2.86 HR (95% CI 1.77–4.62, P < 0.001) and the multivariate 1.62 (1.00–2.62, P = 0.052) for those with abnormal ECG. The strongest predictors of SCD in multivariate analysis were QRS ≥ 110 ms (HR 3.09, 95% CI 1.27–7.52, P = 0.013), QRST-angle > 100° (HR 3.4, 95% CI 1.37–8.44, P = 0.009), LVH (HR 2.67, 95% CI 1.42–5.01, P = 0.002) and T-wave inversions (HR 2.98, 95% CI 1.30–6.79 P = 0.010). Subjects with ER ≥0.2 mV had no SCD events during this period, furthermore prevalence being only 40. The survival curves for SCD events is presented in Fig. 1. For predicting non-sudden cardiac death, abnormal ECG did not increase the risk in multivariate adjusted regression model (HR 1.10, 95% CI 0.87–1.38, P = 0.440).
Table 2.
Competing risk regression model and hazard ratios (HR), 10-year follow-up.
| Univariate HR | P-value | Multivariate HRa | P-value | |
|---|---|---|---|---|
| Normal ECG (N = 7963) | 1.0 | 1.0 | 1.0 | 1.0 |
| Any ECG abnormality (N = 1548, 16.3%) | 2.86 (1.77–4.62) | <0.001 | 1.62 (1.00–2.62) | 0.052 |
| QRS duration > 110 ms (N = 110, 1.2%) | 6.44 (2.59–16.00) | <0.001 | 3.09 (1.27–7.52) | 0.013 |
| QTc (N = 534, 5.6%) | 2.68 (1.38–5.22) | 0.004 | 1.26 (0.64–2.48) | 0.500 |
| QRST-angle > 100° (N = 125, 1.3%) | 5.61 (2.26–13.90) | <0.001 | 3.40 (1.37–8.44) | 0.009 |
| LVH (N = 395, 4.2%) | 5.07 (2.78–9.23) | <0.001 | 2.67 (1.42–5.01) | 0.002 |
| ER ≥ 0,1 mV (N = 351, 3.7%) | 1.12 (0.35–3.55) | 0.850 | 0.86 (0.27–2.72) | 0.800 |
| ER ≥ 0,2 mV (N = 40, 0,4%) | 0.00 (0–0) | N/A | 0.00 (0–0) | N/A |
| T-wave inversion (N = 284, 3,0%) | 4.05 (1.94–8.45) | <0.001 | 2.98 (1.30–6.79) | 0.010 |
Adjusted for age, gender, systolic blood pressure, diabetes, BMI and cholesterol. ECG = electrocardiogram, ER = early repolarization, HR = hazard ratio, LVH = left ventricular hypertrophy, QTc = heart rate corrected QT interval. HRs for ER > 0.2 mV were not possible to analyze as no events occurred in this group during the 10-year follow-up.
Fig. 1.
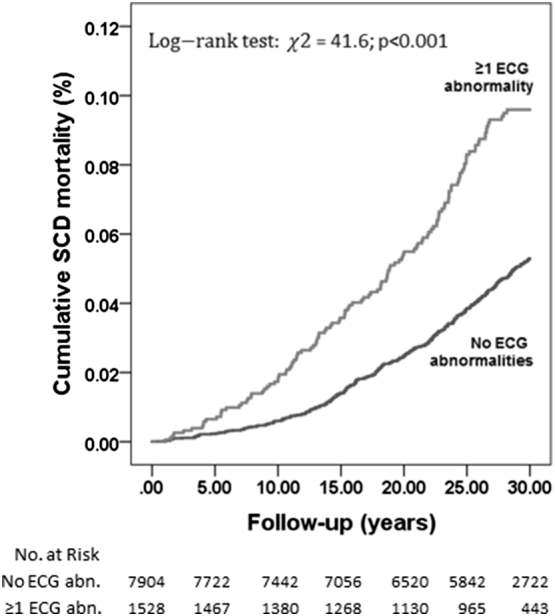
Mortality rates in subject with and without ECG abnormalities for sudden cardiac death.
