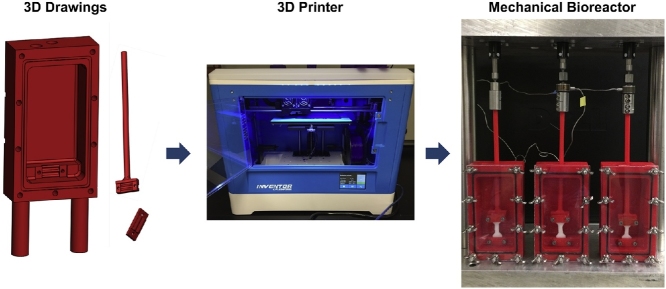
Graphical abstract
Method name: Design and fabrication of a 3D printed mechanical bioreactor system and small-scale tensile load frame
Abbreviations: 3DP, 3-dimensional printing; ABS, acrylonitrile butadiene styrene; CAD, computer-aided design; DAPI, 4’,6-diamidino-2-phenylindole; DAQ, data acquisition device; D, dimensional; DMEM, Dulbecco’s Modified Eagle’s Medium; FBS, fetal bovine serum; MSCs, mesenchymal stem cells; MSC-constructs, MSC-seeded collagen sponges; MTTFs, mouse tail tendon fascicles; NI, National Instruments; PBS, phosphate buffered saline
Keywords: Mechanical bioreactor, 3D printing, Stem cells, Mechanobiology, Soft tissue biomechanics, Tissue engineering
Abstract
Mechanical loading is an important cue for directing stem cell fate and engineered tissue formation in vitro. Stem cells cultured on 2-dimensional (D) substrates and in 3D scaffolds have been shown to differentiate toward bone, tendon, cartilage, ligament, and skeletal muscle lineages depending on their exposure to mechanical stimuli. To apply this mechanical stimulus in vitro, mechanical bioreactors are needed. However, current bioreactor systems are challenged by their high cost, limited ability for customization, and lack of force measurement capabilities. We demonstrate the use of 3-dimensional printing (3DP) technology to design and fabricate a low-cost custom bioreactor system that can be used to apply controlled mechanical stimuli to cells in culture and measure the mechanical properties of small soft tissues. The results of our in vitro studies and mechanical evaluations show that 3DP technology is feasible as a platform for developing a low-cost, customizable, and multifunctional mechanical bioreactor system.
• 3DP technology was used to print a multifunctional bioreactor system/tensile load frame for a fraction of the cost of commercial systems.
• The system mechanically stimulated cells in culture and evaluated the mechanical properties of soft tissues.
• This system is easily customizable and can be used to evaluate multiple types of soft tissues.
Method details
Introduction
Mechanobiology and tissue engineering approaches require a mechanical bioreactor system that can apply user-defined cyclic strains to cells and tissues in vitro and mechanically evaluate the developing tissues. The objective of this study was to design, build, and evaluate a low-cost, customizable, and multifunctional mechanical bioreactor system. To achieve this, we focused on developing a tensile bioreactor with potential applications for soft tissues such as tendons, ligaments, or skin. Here, we utilized 3-dimensional printing (3DP) to build a low-cost culture chamber for maintaining cells and engineered tissues in culture medium and custom grips for mounting 3D engineered tissue constructs and soft tissues. Additionally, we developed custom software to control three actuators and monitor load cells independently to conduct high-throughput loading experiments and evaluate the mechanical properties of developing tissues. Our results show that 3DP is a promising technology that can be used to fabricate a multifunctional, low-cost, mechanical bioreactor system.
