Abstract
Marine organisms experience abiotic stressors such as fluctuations in temperature, UV radiation, salinity, and oxygen concentration. Heat shock proteins (HSPs) assist in the response of cells to these stressors by refolding and maintaining the activity of damaged proteins. The well-conserved Hsp70 chaperone family is essential for cell viability as well as the response to stress. Organisms possess a variety of Hsp70 isoforms that differ slightly in amino acid sequence, yet very little is known about their functional relevance. In this study, we undertook analysis of three principal Hsp70 isoforms NvHsp70A, B, and D from the starlet sea anemone Nematostella vectensis. The functionality of Hsp70 isoforms in the starlet sea anemone was assessed through transcriptional analysis and by heterologous expression in budding yeast Saccharomyces cerevisiae. Interestingly, these isoforms were found to not only differ in expression under stress but also appear to have functional differences in their ability to mediate the cellular stress program. These results contribute to an understanding of Hsp70 isoform specificity, their shared and unique roles in response to acute and chronic environmental stress, and the potential basis of local adaptation in populations of N. vectensis.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0900-7) contains supplementary material, which is available to authorized users.
Keywords: Hsp70, Molecular chaperone, Nematostella vectensis, Isoform diversity
Introduction
Abiotic factors like temperature, salinity, ultraviolet (UV) radiation, and oxygen concentration are critical environmental conditions that affect physiology, survival, and distribution of most organisms, especially marine species, by affecting efficacy and rates of biochemical and physiological reactions (Hochachka and Somero 2002; Portner 2002; Parmesan 2006; Somero 2012). The consensus among climatic models is that temperatures will steadily increase in the coming decades, leading to higher average annual temperature as well as increases in seasonal fluctuations (Solomon et al. 2007; Lima and Wethey 2012). Temperature shifts affect rainfall patterns, biological productivity, and nutrient availability and thus influence the abiotic environment directly and indirectly, particularly in isolated coastal habitats like bays and estuaries. These changes to the environment physiologically challenge resident organisms with limited migratory ability, who therefore must either rely on existing physiological plasticity or genetically adapt to the shifting environment (Parmesan 2006; Visser 2008). The mechanisms by which individuals acclimate and adapt to acute and chronic environmental variation are critical to understand in order to assess the health of current population as well as predict how they will respond to future climate change.
Heat shock proteins (HSPs) have been broadly studied as biological markers in the ecology and evolution of organismal physiology and stress (Feder and Hofmann 1999). HSPs are categorized into different classes based on protein size and of these different size classes; Hsp70s and Hsp90s have been the most commonly utilized classes for studies of environmental stress in both laboratory and field research. These larger HSP size classes are ancient, with broad conservation in most eukaryotes, and diverse, with multiple genes per size class with distinct cellular localization and inducibility (Georgopoulos and Welch 1993). Over the past few decades, Hsp70 has developed as an early response biomarker for determination of organismal stress in numerous animals (Kultz 2005), particularly for marine invertebrates including cnidarians. The use of Hsp70 as a biomarker in cnidarians has resulted from comparative studies showing upregulation of various Hsp70 isoforms in reef building corals (Nakamura et al. 2012; Louis et al. 2017), sea anemones (Meyer and Weis 2012), and hydrozoans (Gellner et al. 1992) in response to a suite of stressors. Hsp70 has also drawn interest for understanding cnidarian evolutionary ecology because populations of particular species living in different locales exhibit distinct expression patterns for particular Hsp70s that are consistent with adaptation to surviving in more physiologically challenging conditions (Bellantuono et al. 2012; Barshis et al. 2013). Hsp70 has been used as an early response biomarker for physical stress for several marine organisms (Feder and Hofmann 1999), including cnidarians such as Nematostella vectensis, or the starlet sea anemone (Tarrant et al. 2014). The starlet sea anemone resides in coastal marine waters on the Atlantic and Pacific coasts of the USA and Canada, as far south as Florida and as far north as Nova Scotia. Populations of starlet sea anemone have been introduced to the coast of the United Kingdom (Hand and Uhlinger 1994; Reitzel et al. 2008) and Brazil (Silva et al. 2010). Prior studies of Hsp70 in cnidarians have determined that upregulation of various isoforms of the chaperone occurs in response to different abiotic stressors (Nakamura et al. 2012; Louis et al. 2017). Additionally, populations of a cnidarian species existing in different locations show distinct expression patterns for specific Hsp70s that are consistent with adaptation to surviving in more physiologically challenging conditions (Barshis et al. 2013). For this reason, Hsp70 regulation is particularly useful in understanding cnidarian ecology and comparative biology.
At a cellular level, Hsp70 proteins are essential to proteostasis, assisting in folding of newly synthesized proteins and denatured proteins, and if necessary, degradation of aggregated proteins (Hartl et al. 2011). Structurally, Hsp70 has two major domains: a 44-kDa N-terminal nucleotide binding domain (NBD) and an 18-kDa substrate binding domain (SBD) joined together by a flexible conserved linker region (Flaherty et al. 1990). The binding of ATP and subsequent hydrolysis to ADP drives a conformational change that is transmitted from the NBD, through the linker to the SBD promoting the correct folding of bound proteins known as “clients” (Sharma and Masison 2009). Modifications at either the N-terminal domain or the C-terminal domain of these proteins can affect regulation of their function (Chirico et al. 1998; Truman et al. 2012; Nitika and Truman 2017).
