Abstract
High expression of Hsp27 in glioma cells has been closely associated with tumor cell proliferation and apoptosis inhibition. The aim of the present study was to asses the effects of rosmarinic acid (RA) on Hsp27 expression and apoptosis in non-transfected and transfected human U-87 MG cells. The effect of rosmarinic acid was compared to quercetin, which is known to be a good Hsp27 inhibitor. In order to block the expression of Hsp27 gene (HSPB1), transfection with specific siRNAs was performed. Western blotting technique was used to assess the Hsp27 expression, and caspase-3 colorimetric activity assay was performed to determine apoptosis induction. According to the results, it was found that RA and quercetin effectively silenced Hsp27 and both agents induced apoptosis by activating the caspase-3 pathway. Eighty and 215 μM RA decreased the level of Hsp27 by 28.8 and 46.7% and induced apoptosis by 30 and 54%, respectively. For the first time, we reported that rosmarinic acid has the ability to trigger caspase-3 induced apoptosis in human glioma cells. As a result of siRNA transfection, the Hsp27 gene was silenced by ~ 50% but did not cause a statistically significant change in caspase-3 activation. It was also observed that apoptosis was induced at a higher level as a result of Hsp27 siRNA and subsequent quercetin or RA treatment. siRNA transfection and 215 μM RA treatment suppressed Hsp27 expression level by 90.5% and increased caspase-3 activity by 58%. Herein, we demonstrated that RA administered with siRNA seems to be a potent combination for glioblastoma therapy.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0896-z) contains supplementary material, which is available to authorized users.
Keywords: Rosmarinic acid, Quercetin, siRNA, Hsp27, Glioma, Apoptosis
Introduction
Glioblastomas (GBM-grade IV astrocytoma) are the most malignant primary brain tumors with fatal consequences worldwide. For these malignant astrocytic tumors, average survival rates are less than 15 months and the mortality rate is over 50%. Despite surgical, radiological, and chemotherapeutic interventions, these rates have not changed much (Graner et al. 2009; Jakubowicz-Gil et al. 2010; Combs et al. 2016). Therefore, scientists and clinicians are still searching for better therapies for malignant gliomas. Since the therapeutic potential of reducing the abnormal levels of stress proteins (also known as heat shock proteins, Hsps) in diverse tumor cells have been revealed, Hsp-based therapies have become a significant target for new approaches against cancer (Jakubowicz-Gil et al. 2010; Lianos et al. 2015; Önay-Uçar 2015).
Hsps are a group of highly evolutionary conserved proteins. In normal cells, many of Hsps act as molecular chaperones and they are responsible for maintaining protein homeostasis. Conversely, during disease, their function aids the advancement of disease (McConnell and McAlpine 2013). Especially in cancer, high level expression of many Hsps has been observed (Lianos et al. 2015; Önay-Uçar 2015). It is known that Hsp overexpression in tumor cells is caused by resistance to cell death (Samali and Cotter 1996). One of the best-studied of these proteins is Hsp27, which is highly expressed in different brain tumors such as astrocytomas, gliomas, and oligodendrogliomas (Zhang et al. 2003). High levels of Hsp27 induce tumor cell proliferation, differentiation, and metastasis and inhibit apoptosis. It is also associated with drug resistance and survival of the cell in lethal conditions. All these studies suggest that Hsp27 stimulates the process of the carcinogenesis (Garrido et al. 2006; Kim and Kim 2011; Khalil et al. 2011; Jego et al. 2013).
During the past decade, antioxidant agents have been studied in cancer researches as potential therapeutic agents because of their free radical-scavenging capacity, modulation of gene expression, and interaction with cell-signaling pathways. One of the best known Hsp inhibitors as an antioxidant agent is quercetin (3,3′,4′,5–7-pentahydroxyflavone). It is a natural flavonoid and found in many edible fruits and vegetables such as apple, various berries, grapes, lemon, onions, dill, tomato, and beverages such as coffee and tea. Quercetin is known to possess an anticarcinogenic potential. The anticancer property of it is due to ability to inhibit various proteins that are effective in carcinogenesis (Jakubowicz-Gil et al. 2005; Gupta et al. 2010; Khan et al. 2016). In particular (especially today), quercetin is known as Hsp27 inhibitor (Li et al. 2016) and also is known to facilitate apoptosis of tumor cells by caspase-3 and caspase-9 activations (Chowdhury et al. 2005; Jakubowicz-Gil et al. 2008; Kim et al. 2008; Vargas and Burd 2010; Jakubowicz-Gil et al. 2013a; Bądziul et al. 2014).
Rosmarinic acid (RA) is a phenolic carboxylic acid and is naturally found in many Lamiaceae species such as lemon balm, oregano, peppermint, rosemary, sage, and thyme. RA has many beneficial biological effects. These are mainly antioxidant, antidiabetic, antitumoral, and neuroprotective effects (Petersen and Simmonds 2003; Tepe et al. 2007; Airoldi et al. 2013; Furtado et al. 2015; Runtuwene et al. 2016; Venkatachalam et al. 2016). However, the potential effect of RA on the expression of Hsp27 in cancer cells and its mechanism has not yet been found.
Today, RNA interference (RNAi) based on silencing of targeted gene expression, e.g., small interfering (si)RNA-mediated silencing, has a potential therapeutic strategy against cancer. The modulation of gene expression by siRNAs is a powerful tool (Elbashir et al. 2001; Wang et al. 2011; Wu et al. 2014; Sun et al. 2015). Furthermore, the specificity and potency of Hsp-spesific siRNAs in cell culture for inhibit Hsp gene are shown in many studies (Hosaka et al. 2006; Rocchi et al. 2006; Kumano et al. 2012; Behnsawy et al. 2013; Jakubowicz-Gil et al. 2013a; Bądziul et al. 2014; Li et al. 2016).
The initial purpose of the presented study is to exhibit the effect of RA on the Hsp27 expression and apoptosis in U-87 MG cells. The second purpose is to compare the effects of RA with quercetin and siRNA-mediated silencing and to determine effect of combined therapy.
Materials and methods
Reagents and siRNAs
Rosmarinic acid was from Sigma-Aldrich, and quercetin was from Santa Cruz Biotechnology. They were dissolved in dimethyl sulfoxide (DMSO). Double-stranded siRNA molecules were purchased from Ambion and dissolved in ultrapure DNase/RNase-free distilled water to a final concentration of 10 μM. Two different Hsp27 siRNA molecules were used in this study. The sense strand of Hsp27 siRNA 1 (Hsp27 si1) was 5′-GCGUGUCCCUGGAUGUCAAtt-3′, and the antisense strand was 5′-UUGACAUCCAGGGACACGCgc-3′; the sense strand of Hsp27 siRNA 2 (Hsp27 si2) was 5′-GCCGCCAAGUAAAGCCUUAtt-3′, and antisense strand was 5′-UAAGGCUUUACUUGGCGGCag-3′. Scrambled non-silencing siRNA (NS si) was used as a negative control. DharmaFECT 4 transfection reagent was from Dharmacon, and Opti-MEM transfection medium was from Invitrogen. Standard tissue culture reagents were obtained from Gibco. Caspase-3 Colorimetric Activity Assay Kit and polyvinylidene fluoride (PVDF) membrane were purchased from Millipore. Mouse anti-Hsp27 monoclonal antibody and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG were obtained from Enzo Life Sciences. HRP-conjugated GAPDH Loading Control Monoclonal Antibody was from ThermoFisher Scientific. ECL Plus Western Blotting Detection System was purchased Amersham. SMART™ BCA Protein Assay Kit was from iNtRON Biotechnology. EDTA-free protease inhibitor cocktail was from Roche. All the other chemicals were obtained from Sigma.
