Highlights
-
•
MCH neurons contain neither VGAT nor VGLUT2.
-
•
Majority of orexin neurons contain VGLUT2.
-
•
MCH neurons do not contain orexin.
Abbreviations: CeA, central nucleus of amygdala; GABA-γ, Aminobutyric acid; Gad1, Glutamate decarboxylase 1; GAD65, glutamic acid decarboxylase-65; GAD67, glutamic acid decarboxylase-67; MCH, melanin concentrating hormone; NREM, non-rapid eye movement; REM, rapid eye movement; RTN, reticular thalamic nucleus; SSC, somatosensory cortex; VGAT, vesicular GABA transporter; VGLUT2, vesicular glutamate transporter-2
Keywords: GABA, Glutamate, Sleep, Arousal
Abstract
The neuropeptides orexin and melanin-concentrating hormone (MCH), as well as the neurotransmitters GABA (γ-Aminobutyric acid) and glutamate are chief modulators of the sleep-wake states in the posterior hypothalamus. To investigate co-expression of vesicular GABA transporter (VGAT, a marker of GABA neurons) and the vesicular glutamate transporter-2 (VGLUT2, a marker of glutamate neurons) in orexin and MCH neurons, we generated two transgenic mouse lines. One line selectively expressed the reporter gene EYFP in VGAT+ neurons, whereas the other line expressed reporter gene tdTomato in VGLUT2+ neurons. Co-localization between these genetic reporters and orexin or MCH immunofluorescent tags was determined using 3D computer reconstructions of Z stacks that were acquired using a multiphoton laser confocal microscope. Our results demonstrated that MCH neurons expressed neither VGAT nor VGLUT2, suggesting MCH neurons are a separate cluster of cells from VGAT+ GABAergic neurons and VGLUT2+ glutamatergic neurons. Moreover, most orexin neurons expressed VGLUT2, indicating these neurons are glutamatergic. Our data suggested that in the posterior hypothalamus there are four major distinct groups of neurons: VGAT+, orexin+/VGLUT2+, orexin-/VGLUT2+, and MCH neurons. This study facilitated our understanding of the role of these neurotransmitters and neuropeptides in relation to sleep/wake regulation.
Introduction
Neurons containing the neuropeptide orexin (also known as hypocretin) and melanin concentrating hormone (MCH) are solely located in the posterior hypothalamus (De Lecea et al., 1998; Broberger, 1999). The orexin and MCH neurons are distinct cell groups, but they share similar target regions (Elias et al., 1998, Elias et al., 2008; Peyron et al., 1998). The orexin neurons are implicated in regulating arousal because loss of the peptide or ablation of the neurons results in excessive sleepiness and the neurodegenerative sleep disorder narcolepsy (Nishino et al., 2000). Administration of orexin into the brain induces waking whereas pharmacological blockade of both receptors of orexin induces sleep (Mieda et al., 2004; Mang et al., 2012). In addition, optogenetic stimulation of orexin neurons rapidly induces arousal (Adamantidis et al., 2007). Orexin neurons are active during waking and silent in sleep, which is consistent with their role in maintaining arousal (Lee et al., 2005; Mileykovskiy et al., 2005). By contrast, MCH neurons are quiet during waking but active in sleep, especially rapid-eye movement (REM) sleep (Hassani et al., 2009). Moreover, optogenetic stimulation of MCH neurons during waking induces sleep in mice and rats (Jego et al., 2013; Konadhode et al., 2013; Blanco-Centurion et al., 2016).
Since orexin and MCH neurons are important triggers of arousal and sleep, it is crucial to determine whether or not other neurotransmitters are co-expressed in these neurons as well. Of particular interest is the presence of the fast neurotransmitters glutamate and GABA. The release of glutamate and GABA could modulate the action of orexin and MCH on downstream targets (Gao and Van den Pol, 2012). For instance, it has been reported that glutamate is co-expressed by orexin neurons (Torrealba et al., 2003). Orexin has a depolarizing effect and the co-release of glutamate could prolong the excitatory effect of the peptide (Schone et al., 2014). By contrast, the peptide MCH hyperpolarizes neurons (Rao et al., 2008; Jego et al., 2013) and co-release of GABA may prolong its inhibitory effect (Gao and Van den Pol, 2001). GABA (Elias et al. 2008; Del Cid-Pellitero et al., 2012) and the GABA-synthesizing enzyme GAD67 are present in MCH neuron. Also, it has been shown that GABA is released in response to optogenetic stimulation of MCH terminals in the tuberomammillary nucleus (TMN) area (Jego et al., 2013). However, a recent study found that MCH terminals at the lateral septal nucleus release glutamate, suggesting that glutamate is also expressed in MCH neurons (Chee et al., 2015).