Organisms express a large complement of Hsp70 isoforms, typically varying in expression and localization. For example in humans, Hsc70 is constitutively expressed while Hsp70 levels are stress induced. The rational for these expression differences is that Hsc70 provides essential cell function, folding synthesized proteins. Under times of stress, when a larger number of unfolded proteins are present, Hsp70 is induced to compensate (Meimaridou et al. 2009). There are also specialized Hsp70 isoforms that are localized to specific cellular compartments (Evans et al. 2010). There are 14 Hsp70 isoforms in budding yeast: 9 isoforms are cytosolic, 3 are mitochondrial, and 2 are restricted to the ER (Walsh et al. 2004). Hsp70 isoform localization is typically determined by C-terminal signal sequences—cytosolic Hsp70 contains a EEVD motif, mitochondrial Hsp70 utilizes a PEAEYEEAKK motif, and the plastid motif is PEGDVIDADFTDSK (Guy and Li 1998). The reason for the large numbers of Hsp70 isoforms present in cells remains unexplained, but it has been suggested that different isoforms bind and fold a select subset of Hsp70-interacting proteins.
Assessments of Hsp isoform diversity in Nematostella remain largely uncharacterized.
Early genomic surveys suggested perhaps a dozen members of Hsp20 family, five Hsp70s, and three Hsp90s (Goldstone 2008; Reitzel et al. 2008). Transcriptome-wide surveys from adult Nematostella cultured under various stressors have shown particular Hsps in each size class to have significant changes in expression (Elran et al. 2014; Oren et al. 2015; Tarrant et al. 2018). Despite the increased taxonomic sampling and range of environments tested, the lack of in vivo protein technologies in non-model organisms has limited previous studies of Hsp70 function in marine organisms to transcriptional analysis. The resulting absence of information of isoform-specific functions and interactions of Hsp70 at the protein level limit the value of HSPs as informative or predictive biomarkers for understanding the ecology and evolution of species.
In this study, we compared the basal and stress-induced transcription of three main Nematostella cytosolic Hsp70 isoforms. In addition, we expressed these isoforms as the sole isoform in the model organism budding yeast at constitutive levels to tease apart functional differences between these isoforms. Understanding the roles of the Hsp70 family in this organism may allow ecologists to make predictions on marine fauna’s response to the climate change that exists both in the present and the future.
Materials and methods
Animal cultures and abiotic exposures
Adult N. vectensis from three populations (Saco, ME; Sippewissett, MA; Wilmington, NC), which represent a large part of the species’ latitudinal distribution along the Atlantic coast of the USA (Reitzel et al. 2008), were collected and transported to UNC Charlotte. Individuals from each location were cultured under standard conditions (20 °C, 13% artificial seawater, fed Artemia three times weekly) for approximately 1 year prior to beginning experiments.
Identification of Hsp70 genes in the Nematostella genome
Candidate Hsp genes for Nematostella have been previously identified through similarity searches using BLAST searches of the reference genome and transcriptome (see the “Introduction” section). In an earlier study, three Hsp70 isoforms were identified, named NvHsp70A, B, and D, from N. vectensis that likely grouped with other cytosolic forms from other animals that also showed inducible expression under acute temperature stress (e.g., 40 °C) (Reitzel et al. 2013). We focused on these three Hsp70s for this study to (1) determine transcriptional dynamics under temperature changes that mirror natural oscillations and to then compare these changes for individuals from each of three locations and (2) determine similar and unique properties for each Hsp70 isoform when heterologously expressed in yeast.
Phylogenetic analysis of NvHsp70 isoforms
Yeast and human Hsp70 sequences were obtained from the Saccharomyces Genome Database (SGD; http://www.yeastgenome.org/) and Universal Protein Resource (UniProt; http://www.uniprot.org/), respectively. Sequences were aligned using Clustal Omega Multiple Sequence Alignment (http://www.ebi.ac.uk/Tools/msa/clustalo/). Using these aligned sequences and the software program Mega7, a phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrap replications.
Expression of Hsp70 isoforms in Nematostella populations
Adult Nematostella from each location were exposed to two temperature regimes (“acute” and “chronic”) in order to determine the impacts of thermal environment on Hsp70 transcription. In the acute experiment, adults from each population were divided into four replicate bowls per population (12 bowls total, 200 ml volume) at 20 °C overnight. At the beginning of the experiments, two to three adults cultured at 20 °C were sampled and immediately preserved in RNAlater. The temperature was increased 2 °C per hour for the next 8 h to a final temperature of 36 °C. Adults were sampled from each bowl at 28 °C (4 h) and 36 °C and preserved. For the chronic temperature treatment, half of the remaining adults in each bowl were cultured for an additional 14 h at 36 °C, while the other half were cultured at 20 °C. Adults sampled from each replicate and preserved in RNAlater. RNA was extracted from all samples using the RNAqueous® Total RNA Kit (Ambion), DNase treated, and then quantified with a NanoDrop. Complementary DNA (cDNA) was synthesized from 200 ng of total RNA in a 20 μl reaction using the iScript cDNA Synthesis Kit (Bio-Rad). Expression of each Hsp70 was determined following methods by Reitzel and Tarrant (2014) using an Applied Biosystems® 7500 Real-Time system. Briefly, a 20 μl reaction consisted of 10 μl of Power SYBR® Green Master Mix (Thermo), 1 μl of cDNA, and 400 nM of gene-specific primers. Expression was calculated by comparing the threshold cycle of amplification against a standard curve constructed from a serially diluted plasmid standard containing the amplicon of interest. The PCR conditions were 95 °C for 2 min followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. After 40 cycles, a melt curve analysis was used to verify single amplicon during the amplification steps.