Mechanical bioreactor design
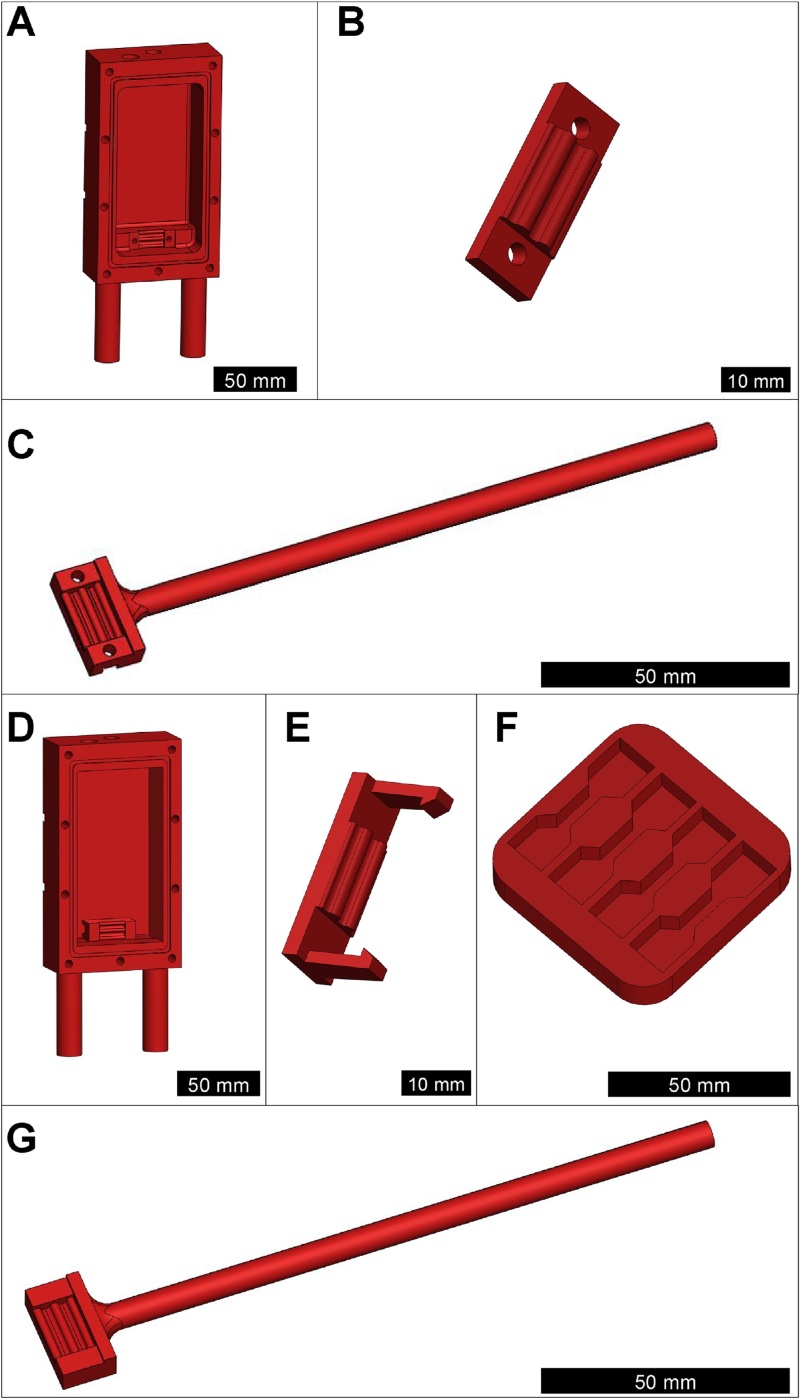
The bioreactor system design described in this study was modeled after a system developed by Kluge et al. [1] and used by colleagues [1,2], but has several significant modifications. Here, the system was designed to be vertically orientated to reduce the potential for off-axis forces. This vertical orientation also eliminated the need for rubber gaskets associated with the moving actuator connectors, which reduced the potential for friction in the system and errors in force measurements. In addition, the bottom grip (i.e., the static grip) was fully integrated into the culture chamber to reduce the number of moving parts. The culture chambers, soft tissue grips, and actuator arm were designed in a computer-aided design (CAD) software package (SolidWorks Corp., Waltham, MA) (Fig. 1, and see supplemental data for the SolidWorks drawing files and STL files). The rectangular culture chambers were 71 × 128 × 29 mm with inner chamber dimensions of 51 × 98 × 26 mm. The grips have a custom waveform pattern that secures a wide range of soft tissues and minimizes slipping during tensile loading. Two custom soft tissue grips were developed, one for mechanical evaluations and the other for use with soft 3D cell-seeded scaffolds in vitro. For mechanical testing, through-holes in the fixed grip and actuator arm grip allowed for tissues to be mounted and secured with stainless steel cap screws and nuts (Fig. 1A–C). For in vitro culture, the grips were designed to have snap-hooks at either end in place of through-holes. Corresponding snap-hook grooves were developed for the actuator arm and static culture chamber grip. This modification eliminated the need for through-holes spanning the depth of the chamber, which reduced the potential for culture medium leakage and allowed for more rapid and sterile mounting of cell-seeded scaffolds (Fig. 1D–G). The culture chambers were equipped with mounting posts to quickly load and secure chambers into the bottom plate of an aluminum load frame using two-piece shaft collars. A through-hole for the moving actuator arm allowed the arm to move with minimal friction. A separate port in the chambers was designed for adding and exchanging cell culture medium. A syringe filter covered the cell culture medium port during in vitro culture, maintaining a sterile environment while allowing for gas exchange within the culture chamber. The front of the chamber was designed to be sealed with a transparent polycarbonate cover that compresses against a rubber o-ring using stainless steel wing-nuts and bolts for quick, tool-free mounting. A list of materials is found in Table 1.
Fig. 1.
