Abstract
The reduction of the particle size of drugs of pharmaceutical interest down to the nano-sized range has dramatically changed their physicochemical properties. The greatest disadvantage of nanocrystals is their inherent instability, due to the risk of crystal growth. Thus, the selection of an appropriate stabilizer is crucial to obtain long-term physicochemically stable nanocrystals. High pressure homogenization has enormous advantages, including the possibility of scaling up, lack of organic solvents and the production of small particles diameter with low polydispersity index. The sequential use of high shear homogenization followed by high pressure homogenization, can modulate nanoparticles’ size for different administration routes. The present study focuses on the optimization of the production process of two formulations composed of different surfactants produced by High Shear Homogenization followed by hot High Pressure Homogenization. To build up the surface response charts, a 22 full factorial design experiment, based on 2 independent variables, was used to develop optimized formulations. The effects of the production process on the mean particle size and polydispersity index were evaluated. The best ibuprofen nanocrystal formulations were obtained using 0.20% Tween 80 and 1.20% PVP K30 (F1) and 0.20% Tween 80 and 1.20% Span 80 (F2). The estimation of the long-term stability of the aqueous suspensions of ibuprofen nanocrystals was studied using the LUMISizer. The calculated instability index suggests that F1 was more stable when stored at 4 °C and 22 °C, whereas F2 was shown to be more stable when freshly prepared.
Keywords: Factorial design, Nanocrystals, High pressure homogenization, Physicochemical stability, Ibuprofen, Surfactants
1. Introduction
Ibuprofen, ((RS)-2-(4-(2-methylpropyl) phenyl) propanoic acid), is a non-steroidal anti-inflammatory drug (NSAID), with recognised low oral bioavailability attributed to its insolubility in water (0.12 mg/ml R-(-)-enantiomer; 0.08 mg/ml S-(+)-enantiomer) Nerurkar et al., 2005. It is an acidic compound (pKa = 4.49), showing therefore higher solubility in neutral-basic environment (Brigo et al., 2016), and in a set of organic solvents e.g. ethanol (0.20 mg/ml) Rashid et al., 2014, chloroform (618 mg/ml) n-octanol (394 mg/ml) and cyclohexane (193 mg/ml) Garzón and Martínez, 2004. Ibuprofen has been frequently used as a model drug for sustained/controlled release. It is classified as Biopharmaceutical Classification System (BCS) class II compound (Oner and Uysal, 2013), because of its low solubility (intrinsic solubility of approximately 0.06 mg/mL) (Shaw et al., 2005), and high permeability. While existing as racemic mixture of (R)-(-)- and (S)-(+)-enantiomers, only the latter (and that of even lower water solubility) is active, both in vitro and in vivo.
As a NSAID, it inhibits the production of prostaglandins from arachidonic acid by the cyclooxygenase (COX), followed by the suppression of inflammation in most patients (Bleumink et al., 2003). The drug is commonly recommended in rheumatoid arthritis, post-operative pain, chronic pain associated with cancer and to treat fever. Ibuprofen has good therapeutic effect and, at high doses, it can cause gastric irritation, therefore it should be delivered via parenteral route only on the site of the infection (Jarosz et al., 2016).
The drug is formulated in several pharmaceutical dosage forms (Rudy et al., 1991). In the liver, approximately 65% of the R-(-)-enantiomer is transformed in the (S)-(+) – ibuprofen. Some of it is pre-systemically transformed in the gut in the presence of acyl CoA thioester, where alpha-methylacylcoenzyme A racemase acts as the catalyst. Both enantiomers are quickly metabolised by phase I detoxification enzymes in human liver (Woodman et al., 2011). However, the metabolic pathways of its enantiomers differ significantly. Although the (S)-(+)- enantiomer is frequently metabolised by CYP2C9, the (R)-(-)-ibuprofen is metabolised mostly via CYP2C8 (Neunzig et al., 2012). Most of ibuprofen is metabolised and the main route of ibuprofen excretion is through the kidney, while only a small percentage of the drug is excreted without alteration in urine.
The risk of toxicity is associated with the binding of ibuprofen-glucuronide to plasma proteins, the highest risk is related to patients with renal impairment (Castillo et al., 1995). Ibuprofen was shown to be beneficial in reducing the colorectal cancer risk (Krasniqi et al., 2016). In the clinical practice, ibuprofen seems to be the first choice, due do its higher safety profile, as it is associated with rarer gastrointestinal and renal side effects when compared to others drugs, e.g., indomethacin (Oncel and Erdeve, 2016).
