Abstract
The current study aimed to test the effect of Moringa oleifera extract (MOE), vitamin (Vit) C, and sodium bicarbonate (NaHCO3) on heat stress (HS)-induced alterations in rabbits. Five groups of rabbits were designed as control, HS, HS + MOE, HS + Vit C, and HS + NaHCO3. HS groups were exposed to high temperatures, while treatments were given in drinking water for 6 weeks. Levels of blood cortisol, leptin, IFN-γ, TNF-α, and IL-10 were assayed using ELISA, while adrenaline was assayed calorimetrically. Expression of HSP70, FOXP3, T cell receptor (TCR) γ, and δ mRNA was tested using real-time (RT)-PCR, while HSP70 protein expression was tested using western blotting in liver and kidney tissues. Infiltration of regulatory T cells (Treg; CD25+) and NK (CD56+) cells were tested using immunohistochemistry (IHC). The levels of liver enzymes (ALT & AST), urea, and creatinine were assayed calorimetrically, while body weight gain (BWG) and feed conversion ratio (FCR) were calculated. The results showed increased levels of cortisol, adrenaline, leptin, IFN-γ, TNF-α, ALT, AST, urea, and creatinine but decreased IL-10 in the HS group. Increased expression of HSP70 on both mRNA and protein levels was associated with increased NK and γδ T cells versus decreased Treg cell infiltration in liver and kidney tissues of the HS group. In the same group, BWG was decreased, while FCR was increased with respect to the control group. All treatments used in this study reversed the effects of HS significantly. In conclusion, MOE, Vit C, and NaHCO3 can be added to rabbit diets for the amelioration of HS-induced symptoms.
Keywords: Moringa, Rabbit, HSP70, Immune cells
Introduction
In summer, rabbits are usually exposed to higher temperatures, causing heat stress. This stress affects negatively their metabolism, reproduction, and resistance to disease, leading in turn to economic losses (Marai et al. 2002). Rabbits are highly susceptible to heat stress because they do not have sweat glands or any other means of eliminating excess body heat. Heat stress has adverse physiological effects such as a decrease in blood glucose and thyroid hormones (T3) and an increase in adipokines, stress hormones, urea, and creatinine (Brenner et al. 1998; Marai et al. 2008; Morera et al. 2012).
The exposure of living animals to heat stress induced HSPs synthesis in body tissues to act as molecular chaperons which assisted in protein folding, assembly or disassembly (Wu, 1995). According to the molecular weights of HSPs, different families were identified as HSP105/110, HSP90, HSP70, HSP60, HSP40, and other small HSPs. HSP70 expression on mRNA and protein levels was found to increase in response to heat stress (Pei et al. 2012).
While intracellular HSP70 was found to be cytoprotective against apoptosis, the released extracellular HSP70 was found to activate both innate and adaptive immunity through its interaction with TLR2/4 and CD14 receptors (Basu et al. 2000; Mansilla et al. 2014). HSPs constitute carriers of antigens to antigen-presenting cells provoking production of proinflammatory cytokines. In addition, HSPs were found to induce maturation of NKs and dendritic cells (Joly et al. 2010). However, HSP70 was reported previously to downregulate the immune response because it activates IL–10-producing regulatory T cells (Wachstein et al. 2012).
MOE, Vit C, and NaHCO3 were found to improve oxidative stress and heat tolerance by immunomodulation (Peart et al. 2013; Bin-Meferij and El-Kott 2015; Dawood et al. 2016). The current study aimed to investigate the effect of MOE, Vit C, and NaHCO3 on heat stress-induced physiological and immunological changes in rabbit. Expression of HSP70 in both liver and kidney and related cellular (NK, regulatory T cell, and γδ+ T cells) infiltrations were investigated.
Experimental section
Experimental animals and housing
Forty-five New Zealand white rabbits (aged 6 weeks and with an average body weight of 1.29 ± 0.064) were used as experimental animals in this investigation. Animals were kept under observation for about 10 days before the onset of the experiment to exclude any underlying infections. Rabbits were housed in galvanized metal rabbit wire cages (60 × 50 × 40 cm) arranged in a flat-deck system with similar hygienic conditions. All cages were supplied with separated feeders. Diets were offered in pellets ad libitum and fresh water was available at all times from automatic nipple drinkers. The formulation and chemical analysis of the basal diet were constructed as previously described (Al-Sagheer et al. 2017). All protocols were approved by the Animal Ethics Committee within the University “Beni-Suef” in accordance with the International Guiding Principles for Biomedical Research Involving Animals, as issued by the Council for International Organizations of Medical Sciences.
Hydrothermographs TES-1361C (TES electrical electronic Corp, Taipei, Taiwan) were used to record temperature and relative humidity every 5 min. From these data, mean daily air temperature and relative humidity were calculated. Temperature–humidity index (THI) was estimated according to the previously developed equation for rabbits (Tuorkey 2016): THI = db (in °C) − {(0.31 − 0.31 RH) × [db (in °C) − 14.4]}, where db is dry bulb temperature in degrees Celsius and RH is the relative humidity percentage/100. Calculated THI values were subsequently classified as follows: < 27.8 = absence of heat stress, 27.8–28.9 = moderate heat stress, 28.9–30.0 = severe heat stress, and > 30.0 = extremely severe heat stress.
Preparation of M. oleifera aqueous extract
Moringa leaves were obtained from The Egyptian Scientific Association of Moringa, National Research Center. The leaves were harvested, air-dried under shade until the moisture of the collected leaves reached 10%. The dry leaves were finally milled, sieved (1 mm mesh), and stored in an airtight polyethylene bags at room temperature 25 °C. The aqueous extract was prepared according to (Berkovich et al. 2013) by mixing 1 g dried powdered leaves of M. oleifera with 10 mL boiling water for 5 min. The mixture was then filtered twice through a 2-μm pore sterile filter paper into a sterile tube. The aqueous extract stock solution (100 mg/mL) was freshly prepared for each set of experiments and stored at 4 °C for up to 5 days.
Experimental design
Rabbits were randomly allocated into five groups (nine per group). The first group acted as a negative control where the rabbits were housed at a constant 23 °C with 60% relative humidity. The second group acted as a heat-stressed group and was housed in temperatures that fluctuated between 28 and 39 °C on a daily basis. The third, fourth, and fifth groups were heat stressed but treated daily for 6 weeks with 100, 200, and 300 mg of MOE (Tuorkey 2016), Vit C (Jang et al. 2014), and NaHCO3 (Peart et al. 2013)/body weight in drinking water, respectively.