Cells and culture conditions
Human glioma U-87 MG cells were grown in Dulbecco’s modified Eagle’s medium/high glucose (DMEM/High) supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 1% (v/v) antibiotic-antimycotics solution (100 U/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin B), and 1% (v/v) non-essential amino acids at 37 °C in a humidified incubator containing 5% CO2. The cells were passaged every 3 days.
Cell viability test
MTT (3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl-tetrazolium bromide) assay with minor modifications was used for detecting cytotoxic activities of antioxidant agents (rosmarinic acid and quercetin), siRNA transfections, and combined therapies (Mosmann 1983; Uçar et al. 2012). Cells were seeded in a flat-bottomed 96-well plate at a density of 1 × 104 per well 24 h prior to treatment. For detecting cytotoxic activity of antioxidant agents, the cells were treated with increasing concentrations of agents diluted with DMEM/High (0–200 μM for quercetin, 0–1000 μM for RA). To determine the effects of cell transfection and combined therapy applications on cell viability, transfection reagent/siRNA complex was prepared. DharmaFECT 4 transfection reagent was used different final concentrations (1.5, 2.5, 3.5, and 4.5 μL/mL), and the cells were transfected with 25 and 50 nM siRNAs. siRNA transfection and combined therapy applications were performed as described in the title cell transfection and combined therapy. After 48 h post-incubation at 37 °C, the adherent cells were washed with Dulbecco’s phosphate-buffered saline (D-PBS) and 30 μL of MTT (5 mg/mL in sterile D-PBS) was added to each well. Then, the cells were incubated for 4 h to allow formation of formazan crystals. After 4 h, 150 μL of DMSO was added to each well to dissolve the formazan crystals, and the absorbance of formazan products was measured at 540 nm in a microplate reader (EON, BioTek Instruments, Inc.). Samples were analyzed in triplicate in three independent assays.
Rosmarinic acid and quercetin treatments
For cell treatment with rosmarinic acid and quercetin, U-87 MG cells were plated in a six-well plate at a density of 2.8 × 105 and the cells kept overnight. According to cell viability test results, quercetin at the final concentrations of 10 and 30 μM and rosmarinic acid at the final concentrations of 80 and 215 μM were applied to the cells. The final concentration of DMSO in the culture medium did not exceed 0.2%. Assays were done at 48 h after treatments.
Cell transfection and combined therapy
To block the expression of Hsp27, transfection of U-87 MG cells with specific siRNAs was performed according to the manufacturer’s protocol. The amounts of specific siRNAs and transfection reagent were selected experimentally via MTT assay. For transfection, U-87 MG cells at the density 2.8 × 105 were seeded in a six-well cell culture plate, and the cells were incubated at 37 °C in a CO2 incubator for 24 h until cell confluence reached 60–80%. Prior to transfection, transfection reagent/siRNA complex was prepared. DharmaFECT 4 transfection reagent was added to Opti-MEM transfection medium for a final concentration of 2.5 μL/mL and incubated at room temperature for 5 min. Hsp27 or NS siRNA was added at final concentrations of 25 and 50 nM, and incubated for another 20 min. During this time, the cells were washed with D-PBS, and then fresh growth medium without antibiotics was added each well of plate. Next, the transfection reagent/ Hsp27 or NS siRNA complex (first and second set of transfections) was added to the wells and incubated at 37 °C for 5 h. A third set of transfections was done without any siRNA (mock transfection). And also, non-transfected cells were used as a control. Five hours later, the medium was replaced with complete fresh medium. Additively for combined therapy groups, the transfected U-87 MG cells were exposed to quercetin or rosmarinic acid treatment at the end of the 5-h transfection period. For this, the medium was replaced with complete fresh medium containing these agents. The effectiveness of Hsp27 gene silencing was assessed at the protein level by immunoblotting. Assays were done at 48 h after transfection. Three independent experiments were performed.
Immunoblotting assays
At 48 h after treatments, the cells were collected by trypsinization and washed with D-PBS. The cells were lysed in lysis buffer containing 0.02 M Tris-HCl (pH 6.8), 0.04% (w/v) ethylenediaminetetraacetic acid (EDTA), 1% (v/v) Triton X-100, EDTA-free protease inhibitor cocktail (1 tablet/50 mL), and 1 mM phenylmethanesulfonyl fluoride (PMSF). The extract was centrifuged at 20,000×g for 20 min at 4 °C to remove insoluble materials. The protein concentration of the supernatants was determined using the SMART™ BCA Protein Assay Kit. Cell lysates were solubilized in sample buffer [25 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 10% 2-mercaptoethanol, and 0.002% bromophenol blue] and boiled for 4 min. Equal amounts of protein (30 μg/well) were analyzed with 10% sodium dodecyl-sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, Trans-Blot Turbo Transfer System (Bio-Rad) was used for electrotransfer of the separated proteins to PVDF membrane. Following transfer, immunodetection analysis was performed. Firstly, membranes were blocked with 5% (w/v) non-fat dry milk [in PBS-(0.05% (v/v) Tween 20] at room temperature for 1 h. Then, membranes were incubated overnight at 4 °C with mouse anti-Hsp27 monoclonal antibody diluted 1:1000. The membranes were washed four times for 10 min with PBS containing 0.05% Tween 20. After the wash period, the membranes were incubated for 2 h at room temperature with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG diluted 1:5000. The data were normalized relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the HRP-conjugated GAPDH Loading Control Monoclonal Antibody (working dilution 1:2000). Proteins were visualized with chemiluminescence luminol reagents (ECL Plus Western Blotting Detection System). The levels of protein expression were determined using the ImageLab 5.2.1 software (Bio-Rad). At least three independent experiments were performed.
Caspase-3 activity assays
Caspase-3 activity was measured using the Caspase-3 Colorimetric Activity Assay Kit according to the manufacturer’s protocol. Briefly, cells were resuspended in cell lysis buffer and incubated on ice for 10 min. After incubation period, the cell lysates were centrifuged at 10,000×g for 5 min at 4 °C. The supernatants were incubated with 1 mM caspase-3 substrate (Ac-DEVD-pNA) for 2 h at 37 °C, and caspase-3 activity was measured at 405 nm in a microplate reader. Samples were analyzed in triplicate in three independent assays.
Statistical analysis
The quantitative data were presented as mean ± standard deviation (SD) with n denoting the number of experiments. Statistical analysis and graph generation were performed using the GraphPad Prism Software version 5.01. The statistical evaluation was performed with one-way ANOVA test followed by Dunnet’s multiple comparison test. P value of < 0.05 was taken as the criterion of statistically significance.