To expedite studies on the role of orexin and MCH in sleep, we generated genetically engineered mice to identify GABAergic neurons using the vesicular GABA transporter (VGAT) and glutamatergic neurons expressing vesicular glutamate transporter-2 (VGLUT2). VGAT is functionally coupled with GAD65 and GABA, and is considered a reliable marker of neurons containing GABA (Jin et al., 2003; Venner et al., 2016; Herrera et al., 2016). Similarly, VGLUT2 is a reliable marker of glutamatergic neurons (Borgius et al., 2010). The aim of this study was to investigate the co-localization of VGLUT2 and VGAT in orexin and MCH neurons.
Experimental procedures
Ethics statement
All manipulations performed on the mice adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Medical University of South Carolina (protocol #3267) and the Ralph H. Johnson VA Institutional Animal Care and Use Committee (protocol #537).
Transgenic mice generation
All mice used in this study were obtained from the Jackson Laboratory (Bar Harbor, ME). VGAT-EYFP mice were created by crossing R26R-EYFP mice (stock #006148) with VGAT-ires-Cre Knock-In mice (stock #016962). R26R-EYFP mice have a loxP-flanked STOP sequence followed by the EYFP gene. When R26R-EYFP mice were bred with VGAT-ires-Cre knock-in mice, Cre recombinase deleted the STOP sequence allowing EYFP expression among VGAT+ neurons in the offspring. Similarly, VGLUT2-tdTomato mice were created by crossing VGLUT2-ires-Cre Knock-In mice (stock #016963) with Ai14 mice (stock #007914). Ai14 mice harbor a loxP-flanked STOP cassette preventing the expression of the fluorescent protein tdTomato. When Ai14 mice were bred with VGLUT2-ires-Cre mice, tdTomato was observed in all VGLUT2+ neurons of the offspring. Four VGAT-EYFP mice (two male and two female) and four VGLUT2-tdTomato mice (two male and two female) were used in this study. All mice used were 3-6 months old. They were fed ad libitum and kept on a 12/12 h light/dark cycle.
Immunofluorescent staining and multiphoton laser scanning microscopy
The mice were deeply anesthetized with overdose isoflurane (5–10%) and perfused transcardially with 20 ml PBS followed by 50 ml 10% formalin during the day (lights-on cycle) between 10AM-12PM (Zeitgeber time 4–6). Brains from these mice were sectioned at 40 μm thickness on a compresstome instrument (Precisionary Instruments, Greenville, NC). One-in-four series of coronal sections of the brain were incubated at room temperature for 24 hours with goat anti-orexin antibody (1:500, Santa Cruz Biotechnology, Dallas TX) and rabbit anti-MCH antibody (1:500, Phoenix Pharmaceuticals, Inc. CA), followed by 1 h incubation with two distinct Alexa Fluor secondary antibodies (1:500; Invitrogen, Carlsbad, CA). In VGAT-EYFP mice orexin neurons were labeled with Donkey anti-goat Alexa Fluor-350, and MCH neurons were labeled with donkey anti-rabbit Alexa Fluor-568. In VGLUT2-tdTomato mice orexin neurons were labeled with Donkey anti-goat Alexa Fluor-488 and MCH neurons were labeled with donkey anti-rabbit Alexa Fluor-350. Immuno-reactive neurons and genetic marker expression in the hypothalamus were imaged with a multiphoton laser scanning microscope at a magnification of 20X (Leica TCS SP8-MP). Co-localization was determined using computer reconstructions of Z stacks with an optical section of 1 μm. Immuno-reactive orexin and MCH neurons were counted on digitized images of one-in-four series of sections containing posterior hypothalamus regions with MCID image analysis software (St. Catharines, ON, Canada).