Cloning of NvHsp70 isoforms into yeast expression plasmids
We assembled the open reading frame from each Hsp70 (A, B, D) using sequence resources available through Nematostella JGI genome portal. Each full-length Hsp70 was amplified from cDNA synthesized from RNA isolated from Nematostella originally collected from Sippewissett, MA, and inserted into the pAG415GPD-ccdB vector for expression in yeast using in-fusion cloning. All inserts were sequence confirmed with Sanger sequencing.
Expression of NvHsp70 isoforms in Saccharomyces cerevisiae
Yeast cultures were grown in YPD (1% yeast extract, 2% peptone, 2% glucose) or in SD (0.67% yeast nitrogen base, 2% glucose) supplemented with the appropriate nutrients to select for plasmids and gene replacements. NvHsp70 isoforms in pAG415GPD-ccdB and the control empty pAG415GPD-ccdB plasmid were transformed into yeast strain ssa1–4∆ (Jaiswal et al. 2011) using PEG/lithium acetate. After restreaking onto media lacking leucine, transformants were further restreaked onto media lacking leucine and containing 5-fluoro-orotic acid (5-FOA).
Serial dilution plates to test functionality of isoforms
Ssa1–4∆ cells expressing NvHsp70 isoforms as their sole cytosolic Hsp70 were grown to mid-logarithmic phase and were 10-fold serially diluted (full concentration, 1/10 concentration, 1/100 concentration, and 1/1000 concentration) in a 96-well plate. Cells were replica plated onto solid media containing chemicals or exposed to abiotic stressors such as heat shock at 37 °C or UV radiation. After 3 days of growth, the phenotypes of the experimental plates were compared to the control plate in order to determine functional differences between isoforms.
Results
Molecular cloning and sequence analysis of Hsp70 isoforms from Nematostella
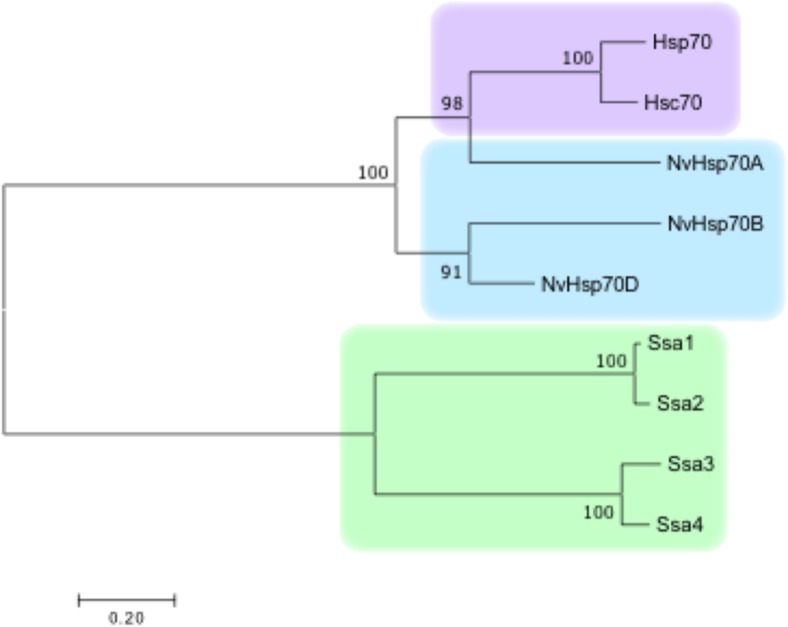
CLUSTAL analysis revealed a high-sequence similarity between the NvHsp70 A, B, and D isoforms (Fig. 1). Although individual amino acid differences were present sporadically throughout the sequences, the most differences between sea anemone isoforms occur in the substrate-binding region near the C terminal (amino acid positions 555–640). In an effort to place the NvHsp70 isoforms in a broader evolutionary context, we constructed a phylogenetic tree containing NvHsp70 isoforms (A, B, and D), the major human Hsp70 isoforms (Hsc70 and Hsp70), and budding yeast Hsp70s (Ssa1, Ssa2, Ssa3, and Ssa4). The sequences clustered into two distinct groups, one containing all of the cytosolic yeast isoforms and the other containing both human and NvHsp70 (Fig. 2). Interestingly, NvHsp70A is most closely related to human Hsp70, sharing a common evolutionary ancestor with the human isoforms. In contrast, NvHsp70B and NvHsp70D are most closely related to each other, sharing a common ancestor with the branch containing Hsp70, Hsc70, and NvHsp70A (Fig. 2).