Growth performance
Initial body (IBW) and final body weights (FBW) were individually taken at the beginning and at the end of the experiment, respectively. BWG and FCR were calculated as previously described (Berger and Halver 1987). Dead rabbits were weighed and the weight was included in calculating the feed conversion ratio.
Collection of blood and tissue samples
After rabbits were euthanized, 5 mL blood samples were collected into a sterile vacutainer tube and allowed to coagulate for serum preparation by centrifugation at 3500×g for 15 min, and then transferred into sterilized tubes and stored at − 20 °C.
Parts of liver and kidney tissues were homogenized in PBS using Potter–Elvehjem homogenizer (Braun, Melsungen, Germany) with a loose-fitting Teflon pestle at 1000 rpm with eight up and down strokes. After filtration, the homogenate was centrifuged at 600×g for 10 min at 4 °C in a Beckman TJ-6 centrifuge (Beckman Instruments; Munich, Germany). Other parts of liver and kidney tissues were fixed in 10% buffered formalin (Sigma, St. Louis, USA), dehydrated in alcohol series, and then embedded in paraffin wax for immunohistochemical analysis. Pieces of liver and kidney were kept in sterilized Eppendorf tubes at − 70 °C until they were used for RNA isolation and RT-PCR analysis.
Immunohistochemistry
The protocol was performed as previously described (Abdel-Latif et al. 2016). Paraffin-embedded liver and kidney tissues were cut into 5 μm sections. The slides were then incubated in 3% H2O2 for 10 min at room temperature to block the endogenous peroxidase activity. Slides were treated with 1.5% normal serum obtained from the same species in which the secondary antibody was developed for 30 min to block non-specific staining. Subsequently, slides were incubated with monoclonal antibodies against rabbit CD25 (1:100; antibodies—online, NewYork, USA) and CD56 (1:100; Antigenix America Inc., New York, USA) for overnight at 4 °C. Then, slides were treated with a biotin-conjugated secondary antibody for 10 min followed by incubation with peroxidase-conjugated streptavindin for 10 min at room temperature according to the instructions of IHC Detection Kit (Dako, Glostrup, Denmark). All the above steps were followed by washing three times in Tris buffer (pH 7.4). Immunolabeling was detected by incubation with 0.06% diaminobenzidine (Sigma) dissolved in tap water containing 0.01% H2O2 for 3–5 min, followed by washing and staining with Mayer’s hematoxylin. CD25+ and CD56+ cells were counted per higher power field (HPF) using an Axioplan® microscope (Carl Zeiss, Jena, Germany).
Quantitative RT-PCR of mRNA
The protocol was performed as described (Delic et al. 2010). Total RNA was isolated from parts of the liver and kidney using SV Total RNA Isolation system (Promega, Madison, WI, USA). Contaminating genomic DNA was digested with the DNA-free™ kit (Applied Biosystems, Darmstadt, Germany), before cDNA was synthesized using a Reverse Transcription kit (Stratagene, USA). RT-PCR was performed in a TaqMan7500 (Applied Biosystems) using the QuantiTect™ SYBR® Green PCR kit (Qiagen) according to the manufacturer’s instructions. Qiagen (Hilden, Germany) delivered the primers for rabbit HSP70, FOXP3, TCR gamma and delta chains and β-actin (Table 1). Initial incubation was done at 50 °C for 2 min, followed by Taq polymerase activated at 95 °C for 10 min, 1 cycle followed at 95 °C for 10 min, at 60 °C for 35 s, and for 30 s at 72 °C. All PCR reactions yielded only a single product of the expected size as detected by melting point analysis and gel electrophoresis. Quantitative evaluation was performed with TaqMan7500 system software (Applied Biosystems). Expression of genes was normalized to that of β-actin.
Table 1.
Primer sequences of detected rabbit genes in RT-PCR
| Target gene | Primer sequence |
|---|---|
| HSP70 | F: 5′-CTCCAGCATCCGACAAGAAGC-3′ R:5′- ACGGTGTTGTGGGGGTTCAGG-3′ |
| FOXP3 | F: 5′-TTTCACCTACGCCACGCTCA-3′ R: 5′-CCAGCTCATCCACGGTCCA-3′ |
| TCR γ | F: 5′-CAGTGGTCACCAAGCCAACT-3′ R: 5′-GTCGCGCTGGCACAGTAATA-3′ |
| TCR δ | F: 5′-ACTGCACGTACGACACTAGG-3′ R: 5′-CTGTGTTGCATTCTGTGGCT-3′ |
| β-Actin | F: 5′-GAAATCGTGCGTGACATTAAG-3′ R:5′-CTAGAAGCATTTGCGGTGGAC-3′ |
ELISA
Rabbit serum cortisol, leptin, IFN-γ, TNF-α, and IL-10 were assayed using ELISA kit (MyBiosource, California, San Diego, USA). The procedures were carried out according to the instructions provided with the kits.
Assay of adrenaline
The estimation of adrenaline in rabbit serum was carried out according to the fluorometric method described (Ciarlone 1978; Ezzeldin et al. 2014). In brief, 3 mL of acidified N-butanol was added to 0.3 mL of plasma. Duplicate internal standard tubes were carried in parallel with the sera samples. Sera were centrifuged at 2500×g for 5 min. Subsequently, 2.5 mL of the supernatant fluid was transferred to tubes, placed on a vortex mixer for 30 s, and the phases were separated by centrifugation at 2500×g for 5 min. The aqueous phase (1 mL) was transferred to a tube for the assay of adrenaline. Standards were prepared for adrenaline in duplicate in 0.2-N acetic acid and a total volume of 1.6 mL per tube. Acidified N-butanol (2.5 mL) and heptanes (5 mL) were added to the tube. All tubes were placed on a vortex mixer for 30 s and centrifuged at 2500×g for 5 min. The organic supernatant phase was discarded and 1 mL of the aqueous phase was transferred to a clean, dry test tube. EDTA reagent (0.2 mL) was added to all tubes (sample, standard, and reagent blank (1 mL of 0.2-N acetic acid) and mixed. Subsequently, 0.1 mL of 0.1-N iodine was added and the solution was mixed again. Two minutes later, 0.2 mL of alkaline sulfite reagent was added and mixed. The tubes were allowed to stand exactly 2 min, followed by the addition of 0.2 mL of 5-N acetic acid and mixing. All tubes were placed in a boiling water bath for 2 min, cooled under tap water, and analyzed for adrenaline fluorescence at excitation and emission wavelengths of 360 nm and emission 480 nm using spectrophotofluorometer (Hitachi, California, USA).