Results
Cytotoxic analyses
The effects of quercetin and rosmarinic acid on U-87 MG cell viability
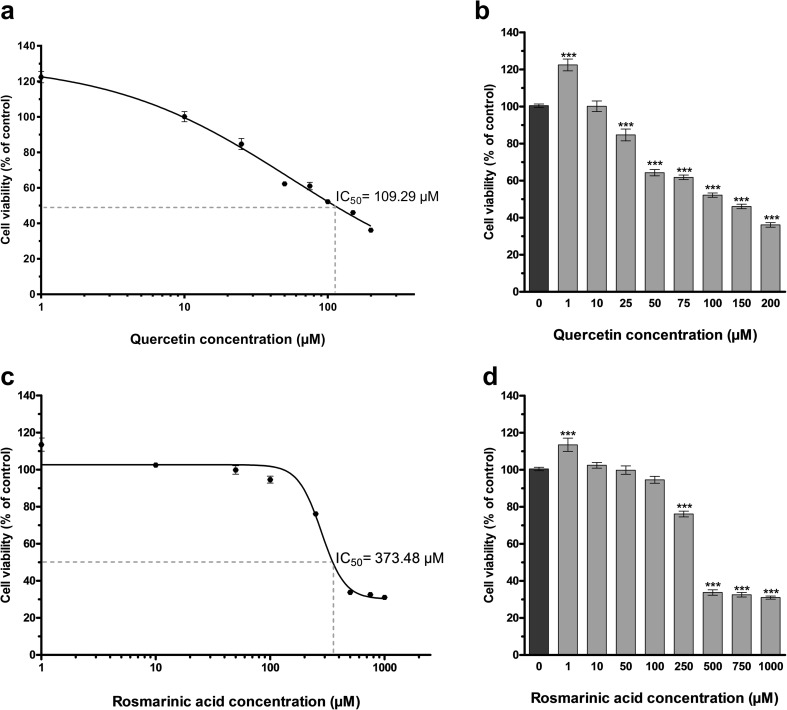
The cytotoxic activity of quercetin and rosmarinic acid was examined in the presence of different concentrations. To determine the cytotoxicity, tested compounds were applied singly, and a modified MTT assay was used. Cells were treated with 0–200 μM of quercetin or 0–1000 μM RA for 48 h. The final concentration of DMSO in the culture medium did not exceed 0.2% which this rate did not affect cell (data not shown). Cytotoxicity data are presented as mean percentages of control ± standard deviation (SD). The half-maximal inhibitory concentration (IC50) was determined using GraphPad Prism® 5.01 software, and sigmoidal dose-response (variable slope) curve fit analysis was used to calculate IC50 value. The cytotoxic activity data showed that quercetin and RA induced death in U-87 MG cell line in a dose-dependent manner after 48 h incubation (Fig. 1). For U-87 MG cells, the dose of quercetin yielding 50% mortality (IC50) was 109.29 μM and the IC50 value of RA was 373.48 μM. According to cytotoxicity results, doses of all agents used in experiments were selected according to cell viability ≥ 80%. Therefore, quercetin at the final concentrations of 10 and 30 μM and RA at the final concentrations of 80 and 215 μM were used in the experiments.
Fig. 1.
Determination of quercetin and rosmarinic acid effect on cell viability. a Quercetin dose-response curve for cell viability (R2 = 0.90). For U-87 MG cells, IC50 value of quercetin was 109.29 μM. Graph [sigmoidal dose-response (variable slope) curve fit] represents the mean ± SD of three independent experiments analyzed together (n = 18). b Quercetin dose-response for control (0 μM) U-87 MG viability. Cell viability was measured as in a. Graph represents the mean ± SD (n = 18). ***P < 0.001 determined by one-way ANOVA using Dunnet’s multiple comparison test. c Rosmarinic acid dose-response curve for cell viability (R2 = 0.92). For U-87 MG cells, IC50 value of rosmarinic acid was 373.48 μM. Graph [sigmoidal dose-response (variable slope) curve fit] represents the mean ± SD of three independent experiments analyzed together (n = 18). d Rosmarinic acid dose-response for control (0 μM) U-87 MG viability. Cell viability was measured as in c. Graph represents the mean ± SD (n = 18). ***P < 0.001 determined by one-way ANOVA using Dunnet’s multiple comparison test
The effects of siRNA transfection and combined therapy on U-87 MG cell viability
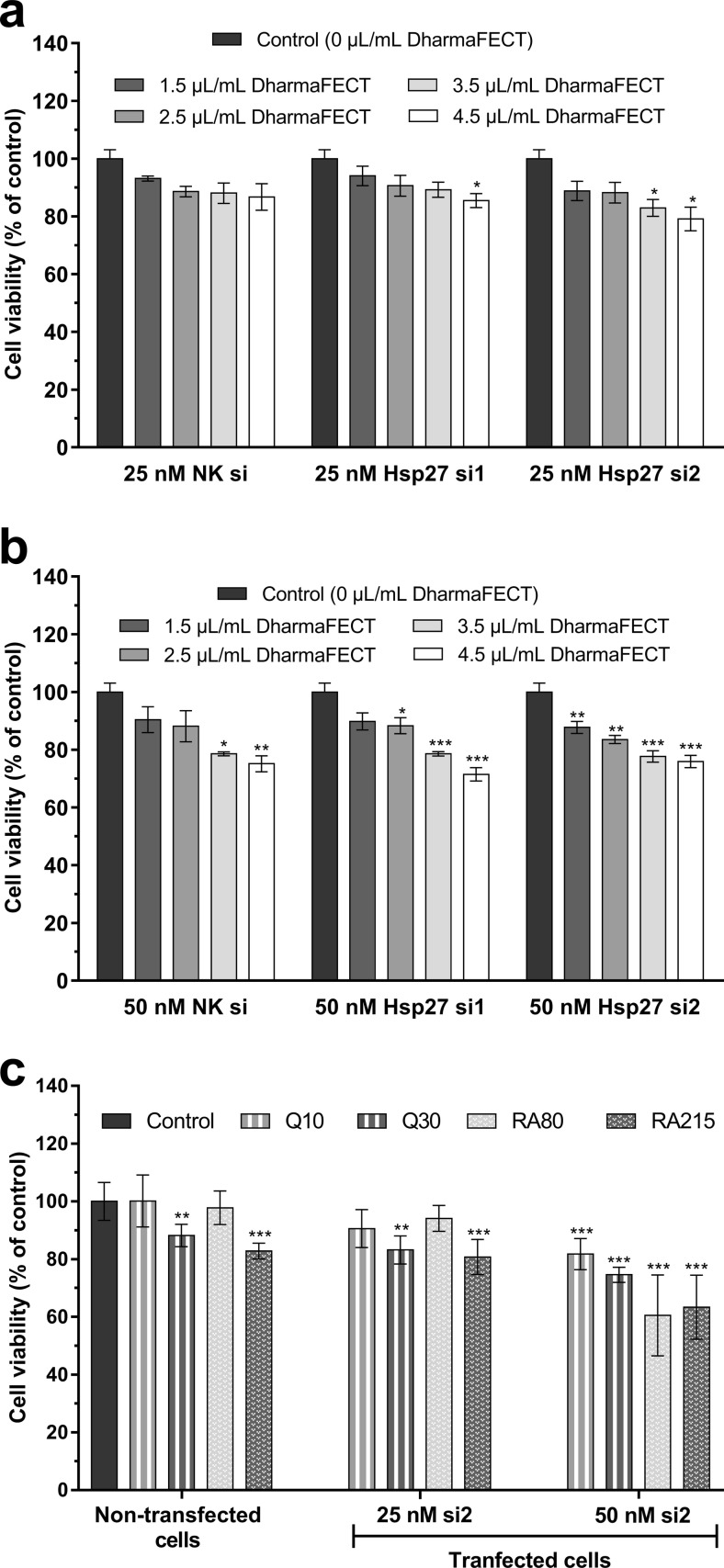
The cytotoxic effects of siRNA transfection were examined in the presence of 25 and 50 nM siRNAs and four different final concentrations of transfection reagent (1.5, 2.5, 3.5, and 4.5 μL/mL). A modified MTT assay was used for determining the cytotoxicity of transfection reagent/siRNA complexes. Cell viability was measured with one-way ANOVA test followed by Dunnet’s multiple comparison test. Cytotoxicity data are presented as mean percentages of control ± standard deviation (SD). According to cytotoxicity results (Fig. 2), transfection reagent/siRNA complexes and combined therapy groups that show ≥ 80% viability were used in the experiments. Therefore, transfection reagent at the final concentration of 2.5 μL/mL for cell transfection with both 25 and 50 nM siRNAs was used in the experiments. It was observed that si2, which was determined to be more effective in Hp27 silencing according to the Western blot analysis (Fig. 4), decreased cell viability by more than 20% at 50 nM final concentration in combined therapy groups. There was only one exception; cell viability was > 80% in the combined therapy group in which 50 μM si2 and 10 μM quercetin was administered. According to these results, quercetin and rosmarinic acid treatments were applied to the cells transfected with 25 nM si2 in the combined therapy applications.