Engineering drawings of the custom mechanical bioreactor chambers, grips, and scaffold seeding wells. (A) Chamber, (B) grips, and (C) actuator arm designed for mechanical evaluation of soft tissues. Through-holes in the grips allow for secure mounting of tissues using stainless steel cap screws and nuts to prevent slipping during a pull-to-failure. (D) Cell culture chamber, (E) snap hook grip, and (G) actuator arm for sterile cell culture. Through-holes are eliminated, and the snap hook system successfully secures the cell-seeded scaffolds. (F) Custom wells for seeding scaffolds with cells.
Table 1.
List of materials for the bioreactor system.
| Name | Vendor | Catalog Number | Description |
|---|---|---|---|
| Inventor Dual Extrusion 3D Printer | Flashforge USA | 6970152950192 | 3D printer |
| ABS filament | Flashforge USA | n/a | ABS filament for 3D printer |
| Acetone | Macron Chemicals | n/a | Acetone for waterproofing 3D printed parts |
| Linear Actuator | Haydon Kerk | 35H4N-2.33-915 | Size 14, captive, stepper motor linear actuator |
| Stepper Motor Controller | Peter Norberg Consulting, Inc | AR-BC4E20EU | USB, four stepper motor controller |
| DAQ Chassis | National Instruments | 781425-01 | DAQ-9171, CompactDAQ Chassis |
| DAQ Universal Module | National Instruments | 779781-01 | NI 9219 Universal AI Module |
| Load Cells | Honeywell Sensing & Control | n/a | Model 31 Load cell |
| Polycarbonate | McMaster-Carr | 8574K26 | Clear polycarbonate sheet |
| Wing nuts | McMaster-Carr | 94545A220 | 18-8 Stainless Steel Wing Nut, M4 × 0.7 mm |
| Hex Head Screw | McMaster-Carr | 91287A053 | 18-8 Stainless Steel Hex Head Screw, M4 × 0.7 mm Thread, 40 mm Long |
| O-rings | McMaster-Carr | 9262K716 | Buna-N O-Ring, 2 mm Wide, 100 mm |
| Shaft Coupling | McMaster-Carr | 61005K411 | Clamp-on Rigid Shaft Coupling Type 303 Stainless Steel |
| Thread Adaptor | McMaster-Carr | 98434A126 | 18-8 Stainless Steel Female Hex Thread Adapter 6-32 to M4 × 0.7 mm |
| Female threaded round standoff | McMaster-Carr | 91125A442 | 18-8 Stainless Steel, 1/4" OD, 5/16" Long, 6-32 Thread Size |
| Shaft Collar | McMaster-Carr | 9520T8 | Clamping Two-Piece Shaft Collar for 14 mm Diameter, 2024 Aluminum |
| Quick-Disconnect wire terminals | McMaster-Carr | 72625K74/ 72625K75 | Fully Insulated Heat-Shrink Quick-Disconnect Terminals Male/Female, for 22-18 Wire Gauge |
| Hex Head Cap Screw | McMaster-Carr | 93635A025 | 316 Stainless Steel, M3 × 0.5 mm Thread, 30 mm Long |
| Thin Hex nut | McMaster-Carr | 93935A320 | 316 Stainless Steel, M3 × 0.5 mm Thread |
| Socket Head Cap Screw | McMaster-Carr | 91292A114 | 18-8 Stainless Steel, M3 × 0.5 mm Thread, 12 mm Long |
Fabrication
The 3D drawings (SolidWorks) were sliced into 2D layers using Flashforge Flashprint (Flashforge USA, City of Industry, CA) software with a slice resolution of 2.5 μm. The chambers, grips, and actuator arms were printed with 1.75-mm diameter acrylonitrile butadiene styrene (ABS) plastic filament (Flashforge) using a FlashForge Inventor 3D printer. ABS is an appealing material for use with cell culture as it is chemically and biologically inert [3], and can be sterilized using ethanol [4]. The extruder nozzle was heated to 230 °C and the platform was heated to 110 °C. Each print had 5 shells and a print speed