Biochemical analyses
Serum alanine and aspartate transaminase (ALT and AST, respectively) activities were determined (Reitman and Frankel 1957). Creatinine levels were evaluated using the quantitative kinetic colorimetric method using kits obtained from Roche Diagnostics (Mannheim, Germany). Urea was determined using urea kits of Diamond Diagnostic (Hanover, Germany). Sera of nine animals were used to evaluate the above-mentioned biochemical parameters. A UV 160 spectrophotometer (Shimadzu, Kyoto, Japan) was used to measure the values of these parameters.
Western blotting
The method was carried out as previously described (Abdel-Latif 2015). The total proteins were extracted using radioimmunoprecipitation buffer containing a protease inhibitor cocktail (Sigma). The protein quantity was determined using the Bicinchoninic acid kit (Sigma). Twenty micrograms protein for detection of HSP70 was separated in SDS-10% polyacrylamide gels and transferred to nitrocellulose membranes (0.45 μm; Heidelberg; Serva Electrophoresis GmbH, Germany) by electroblotting. Membranes were washed in PBS/Tween buffer (PBS containing 0.3% Tween-20) and incubated for 1 h at room temperature (RT) in blocking buffer containing 5% non-fat milk in PBS/Tween-20, followed by washing and incubation with the anti-rabbit HSP70 (1:500; Novus Biologicals, Littleton, CO, USA) in the same buffer overnight at RT. The immunocomplexes were detected by using horseradish peroxidase-labeled goat anti-rabbit antibody (1:5000; KPL, Gaithersburg, MD, USA). After 2 h of incubation at room (RT), bands were developed by adding substrate (50 mg 3,3′-diaminobenzidine tetrahydrochloride and 100 μl H2O2 in 100 mL PBS). The intensity of immunoreactive bands at molecular weight 70 kDa was quantified by densitometry using NIH-Image software (version 1.59; National Institutes of Health, Bethesda, MD).
Statistical analysis
SPSS (version 20) statistical program (SPSS Inc., Chicago, IL) was used to carry out a one-way analysis of variance (ANOVA) on our data. When significant differences by ANOVA were detected, analysis of differences between the means of the treated and control groups was performed by using Dunnett’s t test.
Results
Effect of MOE, Vit C, and NaHCO3 on HS-induced HSP70 expression
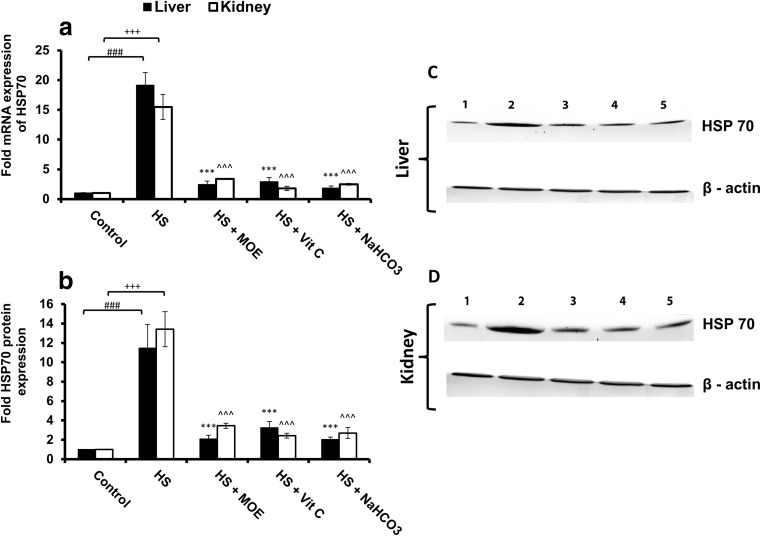
In respect to the control group, HSP70 mRNA and protein expressions were significantly (p < 0.001) increased in both liver and kidney tissues of the HS group (Fig. 1). Treatment of heat-stressed rabbits with MOE, Vit C, and NaHCO3 decreased significantly (p < 0.001) these expressions in comparison to the HS group and restored them to normal levels in comparison with the control group.
Fig. 1.
Effect of MOE, Vit C, and NaHCO3 on HS-induced mRNA (a; n = 9) and protein expressions of HSP70 in both of liver (c) and kidney (d) tissues. The band intensities in western blotting were also represented (b; n = 9). HS increased (p < 0.001) the expression of mRNA and protein in both tissues versus control group, while MOE, Vit C, and NaHCO3 decreased (p < 0.001) the expression of HSP70 on both mRNA and protein levels versus HS group. # & + represent the degree of significance between the liver and kidney in HS group, respectively, and control, while * & ^ represent the difference between treatments in the liver and kidney, respectively, with HS group
Effect of MOE, Vit C, and NaHCO3 on HS-induced changes in stress hormones, leptin, and cytokines
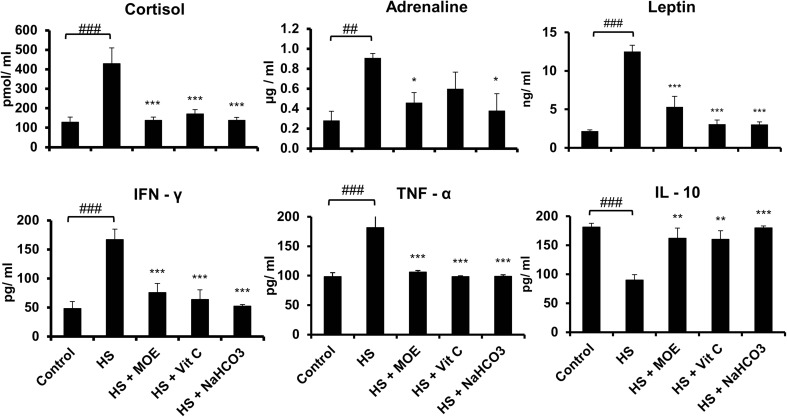
HS upregulated the levels of cortisol, leptin, and adrenaline significantly (p < 0.001) in comparison with the control (Fig. 2). Treatment of heat-stressed rabbits with MOE, Vit C, and NaHCO3 restored both cortisol and leptin to normal levels (p < 0.001) in comparison with the control and HS groups. For adrenaline, MOE and NaHCO3 restored its levels to normal significantly (p < 0.05). For cytokines, HS induced an increase in both IFN-γ and TNF-α but a decrease in IL-10 (p < 0.001) versus the control group. In contrast, all treatments restored the levels of IFN-γ and TNF-α (p < 0.001) and IL-10 (p < 0.01 for MOE and Vit C and p < 0.001 for NaHCO3) to normal versus the HS and control groups.
Fig. 2.