Fig. 2.
Determination of siRNA transfection and combined therapy effects on cell viability. a Transfection reagent/25 nM siRNA. b Transfection reagent/50 nM siRNA complexes for control U-87 MG viability. c combined therapy applications for control U-87 MG viability. Cell viability was determined by MTT assay. Graphs represents the mean ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 determined by one-way ANOVA using Dunnet’s multiple comparison test
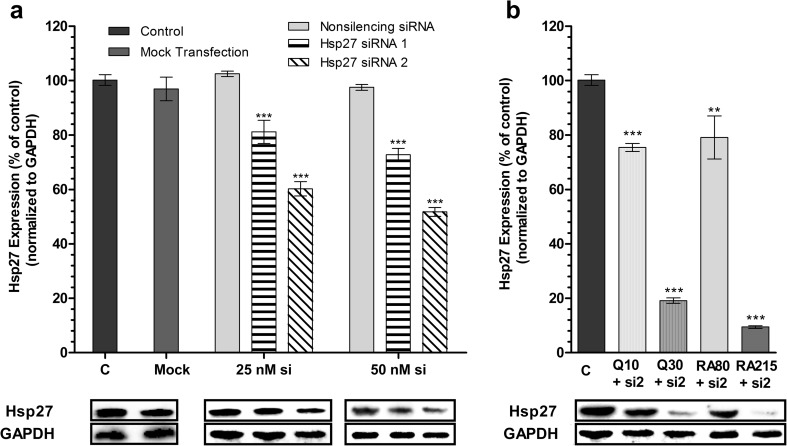
Fig. 4.
Hsp27 levels after siRNA transfection and combined therapy in U-87 MG cells. a Western blot analysis demonstrates dose-dependent and sequence-specific Hsp27 silencing by siRNAs [mean ± SD, n = 7, ***P < 0.001 vs control (non-transfected cells)] b. Western blot analysis demonstrates effects of quercetin (at final concentrations of 10 and 30 μM) and rosmarinic acid (at final concentrations of 80 and 215 μM) on the Hsp27 expression in transfected U-87 MG cells [mean ± SD, n = 7, **P < 0.01, ***P < 0.001 vs control (non-transfected cells)]. C control, Q quercetin, RA rosmarinic acid, si siRNA. All data were normalized to GAPDH
Hsp27 expression in U-87 MG cells
Effects of quercetin and rosmarinic acid on the Hsp27 expression in U-87 MG cells
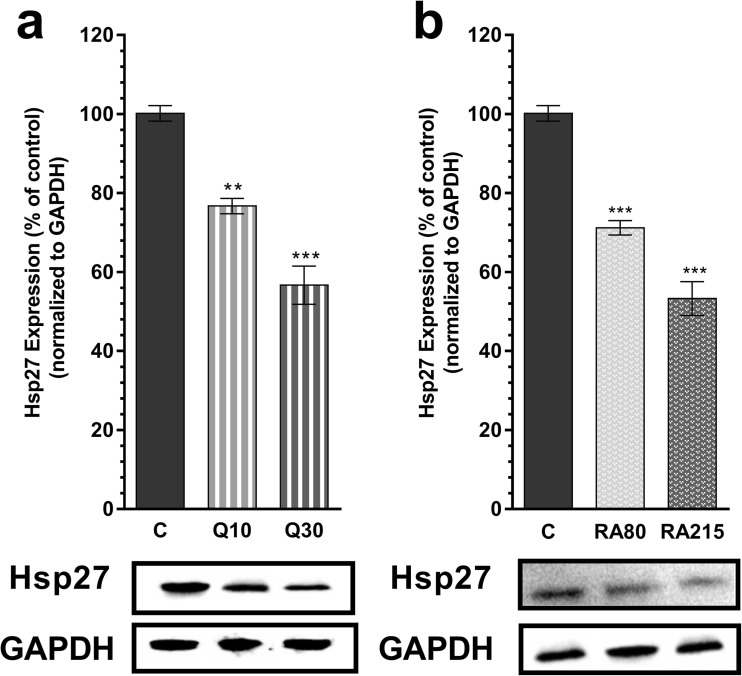
Western blot analysis showed that separately quercetin and RA administrations are effective inhibitors of Hsp27 expression in U-87 MG cells (Fig. 3). Quercetin at 10 and 30 μM final concentrations reduced the expression of Hsp27 by 23.3 and 43.3%, respectively. Similarly, 80 and 215 μM rosmarinic acid decreased level of Hsp27 by 28.8 and 46.7%, respectively.
Fig. 3.
Hsp27 levels after quercetin or rosmarinic acid treatments in U-87 MG cells. a Western blot analysis demonstrates that quercetin (at final concentrations of 10 and 30 μM) dose dependently decreased Hsp27 expression (mean ± SD, n = 7, **P < 0.01, ***P < 0.001 vs control (C)) b. Western blot analysis demonstrates that rosmarinic acid (at final concentrations of 80 and 215 μM) dose dependently decreased Hsp27 expression (mean ± SD, n = 7, ***P < 0.001 vs control). C control, Q quercetin, RA rosmarinic acid. All data were normalized to GAPDH
Effects of quercetin and rosmarinic acid on the Hsp27 expression in transfected U-87 MG cells
To block the expression of Hsp27, the U-87 MG cells were transfected with different concentrations of two commercial specific siRNA compounds (si1 and si2) as described in the “Materials and methods” section, and the efficiency of blocking the Hsp27 gene was examined by immunoblotting analysis. Our experiments showed that transfection of U-87 MG cells was successful and the level of Hsp27 expression was markedly reduced. According to immunoblotting analysis results, there was no statistically significant effect of non-silencing siRNA (NS si) or transfection agent (mock) on Hsp27 expression (P > 0.05). Although both si1 and si2 treatments reduced Hsp27 expression, Hsp27 si2 was found to be more effective in gene silencing. Hsp27 si1 at 25 and 50 nM final concentrations reduced the expression of Hsp27 by 19 and 28%, respectively, whereas Hsp27 si2 at 25 and 50 nM decreased level of Hsp27 by 40 and 49%, respectively (Fig. 4a). As shown in the Western blot analysis (Fig. 4), two different siRNA targeting different regions of Hsp27 mRNA were tested in U-87 MG cells, and si2 was identified as the most potent.
For combined therapy applications, after cell transfection with siRNA, cells were exposed to quercetin or rosmarinic acid treatment as described in the “Materials and methods” section, and the level of repressing Hsp27 expression was determined by immunoblotting. It was observed that the Hsp27 gene was very effectively silenced in the combined therapy groups, in which the transfected U-87 MG cells with 25 nM Hsp27 si2 were treated with quercetin or rosmarinic acid. The application of 50 nM siRNA in combined therapy groups was not studied for the analysis of Hsp27 expression, because the cell viability ratio in these combinations was found to be less than 80%. Thirty micromolars of quercetin and 25 nM si2 combination resulted in a decrease in the level of Hsp27 to 80.9%. The rosmarinic acid treatment to the transfected cells resulted in a higher level of Hsp27 silencing. Combination of 215 μM rosmarinic acid and 25 nM si2 reduced the Hsp27 expression by 90.5% (Fig. 4b). Among all treatments, this was the highest level of Hsp27 silencing when compared to the control.