To determine the risk prediction value of abnormal ECG further, we used the C-index and IDI analysis. Original model included known risk factors of cardiac disease: age, gender, systolic blood pressure, diabetes and smoking. After adding abnormal ECG variable to the original model, the C-index showed no significant improvement in risk prediction for SCD, whereas the IDI analysis showed a minor improvement in risk prediction (IDI 0.0033, P = 0.032).
3.2. 30-year analysis
The results of competing risk regression model for 30-year follow-up is presented in Table 3. In the univariate model, subjects with ECG abnormalities had a risk of 1.67 (95% CI 1.39–2.00, P < 0.001) compared to the reference group. In multivariate analysis the risk of SCD was 1.30 (95% CI 1.07–1.57, P = 0.007). In effort to evaluate the predictive value of individual abnormal ECG findings, QRST-angle > 100° (HR 1.79, 95% CI 1.08–2.95, P = 0.023), LVH (HR 1.52, 95% CI 1.12–2.05, P = 0.007), ER ≥ 0,1 mV (HR 1.60, 95% CI 1.15–2.21, P = 0.005) and ER ≥ 0,2 mV (HR 2.60, 95% CI 1.28–5.29, P = 0.009) showed significant association to SCD in 30-year follow-up. Abnormal ECG did not predict non-sudden cardiac death in multivariate analysis (HR 0.99, 95% CI 0.91–1.08, P = 0.820). The C-index for SCD showed a minor, but not significant improvement in risk prediction (original model 0.708; model with abnormal ECG 0.710, P = 0.583). IDI analysis yielded a minor improvement in risk prediction (IDI 0.0027, P = 0.003).
Table 3.
Competing risk regression model, 30-year follow-up.
| Univariate | P-value | Multivariatea | P-value | |
|---|---|---|---|---|
| Normal ECG (N = 7963) | 1.0 | 1.0 | 1.0 | 1.0 |
| Any ECG abnormality (N = 1548, 16.3%) | 1.67 (1.39–2.00) | <0.001 | 1.30 (1.07–1.57) | 0.007 |
| QRS duration > 110 ms (N = 110, 1.2%) | 2.20 (1.30–3.72) | 0.003 | 1.57 (0.91–2.72) | 0.110 |
| QTc (N = 534, 5.6%) | 1.56 (1.18–2.07) | 0.002 | 1.06 (0.79–1.43) | 0.670 |
| QRS-T angle > 100° (N = 125, 1.3%) | 2.33 (1.45–3.77) | 0.001 | 1.79 (1.08–2.95) | 0.0230 |
| LVH (N = 395, 4.2%) | 2.01 (1.50–2.71) | <0.001 | 1.52 (1.12–2.05) | 0.007 |
| ER ≥ 0,1 mV (N = 351, 3.7%) | 1.81 (1.31–2.49) | <0.001 | 1.60 (1.15–2.21) | 0.005 |
| ER ≥ 2 mV (N = 40, 0,4%) | 3.29 (1.63–6.67) | 0.001 | 2.60 (1.28–5.29) | 0.009 |
| T-wave inversion (N = 284, 3,0%) | 1.41 (0.948–2.08) | 0.090 | 1.33 (0.88–2.02) | 0.170 |
Multivariate model included age, gender, systolic blood pressure, diabetes, BMI and cholesterol. ECG = electrocardiogram, ER = early repolarization, HR = hazard ratio, LVH = left ventricular hypertrophy, QTc = heart rate corrected QT interval. Adjusted for age, gender, systolic blood pressure, diabetes, BMI and cholesterol.
3.3. Cumulative risk of combined ECG abnormalities
The number of ECG abnormalities detected had an impact on the resulting risk for SCD, as shown in Fig. 2. The risk for SCD was 3-fold for both 10-year and 30-year follow-up when two or more ECG abnormalities were observed (Table 4). Comparing those with normal ECG and subjects with ≥2 ECG abnormalities, the C-index showed a minor but not significant improvement for SCD risk prediction (10-year analysis 0.821 vs 0.823, P = 0.662; 30-year analysis 0.755 vs 0.758, P = 0.153, respectively).