ranging from 50 to 70 mm/s. The culture chambers were printed with a 0.12 mm layer height and 15% infill, while the grips and actuator arms had a 0.12 mm layer height and 30% infill. The ABS plastic culture chambers were waterproofed by treating them with an acetone vapor bath. The bottom of a glass 3 L beaker was covered with acetone (Macron Chemicals, Center Valley, PA) to a height of 3 to 4 mm and heated until boiling. The culture chambers were then lowered into the beaker and covered with Parafilm M (Bermis NA, Neenah, WI) to seal in the acetone vapor. After 5 min, the chambers were removed from the beaker and air-dried for 12 h. The acetone vapor-treated chambers had a smooth and waterproof finish.
Data acquisition and control
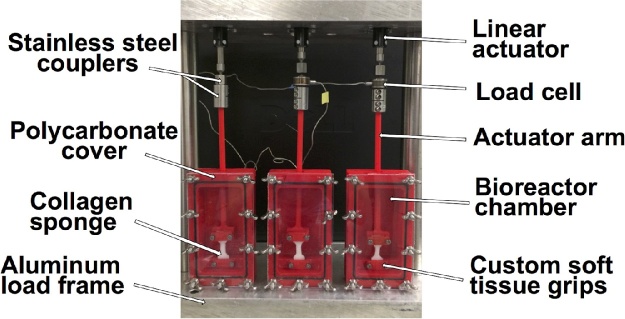
Three size 14 stepper motor linear actuators (Haydon Kerk, Waterbury, CT) were mounted to the top-plate of an aluminum frame (Fig. 2). Stainless steel mounting hardware was used to connect the load cells and actuator arms to each of the linear actuators. Heat-shrink quick-disconnect wire terminals were used to connect the actuator wiring and allow the entire bioreactor system to be easily inserted into a standard cell culture incubator through the sampling port. A 1000 g (9.81 N) capacity load cell (Model 31, Honeywell Sensing and Controls, Columbus, OH) was attached to each linear actuator. The load cells collect force data through a National Instruments (NI) data acquisition device (DAQ) (NI, Austin, TX). Calibration of the load cells was conducted using 18 different calibrated masses. Three consecutive load cell readings were taken for each calibrated mass and a calibration curve was generated. Two additional load cells with 150 g and 500 g capacities (Honeywell) were calibrated using the same procedure, but were not used in the validation experiments described below. However, the load cells can be easily interchanged in the system as needed for different tissues and force capacities. A stepper motor controller board (Peter Norberg, Ferguson, MO) and custom LabView™ programs control the movement of the actuators. To calibrate the actuators, digital images of the actuator grips were taken following actuator movements to 15 different displacement locations. ImageJ (NIH, Bethesda, MD) was used to measure the grip-to-grip displacement from 3 images taken at each location, moving the motor back to a predetermined zero position between captures. Using these calibrated load cells, actuators, and custom LabView™ controls [1], precise cyclic tensile strains can be applied and force and displacement data can be collected. The cyclic program (LabView™) provides user control over strain magnitude, strain rate, number of repetitions (cycles), frequency, and dwell time between stretches. A separate ramp control program can perform pull-to-failure tests to measure tensile mechanical properties of soft tissues and provides user control over strain magnitude, strain rate, and data collection rate.