To improve ibuprofen solubility and thereby its bioavailability, this work reports the development and optimization of a new nanocrystals’ formulation by 22 factorial design. The estimation of the long-term stability of the aqueous suspensions of ibuprofen nanocrystals was carried out using the LUMISizer. The LUMISizer allows the analysis of particle and droplet velocity distributions for creaming and sedimentation phenomena. With this equipment it is possible to run 12 samples at a time under a wide range of viscosities, temperatures, and concentrations.
2. Materials and methods
2.1. Materials
Polysorbate 80 (Tween 80®) was purchased from Uniqema (Everberg, Belgium). Ibuprofen was kindly donated from Medinfar (Amadora, Portugal). Polyvinylpyrrolidone (PVP) K30 was purchased from Fluka (Switzerland). Phosphate buffered saline, pH 7.4 and sorbitan monooleate (Span 80®) were obtained from Sigma-Aldrich (Steinheim, Germany). Ultra-purified water was obtained from Milli® Q Plus system, home supplied.
2.2. Production of nanosuspensions by melt emulsification
The melt-emulsification process was used to produce nanosuspensions composed of 0.25% (m/V) ibuprofen and aqueous solution of surfactants. Ibuprofen was added to the aqueous solution of surfactants, polysorbate 80 (Tween 80®) and Polyvinylpyrrolidone (PVP) K30, or polysorbate 80 (Tween 80®) and sorbitan monooleate (Span 80®). The surfactants should exhibit sufficient affinity for the droplet surface to enable preparation of hot emulsion and should present affinity to the particle surface in order to stabilize the freshly prepared nanocrystals. In preliminary experiments, different concentrations of surfactants were used to stabilize the nanosuspension during the production process. Briefly, a drug suspension was firstly heated up to 80 °C to melt ibuprofen, followed by high speed stirring using the high shear homogenization (Ultra-Turrax ®, T25, IKA) for 10 min to obtain a coarse emulsion. This emulsion was then transferred to a high pressure homogenizer (EmulsiFlex®-C3, Avestin), and processed at 1000 bar for 20 min in the continuous mode, operated at 80 °C. The hot emulsion was then cooled down, by placing it in an ice-bath for, approximately, more 20 min to allow the recrystallization of the drug.
2.3. Particle size analysis
The particle size and polydispersity index (PDI) were determined by dynamic light scattering (DLS) using a particle size analyzer (DelsaNano C Submicron, Beckman Coulter Delsa, Krefeld, Germany). Mean diameter and PdI of nanosuspensions were determined in triplicate. Values are presented as the mean of triplicate runs per sample. For each measurement, the nanosuspension was diluted in Milli Q water to an appropriate concentration to avoid multiple scattering.
2.4. Zeta potential analysis
Zeta potential measurements were taken by electrophoretic light scattering (ELS) using a Nano Zeta Potential Analyzer (DelsaNano C Submicron, Beckman Coulter Delsa, Krefeld, Germany). Measurements were taken in a Flow Cell (Beckman Coulter Delsa) at 25 °C, and Milli-Q water was used to dilute the nanosuspensions to a proper concentration. The zeta potential was calculated using the Helmholtz-Smoluchowsky equation included in the software of the system. Values are presented as the mean of triplicate runs per sample.
2.5. Factorial design study
The influence of the concentration of both surfactants in both mixtures was evaluated using a 22 factorial design with triplicate of central point for estimating the experimental error, composed of 2 variables for each formulation which were set at 2-levels each. The dependent variables were the mean particle size, polydispersity index and zeta potential. The design required a total of 7 experiments for each formulation. Each factor, the lower and higher values of the lower and upper levels, were represented by (−1) and a (+1), respectively, and the central point was represented by (0). These values were chosen based on the tested lower and upper values for each variable according to literature research (Table 1). A factorial design approach was applied to maximize the yield of production based on the production possibility curves. The data were analysed using STATISTICA 7.0.
Table 1.