Effect of MOE, Vit C, and NaHCO3 on HS-induced changes in the blood levels of stress hormones (cortisol and adrenaline), leptin, and cytokines (IFN-γ, TNF-α, and IL-10). HS upregulated the production of stress hormones, leptin, IFN-γ, and TNF-α but downregulated IL-10 (p < 0.001). MOE, Vit C, and NaHCO3 reversed the levels of stress hormones, leptin, and cytokines to normal in comparison to both control and HS groups. # represents the degree of significance between HS and control, while * represents the difference between treatments and HS group
Effect of MOE, Vit C, and NaHCO3 on HS-induced decrease in infiltration of Treg cells
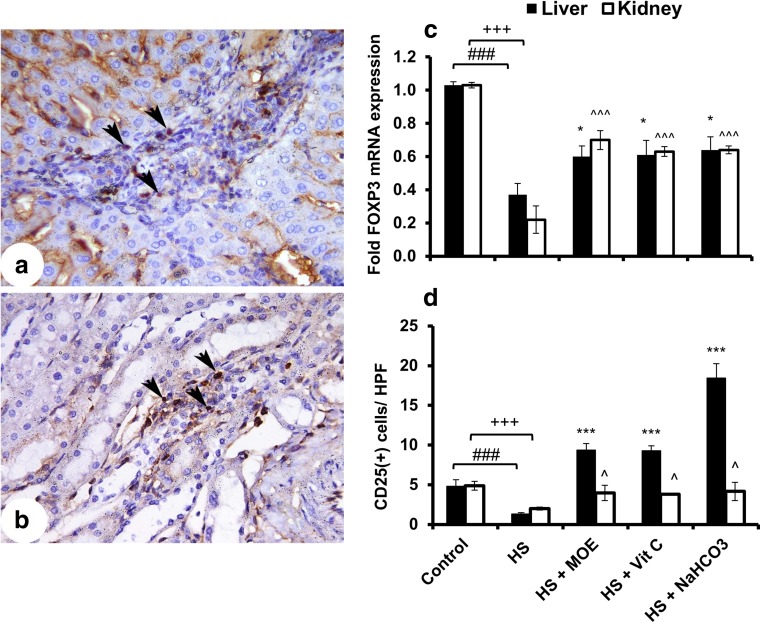
In comparison to the control group, FOXP3 mRNA expression and infiltrated Treg (CD25+) cells were decreased (p < 0.001) in the HS group (Fig. 3). FOXP3 expression was increased in both the liver (p < 0.05) and kidney (p < 0.001) with treatments in respect to the HS group. Similarly, infiltration of CD25+ T cells was significantly increased in the liver (p < 0.001) and kidney (p < 0.05) after treatments. Both RT-PCR and IHC confirmed a HS-induced decrease of Treg cells in liver and kidney tissues and their reversed increase after treatments.
Fig. 3.
Effect of MOE, Vit C, and NaHCO3 on HS-induced infiltration of Treg cells in both of liver and kidney tissues. The figure shows mRNA expression (n = 9; mean ± SE) of FOXP3 (c) as well as the images of CD25+ cells in liver (a) and kidney (b) tissues. It shows also the mean count of stained cells ± SE/HPF (d). HS decreased (p < 0.001) the infiltration of Treg cells in both tissues in comparison to control, while treatments showed an increase in liver and kidney tissues versus HS group. The black arrow heads denote to the stained cells. # & + represent the degree of significance between liver and kidney HS, respectively, and control, while * & ^ represent the difference between treatments in the liver and kidney, respectively, with HS group
Effect of MOE, Vit C, and NaHCO3 on HS-induced increase in infiltration of NK cells
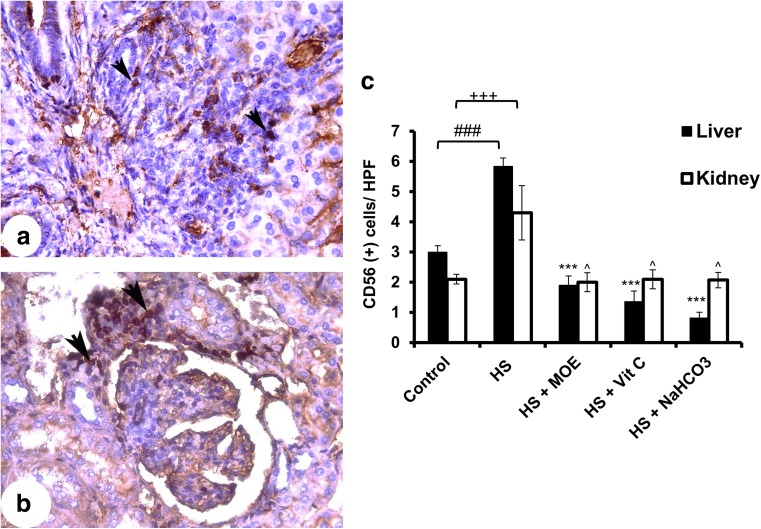
In liver and kidney tissues, the infiltration of CD56+ cells was increased in the HS versus the control group (Fig. 4). Particularly in the liver, the decreased infiltration was highly significant (p < 0.001), while the infiltration in kidney decreased less significantly (p < 0.05) after treatment with MOE, Vit C, or NaHCO3 in comparison to the HS group. Such treatments could restore the infiltrated NK cells into normal levels.
Fig. 4.
