Abstract
Sevoflurane, an inhaled ether general anesthetic agent, exerts a variety of neurotoxic effects, including oxidative stress, mitochondrial dysfunction, and neuronal apoptosis. However, the underlying molecular mechanisms remain to be elucidated. DJ-1 is a protein that exerts neuroprotective effects against different kinds of stress through multiple pathways. This study aimed to investigate the neuroprotective effects of DJ-1 against sevoflurane-induced neurotoxicity. Here, we found that sevoflurane treatment significantly increased DJ-1 expression in human neuroblastoma M17 cells in a dose-dependent manner at both the mRNA and protein levels. Interestingly, we found that overexpression of wild-type (WT) DJ-1 prevented sevoflurane-induced generation of reactive oxygen species (ROS) and nitric oxide (NO), deletion of reduced GSH, reduction of adenosine triphosphate (ATP), and mitochondrial membrane potential. Interestingly, we found that WT DJ-1 could inhibit sevoflurane-induced apoptosis by modulating the mitochondrial pathway. However, its “loss of function” mutation DJ-1(L166P) exacerbated sevoflurane-induced neurotoxicity in M17 cells. Our findings suggest that WT DJ-1 protects neuronal cells against sevoflurane-induced neurotoxicity.
Keywords: Sevoflurane, DJ-1, Oxidative stress, Mitochondria, Apoptosis
Introduction
Sevoflurane, a common inhaled volatile anesthetic, has been widely used in clinical surgeries (Wilder et al. 2009). However, multiple lines of evidence have shown that administration of volatile anesthetics in rodents induces impairment in learning and memory (Liu et al. 2010). Exposure to sevoflurane results in a widespread increase in brain apoptosis (Satomoto et al. 2009; Satomoto 2009). It was recently reported that sevoflurane treatment resulted in decreased cell viability and increased apoptosis by modulating caspase-3, Bcl-2, and MEK/ERK1/2 MAPK signaling pathways in primary hippocampal neuronal cultures (Wang et al. 2013). Furthermore, sevoflurane exposure has been shown to induce apoptosis mediated by endoplasmic reticulum (ER) stress in the hippocampus by increasing the expression of CHOP and caspase-12, which ultimately leads to cognitive impairment (Chen et al. 2013). Increased oxidative stress and mitochondrial dysfunction are other molecular mechanisms involved in sevoflurane-induced neurotoxicity (Yi et al. 2015). However, the underlying mechanism remains unclear.
DJ-1, a small 20 kDa protein encoded by the gene Parkinson’s disease protein-7 (PARK7) is highly conserved and ubiquitously expressed across diverse species (Lucas and Marin 2007). Mutations in the PARK7 gene account for ∼ 1–2% of early-onset recessive Parkinson’s disease (PD). DJ-1 was originally identified as an oncogene product (Nagakubo et al. 1997). Although the biological functions of DJ-1 still need to be elucidated, increasing evidence has shown that DJ-1 could protect cells from cytotoxicity caused by oxidative stress by oxidizing itself into a more acidic form (Taira et al. 2004). Both in vivo and in vitro studies have shown that overexpression of DJ-1 in rodents or cultured cells prevents cell death, which is attributed to its anti-oxidative stress capacities (Martinat et al. 2004). In contrast, inhibition of DJ-1 promotes the susceptibility of cells to oxidative stress (Yokota et al. 2003). Interestingly, DJ-1 can prevent neurotoxin-induced mitochondrial fragmentation in neuronal cells (Thomas et al. 2011). Oxidative stress causes accumulation of DJ-1 in mitochondria, which thereby exerts a neuroprotective effect (Junn et al. 2009), implicating that mitochondria might be a site of neuroprotective action for DJ-1. It is well-documented that wild-type (WT) DJ-1, but not DJ-1 mutants, scavenges oxidative stress. A homozygous point mutation (L166P) of DJ-1 has been studied as a “loss-of-function” mutation of DJ-1, which is associated with early-onset familial Parkinson’s disease (PD). The L166P mutant of DJ-1 increases susceptibility to cell death in response to various oxidative stress stimuli (Deeg et al. 2010). The chaperone activity of DJ-1 has been demonstrated in previous studies. Abnormal aggregation of proteins such as α-synuclein is an important characteristic feature of PD. Interestingly, it has been reported that WT DJ-1, but not its L166P mutant, functions as a redox-sensitive chaperone and inhibits aggregation of α-synuclein in neuroblastoma cells (Shendelman et al. 2004). DJ-1 and Escherichia coli Hsp31 are the two most important members of the ThiJ/PfpI superfamily and contain a conserved domain. Interestingly, Hsp31 also has chaperone and detoxifying enzyme activities and manages misfolded proteins, thus rescuing α-synuclein toxicity by attenuating α-synuclein aggregation (Zondler et al. 2014; Aslam and Hazbun 2016).