Results
Orexin neurons and MCH neurons do not contain VGAT
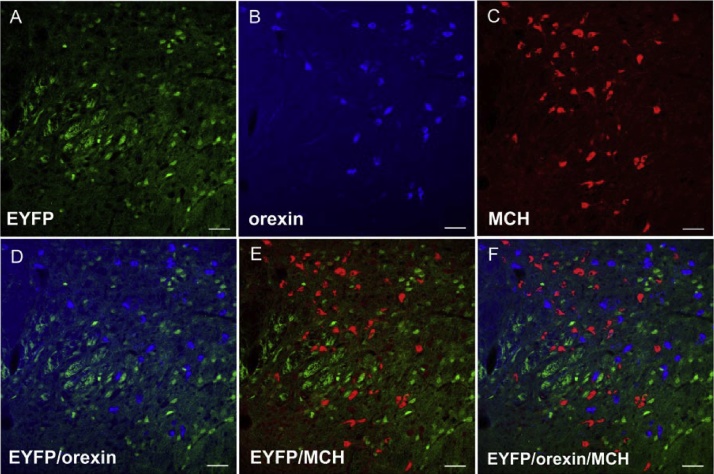
EYFP, the genetic reporter linked to VGAT, was extensively expressed within brain regions previously known to contain abundant GABAergic neurons, such as the central nucleus of amygdala (CeA) and the reticular thalamic nucleus (RTN) (Fig. 1A and B). EYFP was also observed in our region of interest: the posterior hypothalamus. The distribution of EYFP in the posterior hypothalamus of VGAT-EYFP mice is shown in Fig. 2: orexin neurons are illustrated in blue and MCH neurons are illustrated in red. EYFP+ neurons were tightly intermingled with orexin and MCH neurons but there was very little overlap among them (Table 1; Fig. 2D–F).
Fig. 1.
EYFP+ neurons in CeA (A) and RTN (B) in VGAT-EYFP transgenic mice; Tdtomato+ neurons in somatosensory cortex (C), and CeA (D) of VGLUT2-tdTomato transgenic mice. Scale bar = 45 μm.
Fig. 2.
Distribution of VGAT+ (EYFP+, A), orexin+ (B) and MCH+ (C) neurons in posterior hypothalamus of VGAT-EYFP mice. No co-localization was found between EYFP and orexin (D), EYFP and MCH (E), orexin and MCH (F). Scale bar = 45 μm.
Table 1.
Cell counts and co-expression percent in VGAT-EYFP and VGLUT2-tdTomato groups. Orexin and MCH neurons were counted in both hemispheres and the numbers presented as the average counts per section of each mouse (Mean ± SEM, n = 4).
| (MCH + EYFP)/MCH | (Orexin + EYFP)/orexin | (MCH + orexin)/MCH | (MCH + tdTomato)/MCH | (orexin + tdTomato)/orexin | |
|---|---|---|---|---|---|
| VGAT-EYFP mice (n = 4) | 0.82 ± 0.11/114.25 ± 7.24 (0.71%) | 1.04 ± 0.12/131.00 ± 8.55 (0.79%) | 0.58 ± 0.12/114.25 ± 7.24 (0.51%) | – | – |
| VGLUT2-tdTomato mice (n = 4) | – | – | 1.08 ± 0.07/110.75 ± 10.16 (0.98%) | 1.03 ± 0.05/110.75 ± 10.16 (0.93%) | 108.85 ± 9.33/126.28 ± 8.74 (86.20%) |
Orexin neurons contain VGLUT2
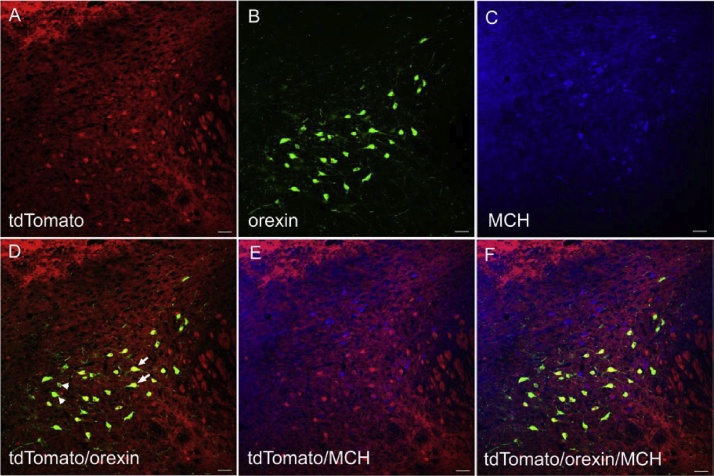
TdTomato, the reporter linked to VGLUT2, was heavily expressed within regions containing ample glutamatergic neurons, such as the somatosensory cortex (SSC, mainly in layer III through V) (Ziegler et al., 2002; Liguz-Lecznar and Skangiel-Kramska, 2007) while tdTomato+ cells were rarely found in CeA, where GABAergic neurons are prevalent (Fig. 1C and D). TdTomato was also observed in the posterior hypothalamus of VGLUT2-tdTomato mice although the density of tdTomato+ neurons was much lower compared to the EYFP labeled VGAT+ neurons seen in the VGAT-EYFP mice (Fig. 3A). We found that 86.20% of orexin neurons were also tdTomato+, indicating those neurons express VGLUT2 (Fig. 3D). By contrast, co-expression of MCH and tdTomato were rarely observed (0.93%, Table 1; Fig. 3E).