Effect of MOE, Vit C, and NaHCO3 on HS-induced infiltration of NK cells in both of liver and kidney tissues. The figure shows the images of CD56+ cells in liver (a) and kidney (b) tissues. It shows also the mean count of stained cells ± SE/HPF (c). HS increased (p < 0.001) the infiltration of NK cells in both tissues in comparison to control, while treatments showed a decrease in liver and kidney tissues (p < 0.001 and p < 0.05, respectively) versus HS group. The black arrow heads denote to the stained cells. # & + represent the degree of significance between liver and kidney HS, respectively, and control, while * & ^ represent the difference between treatments in the liver and kidney, respectively, with HS group
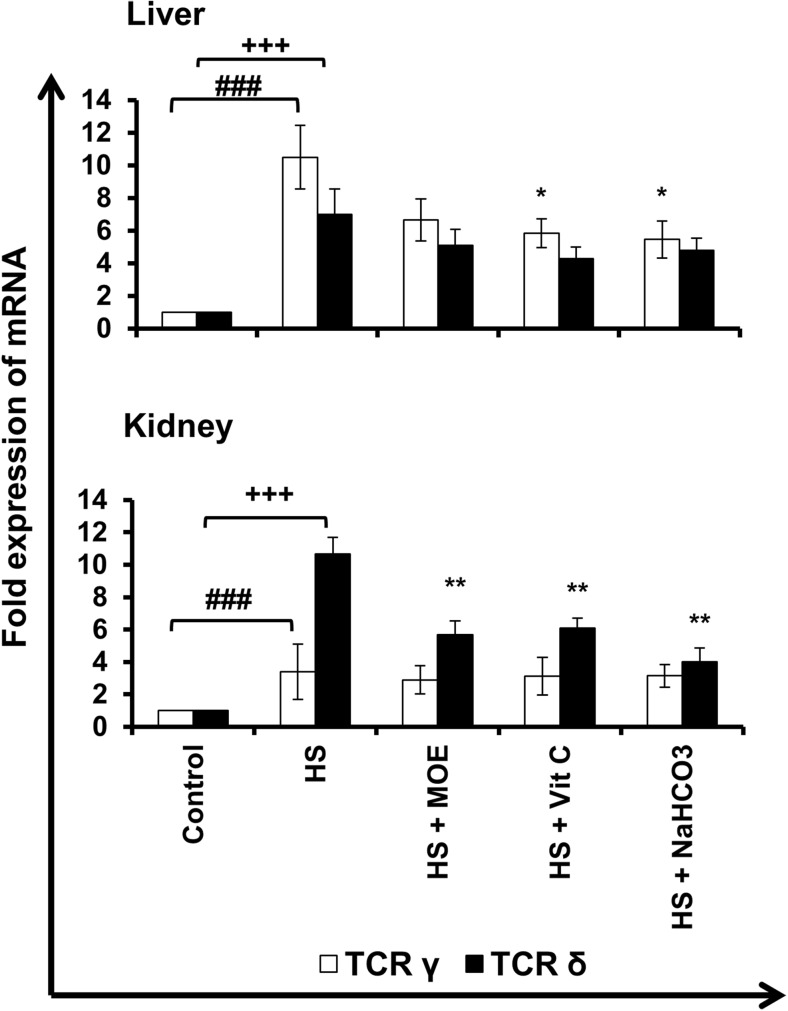
Effect of MOE, Vit C, and NaHCO3 on HS-induced increase in infiltration of γδ T cells
In liver and kidney tissues, TCR γ and δ mRNA expressions (γδ T cell infiltration) increased (p < 0.001) in HS versus control group (Fig. 5). Expression of γ mRNA decreased significantly (p < 0.05) in the liver after both Vit C and NaHCO3 treatments but did not show changes in kidney (p > 0.05). However, expression of δ mRNA decreased significantly (p < 0.01) in the kidney after all treatments but did not show changes in the liver (p > 0.05). Generally, decreased γ and δ in liver and kidney tissues were indicators for less γδ T cell infiltration after treatments with respect to the HS group.
Fig. 5.
Effect of MOE, Vit C, and NaHCO3 on HS-induced infiltration of γδ T cells in both of liver and kidney tissues. The figure shows mRNA expression (n = 9; mean ± SE) of TCR γ and δ chains in both tissues. HS increased (p < 0.001) mRNA expression of both γ and δ in both tissues versus control group, while treatments reversed such increased expression in comparison to HS group. # & + represent the degree of significance between the expression of γ and δ in HS, respectively, and control, while * represents the difference between treatments and HS group
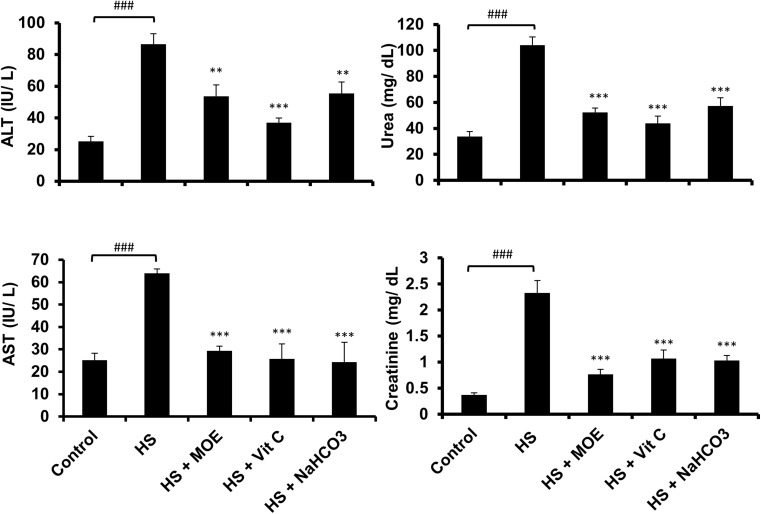
Effect of MOE, Vit C, and NaHCO3 on HS-induced liver and kidney dysfunctions
Tests of liver enzymes (ALT and AST) and kidney functions (urea and creatinine) showed increased (p < 0.001) levels in the HS versus the control group (Fig. 6). All treatments reduced levels almost to normal versus the control and HS groups. Thus, MOE, Vit C, and NaHCO3 could reverse both liver and kidney dysfunctions caused by heat stress in rabbits.
Fig. 6.
Effect of MOE, Vit C, and NaHCO3 on HS-induced liver and kidney dysfunctions. HS induced (p < 0.001) the release of liver enzymes (ALT& AST), urea, and creatinine in the blood in comparison to the control group. Treatments reversed their levels to normal in comparison to both HS and control groups. The data are represented by mean ± SE (n = 9). # represents the degree of significance between HS and control, while * represents the difference between treatments and HS group
Effect of MOE, Vit C, and NaHCO3 on HS-induced alterations in growth performance
HS caused alterations in the growth performance of rabbits because the calculated FBW and hence BWG were decreased (p < 0.05 and p < 0.001, respectively) in comparison to the control group (Table 2). Moreover, the calculated FCR revealed a highly significant increase (p < 0.001). Treatment with NaHCO3 showed a significant increase (p < 0.05) in BWG, while all treatments showed highly significant improvement (p < 0.001) in the FCR versus the HS group.
Table 2.