As one of the causative genes of familial PD, defects and “loss of function” mutations in DJ-1 including L166P lead to autosomal recessive early-onset PD. Indeed, the use of inhaled anesthetics has been linked with PD in previous studies. Mason and colleagues showed that administration of sevoflurane changed the concentration of dopamine (DA) in the brain by impairing the transport synaptosomes of DA, thus leading to further damage of the dopaminergic system and locomotor dysfunction (Mason et al. 1996). Additionally, sevoflurane severely aggravated the prognosis of PD in another causative gene LRRK2-associated Drosophila model by means of synaptic cholinergic deficits and impairment of locomotor abilities (Shan et al. 2015). Interestingly, a recent study demonstrated that DJ-1 has the potential to protect neuronal cells against oxidative stress, mitochondrial dysfunction, and apoptosis induced by the inhaled anesthetic gas isoflurane (Liu et al. 2015). However, little information regarding the effects of DJ-1 on neurotoxicity induced by the inhaled volatile anesthetic sevoflurane has been reported before.
Materials and methods
Cell cultures and treatment
Human M17 neuroblastoma cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/Opti-MEM (1:1) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) at 37 °C in a humidified atmosphere of 5% CO2. Stable cell lines overexpressing WT DJ-1 or DJ-1 L166P mutant were generated as previously described (Li et al. 2013a). Cells were exposed to 1, 2, or 3% sevoflurane for 36 h in one chamber.
MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide) assay
MTT reduction assay was used to determine cell proliferation in response to the indicated treatment. Briefly, upon completion of the indicated treatment, MTT was added into the culture medium without FBS at a final concentration of 1 mg/mL and incubated for 4 h in a CO2 incubator at 37 °C. The reaction product formazan crystals were dissolved using dimethyl sulfoxide (DMSO) on a shaker for 10 min in darkness. OD values were recorded at 570 nm to calculate the rate of cell proliferation.
Determination of LDH release
The release of LDH from cytosol into the culture medium was determined using a commercial kit (Thermo Fisher Scientific, USA). Briefly, after the necessary treatment, 50-μl supernatant per well of the culture was transferred into a 96-well microplate and mixed with 50-μl substrate. After incubation for 30 min, the reaction was stopped using stop buffer. OD values were recorded at 490 nm to calculate the level of LDH.
Measurement of intracellular reactive oxygen species
Intracellular levels of ROS production were assessed using the DCFH-DA assay. After the necessary treatment, cells were gently washed with sterile PBS and loaded with DCFH-DA at a final concentration of 10 μM. After incubation for 30 min at 37 °C in darkness, cells were washed five times with PBS. Fluorescence signals were captured by a fluorescence microscope and a digital camera. Statistical analysis was performed using Image-Pro Plus software.
Determination of intracellular nitric oxide
Intracellular levels of nitric oxide (NO) production were assessed using the DAF-FM DA assay. After the necessary treatment, cells were gently washed with sterile PBS and loaded with DAF-FM DA at a final concentration of 10 μM. After 30-min incubation at 37 °C in darkness, cells were washed five times with PBS. Fluorescence signals were captured by a fluorescence microscope and a digital camera. Statistical analysis was performed using Image-Pro Plus software.
Determination of mitochondrial membrane potential
Intracellular MMP levels were assayed using tetramethylrhodamine methyl ester (TMRM) fluorescent dye. After the necessary treatment, cells were gently washed with sterile PBS and loaded with TMRM at a final concentration of 20 μM. After incubation for 15 min at 37 °C in darkness, cells were washed five times with PBS. Fluorescence signals were captured by a fluorescence microscope and a digital camera. Statistical analysis was performed using Image-Pro Plus software.
Measurement of intracellular ATP levels
Levels of intracellular ATP in M17 cells were assayed using a bioluminescence assay with a commercial kit (Life Technologies, USA). After the necessary treatment, cells were lysed with a lysis buffer. Cell lysates were centrifuged at 12,000×g for 5 min at 4 °C. Fifty microliters of supernatant was mixed with an equal amount of luciferase reagent. The emitted light was captured using a microplate luminometer and used to calculate ATP concentrations.
Cytochrome C assay
After the necessary treatment, cells were washed three times with cold PBS. Cells were then resuspended in ice-cold cytosolic extraction buffer and gently homogenized with a glass Dounce homogenizer. Cells were then centrifuged at 2500 rpm at 4 °C for 10 min. The supernatant was then centrifuged at 12,000×g at 4 °C for 30 min to yield a cytosolic extract, which was subjected to Western blot analysis to determine cytochrome C levels.