Induction of apoptosis in U-87 MG cells
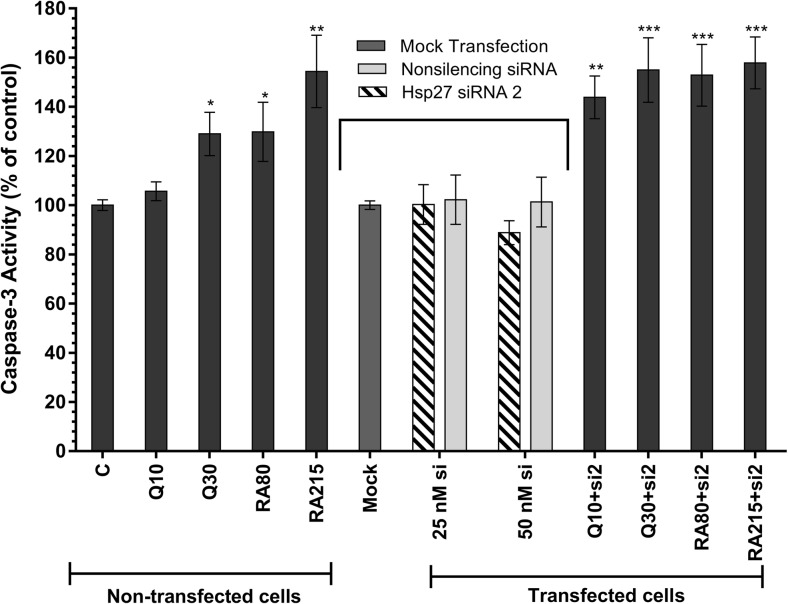
Caspase-3 activity was used to estimate apoptosis induction in U-87 MG cells (Fig. 5). Caspase-3 activity analysis has showed that separately quercetin and rosmarinic acid administration (only 10 μM quercetin exception) are statistically effective induction of apoptosis in U-87 MG cells. Quercetin at 30 μM final concentrations increased the apoptosis rate by 29%, whereas 80 and 215 μM rosmarinic acid increased caspase-3 activity by 30 and 54%, respectively.
Fig. 5.
Caspase-3 activity in non-transfected and transfected U-87 MG cells. The effect of quercetin (at final concentrations of 10 and 30 μM) and rosmarinic acid (RA, at final concentrations of 80 and 215 μM) separately on the activation of caspase-3 was shown in the figure in non-transfected cells group. The effect of siRNA transfection and combined therapy on the activation of caspase-3 was shown in the figure in transfected cells group. Graph represents the mean ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 determined by one-way ANOVA using Dunnet’s multiple comparison test. C control, Q quercetin, RA rosmarinic acid, si siRNA
To verify whether the blocking of Hsp27 gene expression is associated with apoptosis induction, the cells were transfected with 25 nM si2. Our experiments showed that blocking the Hsp27 gene with commercial specific siRNA in U-87 MG cells did not alter caspase-3 activity—a mediator of apoptosis induction—when compared to control group. According to caspase-3 activity analysis results, there was no statistically significant effect of non-silencing siRNA (NS si), transfection agent (mock), and transfection with Hsp27 si2 on caspase-3 activity (P > 0.05).
It was observed that the caspase-3 activity was very effectively increased in the combined therapy groups, in which the transfected U-87 MG cells with 25 nM Hsp27 si2 were treated with quercetin or rosmarinic acid. In the combined therapy groups, 50 nM siRNA plus antioxidant agents were not studied for the analysis of apoptosis induction, because the cell viability ratio in these combinations was found to be less than 80%. Caspase-3 activity was increased respectively by 44% and 55% in cells that were treated with both siRNA and quercetin at 10 and 30 μM final concentrations. Combination of 80 and 215 μM rosmarinic acid and siRNA increased caspase-3 activity by 53 and 58%, respectively. Two hundred and fifteen micromolars of RA and si2 combined group was the highest rate of increasing among treatments.
Discussion
Among the various human cancer types, GBM is known to be one of the most malignant tumors with fatal consequences at high levels worldwide (Graner et al. 2009; Jakubowicz-Gil et al. 2010; Combs et al. 2016). Hsps are highly evolutionary conserved proteins and function as molecular chaperones in normal cells. However, they overexpressed most cancers such as human gliomas. Some Hsp levels are high in abnormal levels in tumor cells compared with normal cells, and they provide an advantage to these cells for cell death resistance. In addition, they are responsible for increased chemotherapy resistance and poor prognosis (Samali and Cotter 1996; Bądziul et al. 2014; Lianos et al. 2015; Önay-Uçar 2015). Therefore, they act as negative prognostic markers in tumor cells. In a study conducted by Khalil (2007), it has been shown that Hsp27 can be used as a biomarker for GBM. High levels of Hsp27 stimulate the process of the carcinogenesis by inducing tumor cell proliferation, differentiation and metastasis, and inhibiting apoptosis. It is also associated with drug resistance and survival of the cell in lethal conditions (Garrido et al. 2006; Kim and Kim 2011; Khalil et al. 2011; Jego et al. 2013). Hence, the inhibition of Hsp27 expression and induction of apoptosis have become a novel strategy against cancer.
Today, siRNA-based silencing of targeted gene expression has a potential therapeutic strategy and powerful tool against cancer (Elbashir et al. 2001; Wang et al. 2011; Wu et al. 2014; Sun et al. 2015). The specificity and potency of Hsp-spesific siRNAs in cell culture to inhibit different Hsp genes are shown in many studies (Hosaka et al. 2006; Rocchi et al. 2006; Kumano et al. 2012; Behnsawy et al. 2013; Jakubowicz-Gil et al. 2013a; Bądziul et al. 2014; Li et al. 2016). In the present study, Hsp27 siRNAs were used to knock-down of the Hsp27 mRNA and to determine the effect of Hsp27 inhibition on caspase-3-induced apoptosis in U-87 MG cells. The Western blot analysis showed that siRNA transfection was successful, and Hsp27 expression was significantly reduced in U-87 MG cells. Twenty-five and 50 nM Hsp27 si1 reduced the expression of Hsp27 by 19 and 28%, respectively, whereas Hsp27 si2 at 25 and 50 nM decreased level of Hsp27 by 40 and 49%, respectively. However, apoptotic analysis indicated that caspase-3 activity did not change compared with control (P > 0.05).
Quercetin, the most frequently studied flavonoids, is known to possess an anticarcinogenic potential, and it has emerged as a potential therapeutic agent against cancer. Quercetin is known to induce growth inhibition and cell death in many cancer cells, including glioma cells. Several articles have suggested that quercetin is an effective inhibitor of Hsp, especially Hsp27 and Hsp72, and an apoptosis inducer by caspase-3 and caspase-9 activations. Quercetin inhibits Hsp expression at the transcriptional level by blocking the heat shock factors 1 and 2 (HSF1, HSF2) (Chowdhury et al. 2005; Jakubowicz-Gil et al. 2008; Kim et al. 2008; Vargas and Burd 2010; Jakubowicz-Gil et al. 2013a; Jakubowicz-Gil et al. 2013b; Bądziul et al. 2014; Li et al. 2016). In this study, the Western blot analysis indicated that quercetin, used as a positive control, was an effective Hsp27 inhibitor in U-87 MG cells at the 30 μM final concentration. It reduced the expression of Hsp27 by 43.3%. Also, performed caspase-3 activity analysis indicated that 30 μM quercetin increased the apoptosis rate by 29%. Ten micromolars of quercetin silenced Hsp27 by 23.3%, but caspase-3 activity did not change at a statistically significant (P > 0.05). It is known that low doses of quercetin do not induce apoptosis as indicated in the various literatures (Jakubowicz-Gil et al. 2008; Vargas and Burd 2010; Jakubowicz-Gil et al. 2013b). Our experiments also demonstrated that siRNA (25 nM) and quercetin (10 and 30 μM) applied in combinations lead to a potent reduction of the Hsp27 level. These combinations resulted in a decrease in the level of Hsp27 to 24.6 and 80.9% and in an activation in the caspase-3-dependent apoptosis to 43.9 and 55%, respectively. The combined therapy was determined to be more effective in inhibition of Hsp27 and in apoptosis induction than the single application of quercetin was.