Initial full factorial design of both formulations, providing the lower (−1), upper (+1) and (0) central point level for each variable.
| Variables | Levels |
||
|---|---|---|---|
| −1 | 0 | +1 | |
| Formulation 1 | |||
| Tween 80 | 0.125 | 0.25 | 0.50 |
| PVP K 30 | 0.125 | 0.25 | 0.50 |
| Formulation 2 | |||
| Tween 80 | 0.125 | 0.25 | 0.50 |
| Span 80 | 0.125 | 0.25 | 0.50 |
2.6. Long-term stability study
Simulated long-term physical stability of nanocrystals was assessed by analytic centrifuge, LUMiSizer (LUM GmbH, Dias de Sousa, Portugal), which accelerates the occurrence of destabilization phenomena. For the analysis of long-term stability of nanocrystals, native samples, i.e. without prior dilution, were placed in rectangular test-tubes (optical path of 2 mm) and exposed to centrifugal force under 4000 rpm, 25 °C, for 1 h. These experiments allowed differentiating between various instability mechanisms at an accelerated rate. Extrapolated results were used to estimate dispersion shelf-life in minutes. The samples were periodically analysed with the interval of 30 s, speed 4000 r.p.m, until 850 different profiles were obtained over time. During the assay, samples were kept at 25 °C.
3. Results and discussion
In the production of nanosuspensions by melt emulsification method, the drug droplets are forced through a gap in the micronizing zone, which creates conditions of high turbulence and shear, combined with compression, acceleration, pressure drop and impact, contributing for the reduction of the particle size (Loh et al., 2015). The first step is the preparation, by high shear homogenization (Ultra-Turrax) of a hot emulsion with melted drug (melting point of ibuprofen is approximately 75–78 °C (States, 2000) as the dispersed phase. The primary emulsion is homogenized in a high pressure homogenizer and cooled down to solidify the droplets of melted drug. In this technique, particle formation is the result of the transformation of the melted drug into the solid state.
The size of the drug particles is dependent mostly on the size of dispersed droplets, which is influenced by the composition and concentration of surfactants used. To prepare smaller drug particles from hot emulsion, the collision of droplets can be prevented by fast cooling once it causes instantaneous solidification of melted drug droplets, resulting in smaller drug nanocrystals.
In preliminary experiments, different concentrations and composition of surfactants were used to stabilize the nanosuspension during the production process and their influence on mean particle size and polydispersity index was studied by DLS. Before the measurements, Milli-Q water was used to dilute the nanosuspensions to a proper concentration. The preliminary factorial design study, a total of 7 experiments was required for each formulation. The influence of the concentration of each surfactant on the mean particle size and polydispersity of ibuprofen nanocrystals (fixed concentration of 0.25%), produced with the constant pressure of 1000 bar and 20 min in HPH, are shown in Table 2 (Formulation 1, F1) and Table 3 (Formulation 2, F2).
Table 2.
Mean particle size and polydispersity index of different concentrations of the mixture of Tween 80 and PVP K30 (Formulation 1 – F1).
| Formulation Codes | TWEEN 80 (%, m/V) | PVP K 30 (%, m/V) | Z-Ave (nm) | PdI |
|---|---|---|---|---|
| P1 | 0.125 | 0.125 | 1204.0 | 0.971 |
| P2 | 0.500 | 0.125 | 120.6 | 0.278 |
| P3 | 0.125 | 0.500 | 157.6 | 0.193 |
| P4 | 0.500 | 0.500 | 83.7 | 0.198 |
| P5 | 0.250 | 0.250 | 157.3 | 0.339 |
| P6 | 0.250 | 0.250 | 145.5 | 0.203 |
| P7 | 0.250 | 0.250 | 68.8 | 0.155 |
Table 3.
Mean particle size and polydispersity index of different concentrations of the mixture of Tween 80 and Span 80 (Formulation 2 – F2).
| Formulation Codes | TWEEN 80 (%, m/V) | SPAN 80 (%, m/V) | Z-Ave (nm) | PdI |
|---|---|---|---|---|
| S1 | 0.125 | 0.125 | 169.6 | 0.192 |
| S2 | 0.500 | 0.125 | 150.7 | 0.349 |
| S3 | 0.125 | 0.500 | 160.3 | 0.196 |
| S4 | 0.500 | 0.500 | 160.9 | 0.264 |
| S5 | 0.250 | 0.250 | 149.5 | 0.177 |
| S6 | 0.250 | 0.250 | 150.9 | 0.205 |
| S7 | 0.250 | 0.250 | 146.4 | 0.197 |
To design a new formulation, it is of paramount importance to identify the influencing parameters, since these will affect the properties of the final dosage form. The experimental design method analyses the influence of different variables on the properties of the drug delivery system. To evaluate the optimum experimental conditions for both formulations of nanocrystals produced with different concentrations of surfactants (independent variables), a factorial design approach was used to assess the effects of the independent variables over the dependent factors which ultimately define the physicochemical properties of nanocrystals.