Fig. 2.
3D printed cell culture chambers mounted into the bioreactor system. Stainless steel couplers attach the actuator arms to the linear actuators and load cells. Clear polycarbonate covers seal the chambers. Custom soft tissue grips secure the scaffolds and prevent slipping during loading. The entire system fits inside a standard cell culture incubator.
Methods validation and results
In vitro bioreactor validation - dynamic mechanical stimulation of stem cells in 3D scaffolds
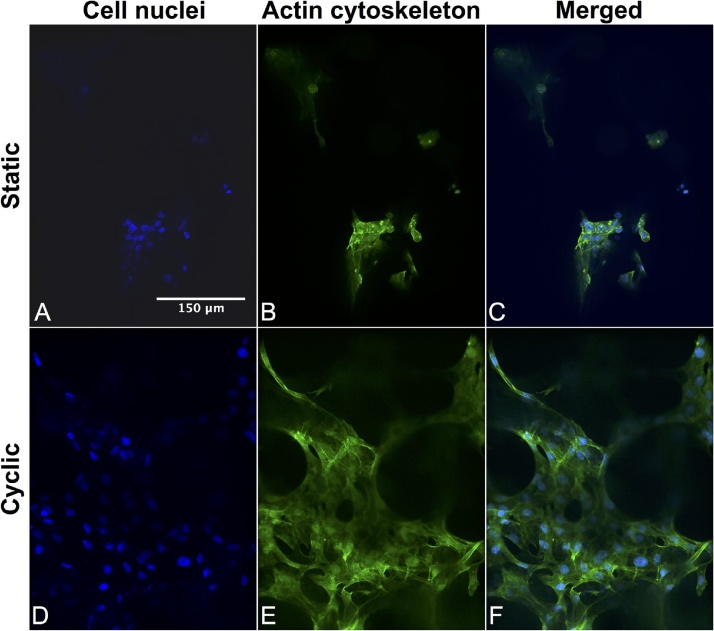
To evaluate the bioreactor for in vitro cell culture, 3D collagen type I sponges (DSM Biomaterials, Exton, PA) were prepared using a protocol previously described [5]. The collagen sponges were cut into dumbbell-shaped tensile specimens (12 mm gauge length and 4 mm width), sterilized overnight on a rocker in 70% ethanol, washed in sterile phosphate buffered saline (PBS) 6 times for 30 min each, and placed into custom 3D printed culture wells for cell seeding (Fig. 1F). Murine mesenchymal stem cells (MSCs) (C3H10T1/2, ATCC, Manassas, VA), a model MSC used in prior tendon tissue engineering studies of cyclically loaded cells [6,7], were cultured in standard growth medium (Dulbecco’s Modified Eagle’s Medium (DMEM), 10% fetal bovine serum (FBS), and 1% Penicillin/Streptomycin) until 70% confluent, and used between passage 3 and 9. MSCs were trypsinized, and then 1 × 106 cells were seeded into each collagen sponge, and incubated for 24 h in the 3D printed culture wells for initial cell attachment. The MSC-seeded collagen sponges (MSC-constructs) were mounted into the bioreactor culture chambers (Fig. 1D, E, G). The chambers were initially sterilized by soaking them overnight in a bath of 70% ethanol. The culture chambers were filled with 40 mL of fresh cell culture medium to ensure the MSC-constructs were fully submerged, even when stretched. MSC-constructs were preloaded to 0.02 N to remove the slack, and the grip-to-grip length of each MSC-construct was measured using digital calipers. Independent displacement control of each actuator ensured that each MSC-construct was cyclically loaded to a peak tensile strain of 10% at a strain rate of 1%/s for 720 cycles/day (0.05 Hz) for three days (n = 3), despite the slight differences in construct lengths after the initial preload was applied. MSC-constructs mounted in the culture chambers and statically loaded (e.g., 0 cycles) were used as controls (n = 3). On day 4, the MSC-constructs were fixed in 10% formalin, stained with FITC-phalloidin (Life Technologies) and 4′,6-diamidino-2-phenylindole (DAPI) to observe the actin cytoskeleton and cell nuclei, respectively, and then imaged on a spinning-disk confocal microscope (Nikon/Andor, Melville, NY). The staining showed that MSCs were present in both the cyclically loaded and static control groups. Furthermore, cyclic tensile strain appeared to increase actin cytoskeleton network formation, intercellular connections, and proliferation by the MSCs when compared to the static controls (Fig. 3), which is consistent with other studies [1,[8], [9], [10]]. These results demonstrate this bioreactor is useful for applying mechanical stimuli to cells in culture.