Fig. 2.
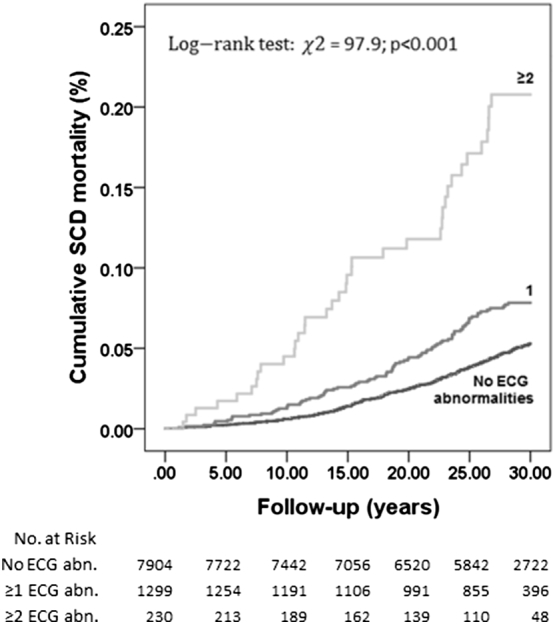
Cumulative effect of abnormal ECG findings for sudden cardiac death.
Table 4.
Association between the number of ECG abnormalities and risk for sudden cardiac death.
| Number of ECG abnormalities | Number of SCDs/subjects at risk | Univariate | P value | Multivariatea | P value |
|---|---|---|---|---|---|
| 10-year follow-up | |||||
| 1 ECG abnormality | 16/1313 | 2.10 (1.19–3.69) | 0.011 | 1.29 (0.72–2.30) | 0.393 |
| ≥2 ECG abnormalities | 10/186 | 7.62 (3.85–15.09) | <0.001 | 3.22 (1.57–6.62) | 0.001 |
| 30-year follow-up | |||||
| 1 ECG abnormality | 87/1313 | 1.55 (1.23–1.96) | <0.001 | 1.12 (0.89–1.43) | 0.334 |
| ≥2 ECG abnormalities | 37/186 | 4.57 (3.26–6.41) | <0.001 | 2.97 (2.09–4.20) | <0.001 |
Adjusted for age, gender, systolic blood pressure, diabetes, BMI and cholesterol. ECG = electrocardiogram.
In addition, when prolonged QRS complex (QRSd ≥ 110 ms) was accompanied by any of the measured ECG abnormalities (N = 110), the risk for SCD was 6.6-fold (95% CI: 2.6–16.3; P < 0.001) in the 10-year follow-up and 2.9-fold (95% CI: 1.7–5.0) in the 30-year follow-up. After adjustments for clinical covariates these risks decreased to 2.9-fold (95% CI: 1.2–7.4; P = 0.022) and 1.8-fold (95% CI: 1.1–3.1; P = 0.029), for 10-year and 30-year follow-ups, respectively.
4. Discussion
In this study setting of middle-aged subjects without a known cardiac disease, several ECG abnormalities were found to possess predictive value for SCD events. The predictive power of various ECG markers was variable during 10-year and 30-year follow-up periods. In summary, results suggest abnormal QRST-angle, LVH and ER ≥ 0.2 mV having the highest predictive value for SCD events, and cumulative impact of multiple ECG risk variants markedly improves the risk prediction of SCD events in subjects with no history of cardiac disease. The IDI analysis showed a minor enhance in risk prediction with abnormal ECG for SCD. However, abnormal ECG did not improve the risk prediction model in C-statistics, probably due to strong variables in the original model (age, gender, BMI, high blood pressure, cholesterol, diabetes, smoking).