Rosmarinic acid (RA) has many beneficial biological effects such as antioxidant, antitumoral, antiapoptotic, and neuroprotective effects (Petersen and Simmonds 2003; Tepe et al. 2007; Airoldi et al. 2013; Furtado et al. 2015; Runtuwene et al. 2016; Venkatachalam et al. 2016). The potential effect of RA on the expression of Hsp27 in cancer cells and its mechanism have not been studied yet. In this study, we evaluated, for the first time, the effect of RA on Hsp27 inhibition and cell death induction in non-transfected and transfected human glioma cells. Herein, we showed that RA had a good potency in the inhibition of Hsp27 and induction of apoptosis in U-87 MG cell after 48 h of treatment. Induction of caspase-3 activity was observed after 80 and 215 μM final concentrations of RA treatment at ratio of 30 and 54%, respectively. The 80 and 215 μM of RA decreased level of Hsp27 by 28.8 and 46.7%, respectively. However, the molecular mechanism underlying RA-mediated inhibition of Hsps has not been elucidated. A few studies have shown that RA inhibits MAPK-mediated cell proliferation pathways and induces apoptosis in cells. In a study, it has been shown that RA inhibits the proliferation by suppressing MAPK/ERK pathway in the human colorectal cell line and induces apoptosis (Xavier et al. 2009). In another study conducted on colon HT-29 and breast MCF-7 cancer cells, it has been shown that RA treatment inhibits the activation of phosphorylated ERK1/2 in the cells (Scheckel et al. 2008). RA has been shown to reduce cell proliferation and migration in the head and neck squamous cell carcinoma cell lines by inhibiting EGFR phosphorylation and MAPK signaling (Tumur et al. 2015). We demonstrated in this study that RA treatment reduces Hsp27 expression and induces caspase-3-dependent apoptosis in U-87 MG cells. The reduction in the levels of Hsp27 expression in the cells treated with RA may have been due to the inhibition of heat shock factor 1 (HSF1), the common transcription factor of Hsps, by suppressing the mitogen-activated protein kinase (MAPK) family members.
Interestingly, while some studies have shown that RA has apoptotic activity in cancer cells, there are also studies on antiapoptotic effect of RA on healthy cells. In a study on myoblast C2C12 cell line, it has been shown that RA has a protective effect on hyperthermia-induced cellular damage and reduces caspase-3 expression (Chen et al. 2014). In another study conducted on H9C2 cardiac muscle cells, RA has been shown to inhibit ROS generation and activation of JNK and ERK and to inhibit adriamycin-induced apoptosis (Kim et al. 2005). The neuroprotective effect of RA via antiapoptotic and antioxidant activity on H2O2-mediated neuronal cell damage has been demonstrated (Gao et al. 2005; Ghaffari et al. 2014). On the contrary, in the literature, there are many studies that demonstrate the effects of RA on apoptosis-inducing and cell proliferation-reducing (Moore et al. 2016). The anticancer effects of RA have been shown in many types of cancer such as breast (Yesil-Celiktas et al. 2010; Berdowska et al. 2013), colorectal (Xavier et al. 2009), leukemia (Moon et al. 2010), liver (Lin et al. 2007), lung, and prostate (Yesil-Celiktas et al. 2010). The molecular mechanism underlying RA-mediated both pro- and antiapoptotic effects has not been well elucidated. A few studies have shown that RA inhibits TNF-α-mediated cell proliferation pathway and induces apoptosis in cells. In a study conducted on human leukemia U937 cells, it has been shown that RA treatment inhibits TNF-α-induced NF-ĸB activation and reactive oxygen species (ROS) and significantly sensitizes the cells to TNF-α-induced apoptosis. It has also been shown that the activation of caspases in response to TNF-α is significantly enhanced by RA treatment (Moon et al. 2010). In another study, RA has been shown to induce apoptose through the TNF-α-induced ROS production in cancer cells. (Kim et al. 2010). It is known that TNF-α plays a key role in apoptosis and cell survival, but the regulation of survival-death balance remains unclear (van Horssen et al. 2006). RA may be demonstrating its pro- or antiapoptotic effect via the TNF-a pathway. Moreover, studies on the effect of RA on brain tumors are inadequate, and the mechanism underlying the effects of RA on brain tumors is not understood. In this study, we demonstrated that RA induces caspase-3-dependent apoptosis and reduces Hsp27 expression in U-87 MG human glioma cells.
Hsp27 is a chaperone protein and plays a helpful role in the recognition and destruction of misfolded proteins. In the present study, the depletion of Hsp27 by siRNA-mediated silencing may have significantly reduced the degradation of misfolded proteins and may have led to endoplasmic reticulum (ER) stress in the cell. It is known that in the ER stress, cells undergo apoptosis via mitochondria-dependent and mitochondria-independent pathways by activating different signal transduction pathways such as transcriptional induction of C/EBP (CCAAT/enhancer binding protein) homology protein (CHOP), activation of the c-Jun N-terminal kinase (JNK) pathway, and activation of caspase-12. However, the mechanism and targets of ER stress-induced apoptosis yet remain exactly to be identified (Oyadomari et al. 2002; Rao et al. 2002; Van der Sanden et al. 2003; Mauro et al. 2006; Szegezdi et al. 2006; Jakubowicz-Gil et al. 2013b; Yadav et al. 2014). In the study, caspase-3 activation was not observed in transfected cells with siRNAs, whereas higher levels of caspase-3 activity were observed in cells treated with siRNA and subsequent quercetin or RA treatment compared to cells treated with antioxidant agent alone. This might be explained by the protection of the cells against ER stress by the antioxidant properties of quercetin or RA which are flavonoids. It has been shown that free radical scavengers protect the cells from ER stress-induced cell death (Mauro et al. 2006). Also, some reports demonstrate that quercetin protects the cells against ER stress by its antioxidant properties (Ben Salem et al. 2015; Jakubowicz-Gil et al. 2013b). There is no evidence directly associated with ER stress markers (such as PERK, IRE1a, XBP-1, BiP, CHOP, and caspase-12) upon rosmarinic acid treatment in the literature. New studies are needed to determine the effect of rosmarinic acid treatment on ER stress and markers in cells. Nevertheless, this information may suggest that cell death in U-87 MG cells only siRNA treatment might be correlated with ER stress-activated apoptosis and quercetin or RA treatment might protect the cells against ER stress.
Our experiments showed that Hsp27 siRNA and subsequent RA treatment in U-87 MG cells would be a great agent against cancer therapy. After cell transfection, treatment of RA at 215 μM suppressed the Hsp27 expression by 90.5% and increased caspase-3 activity by 58%. In the present study, it was determined that the combined therapy with RA and siRNA is the most effective treatment group for both Hsp27 suppression and caspase-3 dependent apoptosis induction, when compared to the control group (untransfected and no agent treated cells). Besides, it was determined that rosmarinic acid inhibits Hsp27 and induces apoptosis at a higher level than quercetin, which is known to be a good Hsp27 inhibitor and in this study used as a positive control. These findings have shown that rosmarinic acid seems to be a potent and promising anticarcinogen agent, and it may be useful for glioma therapy.