Fig. 3.
Representative images (20x magnification) of cell nuclei (A, D) and actin cytoskeleton (B, E) of MSCs seeded into collagen sponges and loaded in tension statically (A, B, C) or cyclically (D, E, F) in the bioreactor for 3 days. The merged images of (C, F) of the cell nuclei (blue) and actin cytoskeleton (green) show that cyclic loading in the bioreactor appeared to increase cell proliferation and actin network formation.
Mechanical validation – tensile load frame
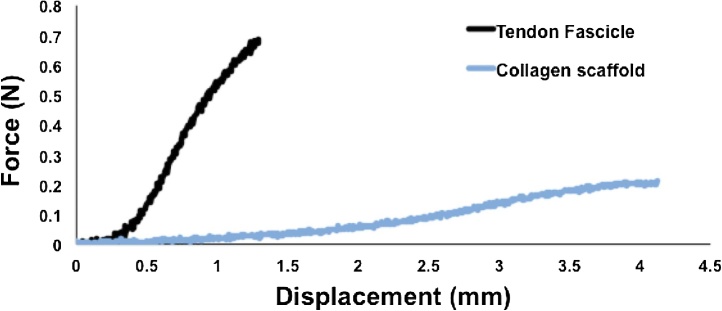
To evaluate the mechanical bioreactor as a small-scale tensile load frame, the mechanical properties of mouse tail tendon fascicles (MTTFs) and collagen sponges (DSM Biomaterials) were measured. MTTFs were isolated from the tails of 2.5-month old wild-type mice (n = 5) with mixed C57BL/6, C3H, 129, and FVB genetic backgrounds that had been used for another University of Idaho IACUC approved study. The MTTFs were removed from the tails in PBS, secured in the mechanical testing grips (Fig. 1A–C) with sandpaper to limit slipping, mounted in the bioreactor, and submerged in PBS. Cross-sectional areas and initial lengths were measured from calibrated digital images using ImageJ (NIH, Bethesda, MD). The MTTFs were preloaded to 0.02 N to remove the slack, and preconditioned for 10 cycles to 5% strain at a strain rate of 1%/s. After preconditioning, the MTTFs were pulled in tension to failure at a strain rate of 1%/s while recording the force and displacement. Results are reported as average ± standard deviation. The custom grips secured the MTTFs (diameter of 216 ± 67 μm) without slipping. The fascicles had a maximum (max) force of 0.59 ± 0.34 N and corresponding displacement of 1.3 ± 0.3 mm, linear-region stiffness of 0.7 ± 0.4 N/mm, max stress of 15.7 ± 4 MPa and corresponding strain of 9.2 ± 2.4%, and linear-region elastic modulus of 266 ± 72 MPa (Table 2). The structural and material properties of MTTFs measured in this study are within the expected range [11,12]. Collagen sponges were also mechanically evaluated using the same mechanical testing protocol. However, the collagen sponges (n = 3) were first cut into dumbbell-shaped specimens with a consistent gauge length of 12 mm and width of 4 mm, as described above for cell culture. The collagen sponges had a max force of 0.24 ± 0.03 N and corresponding displacement of 4.1 ± 0.4 mm, linear-region stiffness of 0.09 ± 0.007 N/mm, max stress of 19 ± 2 kPa and strain of 20.6 ± 2.1%, and linear-region elastic modulus of 143 ± 7 kPa (Table 2). As expected, the collagen sponges are dramatically softer and weaker than the MTTFs, and are consistent with a prior study that reported a tensile elastic modulus of bovine collagen sponges of approximately 50 kPa, and an ultimate stress of 12 kPa [13]. Typical force-displacement curves for the fascicles and collagen sponges are shown in Fig. 4. Overall, we show that our bioreactor is useful as a small-scale tensile load frame.