Sudden cardiac death is considered as a multifactorial event and it is vital to understand the various causes and mechanisms known to increase the risk of SCD [2,15,16]. Majority (80%) of SCD events occur in elderly patients with coronary arterial abnormalities, and minority of SCD cases derive from to cardiomyopathies, valvular/congenital heart diseases and electrophysiological abnormalities. However, stroke, aortic rupture, intoxication and pulmonary embolism are also known triggers of SCD. In 2004, The Framingham Heart Study examined the current trend of SCD, if the mortality rates were in line with notably decreased number of fatal coronary heart disease (CHD) events [17]. They concluded that the incidence of SCD events has decreased by 49% during the past 5 decades. This confirms the importance of primary and secondary intervention of CHD to reduce the incidence of SCD. This phenomenon is also seen in the incidence rates of SCD according to age. The age-related incidence of SCD is demonstrated to form a peak among children under the age of five, remaining low for the age groups of 5 to 44 years, and increasing rapidly from the age of 45 indicating the importance of CHD prevention in effort to decrease SCD events [2].
4.1. ECG risk markers for SCD
In a recent study, ECG risk variants were evaluated in the context of left ventricular ejection fraction (LVEF) for better prediction of future SCD events [18]. They utilized data from the community-based Oregon Sudden Unexpected Death Study with 522 cases of SCD and 736 controls studying a total of 8 abnormalities on ECG. Results indicated progressively greater risk of SCD in subject with multiple ECG abnormalities combined with preserved (>35%) LVEF. Subjects with ≥4 abnormal ECG variants had over a 20-fold risk (95% CI 9.4–47.7, P < 0.001) for SCD. The results suggested that the cumulative effect of ECG risk markers was a strong predictor of future SCD events among subjects with LVEF over 35%. This can be regarded as a marked finding since we lack proper clinical tools and knowledge for decent SCD prediction. From this perspective, our results are parallel for showing remarked elevation of SCD risk for those with multiple risk variants on 12‑lead ECG.
In case of prolonged QRS duration (QRSd) and intraventricular conduction delay (IVCD), the consensus as usable risk variable for SCD is not uniform [6,10,19,20]. Aro et al. in 2011 performed a general population study and concluded QRSd ≥ 110 ms and IVCD as a significant risk marker for SCD with 2- and 3-fold risks, respectively [6]. Our results suggested QRS duration as a risk factor for SCD in 10-year analysis, however no value in risk prediction was found in longer follow-up period. Nonetheless, the segregation between QRSd/IVCD being independent markers for SCD or just a manifestation of more proceeded cardiovascular disease remains debatable.
The total electrical activity of the heart including depolarization and repolarization measured by QT interval is often corrected with Bazett's formula. Patients with long QT syndrome (LQTS), composed of a heterogenic group of patients with genetic variability affecting ion channels of the myocardial cells are known to be at high risk for SCD events [[21], [22], [23]]. However, a clear majority of subjects with prolonged QTc do not have these genetic abnormalities and this abnormal ECG finding is regarded as idiopathic or drug induced. Some studies suggest QTc as a significant risk predictor for SCD in both CAD and general population samples with 3 to 5-fold risk [[24], [25], [26]]. Algra et al. in 1991studied QTc as an independent risk factor for SCD in patients with and without a history of cardiac dysfunction [26]. They utilized 24-h ambulatory electrocardiographs and subject were followed up to 2 years. In subjects without intraventricular conduction defect, QTc > 440 ms was associated to SCD with a 2.3-fold risk (95% CI 1.4–3.9) compared to those with QTc < 440 ms. Prolonged QTc did not increase the risk of SCD in subjects with cardiac dysfunction. In our study setting of middle-aged patients with no history of cardiac disease, prolonged QTc did not show significantly elevated risk for SCD in neither 30-year nor 10-year follow-up periods. As the results remains controversial, the overall risk prediction value of prolonged QTc for SCD needs further scientific verification [20].