Real-time polymerase chain reaction
Total intracellular RNA was isolated from cultured cells using Qiazol reagent (Qiagen, USA). DNAse (Ambion, USA) was used to remove genomic DNA contamination. The quality and concentration of RNA were determined by NanoDrop ND1000 spectrophotometer (Thermo Fisher Scientific, USA). One microgram isolated RNA was used for reverse transcription PCR to synthesize cDNA using the SuperScript III First-Strand Synthesis system. Two microliters of the reaction product was used for real-time PCR on an ABI 7900 system with Taqman gene expression Master Mix (Applied Biosystems, USA). The housekeeping gene GAPDH was used as an internal control.
Western blot analysis
Cells were lysed using RIPA buffer and a commercial cocktail protease inhibitor (Sigma-Aldrich, USA). Protein concentrations were determined using a BCA protein assay (Beyotime, China). Equivalent amounts (20 μg) of total protein were separated on 12% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and electrotransferred onto polyvinylidene difluoride (PVDF) membranes. Blots were blocked in 5% fat-free dry milk in TBST (0.1 M Tris-buffered saline-0.1% Tween-20) buffer for 2 h. Membranes were then sequentially incubated with primary antibodies overnight at 4 °C and secondary antibodies for 1 h at room temperature. Blots were visualized with an enhanced chemiluminescence reagent (Amersham Biosciences, USA). The following antibodies were used in this study: Rabbit monoclonal primary antibody against DJ-1 (1:2000, #2134, Cell Signaling Technology, USA); Rabbit monoclonal primary antibody against cytochrome C (1:1000, #11940, Cell Signaling Technology, USA); Rabbit monoclonal primary antibody against cleaved caspase-3 (1:1000, #9661, Cell Signaling Technology, USA); Rabbit monoclonal primary antibody against β-actin (1:5000, #4970, Cell Signaling Technology, USA); HRP-linked anti-rabbit IgG antibody (1:2000, #7074, Cell Signaling Technology, USA).
Statistical analysis
Experimental data were expressed as means ± SEM. Experiments were repeated at least three times. Statistical analysis was performed by one-way or two-way ANOVA. P values less than 0.05 were considered statistically significant.
Results
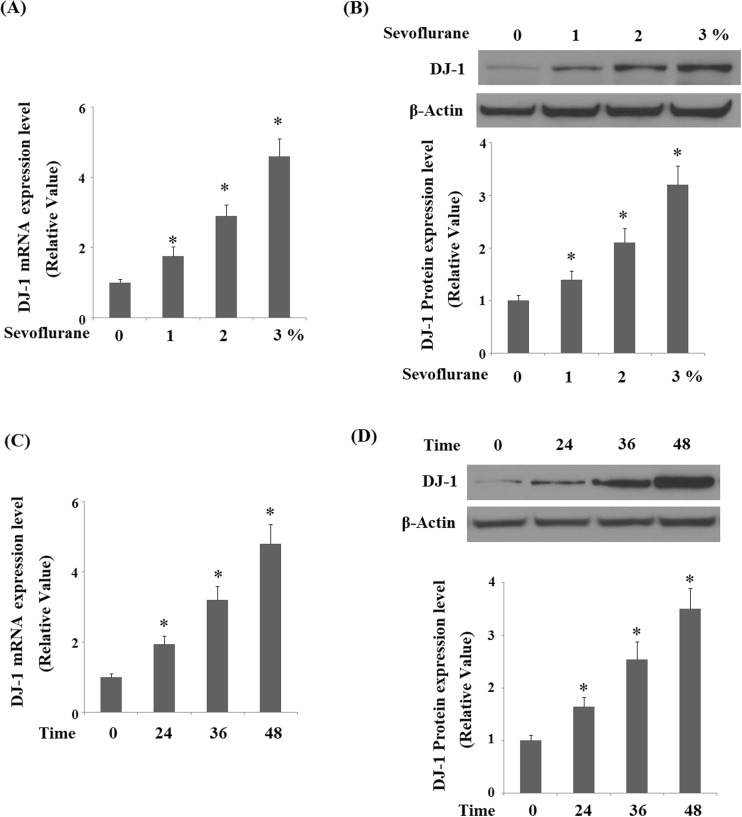
DJ-1 is an essential regulator of oxidative stress and cell survival. We assessed the expression of DJ-1 in response to sevoflurane in human M17 cells. Our results demonstrated that sevoflurane treatment increased the expression of DJ-1 in a dose-dependent manner (1, 2, and 3% for 36 h) at both the mRNA levels (Fig. 1a) and protein levels (Fig. 1b). In addition, treatment with 2% sevoflurane increased the expression of DJ-1 in a time-dependent manner at both the mRNA levels (Fig. 1c) and protein levels (Fig. 1d).