Fig. 1.
Amino acid alignment of Nematostella vectensis Hsp70 isoforms. NvHsp70A, NvHsp70B, and NvHsp70D were aligned using Clustal Omega
Fig. 2.
Phylogenetic comparison of Nematostella, yeast, and human Hsp70 isoforms. Sequences were aligned using Clustal Omega and used to produce a NJ tree with bootstrap using MEGA7. The numbers in the interior nodes represent percentage of replicate trees during the bootstrap test (1000 replicates) in which the associated taxa were clustered together. The branch lengths are proportional to inferred evolutionary distances. These lengths were computed using the Poisson correction method in the units of number of amino acid substitutions per site. Analysis involved a total of 639 positions in the final dataset
Expression of NvHsp70 isoforms under acute and chronic heat stress
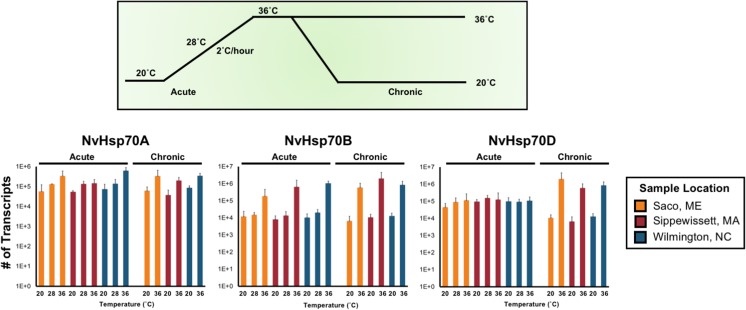
The habitats of N. vectensis experience relatively large daily (> 20 °C) and seasonal (> 25 °C) changes in temperature. Acclimation and adaptation to different environmental regimes may involve adjustments in the threshold and magnitude for induction of an Hsp70 response if the quantity of particular isoforms is specific for particular stressors. Furthermore, because different populations of N. vectensis show divergent organismal-level responses to abiotic variation (Reitzel et al. 2013), we tested if the induction of each of the NvHsp70 isoforms correlates with particular thresholds for each population. Our selected temperatures followed the daily oscillations in temperature for a typical late spring or summer day, where temperatures are recorded by field-deployed loggers at lows of 20 °C and increase to 36 °C or more by mid-day. This temperature increase follows an approximately 2 °C per hour increase (Reitzel et al. 2013, Fraune et al. 2016). Anemones from three populations (ME, MA, and NC) were exposed to acute and chronic high temperature, with the former mimicking a temperature gradient these organisms experience in nature and the latter representing an extreme temperature shift that would activate the heat shock response. All three populations of starlet sea anemone showed similar trends in transcriptional upregulation for each Hsp70 isoform; however, each isoform responded differently to the treatments. NvHsp70A transcription gradually increased during acute heat stress from 28 to 36 °C. Chronic heat stress induced NvHsp70A at the same magnitude as acute stress. In contrast, NvHsp70B transcription was not responsive to exposure at 28 °C, increasing robustly only during the 36 °C acute and chronic heat stress. NvHsp70D showed constitutive transcription when challenged by acute heat stress, only becoming induced during chronic heat stress (Fig. 3).
Fig. 3.
NvHsp70 isoforms display differential expression patterns under chronic and acute heat stress. Quantitative RT-PCR of NvHSP70A, B, and D genes following acute (28 and 36 °C) and chronic (24 h at 36 °C). Results shown are the average and standard deviation of expression from two to four replicates per treatment for each population