A decade ago, the pattern of early repolarization (ER) on 12‑lead ECG was considered as a benign variant. However, Haïssaguerre et al. in 2008 reported that subjects with ER had a significantly higher risk of sudden cardiac arrest and idiopathic ventricular fibrillation than those without this ECG pattern, even without clinical evidence of structural heart disease or evident abnormalities on resting ECG [9]. This study was followed by Tikkanen et al. in 2009, suggesting J-point elevation in inferior leads as a significant predictor of SCD, especially in case of ER ≥ 0.2 mV [14]. Recently, this knowledge has been strengthened and ER needs to be considered as a noteworthy risk marker for SCD events [27]. Our study results are in line with previous conclusions. Early repolarization seems to have value in predicting SCD. However, more high quality clinical trials are needed in effort to discover practical and cost-effective means to recognize and treat these patients.
The spatial and frontal QRS-T angle calculated by using vectorcardiography of depolarization and repolarization has been studied for decades and this field research has reemerged in recent years as it has been associated with increased risk of cardiac death and SCD [28]. The definition of an abnormal QRS-T angle varies (from 100° to 135°) depending on the study [[28], [29], [30], [31]]. In selected studies using large general populations, QRS-T angle was regarded as a significant risk factor for adverse cardiac events. The hazard ratio for cardiac mortality varied from 1.9 to 5.2, and the risk for SCD from 2.3 to 5.6. Our study yielded parallel results. The significance was notable in 10-year risk prediction. In addition, T-wave inversions are suggested as isolated risk factor for SCD [32]. This was also noted in our study. T-wave inversions showed a 3-fold risk in 10-year period but had no significance in 30-year follow-up.
Left ventricular hypertrophy on ECG is regarded as a potential trigger of ventricular arrhythmias and therefore regarded as a risk marker for SCD events [33]. This has been established especially among patient with hypertension induced hypertrophy [34]. This increased risk was noted in this setting of middle-aged subjects with no history of cardiac disease, being one of the strongest individual predictors for SCD.
Currently, we obviously lack decent tools for SCD prediction. As recent studies have implied, a 12‑lead ECG provides evident benefits for this field of research. Wide availability, simple interpretation and good cost-effectiveness makes it important for clinicians and researchers. More prospective studies are needed to validate novel risk models for SCD prediction. From electrocardiographic point of view, future studies should focus on both individual and combined ECG risk variants alongside with other clinical factors.
4.2. Study limitations
As retrospective study, a few limitations are obvious concerning this study. For identifying subjects with and without cardiac disease, the definition was based on self-reported information. This information was obtained by using a questionnaire including data of used medications, symptoms (angina pectoris for example), and was performed by a trained nurse. At that time of pre-digitalization and lacking electronic patient report regimes, this was regarded as the most reliable method for obtaining a large amount of data. We cannot out rule the possibility of a silent cardiac disease developing during the 30-year follow-up.
After the time of baseline study in 1960s' to 1970s', the practice of medicine has gone throw major changes. As the general knowledge of diseases, symptoms and risk prevention is being brought to public awareness via internet and public channels, and this information is added to the constantly growing prosperity and well-being, it is apparent that the utilization of health care has increased substantially. This general knowledge combined with more advanced and novel clinical tools have led to early diagnosis and/or intervention to prevent adverse events. Therefore, comparing the past and the present era, the risk profiles are not completely in line for the risk of SCD. More studies are needed using populations of the present time, rather with various ethnic backgrounds.
4.3. Future directions of SCD prevention
As for risk prediction for SCD events, the current consensus and understanding relies on prevention/treating/diagnostics of known risk factors, such as CHD, congenital heart/valvular diseases and LQTS. Improved knowledge of drugs with potency of QT prolongation educates clinical practitioners for reasonable evaluation of medication to prevent redundant SCD events. Since the all mechanisms of SCD are not completely understood, the risk evaluation of those with no clinical symptoms or known risk factors for SCD still remains challenging and more research is needed. As for future innovation in general practice with constantly increasing knowledge for SCD risk markers, considering cost-effectiveness and availability, the development of automated ECG analysis could involve more advanced algorithms to detect those subjects with multiple known risk factors. This initial risk assessment could lead to further diagnostic inspection with traditional methods such as echocardiogram, magnetic resonance imaging and Holter monitoring. For example, patient with numerous risk variables on ECG combined with low (<35%) left ventricular ejection fraction (LVEF) on ECHO imaging, could benefit primary prevention with ICD therapy, thus improving the “number needed to treat” rate compared to risk assessment using only LVEF values [35]. As genetic testing has grown dramatically over the past decades with decreasing expense, this field of research probably improves the future risk assessment for SCD.