Fig. 3.
Distribution of VGLUT2+ (tdTomato+, A), orexin+ (B) and MCH+ (C) neurons in posterior hypothalamus of VGLUT2-tdTomato mice. Many orexin neurons were labeled with tdTomato (arrows) but some of them were not (arrowheads). Co-localization between tdTomato and MCH (E), orexin and MCH (F) were rarely found. Scale bar = 45 μm. Arrows and arroheads were used to show example neurons only.
Orexin neurons and MCH neurons are separate groups
In both VGAT-EYFP mice and VGLUT2-tdTomato mice, orexin does not co-localize with MCH (Table 1; Figs. Figure 2F and Figure 3F).
Discussion
The primary findings of the present study were that in the posterior hypothalamus of mice MCH neurons were neither VGAT+ nor VGLUT2+, while 86.20% of the orexin neurons were VGLUT2+. Overall, using these double transgenic mice we identified four major separate populations of neurons in the posterior hypothalamus: VGAT+, orexin+/VGLUT2+, orexin-/VGLUT2+, and MCH+ neurons.
VGAT was absent in orexin and MCH neurons
Our results indicate that neither orexin neurons nor MCH neurons contained VGAT. This is not surprising for orexin because orexin is an excitatory neuropeptide (De Lecea et al., 1998) and orexin neurons co-release glutamate, not GABA (Schone et al., 2014). But this does not exclude the possibility that orexin neurons also contain GABA since some orexin neurons have been found to express Gad1 gene which encodes GAD65, which is required for GABA synthesis (Mickelsen et al., 2017). Indeed, Apergis-Schoute et al. (2015) found that optogenetic stimulation of orexin neuron may release GABA to suppress MCH neurons.
Previous studies have noted that MCH neurons are GABAergic and inhibitory. For instance, MCH neurons co-express mRNA of GABA-synthesizing enzymes GAD67 and GAD65 (Harthoorn et al., 2005; Elias et al., 2001). However, in our VGAT-EYFP transgenic mice we did not detect co-expression of MCH and VGAT, indicating MCH neurons may be a distinct group of cells from VGAT+ GABA neurons. Consistent with our finding, recent studies have used similar transgenic mice models and demonstrated that the posterior hypothalamus GAD65+ (Karnani et al., 2013), or VGAT+ neurons (Jennings et al., 2015; Venner et al., 2016; Kosse et al., 2017), do not co-express MCH. Although VGAT, GAD65 and GAD67 are all markers for GABAergic neurons they are not always co-expressed in the same neuron in the hypothalamus (Jin et al., 2003; Romanov et al., 2017). Another study (Kosse et al., 2017) demonstrated that only 50% of GAD65+ hypothalamic neurons are also VGAT+. A recent study using genetic tagging of MCH neurons performed single cell resolution gene profiling corroborating that MCH neurons express GABA synthetic enzyme Gad1 but not VGAT (Mickelsen et al., 2017). Nevertheless, the absence of VGAT or GAD65 in MCH neurons does not definitively indicate that the MCH neurons are not GABAergic. They could potentially still be GABAergic by synthesizing GABA with GAD67 in the absence of a vesicular GABA release pathway. However, since VGAT is an essential component for GABA synaptic transmission MCH neurons likely synthesize GABA for local use only.