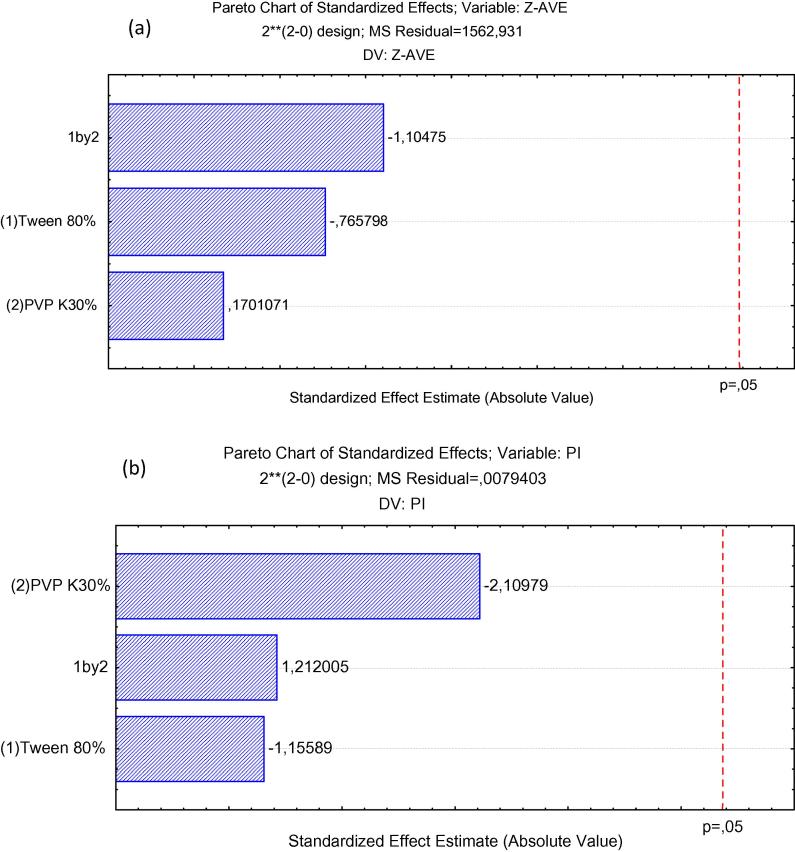
The ZP (i.e., the electrical charge at the nanocrystals surface) reflects the long-term physical stability and translates the tendency for particles aggregation. Higher ZP values, either positively or negatively charged, mean that nanocrystals will have greater long-term stability (Feng and Huang, 2001). Fig. 1 shows the Pareto chart of the standardized effects for F1 and Fig. 2 shows surface response charts of experimental design for the same formulation.
Fig. 1.
Pareto chart of the standardized effects for nanocrystals produced with F1: (a) particle size (Z-Ave (nm)); (b) polydispersity index (PdI).