NvHsp70 isoforms provide essential Hsp70 function when expressed in yeast
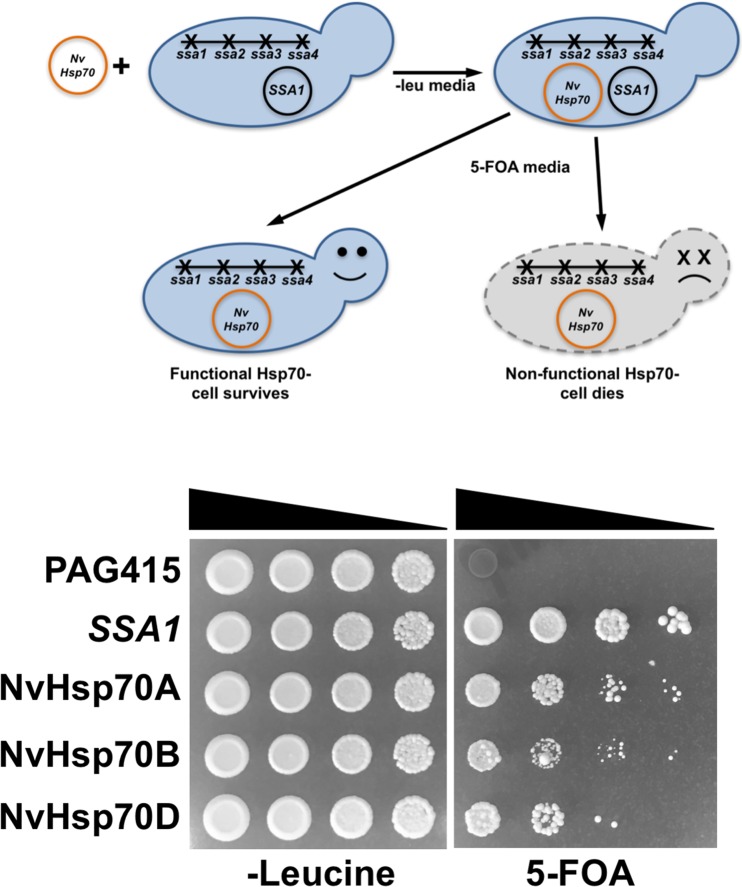
The lack of both efficient gene editing and Nematostella-specific antibody reagents makes the direct study of proteins in Nematostella technically challenging. To examine potential differences in NvHsp70 isoform functionality, we expressed them in a yeast model system. The NvHsp70 isoforms were expressed in yeast strain ssa1–4∆, a strain lacking chromosomal versions of Ssa1, Ssa2, Ssa3, and Ssa4. Although Ssa1–4 function is required for cell viability, the ssa1–4∆ strain is kept viable through expression of Ssa1 on a URA3 plasmid (Jaiswal et al. 2011). After transformation of cells with plasmids expressing NvHsp70 isoforms from a LEU2-based constitutive GPD promoter, the original Ssa1 plasmid was removed using 5-FOA curing. In contrast to cell expressing the empty pAG415GPD-ccdB control, cells expressing NvHsp70 A, B, and D all grew on 5-FOA media (Fig. 4). While these cells were slower growing than cells expressing Ssa1, the data suggest that NvHsp70 A, B, and D at least minimally complement essential Hsp70/Ssa1 function when expressed in yeast.
Fig. 4.
NvHsp70 isoforms provide essential Hsp70 function in yeast. Ssa1–4∆ cells were transformed with control plasmid pAG415-ccDB, Ssa1-expressing, or NvHSP70 isoform-expressing plasmids and then serially diluted onto media lacking leucine or containing 5-FOA. Growth of cells on 5-FOA demonstrates ability of NvHSP70 isoforms to provide essential function when expressed as the sole Hsp70 in the cell. Plates were incubated for 3 days at 30 °C and then photographed
NvHsp70 isoforms are functionally different when expressed in yeast
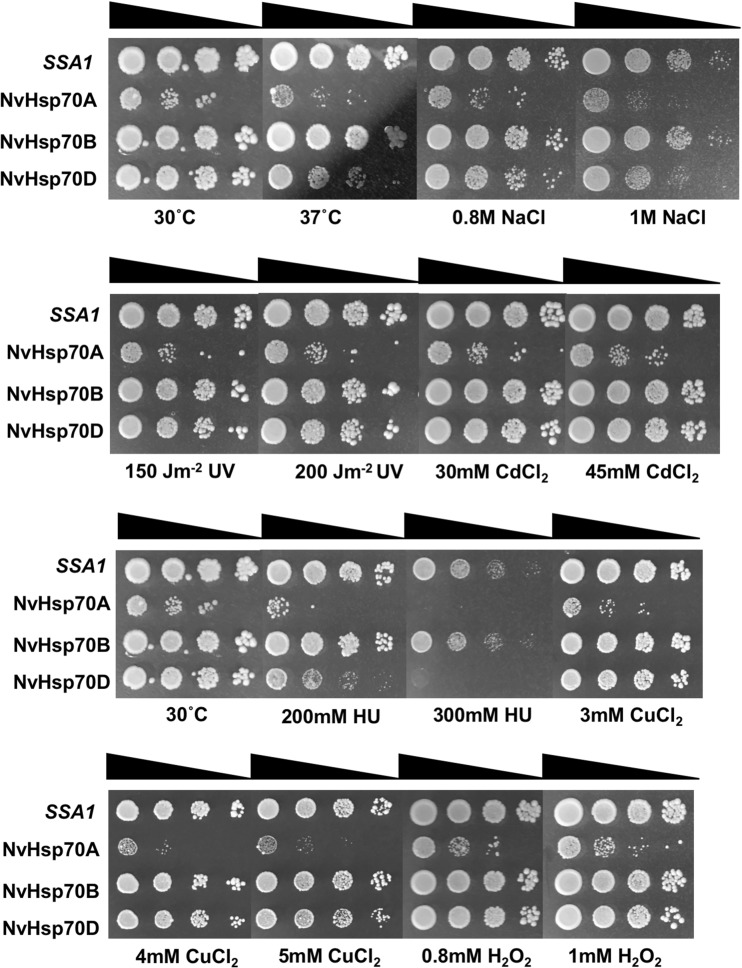
Hsp70 mediates the cellular response to a wide variety of stressors including high temperature, DNA damage, and heavy metal exposure (Verghese et al. 2012; Wang et al. 2012; Truman et al. 2015). Having established that the NvHsp70 variants could complement essential Hsp70 function in yeast, we decided to examine differences between the NvHsp70 isoforms by screening these cells for resistance to NaCl (osmotic stress), UV radiation, H2O2, and hydroxyurea (DNA-damaging agents); CdCl2 and CuCl2 (heavy metal exposure); and high temperature (37 °C). Cells expressing NvHsp70A were the slowest growing and the most sensitive to all stressors tested (Fig. 5). Cells expressing NvHsp70B were the closest to full Ssa1 function, with cells displaying minimal sensitivity to all agents examined. Interestingly, cells expressing NvHsp70D had an intermediate phenotype, falling somewhere between NvHsp70A and B for stress resistance. NvHsp70D cells were sensitive to NaCl and hydroxyurea relative to WT Ssa1- and NvHsp70B-expressing strains (Fig. 5). Taken together, this suggests that even when transcriptional changes are compensated for (by constitutive expression in yeast), NvHsp70A, B, and D display differing functionality in vivo.