Fig. 1.
Sevoflurane increased expression of DJ-1 in human M17 neuroblastoma cells. a, b Human M17 neuroblastoma cells were treated with sevoflurane at concentrations of 1, 2, 3% for 36 h. Real-time PCR analysis and Western blot analysis of DJ-1. c, d Human M17 neuroblastoma cells were treated with 2% sevoflurane for 24, 36, and 48 h. Real-time PCR analysis and Western blot analysis of DJ-1 (*P < 0.01 vs. non-treated control; n = 3–5)
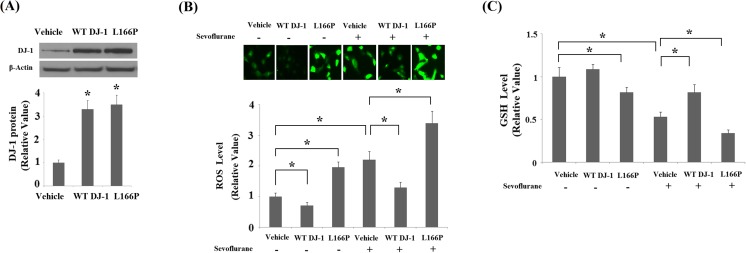
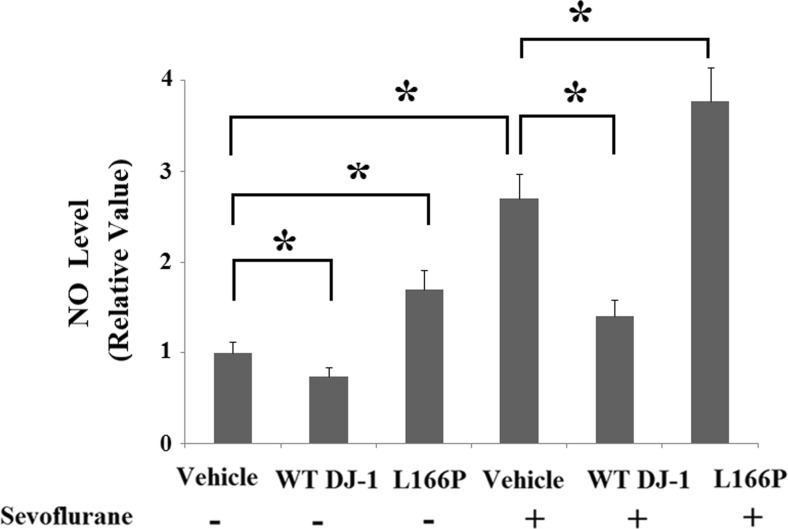
Elevated levels of DJ-1 may be associated with sevoflurane neurotoxicity or a compensatory response for survival. To elucidate the physiological function of DJ-1 in sevoflurane-induced neurotoxicity, we used stable transfection lines of wild-type (WT) DJ-1 and the “loss of function” L166P mutant of DJ-1. Successful overexpression of DJ-1 is shown in Fig. 2a. The potential protective effects of DJ-1 and the toxic effects of L166P in neuronal cells have been widely reported in previous studies (Li et al. 2014; Li et al. 2013b). Consistently, we found that overexpression of WT DJ-1 reduced basal levels of ROS (Fig. 2b) and nitric oxide (NO) (Fig. 3). In contrast, overexpression of DJ-1 L166P mutant promoted the production of basal ROS and NO. Additionally, overexpression of DJ-1 L166P mutant caused mitochondrial dysfunction by reducing MMP levels (Fig. 4a) and ATP production (Fig. 4b). The results in Fig. 2b indicate that overexpression of WT DJ-1 inhibited 2% sevoflurane (36 h)-induced generation of ROS. In contrast, overexpression of DJ-1 L166P mutant exacerbated the production of ROS. Similarly, our results indicate that sevoflurane treatment significantly decreased the levels of reduced GSH, which was prevented by overexpression of WT DJ-1 but exacerbated by the “loss of function” L166P mutant of DJ-1 (Fig. 2c). Additionally, we found that overexpression of WT DJ-1 reduced sevoflurane-induced production of nitric oxide (NO) (Fig. 3). However, DJ-1 L166P mutant exacerbated sevoflurane-induced production of nitric oxide (NO).
Fig. 2.
Stable transfection of wild-type (WT) DJ-1 but not DJ-1 L166P mutant ameliorated sevoflurane-induced oxidative stress. Cells were treated with 2% sevoflurane for 36 h. a Western blot analysis revealed the successful overexpression of DJ-1. b Intracellular ROS was determined by DCFH-DA assay. c Levels of reduced GSH (*P < 0.01, n = 4–5)
Fig. 3.