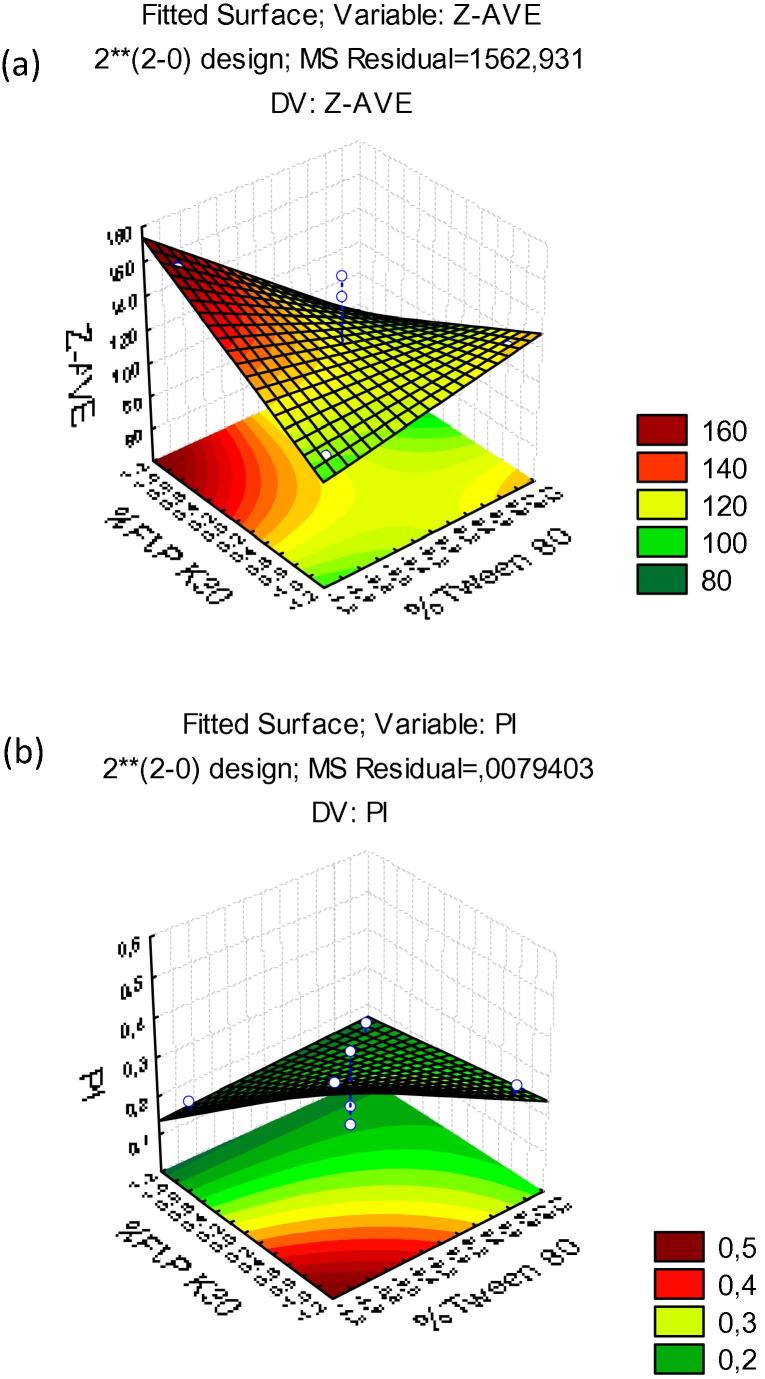
Fig. 2.
Surface response charts of experimental design of nanocrystals produced with F1: (a) particle size (Z-Ave (nm)); (b) polydispersity index (PdI).
As shown in Fig. 1, the size of nanocrystals and their PdI were not significantly influenced by the tested parameters, neither were the interaction between variables. Nevertheless, for the formulation produced with a smaller concentration of Tween 80 (∼0.2%) and higher concentration of PVP K 30, a smaller mean particle size was recorded. Additionally, in this range of concentrations, the PdI was below 0.2 indicating homogeneous size distribution between the particles.
The results of the mean particle size were also not statistically significant. The p value obtained for concentration of Tween 80 was −0.765798, concentration of PVP K30 was 0.1701071 and interaction was −1.10475. The concentration of the surfactants and their interaction were reported not to be statistically significant. For F1, PdI results were not shown to be statistically relevant. The p value obtained for concentration of Tween 80 was −1.15589, concentration of PVP K30 was −2.10979 and interaction was 1.212005. The concentrations and their interaction were reported also not to be statistically significant. Based on these findings, the formulation we selected as optimal to follow in vitro studies has the characteristics summarized in Table 4. For formulation 2, the influence of each independent variable and their interactions were also assessed using Pareto charts (Fig. 3).
Table 4.
Physicochemical characterization of the optimized F1 (0.2% Tween 80 and 1.2% PVP K30).
| Storage time | Z-Ave (nm) | PdI | ZP |
|---|---|---|---|
| Day 0 | 79.00 | 0.126 | −12.7 |
| Day 1 | 82.72 | 0.149 | n.a. |
| Day 7 | 94.58 | 0.077 | n.a. |
n.a., not analysed.