Effect of MOE, Vit C, and NaHCO3 on growth performance of experimental rabbit groups
| Growth performance | Experimental groups | ||||
|---|---|---|---|---|---|
| Control | HS | HS + MOE | HS + Vit C | HS + NaHCO3 | |
| IBW | 1.29 ± 0.064 | 1.33 ± 0.064 | 1.44 ± 0.101 | 1.30 ± 0.098 | 1.27 ± 0.069 |
| FBW | 2.38 ± 0.070 | 2.03 ± 0.065# | 2.22 ± 0.084 | 2.07 ± 0.115 | 2.12 ± 0.100 |
| BWG | 1.09 ± 0.051 | 0.70 ± 0.033### | 0.79 ± 0.059 | 0.77 ± 0.041 | 0.86 ± 0.046# |
| FCR | 2.84 ± 0.117 | 4.61 ± 0.127### | 3.45 ± 0.182*** | 3.18 ± 0.165*** | 2.91 ± 0.175*** |
#Degree of statistical difference between HS and control groups
*Degree of statistical difference between treated and HS groups
Discussion
HSP70 is involved in cellular repair and protective mechanisms against stresses including hyperthermia (Mosser et al. 2000). It is stress-inducible at both mRNA and protein levels (Chen et al. 1996). Similarly, in the current study, HS induced its expression at both mRNA and protein levels in liver and kidney tissues. The liver and kidney showed the greatest expression of HSP70 after rabbit hyperthermia (Manzerra et al. 1997). Giving doses of MOE, Vit C, and NaHCO3 to HS rabbits reduced HSP70 expression on both levels indicating the sensitivity of the latter protein to attenuated cellular stress. It indicated also the great role of HSP70 in thermal tolerance (Liu et al. 2017). M. oleifera gold nanoparticles were found to decrease the expression of HSP70 mRNA and protein level in lung cancer cells (Tiloke et al. 2016). In broiler chicks exposed to HS, the basal diet supplemented with Vit C reduced the expression of HSP70 in the liver (Jang et al. 2014). Doses of NaHCO3 given to exercising individuals attenuated the expression of monocyte HSP72 (Peart et al. 2016). Thus, the three treatments used in this study were previously known to reduce the expression of HSP70.
Cortisol and adrenaline were previously identified as stress hormones and found to increase in HS (Mazlomi et al. 2017). Moreover, HS was found to increase the levels of blood adipokines including leptin (Al-Dawood, 2017). In the current study, serum cortisol, adrenaline, and leptin increased in HS and decreased with treatments. Increased levels of stress hormones during heat stress led to increased mobilization of energy sources and adaptation of the animal to new circumstance (Ranabir and Reetu, 2011). Treatments with MOE, Vit C, and NaHCO3 decreased the later responses as a result of decreased levels of stress hormones. The increased level of leptin during heat stress indicated stimulated satiety signals or prevention of food intake (Schwartz et al. 2000). It was clearly shown that all treatments could reduce leptin levels in the serum with respect to the HS group and led to an amelioration of satiety signals and to stimulation of hunger. Furthermore, it was found that HS could elevate the inflammatory responses through increased levels of TNF-α. Indeed, HS was previously known to induce inflammatory responses including the release of TNF-α combined with increased HSP70 expression (Yun et al. 2012). Indeed, the regulation of inflammatory signals was found to be dependent on the expression of cellular HSPs (Nair et al. 2015). Increased TNF-α was associated with increased IFN-γ and decreased IL-10 in HS. This indicated upregulated Th1 and downregulated Th2 responses. Treatments reduced levels of the cytokines to their normal values with respect to both the HS and control groups. This demonstrated the anti-inflammatory action of treatments which downregulated HSP70 and TNF-α and upregulated Th1 response. The increased levels of cortisol, adrenaline, leptin (a proinflammatory peptide), and TNF-α during heat stress constituted the interaction between nervous, endocrine, and immune systems towards increased inflammatory status, while the treatments used ameliorated such interaction (Koelsch et al. 2016).
The decreased level of IL-10 after HS was associated with decreased infiltration of Treg cells in liver and kidney tissues. Moreover, treatments reversed the levels of IL-10 and Treg cells. This indicated the major role of Tregs in IL-10 secretion to decrease HS-induced inflammation (Teixeira et al. 2012). The results also showed a highly significant infiltration of CD25+ cells in the liver rather than kidney after treatments (especially NaHCO3). However, FOXP3 test indicated higher significance for all treatments in the kidney than liver. In contrast to the decreased infiltration of Treg cells in HS, NK cells were increased in both the liver and kidney. Similarly, the enhanced cytotoxicity effect of NK cells against cancer was related to the exposure of cancer cells to HS (Dayanc et al. 2013). Indeed, the upregulated expression of HSP70 was responsible for activated NK cells against neoplastic cells (Dang et al. 2014). Like NK cells, γδ T cells were also increased in both liver and kidney tissues of the HS group. Likewise, the activation of heat-stressed tumor cell killing by γδ T cells was found to be dependent on highly expressed HSP70 (Wei et al. 1996). All treatments showed a decrease in infiltrated γδ T cells with a more observable decrease for δ and γ expressions in the liver and kidney, respectively. Both NK and γδ T cells belong to an innate immune system (non-specific activation). The activation of these cells during heat stress could be a result of two possible pathways: (1) HSP70 could be introduced into the extracellular environment through cell necroptosis. This led to inflammation process which included the direct binding of HSP70 to antigen presenting, γδ T and NK cell receptors as well as depression of both Treg cell proliferation and anti-inflammatory cytokines like IL-10 (Multhoff 2009; Khandia et al. 2016). (2) The cell surface heat shock cognate (HSC70) which is a member of HSP70 family was previously suggested to present the cellular peptides to NK and γδ T cells for activation of cytotoxicity (Kishi et al. 2001). In this way, highly expressed HSP70 could be a cause of inflammation. However, administration of microbial HSP70 could lead to anti-inflammatory responses (Borges et al. 2012). The decreased infiltration of both NK and γδ T cells after treatments confirmed the decreased cytotoxicity of liver and kidney cells. This reduced infiltration was associated with reduced levels of produced IFN-γ confirming that NK and γδ T cells are IFN-γ-producing cells (Niu et al. 2015). This explains the increased liver and kidney dysfunctions during HS and their recovery after treatments. Although liver and kidney injuries as a result of heat strokes to rats were previously found, overexpression of HSP70 was found to be protective. Thus, multiorgan dysfunction as a result of heat stress was evident (Niu et al. 2007; Lam et al. 2013). The current study showed increased blood levels of liver enzymes, urea, and creatinine during HS. These levels were significantly decreased to normal after treatments indicating the protective effect of those treatments against HS-induced organ dysfunction.
The exposure of rabbits to high temperatures and humidity causes losses in BWG and FCR and thus has a negative economic impact (Marai et al. 2002). In the current study, HS caused a highly significant reduction and increase in BWG and FCR, respectively. This was related to increased levels of blood leptin (Schwartz et al. 2000). In contrast, the treatments (especially NaHCO3) reduced leptin and improved both BWG and FCR. In addition, the best ameliorative effect of NaHCO3 could be assigned to the acid-base balance because of increased metabolic acidosis (Voiculet et al. 2016). Similarly, dietary supplementation with antioxidants could induce an ameliorative effect on HS-induced heath alterations in rabbits (Al-Sagheer et al. 2017).