Fig. 5.
Yeast expressing NvHsp70 isoforms display altered stress resistance phenotypes. Cells expressing either Ssa1 or NvHsp70 isoforms as the sole cytosolic Hsp70 were grown to mid-log phase and then serially diluted onto plates containing the stress agent. Plates were incubated for 3 days at 30 °C (3 days at 37 °C for the high-temperature stress plate) and then photographed
Discussion
Differential transcription of HSPs by various organisms exposed to stressful conditions has resulted in the general conclusion that these are general stress response proteins. Previous experiments with Nematostella have shown that Hsp genes are dynamically expressed under a variety of conditions including diel light exposure, metal stress, and UV exposure (Elran et al. 2014; Tarrant et al. 2014; Oren et al. 2015). In this study, we show that Nematostella Hsp70 isoforms vary both in the way they are induced under stress and their in vivo functionality. We exposed Nematostella to acute stress, a gradual change in temperature in an effort to mimic the temperature changes that Nematostella experience in the wild. Under these conditions, NvHsp70A was induced relatively early at 28 °C, NvHsp70 only after exposure to 36 °C, whereas NvHsp70D was constitutively expressed at all acute temperatures. This more nuanced expression profile for isoforms in one species may indicate tightly orchestrated regulation of transcription dependent on an accumulation of cellular stress over a prolonged environmental exposure. We did not observe dramatic differences in expression profiles of any HSP between populations. As a shallow water estuarine species, Nematostella populations in all locations experience dramatic daily temperature changes even though mean temperatures are higher in lower-latitude locations. Future experiments that study even higher temperatures or lower temperatures may reveal differences in transcription between populations not captured in our experiments.
Although molecular chaperones have been studied for over 50 years, a major question remaining is the evolutionary reason for maintaining multiple isoforms. Previously, it was thought that most isoforms of chaperones were functionally identical, with transcriptional patterns being the only unique separator. Several studies in both model organisms and mammalian cells have uncovered functional differences in chaperones isoforms (Millson et al. 2007; Hasin et al. 2014; Prince et al. 2015). Although generally well conserved, amino acid alignment shows subtle differences between the NvHsp70 isoforms, the Ssa1 isoforms, and the Hsc70 and Hsp70 proteins. NvHsp70 proteins are especially divergent from each other near the C-terminal domain, a region known to be responsible for binding and refolding of client proteins (DeLuca-Flaherty et al. 1990; Zhu et al. 1996). The variations in the client-binding domain of each NvHsp70 isoform may dictate the number and variety of client proteins that can be bound. Under specific stresses, selected Hsp70 isoforms may be induced that bind and stabilize specific clients to deal with that stress.
To negate the possibility that the only difference in NvHsp70 isoforms was the pattern of expression upon stress, we expressed NvHsp70 isoforms from the same plasmid on a constitutive promoter in yeast. While all NvHsp70 isoforms could provide enough basal Hsp70 function to keep cells viable, it is clear that NvHsp70A- and NvHsp70D-expressing cells were compromised for several Hsp70-mediated stress responses, including high temperature growth and exposure to hydroxyurea. It is interesting to note that NvHsp70A aligns more closely with human rather than yeast Hsp70 isoforms. This may be one explanation for the poor growth and stress resistance phenotype when expressed in yeast cells at the sole Hsp70. Temperature resistance involves complex co-ordination of the heat shock transcription factor HSF1 and associated regulator proteins. In yeast and human cells, Hsp70 binds and directly regulates the activity of HSF1 (Verghese et al. 2012). It may be that the HSF1-transcriptional program is controlled by specific NvHsp70 isoforms or that NvHsp70 isoforms have intrinsically different levels of stability, with higher temperature promoting loss of NvHsp70 isoform activity. Hydroxyurea is a replicative stress agent, activating the DNA damage response pathway in cells. Interestingly, hydroxyurea directly targets the enzyme ribonucleotide reductase (RNR), a known client protein of Hsp70 and Hsp90 (Truman et al. 2015). The reduced ability for NvHsp70A and D expressing cells to grow on media containing hydroxurea may reflect loss of chaperone-RNR interaction and possible RNR destabilization. While beyond the scope of this study, we intend to examine this further by characterizing the global binding partners of these isoforms in in vitro and in vivo conditions via mass spectrometry.
Electronic supplementary material
(DOCX 59 kb)
Acknowledgements
This research was supported by the National Science Foundation (NSF DEB1545539 and REU #1359271). We thank our colleagues in both the Truman and Reitzel labs who provided assistance, insight, and comments that greatly assisted both the research and the manuscript.