Table 2.
Mechanical properties of MTTFs and collagen sponges evaluated using the bioreactor system (mean ± standard deviation).
| Material | Max force (N) | Displacement at max force (mm) | Stiffness (N/mm) | Max stress (MPa) | Strain at max stress (%) | Elastic modulus (MPa) |
|---|---|---|---|---|---|---|
| Tendon fascicle | 0.59 ± 0.34 | 1.3 ± 0.3 | 0.7 ± 0.4 | 15.7 ± 4 | 9.2 ± 2.4 | 266 ± 72 |
| Collagen sponge | 0.24 ± 0.03 | 4.1 ± 0.4 | 0.09 ± 0.007 | 0.019 ± 0.002 | 20.6 ± 2.1 | 0.143 ± 0.007 |
Fig. 4.
Representative force-displacement curves for a tendon fascicle and collagen scaffold.
Discussion and conclusion
3DP is rapid, easily customizable, and lower cost in comparison to machined parts. Each chamber assembly (including the actuator arm and grips) costs approximately $4.90 to print. The 3D printed components are reusable and can be sterilized with 70% ethanol between uses, further reducing the cost. As the bioreactor system is currently configured, it can evaluate the tensile mechanical properties of small-scale soft tissues that have maximum failure loads of less than 10 N (currently limited by the maximum force capacity of the load cell). While this limitation could be addressed through use of higher capacity load cells, the linear actuators and ABS plastic actuator arms and grips further limit the maximum force capacity. The size 14 linear actuators used here have a maximum force capacity of 222 N. Given that ABS has a reported Young’s Modulus in tension of about 1600 MPa and tensile yield stress of 39 MPa [14], it is unlikely that the ABS actuator arm noticeably deforms under the small loads applied during in vitro culture or mechanical evaluations. Based on the cross-sectional area of the actuator arm (6-mm diameter) and 30% in-fill used during 3DP, we estimate the strain in the actuator arm at the maximum load cell capacity (10 N) to be approximately 0.0066% and 300 N would be required to reach the yield point. However, for mechanically evaluating larger tissues, a traditional materials testing load frame (e.g., an Instron) would be more appropriate.
In conclusion, we demonstrated a method for the design and fabrication of a functional, low-cost, and highly customizable 3D printed mechanical bioreactor that is useful for applications in mechanobiology and tissue engineering. Our system was able to evaluate the mechanical properties of small soft tissues (tail tendon fascicles) and engineered tissue scaffolds. Additionally, our bioreactor was successfully used to mechanically stimulate stem cells in culture for 3 days, demonstrating its value for in vitro cell culture. Future studies using this bioreactor system will focus on longer-term cell culture and evaluating the influence of mechanical stimuli on engineered tissue formation.
Author disclosure statement
No competing financial interests exist.
Acknowledgements
The authors would like to thank DSM Biomaterials for the generous donation of the collagen sponges. The authors also acknowledge Hee Jun Um (University of Idaho) and Dr. Jonathan Kluge (Vaxess Inc.) for help with the LabView™ codes. This project was made possible through funding from the University of Idaho, Office of Undergraduate Research (Summer Undergraduate Research Fellowship awarded to Abigail R. Raveling) and Honors Program (to Abigail R. Raveling), and the INBRE Program, NIH Grant No. P20 GM103408 (National Institute of General Medical Science).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.mex.2018.08.001.