Stable transfection of wild-type (WT) DJ-1 but not DJ-1 L166P mutant attenuated sevoflurane-induced production of nitric oxide (NO). Cells were treated with 2% sevoflurane for 36 h. Intracellular levels of nitric oxide (NO) were determined by DAF-FM DA assay (*P < 0.01, n = 4–5)
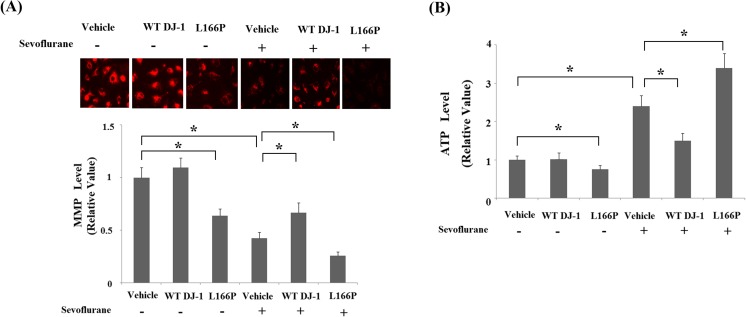
Fig. 4.
Stable transfection of wild-type (WT) DJ-1 but not DJ-1 L166P mutant ameliorated sevoflurane-induced mitochondrial dysfunction. Cells were treated with 2% sevoflurane for 36 h. a Intracellular levels of mitochondrial membrane potential (MMP) were measured by TMRM assay. b Intracellular levels of ATP were measured by bioluminescence assay (*P < 0.01, n = 4–5)
We then assessed the effects of DJ-1 on sevoflurane-induced mitochondrial dysfunction in M17 cells. The results in Fig. 4a indicate that sevoflurane treatment significantly reduced MMP, which was inhibited by overexpression of WT DJ-1, but exacerbated by DJ-1 L166P mutant. Additionally, we found that WT DJ-1 ameliorated sevoflurane-induced reduction of ATP. In contrast, DJ-1 L166P mutant exacerbated sevoflurane-induced reduction of ATP (Fig. 4b). These results imply that DJ-1 plays a key role in protecting mitochondria from sevoflurane-induced insult in M1 neuronal cells.
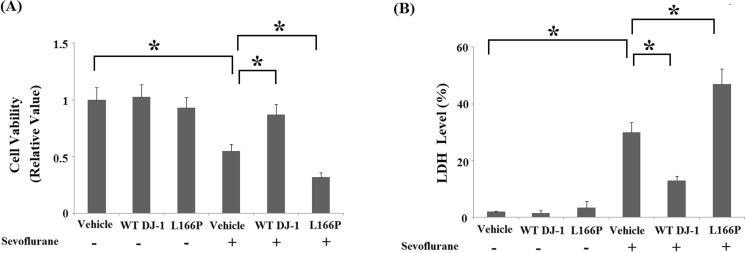
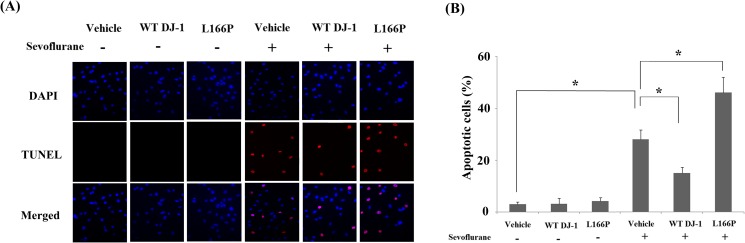
Both MTT (Fig. 5a) and LDH assays (Fig. 5b) indicate that sevoflurane induced significant cell death in M17 cells. Importantly, overexpression of WT DJ-1 caused M17 cells to be more resistant to sevoflurane-induced neurotoxicity. In contrast, overexpression of DJ-1 L166P mutant exacerbated neuronal death. Additionally, TUNEL assay indicated that overexpression of WT DJ-1 reduced sevoflurane-induced apoptosis. In contrast, DJ-1 L166P mutant promoted sevoflurane-induced apoptosis (Fig. 6).
Fig. 5.
Stable transfection of wild-type (WT) DJ-1 but not DJ-1 L166P mutant ameliorated sevoflurane-induced neuronal death. Cells were treated with 2% sevoflurane for 36 h. a Cell viability was determined by the MTT assay. b The release of LDH was determined by a commercial kit (*P < 0.01, n = 4–5)
Fig. 6.