Optogenetic stimulation of MCH terminals evoked GABAA-mediated inhibitory postsynaptic currents (IPSC) in postsysynaptic histaminergic (HA) neurons in brain slices (Jego et al., 2013). One possible explanation as to why intense optogenetic stimulation (20 Hz) increased the number of inhibitory postsynaptic potentials could be that GABA transporters are electrogenic symporters that also require the energy of the Na+ electrochemical gradient for uptaking GABA (Scimemi, 2014). Thus under the conditions of abnormally high stimulation of MCH terminals, a depleted Na+ electrochemical gradient and constant depolarization might have led to the GABA transporter to work as antiporter; e.g. leaking GABA. When MCH stimulation was done at physiological rates, 1 Hz, no increase in the frequency of IPSP was observed (Hassani et al., 2009; Jego et al., 2013), suggesting that MCH neurons under normal physiological conditions do not release GABA synaptically.
VGLUT2 expressed in orexin but not MCH neurons
Here we provided direct evidence that nearly 86.20% of orexin neurons contain VGLUT2, and hence can be considered glutamatergic (Fig. 3D). In situ hybridization studies (Kaneko et al., 2002) have revealed the mRNA expression of the other vesicular glutamate transporter, VGLUT1, within the hypothalamus of rodents. Hence we cannot rule out that the remainder of orexin neurons that did not co-expresses VGLUT2 in our study, could actually contain VGLUT1 and turn out to also be glutamatergic. In any case, resonant modes of excitatory signal may play a crucial role for fine-tuning arousal levels in response to orexin neuronal activation. Postsynaptic targets of orexin neurons might receive two separate (or simultaneous) excitatory signals via orexin receptors and glutamate receptors that may work in different or overlapping timescales.
Recently Mickelsen et al. (2017) found that VGLUT2 mRNA was expressed in virtually every MCH neuron, suggesting MCH neurons are glutamatergic. Another study (Chee et al., 2015) also detected that the majority of MCH neurons expressed VGLUT2 and a subpopulation of MCH neurons released glutamate in the lateral septal nucleus. However, we detected very little co-localization between MCH and VGLUT2 in our VGLUT2-tdTomato transgenic mice. There are a few considerations to examine in order to potentially account for the discrepancy among these studies. Firstly, the specificity of the transgenic animal models used in these studies varies. Based on Mickelsen et al. (2017) study, VGAT-ires-Cre Knock-In mice (stock #016962) and VGLUT2-ires-Cre Knock-In mice (stock #016963), when bred with Ai3 EYFP reporter mice, displayed almost 100% high specificity (no overlapping between VGAT expression and VGLUT2 expression), while the Pmch-Cre mice (stock #014099) used in both Chee’s and Mickelsen’s studies had only 77% specificity, meaning that there is a 23% chances that non-MCH neurons might be stimulated when Pmch-Cre mice were used to express ChR2 in MCH neurons. Secondly, the correlation between mRNA level and actual peptide level varies. The mRNA transcripts do not necessarily correlate with protein expression and there may be a discrepancy between the two (Citri et al., 2011). Moreover, quantifying mRNA content with high sensitivity single-cell qPCR data “may or may not be a faithful, linear representation of translated protein” (Mickelsen et al., 2017). Additionally, antibody’s specificity may be an issue too. In Chee et al. (2015) study, a laboratory-made antibody was used to detect MCH while we used commercial antibody from Phoenix Pharmaceuticals, Inc. CA to detect MCH. Also, Chicken anti-GFP antibody was used to detect the expression of reporter gene in Chee’s study while we used the direct endogenous expression of reporter genes. Lastly, the frequency used for photo-stimulation matters. As we discussed above about the GABA releasing from MCH neurons, a similar situation may also apply to the glutamate releasing from MCH neurons in Chee et al. (2015) study produced by 10 Hz photo-stimulation, which is much higher than the physiological firing rate (1 Hz) of MCH neurons. Nevertheless, further studies at the peptide level are still needed to explore the neurochemical complexity and possible plasticity of the MCH neurons.
Overall, our approach utilizing transgenic mice facilitates future studies that hope to identify and target potential GABA or glutamate neurons in the brain. Results from this study help us to better understand the role of these neurotransmitters and the role of neuropeptides on sleep/wake regulation, as well as other physiological processes including feeding, motor regulation, motivation regulation and pain modulation (Zhang et al., 2013; Diniz and Bittencourt, 2017; Razavi and Hosseinzadeh, 2017; Jang et al., 2018)
Conflict of interest statement
The Authors declare no conflict of interests.