In conclusion, in the present study, caspase-3 activity analysis has shown that quercetin and rosmarinic acid are very good agents for apoptosis induction in U-87 MG cells. It was also observed that apoptosis was induced at a higher level as a result of Hsp27 siRNA and subsequent quercetin or RA treatment, when compared to the control cells. This indicated that siRNA-mediated silencing of Hsp27 expression makes U-87 MG cells vulnerable to apoptosis induction upon quercetin or RA treatment, and also this confirmed that Hsp27, a molecular chaperone, is responsible for glioma cells resistance to apoptosis. In the present study for the first time, we reported that rosmarinic acid has the ability to trigger caspase-3 induced apoptosis in human glioma cells. Moreover, in this study, we provided for the first time the evidence supporting the potential of combinations of Hsp27 siRNA and RA applied to human glioma cells as an effective Hsp27 inhibitor and inducer of apoptosis. We demonstrated that RA administered with siRNA seems to be a potent combination for glioma therapy. The data obtained from this study would contribute to the development of new anticancer drugs and related treatments of brain tumors with observed aggressive growth, poor prognosis, and high mortality rate.
Electronic supplementary material
(DOCX 429 kb)
Acknowledgments
This work was supported by the Istanbul University Research Foundation, Turkey (Project Number: 57959). We are grateful to Yeditepe University for their generous gift of U-87 MG cell line.
Abbreviations
- Hsp27
Heat shock protein 27
- GBM
Glioblastoma
- RA
Rosmarinic acid
- RNAi
RNA interference
- siRNA
Small interfering RNA
- IC50
The half-maximal inhibitory concentration
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
References
- Airoldi C, Sironi E, Dias C, Marcelo F, Martins A, Rauter AP, Nicotra F, Jimenez-Barbero J. Natural compounds against Alzheimer’s disease: molecular recognition of Aβ1-42 peptide by Salvia sclareoides extract and its major component, rosmarinic acid, as investigated by NMR. Chem Asian J. 2013;8(3):596–602. doi: 10.1002/asia.201201063. [DOI] [PubMed] [Google Scholar]
- Bądziul D, Jakubowicz-Gil J, Paduch R, Głowniak K, Gawron A. Combined treatment with quercetin and imperatorin as a potent strategy for killing HeLa and Hep-2 cells. Mol Cell Biochem. 2014;392(1–2):213–227. doi: 10.1007/s11010-014-2032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnsawy HM, Miyake H, Kusuda Y, Fujisawa M. Small interfering RNA targeting heat shock protein 70 enhances chemosensitivity in human bladder cancer cells. Urol Oncol. 2013;31(6):843–848. doi: 10.1016/j.urolonc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Ben Salem I, Prola A, Boussabbeh M, Guilbert A, Bacha H, Abid-Essefi S, Lemaire C. Crocin and Quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress Chaperones. 2015;20(6):927–938. doi: 10.1007/s12192-015-0613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdowska I, Zieliński B, Fecka I, Kulbacka J, Saczko J, Gamian A. Cytotoxic impact of phenolics from Lamiaceae species on human breast cancer cells. Food Chem. 2013;141(2):1313–1321. doi: 10.1016/j.foodchem.2013.03.090. [DOI] [PubMed] [Google Scholar]
- Chen KL, Li HX, Xu XL, Zhou GH. The protective effect of rosmarinic acid on hyperthermia-induced C2C12 muscle cells damage. Mol Biol Rep. 2014;41(8):5525–5531. doi: 10.1007/s11033-014-3429-6. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Kishino K, Satoh R, Hashimoto K, Kikuchi H, Nishikawa H, Shirataki Y, Sakagami H. Tumor-specificity and apoptosis-inducing activity of stilbenes and flavonoids. Anticancer Res. 2005;25(3B):2055–2063. [PubMed] [Google Scholar]
- Combs SE, Schmid TE, Vaupel P, Multhoff G. Stress response leading to resistance in glioblastoma—the need for innovative radiotherapy (iRT) concepts. Cancers (Basel) 2016;8(1):15. doi: 10.3390/cancers8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Furtado RA, Oliveira BR, Silva LR, Cleto SS, Munari CC, Cunha WR, Tavares DC. Chemopreventive effects of rosmarinic acid on rat colon carcinogenesis. J Cancer Prev. 2015;24(2):106–112. doi: 10.1097/CEJ.0000000000000055. [DOI] [PubMed] [Google Scholar]
- Gao LP, Wei HL, Zhao HS, Xiao SY, Zheng RL. Antiapoptotic and antioxidant effects of rosmarinic acid in astrocytes. Pharmazie. 2005;60(1):62–65. [PubMed] [Google Scholar]
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumourigenic properties. Cell Cycle. 2006;5(22):2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- Ghaffari H, Venkataramana M, Jalali Ghassam B, Chandra Nayaka S, Nataraju A, Geetha NP, Prakash HS. Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells. Life Sci. 2014;113(1–2):7–13. doi: 10.1016/j.lfs.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23(5):1541–1557. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta C, Vikram A, Tripathi DN, Ramarao P, Jena GB. Antioxidant and antimutagenic effect of quercetin against DEN induced hepatotoxicity in rat. Phytother Res. 2010;24(1):119–128. doi: 10.1002/ptr.2883. [DOI] [PubMed] [Google Scholar]
- Hosaka S, Nakatsura T, Tsukamoto H, Hatayama T, Baba H, Nishimura Y. Synthetic small interfering RNA targeting heat shock protein 105 induces apoptosis of various cancer cells both in vitro and in vivo. Cancer Sci. 2006;97(7):623–632. doi: 10.1111/j.1349-7006.2006.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Paduch R, Piersiak T, Głowniak K, Gawron A, Kandefer-Szerszeń M. The effect of quercetin on pro-apoptotic activity of cisplatin in HeLa cells. Biochem Pharmacol. 2005;69(9):1343–1350. doi: 10.1016/j.bcp.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Rzeski W, Zdzisińska B, Piersiak T, Weiksza K, Glowniak K, Gawron A. Different sensitivity of neurons and neuroblastoma cells to quercetin treatment. Acta Neurobiol Exp (Wars) 2008;68(4):463–476. doi: 10.55782/ane-2008-1712. [DOI] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Langner E, Wertel I, Piersiak T, Rzeski W. Temozolomide, quercetin and cell death in the MOGGCCM astrocytoma cell line. Chem Biol Interact. 2010;188(1):190–203. doi: 10.1016/j.cbi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W. Silencing of Hsp27 and Hsp72 in glioma cells as a tool for programmed cell death induction upon temozolomide and quercetin treatment. Toxicol Appl Pharmacol. 2013;273(3):580–589. doi: 10.1016/j.taap.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W. Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 2013;34(4):2367–2378. doi: 10.1007/s13277-013-0785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G, Hazoumé A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Lett. 2013;332(2):275–285. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Khalil AA. Biomarker discovery: a proteomic approach for brain cancer profiling. Cancer Sci. 2007;98(2):201–213. doi: 10.1111/j.1349-7006.2007.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816(2):89–104. doi: 10.1016/j.bbcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, Nabavi SM, Bishayee A. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016;8(9):529–548. doi: 10.3390/nu8090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim LS, Kim JH. Heat shock protein as molecular targets for breast cancer therapeutics. Breast Cancer. 2011;14(3):167–174. doi: 10.4048/jbc.2011.14.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Kim HR, Woo ER, Hong ST, Chae HJ, Chae SW. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem Pharmacol. 2005;70(7):1066–1078. doi: 10.1016/j.bcp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Choi CH, Park JY, Kang SK, Kim YK. Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem Res. 2008;33(6):971–979. doi: 10.1007/s11064-007-9416-8. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee SB, Park JK, Yoo YD. TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L) Cell Death Differ. 2010;17(9):1420–1434. doi: 10.1038/cdd.2010.19. [DOI] [PubMed] [Google Scholar]