Stable transfection of wild-type (WT) DJ-1 but not DJ-1 L166P mutant attenuated sevoflurane-induced apoptosis. Cells were treated with 2% sevoflurane for 36 h. a Cell apoptosis was determined by the TUNEL assay. b Quantitation of apoptotic cells (*P < 0.01, n = 4–5)
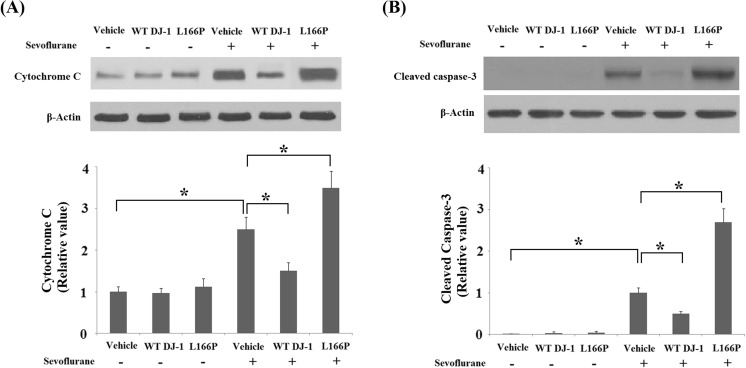
Mitochondrial dysfunction results in the release of cytochrome C from mitochondria into the cytoplasm, thereby activating cleavage of caspase-3 (Fiocchetti et al. 2013). Western blot analysis demonstrated that overexpression of WT DJ-1 reduced the release of cytochrome C and cleavage of caspase-3 induced by sevoflurane. COX4 was used as the control for cytosolic fractions. In contrast, overexpression of DJ-1 L166P mutant exacerbated the release of cytochrome C (Fig. 7a) and cleavage of caspase-3 (Fig. 7b).
Fig. 7.
Stable transfection of wild-type (WT) DJ-1 but not DJ-1 L166P mutant ameliorated sevoflurane-induced release of cytochrome C and cleavage of caspase-3. a The release of cytochrome C was measured by Western blot analysis. b Cleavage of caspase-3 was measured by Western blot analysis (*P < 0.01, n = 4–5)
Discussion
DJ-1 is ubiquitously expressed across diverse tissues. Multifunctional features of DJ-1 have been reported in previous studies (Gao et al. 2017). Although the precise biological functions of DJ-1 remain unclear, DJ-1 has been reported to play key roles in a variety of physiological processes (Xu et al. 2017). It is well-recognized that DJ-1 could act as a redox sensitive adapter protein and play critical roles in regulating the cellular response to toxic stimuli (Piston et al. 2017). However, it should be noted that WT DJ-1, but not DJ-1 L166P mutant, has protective properties against toxic stimuli. Deletion of DJ-1 induces increased cellular susceptibility to neurotoxic insults. For example, DJ-1 knockout mice display nigrostriatal dopaminergic deficits and hypokinesia (Goldberg et al. 2005) and show increased vulnerability to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity (Kim et al. 2005). In contrast, mice with overexpressed DJ-1 display increased resistance to MPTP toxicity (Paterna et al. 2007). The DJ-1 L166P mutant has been associated with the pathogenesis of autosomal recessive early-onset Parkinsonism (Guo et al. 2008). In the current study, we found that sevoflurane could elevate the expression of DJ-1 in human M17 neuronal cells. As far as we know, this is the first time the physiological function of DJ-1 against sevoflurane-induced neurotoxicity has been investigated.
Exposure to sevoflurane caused widespread cerebral neurotoxicity. In vitro studies have shown that exposure to sevoflurane induced excessive production of ROS and reduced the activity of superoxide dismutase (SOD) in neuronal cells (Yi et al. 2015). Here, our results showed that overexpression of WT DJ-1 but not its L166P mutant abolished sevoflurane-induced generation of ROS and deletion of reduced GSH, which is consistent with findings from a previous study showing that WT DJ-1 protected neuronal cells from oxidative stress-induced insults but its L166P mutant promoted such insults (Li and Xu 2013). Sevoflurane treatment can cause apoptosis through the mitochondria-dependent apoptosis pathway (Loop et al. 2005). In this study, our results demonstrated that sevoflurane treatment induced a reduction in MMP and ATP in M17 cells, which could be prevented by treatment with WT DJ-1 and exacerbated by DJ-1 L166P mutant. Similarly, a recent study has shown that overexpression of WT DJ-1 in M17 cells maintained normal mitochondrial morphology but DJ-1 mutants including R98Q, D149A, and L166P induced significant fragmentation of mitochondria. Furthermore, DJ-1 mutants led to mitochondrial dysfunction and increased sensitivity to oxidative stress or neurotoxins (Wang et al. 2012). Here, our findings indicate that DJ-1 mitigated sevoflurane-induced neuronal apoptosis by reducing the release of cytochrome C from mitochondria to cytosol and the subsequent cleavage of caspase-3. We speculated that DJ-1 might protect neuronal cells against sevoflurane-induced neuronal death through modulation of the mitochondria-dependent pathway. Consistently, it has been reported that DJ-1 inhibits apoptosis by decreasing caspase activation and reducing Bax expression, thereby preventing p53 activation (Fan et al. 2008).