5. Conclusion
Electrocardiogram is a valuable and inexpensive tool for predicting SCD events in middle-aged subjects with no history of cardiac disease. There seems to be an obvious correlation between the risk of SCD and the number of observed abnormal ECG findings. The prediction of SCD should include multiple clinical risk factors (age, gender, diabetes, smoking, blood pressure, cardiac function) in addition to observed ECG abnormalities. Future clinical studies on predictive value of ECG should be performed using both traditional and novel statistical methods. Studies providing more exact information about the high-risk combination of ECG variants could offer medical professionals means to prevent majority of SCD events.
Conflict of interest
None.
Footnotes
This study was supported in part by the Sigrid Juselius Foundation, Helsinki, Finland; The Medical Council of The Academy of Finland, Helsinki, Finland; Terttu-Foundation, Oulu, Finland; The Foundation of Ida Montini, Espoo, Finland; The Finnish Medical Foundation, Helsinki, Finland; The Emil Aaltonen Foundation, Tampere, Finland; The Aarne Koskelo Foundation, Helsinki, Finland; The Orion Research Foundation, Espoo, Finland; the Paulo Foundation, Espoo, Finland; Finnish Cultural Foundation, Helsinki, Finland.
References
- 1.Zheng Z.J., Croft J.B., Giles W.H., Mensah G.A. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Adabag A.S., Luepker R.V., Roger V.L., Gersh B.J. Sudden cardiac death: epidemiology and risk factors. Nat. Rev. Cardiol. 2010;7:216–225. doi: 10.1038/nrcardio.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chugh S.S., Reinier K., Teodorescu C. Epidemiology of sudden cardiac death: clinical and research implications. Prog. Cardiovasc. Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adabag A.S., Therneau T.M., Gersh B.J., Weston S.A., Roger V.L. Sudden death after myocardial infarction. JAMA. 2008;300:2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon S.D., Zelenkofske S., McMurray J.J. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 6.Aro A.L., Anttonen O., Tikkanen J.T. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ. Arrhythm. Electrophysiol. 2011;4:704–710. doi: 10.1161/CIRCEP.111.963561. [DOI] [PubMed] [Google Scholar]
- 7.Bardy G.H., Lee K.L., Mark D.B. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 8.Chugh S.S., Kelly K.L., Titus J.L. Sudden cardiac death with apparently normal heart. Circulation. 2000;102:649–654. doi: 10.1161/01.cir.102.6.649. [DOI] [PubMed] [Google Scholar]
- 9.Haissaguerre M., Derval N., Sacher F. Sudden cardiac arrest associated with early repolarization. N. Engl. J. Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 10.Teodorescu C., Reinier K., Uy-Evanado A. Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm. 2011;8:1562–1567. doi: 10.1016/j.hrthm.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reunanen A., Aromaa A., Pyorala K., Punsar S., Maatela J., Knekt P. The Social Insurance Institution's coronary heart disease study. Baseline data and 5-year mortality experience. Acta Medica Scand. Suppl. 1983;673:1–120. [PubMed] [Google Scholar]
- 12.Terho H.K., Tikkanen J.T., Kentta T.V. The ability of an electrocardiogram to predict fatal and non-fatal cardiac events in asymptomatic middle-aged subjects. Ann. Med. 2016;48:525–531. doi: 10.1080/07853890.2016.1202442. [DOI] [PubMed] [Google Scholar]
- 13.Greene H.L., Richardson D.W., Barker A.H. Classification of deaths after myocardial infarction as arrhythmic or nonarrhythmic (the Cardiac Arrhythmia Pilot Study) Am. J. Cardiol. 1989;63:1–6. doi: 10.1016/0002-9149(89)91065-5. [DOI] [PubMed] [Google Scholar]