In the current study, HS was found to cause negative economic effects resulting from poor rabbit health including decreased growth performance and organ (liver and kidney) dysfunctions. This was due to increased levels of stress hormones, leptin, and inflammatory responses including highly infiltrated NK and γδ T cells which play a major role in cytotoxicity. The treatments used in this study can be added to the rabbit diet for the amelioration of HS effects.
Acknowledgements
The authors are grateful for Prof. Dr. Manal Abdul-Hamid and Dr. Rehab Nady (Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt) for their continuous assistance in the experimental study.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Study conception and design: MA, TS, YKB. Experiments performed: MA, DSA. Data analysis: MA. Manuscript writing: MA, DSA.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abdel-Latif M, Abdel-Haleem HM, Abdel-Baki AA. Anticoccidial activities of chitosan on Eimeria papillata-infected mice. Parasitol Res. 2016;115(7):2845–2852. doi: 10.1007/s00436-016-5035-0. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif M. Diethylcarbamazine citrate ameliorates insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. Int Immunopharmacol. 2015;29(2):607–612. doi: 10.1016/j.intimp.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Al-Dawood A. Effect of heat stress on adipokines and some blood metabolites in goats from Jordan. Anim Sci J. 2017;88(2):356–363. doi: 10.1111/asj.12636. [DOI] [PubMed] [Google Scholar]
- Al-Sagheer AA, Daader AH, Gabr HA, Abd El-Moniem EA. Palliative effects of extra virgin olive oil, gallic acid, and lemongrass oil dietary supplementation on growth performance, digestibility, carcass traits, and antioxidant status of heat-stressed growing New Zealand White rabbits. Environ Sci Pollut Res Int. 2017;24(7):6807–6818. doi: 10.1007/s11356-017-8396-8. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12(11):1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Berger A, Halver J. Effect of dietary protein, lipid and carbohydrate content on the growth, feed efficiency and carcass composition of striped bass, Morone saxatilis (Walbaum), fingerlings. Aquac Res. 1987;18(4):345–356. doi: 10.1111/j.1365-2109.1987.tb00323.x. [DOI] [Google Scholar]
- Berkovich L, Earon G, Ron I, Rimmon A, Vexler A, Lev-Ari S. Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med. 2013;13(1):212. doi: 10.1186/1472-6882-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin-Meferij MM, El-Kott AF. The radioprotective effects of Moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. Int J Clin Exp Med. 2015;8(8):12487–12497. [PMC free article] [PubMed] [Google Scholar]
- Borges TJ, Wieten L, van Herwijnen MJ, Broere F, van der Zee R, Bonorino C, Van Eden W. The anti-inflammatory mechanisms of Hsp70. Front Immunol. 2012;3:95. doi: 10.3389/fimmu.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner I, Shek PN, Zamecnik J, Shephard RJ. Stress hormones and the immunological responses to heat and exercise. Int J Sports Med. 1998;19(2):130–143. doi: 10.1055/s-2007-971895. [DOI] [PubMed] [Google Scholar]
- Chen MS, Featherstone T, Laszlo A (1996) Amplification and altered expression of the hsc70/U14 snoRNA gene in a heat resistant Chinese hamster cell line. Cell Stress Chaperones 1 (1): 47–61 [DOI] [PMC free article] [PubMed]
- Ciarlone AE. Further modification of a fluorometric method for analyzing brain amines. Microchem J. 1978;23(1):9–12. doi: 10.1016/0026-265X(78)90034-6. [DOI] [Google Scholar]
- Dang VT, Tanabe K, Tanaka Y, Tokumoto N, Misumi T, Saeki Y, Fujikuni N, Ohdan H. Fasting enhances TRAIL-mediated liver natural killer cell activity via HSP70 upregulation. PLoS One. 2014;9(10):e110748. doi: 10.1371/journal.pone.0110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood MA, Koshio S, Ishikawa M, Yokoyama S. Immune responses and stress resistance in red sea bream, Pagrus major, after oral administration of heat-killed Lactobacillus plantarum and vitamin C. Fish Shellfish Immunol. 2016;54:266–275. doi: 10.1016/j.fsi.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Dayanc BE, Bansal S, Gure AO, Gollnick SO, Repasky EA. Enhanced sensitivity of colon tumour cells to natural killer cell cytotoxicity after mild thermal stress is regulated through HSF1-mediated expression of MICA. Int J Hyperth. 2013;29(5):480–490. doi: 10.3109/02656736.2013.821526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delić D, Gailus N, Vohr HW, Dkhil M, Al-Quraishy S, Wunderlich F. Testosterone-induced permanent changes of hepatic gene expression in female mice sustained during Plasmodium chabaudi malaria infection. J Mol Endocrinol. 2010;45(6):379–390. doi: 10.1677/JME-10-0026. [DOI] [PubMed] [Google Scholar]
- Ezzeldin E, Souror WA, El-Nahhas T, Soudi AN, Shahat AA. Biochemical and neurotransmitters changes associated with tramadol in streptozotocin-induced diabetes in rats. ernational. 2014;2014:238780. doi: 10.1155/2014/238780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IS, Ko YH, Moon YS, Sohn SH. Effects of vitamin C or E on the pro-inflammatory cytokines, heat shock protein 70 and antioxidant status in broiler chicks under summer conditions. Asian-Australas J Anim Sci. 2014;27(5):749–756. doi: 10.5713/ajas.2013.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immunol. 2010;2(3):238–247. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- Khandia R, Munjal AK, Iqbal HM, Dhama K (2016) Heat shock proteins: therapeutic perspectives in inflammatory disorders. Recent Patents on Inflammation & Allergy Drug Discovery 10 (2): 94–104 [DOI] [PubMed]
- Kishi A, Ichinohe T, Hirai I, Kamiguchi K, Tamura Y, Kinebuchi M, Torigoe T, Ichimiya S, Kondo N, Ishitani K, Yoshikawa T, Kondo M, Matsuura A, Sato N. The cell surface-expressed HSC70-like molecule preferentially reacts with the rat T-cell receptor Vdelta6 family. Immunogenetics. 2001;53(5):401–409. doi: 10.1007/s002510100335. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Boehlig A, Hohenadel M, Nitsche I, Bauer K, Sack U (2016) The impact of acute stress on hormones and cytokines, and how their recovery is affected by music-evoked positive mood. Sci Rep 6(23008) [DOI] [PMC free article] [PubMed]