Acknowledgments
This work was supported by Medical Research Service of the Department of Veterans Affairs and the National Institutes of Health (grants number 1R01NS096151 and 1R21NS101469). Ms. Emmaline Bendell, Ms. Bingyu Zou and Dr. Ying Sun performed the animal breeding and histology; Dr. Carlos Blanco-Centurion performed the microscopy and cell counting. Dr. Meng Liu designed the study and drafted the manuscript. Dr. Priyattam Shiromani edited the manuscript. We thank Dr. Siwei Luo for help on proof reading.
Contributor Information
Carlos Blanco-Centurion, Email: blancoce@musc.edu.
Emmaline Bendell, Email: bendell@musc.edu.
Bingyu Zou, Email: zoub@musc.edu.
Ying Sun, Email: sunyi@musc.edu.
Priyattam J. Shiromani, Email: shiroman@musc.edu.
Meng Liu, Email: liumen@musc.edu.
References
- Adamantidis A.R., Zhang F., Aravanis A.M., Deisseroth K., de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apergis-Schoute J., Iordanidou P., Faure C., Jego S., Schöne C., Aitta-Aho T., Adamantidis A., Burdakov D. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. J. Neurosci. 2015;35:5435–5441. doi: 10.1523/JNEUROSCI.5269-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Centurion C., Liu M., Konadhode R.R., Zhang X., Pelluru D., Van den Pol A.N., Shiromani P.J. Optogenetic activation of melanin-concentrating hormone neurons increases non-rapid eye movement and rapid eye movement sleep during the night in rats. Euro J Neurosci. 2016;44:2846–2857. doi: 10.1111/ejn.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 1999;848:101–113. doi: 10.1016/s0006-8993(99)01977-0. [DOI] [PubMed] [Google Scholar]
- Borgius L., Restrepo C.E., Leao R.N., Saleh N., Kiehn O. A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol Cellr Neurosci. 2010;45:245–257. doi: 10.1016/j.mcn.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Chee M.J., Arrigoni E., Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J Neurosci. 2015;35:3644–3651. doi: 10.1523/JNEUROSCI.4187-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A., Pang Z.P., Südhof T.C., Wernig M., Malenka R.C. Comprehensive qPCR profiling of gene expression in single neuronal cells. Nat Protoc. 2011;7:1–10. doi: 10.1038/nprot.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lecea L., Kilduff T.S., Peyron C., Gao X., Foye P.E., Danielson P.E. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cid-Pellitero E., Jones B.E. Immunohistochemical evidence for synaptic release of GABA from melanin-concentrating hormone containing varicosities in the locus coeruleus. Neuroscience. 2012;223:269–276. doi: 10.1016/j.neuroscience.2012.07.072. [DOI] [PubMed] [Google Scholar]
- Diniz G.B., Bittencourt J.C. The melanin-concentrating hormone as an integrative peptide driving motivated behaviors. Front. Syst. Neurosci. 2017;11:32. doi: 10.3389/fnsys.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias C.F., Lee C.E., Kelly J.F., Ahima R.S., Kuhar M., Saper C.B., Elmquist J.K. Characterization of CART neurons in the rat and human hypothalamus. J. Comp. Neurol. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- Elias C.F., Saper C.B., Maratos-Flier E., Tritos N.A., Lee C., Kelly J. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J. Comp. Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- Elias C.F., Sita L.V., Zambon B.K., Oliveira E.R., Vasconcelos L.A., Bittencourt J.C. Melanin-concentrating hormone projections to areas involved in somatomotor responses. J. Chem. Neuroanat. 2008;35:188–201. doi: 10.1016/j.jchemneu.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Gao X.B., Van den Pol A.N. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J. Physiol. 2001;533:237–252. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.B., Van den Pol A.N. Melanin-concentrating hormone depresses L-, N-, and P/Q-type voltage-dependent calcium channels in rat lateral hypothalamic neurons. J. Physiol. 2012;542:273–286. doi: 10.1113/jphysiol.2002.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harthoorn L.F., Sane A., Nethe M., Van Heerikhuize J.J. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol. Neurobiol. 2005;25:1209–1223. doi: 10.1007/s10571-005-8184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani O.K., Lee M.G., Jones B.E. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C.G., Cadavieco M.C., Jego S., Ponomarenko A., Korotkova T., Adamantidis A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 2016;19:290–298. doi: 10.1038/nn.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J.H., Park J.Y., Oh J.Y., Bae S.J., Jang H., Jeon S., Kim J., Park H.J. Novel analgesic effects of melanin-concentrating hormone on persistent neuropathic and inflammatory pain in mice. Sci. Rep. 2018;8:707. doi: 10.1038/s41598-018-19145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego S., Glasgow S.D., Herrera C.G., Ekstrand M., Reed S.J., Boyce R., Friedman J., Burdakov D., Adamantidis A.R. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat. Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J.H., Ung R.L., Resendez S.L., Stamatakis A.M., Taylor J.G., Huang J., Veleta K., Kantak P.A., Aita M., Shilling-Scrivo K., Ramakrishnan C., Deisseroth K., Otte S., Stuber G.D. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160:516–527. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Wu H., Osterhaus G., Wei J., Davis K., Sha D., Floor E., Hsu C.C., Kopke R.D., Wu J.Y. Demonstration of functional coupling between gamma – aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4293–4298. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Fujiyama F., Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J. Comp. Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Karnani M.M., Szabó G., Erdélyi F., Burdakov D. Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin- and melanin-concentrating hormone neurons. J. Physiol. 2013;591:933–953. doi: 10.1113/jphysiol.2012.243493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konadhode R.R., Pelluru D., Blanco-Centurion C., Zayachkivsky A., Liu M., Uhde T. Optogenetic Stimulation of MCH Neurons Increases Sleep. J. Neurosci. 2013;33:10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosse C., Schöne C., Bracey E., Burdakov D. Orexin-driven GAD65 network of the lateral hypothalamus sets physical activity in mice. Proc. Natl. Acad. Sci. U. S. A. 2017;114:4525–4530. doi: 10.1073/pnas.1619700114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.G., Hassani O.K., Jones B.E. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J. Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguz-Lecznar M., Skangiel-Kramska J. Vesicular glutamate transporters VGLUT1 and VGLUT2 in the developing mouse barrel cortex. Int. J. Dev. Neurosci. 2007;25:107–114. doi: 10.1016/j.ijdevneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Mang G.M., Dürst T., Bürki H., Imobersteg S., Abramowski D., Schuepbach E., Hoyer D., Fendt M., Gee C.E. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep. 2012;35:1625–1635. doi: 10.5665/sleep.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen L.E., Kolling F.W., Chimileski B.R., Fujita A., Norris C., Chen K., Nelson C.E., Jackson A.C. Neurochemical heterogeneity among lateral hypothalamic hypocretin/orexin and melanin-concentrating hormone neurons identified through single-cell gene expression analysis. eNeuro. 2017;4(5) doi: 10.1523/ENEURO.0013-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M., Willie J.T., Hara J., Sinton C.M., Sakurai T., Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskiy B.Y., Kiyashchenko L.I., Siegel J.M. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S., Ripley B., Overeem S., Lammers G.L., Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Peyron C., Tighe D.K., van den Pol A.N., de Lecea L., Heller H.C., Sutcliffe J.G., Kilduff T.S. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y., Lu M., Ge F., Marsh D.J., Qian S., Wang A.H., Picciotto M.R., Gao X.B. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J. Neurosci. 2008;28:9101–9110. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi B.M., Hosseinzadeh H. A review of the role of orexin system in pain modulation. Biomed. Pharmacother. 2017;90:187–193. doi: 10.1016/j.biopha.2017.03.053. [DOI] [PubMed] [Google Scholar]
- Romanov R.A., Zeisel A., Bakker J., Girach F., Hellysaz A., Tomer R. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat. Neurosci. 2017;20:176–188. doi: 10.1038/nn.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schone C., Apergis-Schoute J., Sakurai T., Adamantidis A., Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7:697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A. Plasticity of GABA transporters: an unconventional route to shape inhibitory synaptic transmission. Front. Cell Neurosci. 2014;8:128. doi: 10.3389/fncel.2014.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrealba F., Yanagisawa M., Saper C.B. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience. 2003;119:1033–1044. doi: 10.1016/s0306-4522(03)00238-0. [DOI] [PubMed] [Google Scholar]
- Venner A., Anaclet C., Broadhurst R.Y., Saper C.B., Fuller P.M. A novel population of wake-promoting gabaergic neurons in the ventral lateral hypothalamus. Curr. Biol. 2016;26:2137–2143. doi: 10.1016/j.cub.2016.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Y., Yu L., Zhuang Q.X., Zhu J.N., Wang J.J. Central functions of the orexinergic system. Neurosci. Bull. 2013;29:355–365. doi: 10.1007/s12264-012-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D.R., Cullinan W.E., Herman J.P. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J. Comp. Neurol. 2002;448:217–229. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]