- 14.Tikkanen J.T., Anttonen O., Junttila M.J. Long-term outcome associated with early repolarization on electrocardiography. N. Engl. J. Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 15.Huikuri H.V., Castellanos A., Myerburg R.J. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 16.Myerburg R.J., Kessler K.M., Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann. Intern. Med. 1993;119:1187–1197. doi: 10.7326/0003-4819-119-12-199312150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Fox C.S., Evans J.C., Larson M.G., Kannel W.B., Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 18.Aro A.L., Reinier K., Rusinaru C. Electrical risk score beyond the left ventricular ejection fraction: prediction of sudden cardiac death in the Oregon Sudden Unexpected Death Study and the Atherosclerosis Risk in Communities Study. Eur. Heart J. 2017;38:3017–3025. doi: 10.1093/eurheartj/ehx331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamim W., Francis D.P., Yousufuddin M. Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int. J. Cardiol. 1999;70:171–178. doi: 10.1016/s0167-5273(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 20.Goldberger J.J., Cain M.E., Hohnloser S.H. American Heart Association/American College of Cardiology Foundation/heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Heart Rhythm. 2008;5:e1–21. doi: 10.1016/j.hrthm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Moss A.J., Long Q.T. Syndrome. JAMA. 2003;289:2041–2044. doi: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- 22.Medlock M.M., Tester D.J., Will M.L., Bos J.M., Ackerman M.J. Repeat long QT syndrome genetic testing of phenotype-positive cases: prevalence and etiology of detection misses. Heart Rhythm. 2012;9:1977–1982. doi: 10.1016/j.hrthm.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q., Shen J., Splawski I. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 24.Chugh S.S., Reinier K., Singh T. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straus S.M., Kors J.A., De Bruin M.L. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J. Am. Coll. Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 26.Algra A., Tijssen J.G., Roelandt J.R., Pool J., Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Y.J., Li Z.Y., Yao F.J. Early repolarization is associated with a significantly increased risk of ventricular arrhythmias and sudden cardiac death in patients with structural heart diseases. Heart Rhythm. 2017;14:1157–1164. doi: 10.1016/j.hrthm.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Oehler A., Feldman T., Henrikson C.A., Tereshchenko L.G. QRS-T angle: a review. Ann. Noninvasive Electrocardiol. 2014;19:534–542. doi: 10.1111/anec.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aro A.L., Huikuri H.V., Tikkanen J.T. QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace. 2012;14:872–876. doi: 10.1093/europace/eur393. [DOI] [PubMed] [Google Scholar]
- 30.Kardys I., Kors J.A., van der Meer I.M., Hofman A., van der Kuip D.A., Witteman J.C. Spatial QRS-T angle predicts cardiac death in a general population. Eur. Heart J. 2003;24:1357–1364. doi: 10.1016/s0195-668x(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki T., Froelicher V.F., Myers J., Chun S., Wang P. Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm. 2005;2:73–78. doi: 10.1016/j.hrthm.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 32.Laukkanen J.A., Di Angelantonio E., Khan H., Kurl S., Ronkainen K., Rautaharju P. T-wave inversion, QRS duration, and QRS/T angle as electrocardiographic predictors of the risk for sudden cardiac death. Am. J. Cardiol. 2014;113:1178–1183. doi: 10.1016/j.amjcard.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Messerli F.H. Hypertension and sudden cardiac death. Am. J. Hypertens. 1999;12:181S–188S. doi: 10.1016/s0895-7061(99)00106-5. [DOI] [PubMed] [Google Scholar]
- 34.Shenasa M., Shenasa H. Hypertension, left ventricular hypertrophy, and sudden cardiac death. Int. J. Cardiol. 2017;237:60–63. doi: 10.1016/j.ijcard.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Chugh S.S. Sudden cardiac death in 2017: spotlight on prediction and prevention. Int. J. Cardiol. 2017;237:2–5. doi: 10.1016/j.ijcard.2017.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]