- Lam KK, Cheng PY, Lee YM, Liu YP, Ding C, Liu WH, Yen MH. The role of heat shock protein 70 in the protective effect of YC-1 on heat stroke rats. Eur J Pharmacol. 2013;699:67–73. doi: 10.1016/j.ejphar.2012.11.044. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ma D, Zhao C, Xiao Z, Xu S, Xiao Y, Wang Y, Liu Q, Li J. The expression pattern of hsp70 plays a critical role in thermal tolerance of marine demersal fish: multilevel responses of Paralichthys olivaceus and its hybrids (P. olivaceus ♀ × P. dentatus ♂) to chronic and acute heat stress. Mar Environ Res. 2017;129:386–395. doi: 10.1016/j.marenvres.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Mansilla MJ, Costa C, Eixarch H, Tepavcevic V, Castillo M, Martin R, Lubetzki C, Aigrot MS, Montalban X, Espejo C. Hsp70 regulates immune response in experimental autoimmune encephalomyelitis. PLoS One. 2014;9:e105737. doi: 10.1371/journal.pone.0105737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997;170(2):130–137. doi: 10.1002/(SICI)1097-4652(199702)170:2<130::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Marai IFM, Habeb AAM, Gad AE. Performance of New Zealand White and Californian male weaned rabbits in the subtropical environment of Egypt. Anim Sci J. 2008;79(4):472–480. doi: 10.1111/j.1740-0929.2008.00552.x. [DOI] [Google Scholar]
- Marai IFM, Habeb AAM, Gad AE. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: a review. Livestcok Prod Sci. 2002;78(2):71–90. doi: 10.1016/S0301-6226(02)00091-X. [DOI] [Google Scholar]
- Mazlomi A, Golbabaei F, Farhang Dehghan S, Abbasinia M, Mahmoud Khani S, Ansari M, Hosseini M. The influence of occupational heat exposure on cognitive performance and blood level of stress hormones: a field study report. Int J Occup Saf Ergon. 2017;23(3):431–439. doi: 10.1080/10803548.2016.1251137. [DOI] [PubMed] [Google Scholar]
- Morera P, Basiricò L, Hosoda K, Bernabucci U. Chronic heat stress up-regulates leptin and adiponectin secretion and expression and improves leptin, adiponectin and insulin sensitivity in mice. J Mol Endocrinol. 2012;48(2):129–138. doi: 10.1530/JME-11-0054. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20(19):7146–7159. doi: 10.1128/MCB.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G. Activation of natural killer cells by heat shock protein 70. 2002. Int J Hyperth. 2009;25(3):169–175. doi: 10.1080/02656730902902001. [DOI] [PubMed] [Google Scholar]
- Nair S, Arora S, Lim JY, Lee LH, Lim LH. The regulation of TNFα production after heat and endotoxin stimulation is dependent on annexin-A1 and HSP70. Cell Stress Chaperones. 2015;20(4):583–593. doi: 10.1007/s12192-015-0580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C, Jin H, Li M, Xu J, Xu D, Hu J, He H, Li W. Cui J9 in vitro analysis of the proliferative capacity and cytotoxic effects of ex vivo induced natural killer cells, cytokine-induced killer cells, and gamma-delta T cells. BMC Immunol. 2015;16:61. doi: 10.1186/s12865-015-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu KC, Lin MT, Chang CP. Hyperbaric oxygen improves survival in heatstroke rats by reducing multiorgan dysfunction and brain oxidative stress. Eur J Pharmacol. 2007;569(1–2):94–102. doi: 10.1016/j.ejphar.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Peart DJ, Kirk RJ, Hillman AR, Madden LA, Siegler JC, Vince RV. The physiological stress response to high-intensity sprint exercise following the ingestion of sodium bicarbonate. Eur J Appl Physiol. 2013;113(1):127–134. doi: 10.1007/s00421-012-2419-4. [DOI] [PubMed] [Google Scholar]
- Peart DJ, Kirk RJ, Madden LA, Vince RV. Implications of a pre-exercise alkalosis-mediated attenuation of HSP72 on its response to a subsequent bout of exercise. Amino Acids. 2016;48(2):499–504. doi: 10.1007/s00726-015-2103-1. [DOI] [PubMed] [Google Scholar]
- Pei Y, Wu Y, Qin Y. Effects of chronic heat stress on the expressions of heat shock proteins 60, 70, 90, A2, and HSC70 in the rabbit testis. Cell Stress Chaperones. 2012;17(1):81–87. doi: 10.1007/s12192-011-0287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranabir S, Reetu K. Stress and hormones. Indian J Endocrinol Metab. 2011;15(1):18–22. doi: 10.4103/2230-8210.77573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Jr PD, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Teixeira L, Marques RM, Aguas AP, Ferreira PG. Regulatory T cells are decreased in acute RHDV lethal infection of adult rabbits. Vet Immunol Immunopathol. 2012;148(3–4):343–347. doi: 10.1016/j.vetimm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Tiloke C, Phulukdaree A, Anand K, Gengan RM, Chuturgoon AA. Moringa oleifera gold nanoparticles modulate oncogenes, tumor suppressor genes, and caspase-9 splice variants in A549 cells. J Cell Biochem. 2016;117(10):2302–2314. doi: 10.1002/jcb.25528. [DOI] [PubMed] [Google Scholar]
- Tuorkey MJ. Effects of Moringa oleifera aqueous leaf extract in alloxan induced diabetic mice. Interv Med Appl Sci. 2016;8(3):109–117. doi: 10.1556/1646.8.2016.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiculeț C, Zară O, Bogeanu C, Văcăroiu I, Aron G. The role of oral sodium bicarbonate supplementation in maintaining acid-base balance and its influence on the cardiovascular system in chronic hemodialysis patients—results of a prospective study. J Med Life. 2016;9(4):449–454. [PMC free article] [PubMed] [Google Scholar]
- Wachstein J, Tischer S, Figueiredo C, Limbourg A, Falk C, Immenschuh S, Blasczyk R, Eiz-Vesper B. HSP70 enhances immunosuppressive function of CD4(+)CD25(+)FoxP3(+) T regulatory cells and cytotoxicity in CD4(+)CD25(-) T cells. PLoS One. 2012;7(12):e51747. doi: 10.1371/journal.pone.0051747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zhao X, Kariya Y, Fukata H, Teshigawara K, Uchida A. Induction of autologous tumor killing by heat treatment of fresh human tumor cells: involvement of gamma delta T cells and heat shock protein 70. Cancer Res. 1996;56(5):1104–1110. [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Yun SH, Moon YS, Sohn SH, Jang IS. Effects of cyclic heat stress or vitamin C supplementation during cyclic heat stress on HSP70, inflammatory cytokines, and the antioxidant defense system in Sprague Dawley rats. Exp Anim. 2012;61(5):543–553. doi: 10.1538/expanim.61.543. [DOI] [PubMed] [Google Scholar]