Contributor Information
Abigail R. Raveling, Email: rave1579@vandals.uidaho.edu.
Sophia K. Theodossiou, Email: theo4146@vandals.uidaho.edu.
Nathan R. Schiele, Email: nrschiele@uidaho.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Kluge J.A., Leisk G.G., Cardwell R.D., Fernandes A.P., House M., Ward A., Dorfmann A.L., Kaplan D.L. Bioreactor system using noninvasive imaging and mechanical stretch for biomaterial screening. Ann. Biomed. Eng. 2011;39(5):1390–1402. doi: 10.1007/s10439-010-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youngstrom D.W., Rajpar I., Kaplan D.L., Barrett J.G. A bioreactor system for in vitro tendon differentiation and tendon tissue engineering. J. Orthop. Res. 2015;33(6):911–918. doi: 10.1002/jor.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su C.-K., Yen S.-C., Li T.-W., Sun Y.-C. Enzyme-immobilized 3D-printed reactors for online monitoring of rat brain extracellular glucose and lactate. Anal. Chem. 2016;88(12):6265–6273. doi: 10.1021/acs.analchem.6b00272. [DOI] [PubMed] [Google Scholar]
- 4.Neches R.Y., Flynn K.J., Zaman L., Tung E., Pudlo N. On the intrinsic sterility of 3D printing. Peer J. 2016;4(e2661) doi: 10.7717/peerj.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler D.L., Gooch C., Kinneberg K.R.C., Boivin G.P., Galloway M.T., Nirmalanandhan V.S., Shearn J.T., Dyment N.A., Juncosa-Melvin N. The use of mesenchymal stem cells in collagen-based scaffolds for tissue-engineered repair of tendons. Nat. Protoc. 2010;5(5):849–863. doi: 10.1038/nprot.2010.14. [DOI] [PubMed] [Google Scholar]
- 6.Scott A., Danielson P., Abraham T., Fong G., Sampaio A.V., Underhill T.M. Mechanical force modulates scleraxis expression in bioartificial tendons. J. Musculoskelet. Neuronal Interact. 2011;11(2):124–132. [PubMed] [Google Scholar]
- 7.Li Y.H., Ramcharan M., Zhou Z.P., Leong D.J., Akinbiyi T., Majeska R.J., Sun H.B. The role of scleraxis in fate determination of mesenchymal stem cells for tenocyte differentiation. Sci. Rep. 2015;5 doi: 10.1038/srep13149. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Gould R.A., Chin K., Santisakultarm T.P., Dropkin A., Richards J.M., Schaffer C.B., Butcher J.T. Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture. Acta Biomater. 2012;8(5):1710–1719. doi: 10.1016/j.actbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai D., Kii I., Nakagawa K., Matsumoto H.N., Takahashi M., Yoshida S., Hosoya T., Takakuda K., Kudo A. Remodeling of actin cytoskeleton in mouse periosteal cells under mechanical loading induces periosteal cell proliferation during bone formation. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0024847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ralphs J.R., Waggett A.D., Benjamin M. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002;21(1):67–74. doi: 10.1016/s0945-053x(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 11.Reuvers J., Thoreson A.R., Zhao C., Zhang L., Jay G.D., An K.N., Warman M.L., Amadio P.C. The mechanical properties of tail tendon fascicles from lubricin knockout, wild type and heterozygous mice. J. Struct. Biol. 2011;176(1):41–45. doi: 10.1016/j.jsb.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derwin K.A., Soslowsky L.J. A quantitative investigation of structure-function relationships in a tendon fascicle model. J. Biomech. Eng. 1999;121:598. doi: 10.1115/1.2800859. [DOI] [PubMed] [Google Scholar]
- 13.Ghodbane S.A., Dunn M.G. Physical and mechanical properties of linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons. J. Biomed. Mater. Res. A. 2016;104A:2685–2692. doi: 10.1002/jbm.a.35813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arivazhagan A., Masood S.H. Dynamic mechanical properties of ABS material processed by fused deposition modelling. Int. J. Eng. Res. Appl. (IJERA) 2012;2(3):2009–2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.