Abbreviations
- HSP
Heat shock protein
- 5-FOA
5-Fluoro-orotic acid
- Nv
Nematostella vectensis
- UV
Ultraviolet
- NBD
Nucleotide-binding domain
- SBD
Substrate-binding domain
- HS
Heat shock
- HSF
Heat shock factor
- HU
Hydroxyurea
References
- Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, Palumbi SR. Genomic basis for coral resilience to climate change. Proc Natl Acad Sci U S A. 2013;110(4):1387–1392. doi: 10.1073/pnas.1210224110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellantuono AJ, Granados-Cifuentes C, Miller DJ, Hoegh-Guldberg O, Rodriguez-Lanetty M. Coral thermal tolerance: turning gene expression to resist thermal stress. PLoS One. 2012;7(11):e50685. doi: 10.1371/journal.pone.0050685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico WJ, Markey ML, Fink AL. Conformational changes of an Hsp70 molecular chaperone induced by nucleotides, polypeptides, and N-ethylmaleimide. Biochemistry. 1998;37(39):13862–13870. doi: 10.1021/bi980597j. [DOI] [PubMed] [Google Scholar]
- DeLuca-Flaherty C, McKay DB, Parham P, Hill BL. Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis. Cell. 1990;62(5):875–887. doi: 10.1016/0092-8674(90)90263-E. [DOI] [PubMed] [Google Scholar]
- Elran R, Raam M, Kraus R, Brekhman V, Sher N, Plasches I, Chalifa-Caspi V, Lotan T. Early and late response of Nematostella vectensis transcriptome to heavy metals. Mol Ecol. 2014;23(19):4722–4736. doi: 10.1111/mec.12891. [DOI] [PubMed] [Google Scholar]
- Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53(12):4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Flaherty KM, DeLuca-Flaherty C, McKay DB. Three-dimensional structure of the ATPase fragment of a 70 K heat-shock cognate protein. Nature. 1990;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Fraune S, Forêt S, Reitzel AM (2016) Using Nematostella vectensis to study the interactions between genome, epigenome, and bacteria in a changing environment. Front Marine Sci 3(148). 10.3389/fmars.2016.00148
- Gellner K, Praetzel G, Bosch TC. Cloning and expression of a heat-inducible hsp70 gene in two species of Hydra which differ in their stress response. Eur J Biochem. 1992;210(3):683–691. doi: 10.1111/j.1432-1033.1992.tb17469.x. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Goldstone JV. Environmental sensing and response genes in cnidaria: the chemical defensome in the sea anemone Nematostella vectensis. Cell Biol Toxicol. 2008;24(6):483–502. doi: 10.1007/s10565-008-9107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CL, Li QB. The organization and evolution of the spinach stress 70 molecular chaperone gene family. Plant Cell. 1998;10(4):539–556. doi: 10.1105/tpc.10.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand C, Uhlinger KR. The unique, widely distributed, estuarine sea aneome, Nematostella vectensis Stephenson: a review, new facts, and questions. Estuar Coasts. 1994;17(2):501–508. doi: 10.2307/1352679. [DOI] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Hasin N, Cusack SA, Ali SS, Fitzpatrick DA, Jones GW. Global transcript and phenotypic analysis of yeast cells expressing Ssa1, Ssa2, Ssa3, or Ssa4 as sole source of cytosolic Hsp70-Ssa chaperone activity. BMC Genomics. 2014;15:194. doi: 10.1186/1471-2164-15-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Somero GN. Biochemical adaptation: mechanism and process in physiological evolution. New York: Oxford University Press; 2002. [Google Scholar]
- Jaiswal H, Conz C, Otto H, Wolfle T, Fitzke E, Mayer MP, Rospert S. The chaperone network connected to human ribosome-associated complex. Mol Cell Biol. 2011;31(6):1160–1173. doi: 10.1128/MCB.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kultz D. Molecular and evolutionary basis of the cellular stress chaperone. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- Lima FP, Wethey DS. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat Commun. 2012;3:704. doi: 10.1038/ncomms1713. [DOI] [PubMed] [Google Scholar]
- Louis YD, Bhagooli R, Kenkel CD, Baker AC, Dyall SD. Gene expression biomarkers of heat stress in scleractinian corals: promises and limitations. Comp Biochem Physiol C Toxicol Pharmacol. 2017;191:63–77. doi: 10.1016/j.cbpc.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Meimaridou E, Gooljar SB, Chapple JP. From hatching to dispatching: the multiple cellular roles of the Hsp70 molecular chaperone machinery. J Mol Endocrinol. 2009;42(1):1–9. doi: 10.1677/JME-08-0116. [DOI] [PubMed] [Google Scholar]
- Meyer E, Weis VM. Study of cnidarian-algal symbiosis in the “omics” age. Biol Bull. 2012;223(1):44–65. doi: 10.1086/BBLv223n1p44. [DOI] [PubMed] [Google Scholar]