- Kumano M, Furukawa J, Shiota M, Zardan A, Zhang F, Beraldi E, Wiedmann RM, Fazli L, Zoubeidi A, Gleave ME. Cotargeting stress-activated Hsp27 and autophagy as a combinatorial strategy to amplify endoplasmic reticular stress in prostate cancer. Mol Cancer Ther. 2012;11(8):1661–1671. doi: 10.1158/1535-7163.MCT-12-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tang C, Li L, Li R, Fan Y. Quercetin blocks t-AUCB-induced autophagy by Hsp27 and Atg7 inhibition in glioblastoma cells in vitro. J Neuro-Oncol. 2016;129(1):39–45. doi: 10.1007/s11060-016-2149-2. [DOI] [PubMed] [Google Scholar]
- Lianos GD, Alexiou GA, Mangano A, Mangano A, Rausei S, Boni L, Dionigi G, Roukos DH. The role of heat shock proteins in cancer. Cancer Lett. 2015;360(2):114–118. doi: 10.1016/j.canlet.2015.02.026. [DOI] [PubMed] [Google Scholar]
- Lin CS, Kuo CL, Wang JP, Cheng JS, Huang ZW, Chen CF. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J Ethnopharmacol. 2007;112(3):557–567. doi: 10.1016/j.jep.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Mauro C, Crescenzi E, De Mattia R, Pacifico F, Mellone S, Salzano S, de Luca C, D'Adamio L, Palumbo G, Formisano S, Vito P, Leonardi A. Central role of the scaffold protein tumor necrosis factor receptor-associated factor 2 in regulating endoplasmic reticulum stress-induced apoptosis. J Biol Chem. 2006;281(5):2631–2638. doi: 10.1074/jbc.M502181200. [DOI] [PubMed] [Google Scholar]
- McConnell JR, McAlpine SR. Heat shock proteins 27, 40, and 70 as combinational and dual therapeutic cancer targets. Bioorg Med Chem Lett. 2013;23(7):1923–1928. doi: 10.1016/j.bmcl.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DO, Kim MO, Lee JD, Choi YH, Kim GY. Rosmarinic acid sensitizes cell death through suppression of TNF-alpha-induced NF-kappaB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 2010;288(2):183–191. doi: 10.1016/j.canlet.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Moore J, Yousef M, Tsiani E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients. 2016;8(11):E731. doi: 10.3390/nu8110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival application to proliferation and cytotoxicity assay. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Önay-Uçar E. Heat shock proteins and cancer: plant based therapy. In: Asea AAA, Calderwoo SK, editors. Heat shock protein-based therapies. 9. Switzerland: Springer; 2015. pp. 27–48. [Google Scholar]
- Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002;7(4):335–345. doi: 10.1023/A:1016175429877. [DOI] [PubMed] [Google Scholar]
- Petersen M, Simmonds MS. Rosmarinic acid. Phytochemistry. 2003;62(2):121–125. doi: 10.1016/S0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277(24):21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- Rocchi P, Jugpal P, So A, Sinneman S, Ettinger S, Fazli L, Nelson C, Gleave M. Small interference RNA targeting heat-shock protein 27 inhibits the growth of prostatic cell lines and induces apoptosis via caspase-3 activation in vitro. BJU Int. 2006;98(5):1028–1089. doi: 10.1111/j.1464-410X.2006.06425.x. [DOI] [PubMed] [Google Scholar]
- Runtuwene J, Cheng KC, Asakawa A, Amitani H, Amitani M, Morinaga A, Takimoto Y, Kairupan BH, Inui A. Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des Dev Ther. 2016;10:2193–2202. doi: 10.2147/DDDT.S108539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223(1):163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- Scheckel KA, Degner SC, Romagnolo DF. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J Nutr. 2008;138(11):2098–2105. doi: 10.3945/jn.108.090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Yeh SY, Yuan CW, Chiu MJ, Yung BS, Yen Y. Molecular properties, functional mechanisms, and applications of sliced siRNA. Mol Ther Nucleic Acids. 2015;4:e221. doi: 10.1038/mtna.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepe B, Eminagaoglu O, Akpulat HA, Aydin E. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia verticillata (L.) subsp. verticillata and S. verticillata (L.) subsp. amasiaca (Freyn & Bornm.) Bornm. Food Chem. 2007;100(3):985–989. doi: 10.1016/j.foodchem.2005.10.062. [DOI] [Google Scholar]
- Tumur Z, Guerra C, Yanni P, Eltejaye A, Waer C, Alkam T, Henson BS. Rosmarinic acid inhibits cell growth and migration in head and neck squamous cell carcinoma cell lines by attenuating epidermal growth factor receptor signaling. J Cancer Sci Ther. 2015;7(12):367–374. doi: 10.4172/1948-5956.1000376. [DOI] [Google Scholar]
- Uçar EÖ, Arda N, Aitken A. Extract from mistletoe, Viscum album L., reduces Hsp27 and 14-3-3 protein expression and induces apoptosis in C6 rat glioma cells. Genet Mol Res. 2012;11(3):2801–2813. doi: 10.4238/2012.August.24.5. [DOI] [PubMed] [Google Scholar]
- Van der Sanden MH, Houweling M, Van Golde LM, Vaandrager AB. Inhibition of phosphatidylcholine synthesis induces expression of the endoplasmic reticulum stress and apoptosis-related protein CCAAT/enhancer-binding protein-homologous protein (CHOP/GADD153) Biochem J. 2003;369(Pt 3):643–650. doi: 10.1042/bj20020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- Vargas A, Burd R. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr Rev. 2010;68(7):418–428. doi: 10.1111/j.1753-4887.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Gunasekaran S, Namasivayamn N. Biochemical and molecular mechanisms underlying the chemopreventive efficacy of rosmarinic acid in a rat colon cancer. Eur J Pharmacol. 2016;791:37–50. doi: 10.1016/j.ejphar.2016.07.051. [DOI] [PubMed] [Google Scholar]
- Wang Z, Raoi DD, Senzer N, Nemunaitis J. RNA interference and cancer therapy. Pharm Res. 2011;28(12):2983–2995. doi: 10.1007/s11095-011-0604-5. [DOI] [PubMed] [Google Scholar]
- Wu SY, Lopez-Berestein G, Calin GA, Sood AK. RNAi therapies: drugging the undruggable. Sci Transl Med. 2014;6(240):240ps7. doi: 10.1126/scitranslmed.3008362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier CP, Lima CF, Fernandes-Ferreira M, Pereira-Wilson C. Salvia fruticosa, Salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: the role in MAPK/ERK pathway. Nutr Cancer. 2009;61(4):564–571. doi: 10.1080/01635580802710733. [DOI] [PubMed] [Google Scholar]
- Yadav RK, Chae SW, Kim HR, Chae HJ. Endoplasmic reticulum stress and cancer. J Cancer Prev. 2014;19(2):75–88. doi: 10.15430/JCP.2014.19.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum Nutr. 2010;65(2):158–163. doi: 10.1007/s11130-010-0166-4. [DOI] [PubMed] [Google Scholar]
- Zhang R, Tremblay TL, McDermid A, Thibault P, Stanimirovic D. Identification of differentially expressed proteins in human glioblastoma cell lines and tumors. Glia. 2003;42(2):194–208. doi: 10.1002/glia.10222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 429 kb)