In the current study, our results demonstrated that WT DJ-1 but not PD-associated DJ-1 L166P mutant protected neuroblastoma M17 cells against sevoflurane-induced cytotoxicity, which is consistent with a case report showing that long-term exposure to anesthetic gases including sevoflurane may have induced PD in the subject (Mastrangelo et al. 2013). Our results support the hypothesis of association between exposure to anesthetic gases and risk of PD. Here, our results demonstrate that sevoflurane treatment increased expression of DJ-1, which has been reported to be an important chaperone molecule. Interestingly, a previous study demonstrated that sevoflurane induced the expression of another important chaperone protein heat shock protein 70 (HSP70) (Pagel 2008). However, whether sevoflurane influences the chaperone activity of DJ-1 is still unknown. Further investigation is warranted to characterize the underlying mechanism.
Taken together, our findings show that WT DJ-1 protects neuronal cells against sevoflurane-induced oxidative stress and apoptosis via a mitochondria-dependent pathway. In contrast, DJ-1 L166P mutant exacerbates the neurotoxic effect of sevoflurane in neuronal cells.
References
- Aslam K, Hazbun TR. Hsp31, a member of the DJ-1 superfamily, is a multitasking stress responder with chaperone activity. Prion. 2016;10(2):103–111. doi: 10.1080/19336896.2016.1141858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B Li DWY, Xu ZY. Edaravone prevents neurotoxicity of mutant L166P DJ-1 in Parkinson’s disease. J Mol Neurosci. 2013;51(2):539–549. doi: 10.1007/s12031-013-0022-8. [DOI] [PubMed] [Google Scholar]
- Chen G, Gong M, Yan M, Zhang X. Sevoflurane induces endoplasmic reticulum stress mediated apoptosis in hippocampal neurons of aging rats. PLoS One. 2013;8(2):e57870. doi: 10.1371/journal.pone.0057870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg S, Gralle M, Sroka K, Bahr M, Wouters FS, Kermer P. BAG1 restores formation of functional DJ-1 L166P dimers and DJ-1 chaperone activity. J Cell Biol. 2010;188:505–513. doi: 10.1083/jcb.200904103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Ren H, Jia N, Fei E, Zhou T, Jiang P, Wu M, Wang G. DJ-1 decreases bax expression through repressing p53 transcriptional activity. J Biol Chem. 2008;283(7):4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- Fiocchetti M, De Marinis E, Ascenzi P, Marino M. Neuroglobin and neuronal cell survival. Biochim Biophys Acta. 2013;1834(9):1744–1749. doi: 10.1016/j.bbapap.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Gao W, Shao R, Zhang X, Liu D, Liu Y, Fa X. Up-regulation of caveolin-1 by DJ-1 attenuates rat pulmonary arterial hypertension by inhibiting TGFβ/Smad signaling pathway. Exp Cell Res. 2017;361(1):192–198. doi: 10.1016/j.yexcr.2017.10.019. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Guo JF, Xiao B, Liao B, Zhang XW, Nie LL, Zhang YH, Shen L, Jiang H, Xia K, Pan Q, Yan XX, Tang BS. Mutation analysis of Parkin, PINK1, DJ-1 and ATP13A2 genes in Chinese patients with autosomal recessive early-onset parkinsonism. Mov Disord. 2008;23:2074–2079. doi: 10.1002/mds.22156. [DOI] [PubMed] [Google Scholar]
- Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res. 2009;87:123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Geng J, Liu J. Adiponectin offers protection against L166P mutant DJ-1-induced neuronal cytotoxicity mediated by APPL1-dependent AMPK activation. Int J Neurosci. 2014;124(5):350–361. doi: 10.3109/00207454.2013.846340. [DOI] [PubMed] [Google Scholar]
- Liu XS, Xue QS, Zeng QW, Li Q, Liu J, Feng XM, Yu BW. Sevoflurane impairs memory consolidation in rats, possibly through inhibiting phosphorylation of glycogen synthase kinase-3beta in the hippocampus. Neurobiol Learn Mem. 2010;94:461–467. doi: 10.1016/j.nlm.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Liu W, Guo Q, Hu X, Peng L, Zhou B. Induction of DJ-1 protects neuronal cells from isoflurane induced neurotoxicity. Metab Brain Dis. 2015;30(3):703–709. doi: 10.1007/s11011-014-9622-4. [DOI] [PubMed] [Google Scholar]
- Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl HL, Geiger KK, Pannen BH. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102(6):1147–1157. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- Lucas JI, Marin IA. New evolutionary paradigm for the Parkinson disease gene DJ-1. Mol Biol Evol. 2007;24:551–561. doi: 10.1093/molbev/msl186. [DOI] [PubMed] [Google Scholar]
- Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. Sensitivity to oxidative stress in DJ-1-Deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason LJ, Cojocaru TT, Cole DJ. Surgical intervention and anesthetic management of the patient with Parkinson’s disease. Int Anesthesiol Clin. 1996;34:133–150. doi: 10.1097/00004311-199603440-00010. [DOI] [PubMed] [Google Scholar]
- Mastrangelo G, Comiati V, dell'Aquila M, Zamprogno E. Exposure to anesthetic gases and Parkinson's disease: a case report. BMC Neurol. 2013;13:194. doi: 10.1186/1471-2377-13-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Pagel PS. Induction of heat shock protein 70 and preconditioning by sevoflurane: a potent protective interaction against myocardial ischemia-reperfusion injury. Anesth Analg. 2008;107(3):742–745. doi: 10.1213/ane.0b013e31817f6d40. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Leng A, Weber E, Feldon J, Bueler H. DJ-1 and Parkin modulate dopamine-dependent behavior and inhibit MPTP-induced nigral dopamine neuron loss in mice. Mol Ther. 2007;15:698–704. doi: 10.1038/sj.mt.6300067. [DOI] [PubMed] [Google Scholar]
- Piston D, Alvarez-Erviti L, Bansal V, Gargano D, Yao Z, Szabadkai G, Odell M, Puno MR, Björkblom B, Maple-Grødem J, Breuer P, Kaut O, Larsen JP, Bonn S, Møller SG, Wüllner U, Schapira AHV, Gegg ME. DJ-1 is a redox sensitive adapter protein for high molecular weight complexes involved in regulation of catecholamine homeostasis. Hum Mol Genet. 2017;26(20):4028–4041. doi: 10.1093/hmg/ddx294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satomoto M. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- Shan Z, Cai S, Zhang T, Kuang L, Wang Q, Xiu H, Wen J. Gu H, Xu K. Effects of sevoflurane on leucine-rich repeat kinase 2-associated Drosophila model of Parkinson's disease. Mol Med Rep. 2015;11(3):2062–2070. doi: 10.3892/mmr.2014.2966. [DOI] [PubMed] [Google Scholar]
- Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KJ, McCoy MK, Blackinton J, et al. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet. 2011;20:40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Petrie TG, Liu Y, Liu J, Fujioka H, Zhu X. Parkinson's disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem. 2012;121(5):830–839. doi: 10.1111/j.1471-4159.2012.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WY, Yang R, Hu SF, Wang H, Ma ZW, Lu Y. N-stearoyl-L-tyrosine ameliorates sevoflurane induced neuroapoptosis via MEK/ERK1/2 MAPK signaling pathway in the developing brain. Neurosci Lett. 2013;541:167–172. doi: 10.1016/j.neulet.2013.02.041. [DOI] [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110(4):796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CY, Kang WY, Chen YM, Jiang TF, Zhang J, Zhang LN, Ding JQ, Liu J, Chen SD. DJ-1 inhibits α-Synuclein aggregation by regulating chaperone-mediated autophagy. Front Aging Neurosci. 2017;9:308. doi: 10.3389/fnagi.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi W, Zhang Y, Guo Y, Li D, Li X. Elevation of Sestrin-2 expression attenuates sevoflurane induced neurotoxicity. Metab Brain Dis. 2015;30(5):1161–1166. doi: 10.1007/s11011-015-9673-1. [DOI] [PubMed] [Google Scholar]
- Yokota T, Sugawara K, Ito K, Takahashi R, Ariga H, Mizusawa H. Biochem. Biophys Res Commun. 2003;312:1342–1348. doi: 10.1016/j.bbrc.2003.11.056. [DOI] [PubMed] [Google Scholar]
- Zondler L, Miller-Fleming L, Repici M, Goncalves S, Tenreiro S, Rosado-Ramos R, Betzer C, Straat- man KR, Jensen PH, Giorgini F, et al. DJ-1 interactions with a-synuclein attenuate aggregation and cellular toxicity in models of Parkinson’s disease. Cell Death Dis. 2014;5:e1350. doi: 10.1038/cddis.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]