- Millson SH, Truman AW, Racz A, Hu B, Panaretou B, Nuttall J, Mollapour M, Soti C, Piper PW. Expressed as the sole Hsp90 of yeast, the alpha and beta isoforms of human Hsp90 differ with regard to their capacities for activation of certain client proteins, whereas only Hsp90beta generates sensitivity to the Hsp90 inhibitor radicicol. FEBS J. 2007;274(14):4453–4463. doi: 10.1111/j.1742-4658.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Morita M, Kurihara H, Mitarai S. Expression of hsp70, hsp90 and hsf1 in the reef coral Acropora digitifera under prospective acidified conditions over the next several decades. Biol Open. 2012;1(2):75–81. doi: 10.1242/bio.2011036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitika, Truman AW. Cracking the chaperone code: cellular roles for Hsp70 phosphorylation. Trends Biochem Sci. 2017;42(12):932–935. doi: 10.1016/j.tibs.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M, Tarrant AM, Alon S, Simon-Belcher N, Elbaz I, Appelbaum L, Levy O. Profiling molecular and behavioral circadian rhythms in the non-symbiotic sea anemone Nematostella vectensis. Sci Rep. 2015;5:11418. doi: 10.1038/srep11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2006;37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- Portner HO. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol. 2002;132(4):739–761. doi: 10.1016/S1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Prince TL, Kijima T, Tatokoro M, Lee S, Tsutsumi S, Yim K, Rivas C, Alarcon S, Schwartz H, Khamit-Kush K, Scroggins BT, Beebe K, Trepel JB, Neckers L. Client proteins and small molecule inhibitors display distinct binding preferences for constitutive and stress-induced HSP90 isoforms and their conformationally restricted mutants. PLoS One. 2015;10(10):e0141786. doi: 10.1371/journal.pone.0141786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel AM, Tarrant AM 2014 Phylogenetic diversity and transcription dynamics of heat shock proteins in the estuarine cnidarian Nematostella vectensis. Integr Comp Biol 54:E174–E174
- Reitzel AM, Darling J, Sullivan J, Finnerty J. Global population genetic structure of the starlet anemone Nematostella vectensis: multiple introductions and implications for conservation policy. Biol Invasions. 2008;10:1197–1213. doi: 10.1007/s10530-007-9196-8. [DOI] [Google Scholar]
- Reitzel AM, Chu T, Edquist S, Genovese C, Church C, Tarrant AM, Finnerty JR. Physiological and developmental responses to temperature by the sea anemone Nematostella vectensis. Mar Ecol Prog Ser. 2013;484:115–130. doi: 10.3354/meps10281. [DOI] [Google Scholar]
- Sharma D, Masison DC. Hsp70 structure, function, regulation and influence on yeast prions. Protein Pept Lett. 2009;16(6):571–581. doi: 10.2174/092986609788490230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JF, Lima CAC, Perez CD, Gomes PB. First record of the sea anemone Nematostella vectensis (Actiniaria: Edwardsiidae) in southern hemisphere waters. Zootaxa. 2010;23(43):66–68. [Google Scholar]
- Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, 2007. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Somero GN. The physiology of global change: linking patterns to mechanisms. Annu Rev Mar Sci. 2012;4:39–61. doi: 10.1146/annurev-marine-120710-100935. [DOI] [PubMed] [Google Scholar]
- Tarrant AM, Reitzel AM, Kwok CK, Jenny MJ. Activation of the cnidarian oxidative stress response by ultraviolet radiation, polycyclic aromatic hydrocarbons and crude oil. J Exp Biol. 2014;217(Pt 9):1444–1453. doi: 10.1242/jeb.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant AM, Payton SL, Reitzel AM, Porter DT, Jenny MJ. Ultraviolet radiation significantly enhances the molecular response to dispersant and sweet crude oil exposure in Nematostella vectensis. Mar Environ Res. 2018;134:96–108. doi: 10.1016/j.marenvres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Truman AW, Kristjansdottir K, Wolfgeher D, Hasin N, Polier S, Zhang H, Perrett S, Prodromou C, Jones GW, Kron SJ. CDK-dependent Hsp70 phosphorylation controls G1 cyclin abundance and cell-cycle progression. Cell. 2012;151(6):1308–1318. doi: 10.1016/j.cell.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman AW, Kristjansdottir K, Wolfgeher D, Ricco N, Mayampurath A, Volchenboum SL, Clotet J, Kron SJ. Quantitative proteomics of the yeast Hsp70/Hsp90 interactomes during DNA damage reveal chaperone-dependent regulation of ribonucleotide reductase. J Proteome. 2015;112:285–300. doi: 10.1016/j.jprot.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Abrams J, Wang Y, Morano KA. Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol Mol Biol Rev. 2012;76(2):115–158. doi: 10.1128/MMBR.05018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc Biol Sci. 2008;275(1635):649–659. doi: 10.1098/rspb.2007.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P, Bursac D, Law YC, Cyr D, Lithgow T. The J-protein family: modulating protein assembly, disassembly and translocation. EMBO Rep. 2004;5(6):567–571. doi: 10.1038/sj.embor.7400172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gibney PA, West JD, Morano KA. The yeast Hsp70 Ssa1 is a sensor for activation of the heat shock response by thiol-reactive compounds. Mol Biol Cell. 2012;23(17):3290–3298. doi: 10.1091/mbc.e12-06-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272(5268):1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 59 kb)