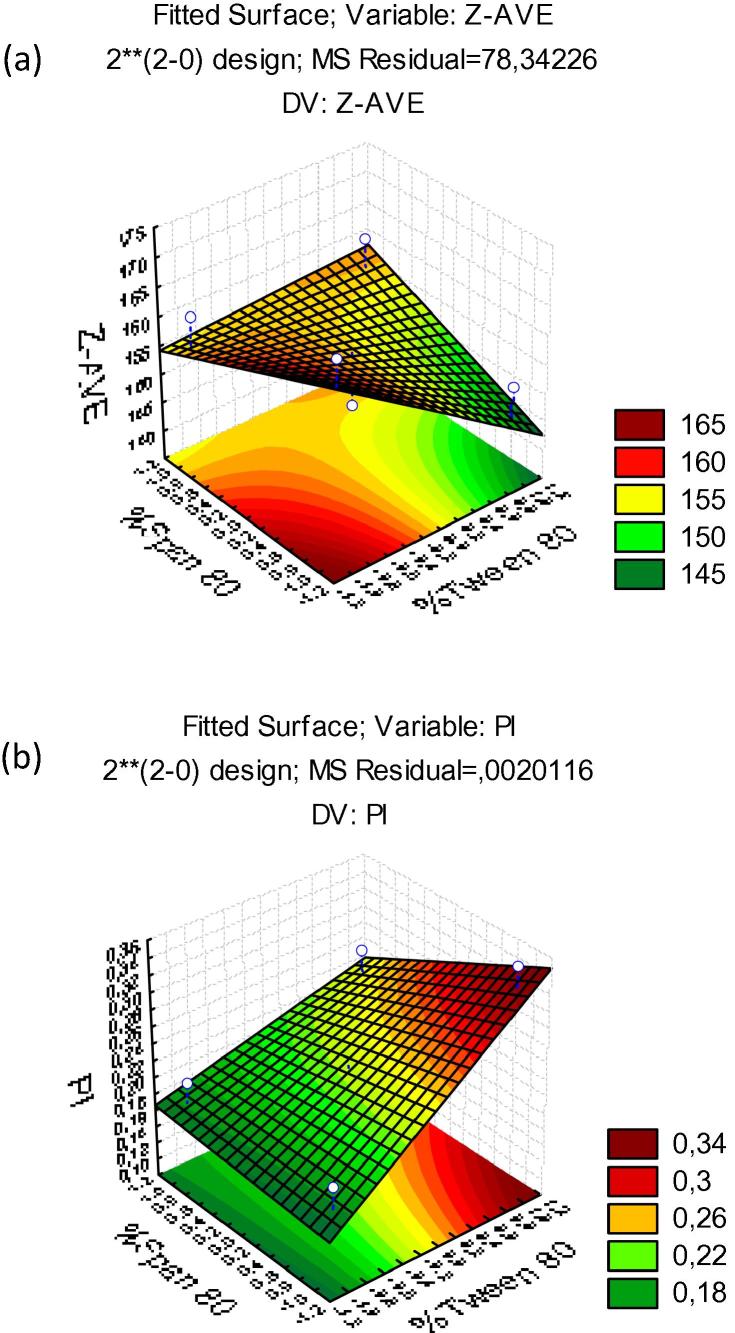
Fig. 3.
Pareto chart of the standardized effects for nanocrystals produced with F2: (a) particle size (Z-Ave (nm)); (b) polydispersity index (PdI).
The results obtained for the influence of the concentration of surfactants in F2 were also not statistically significant (Fig. 3). Similar results obtained with F1 were also observed in F2 (Fig. 4). However, in the surface response charts of experimental design, Fig. 4(b), the PdI is strongly affected by the increase of the concentration of Tween 80.
Fig. 4.
Surface response charts of experimental design of nanocrystals produced with F2: (a) particle size (Z-Ave (nm)); (b) polydispersity index (PdI).
A relationship can be established between the values of Z-Ave and PdI. The response surface graph (Fig. 4(a)) shows that when increasing the percentage of Tween 80 the average size decreased, whereas the PdI increased. This result is attributed to the rearrangements of polysorbate chains on the surface of nanocrystals, while excess of surfactant may produce other colloidal species (e.g. micelles) which increases the PdI. While no variable was shown to be statistically significant, larger nanocrystals (z-AVE 190 nm; PdI < 0.2) were obtained with F2 when produced with lower concentration of Tween 80 and higher concentration of Span 80. For F2, also z-Ave results were not shown to be statistically significant. The p value obtained for concentration of Tween 80 was −1.03377, concentration of Span 80 was 0.050841 and interaction was 1.101556. The concentrations and their interaction were also not statistically significant, neither were the PdI results. The p value obtained for concentration of Tween 80 was 2.508338, concentration of Span 80 was −0.903002 and interaction was −0.992187. Based on these findings, the formulation we selected as optimal to follow in vitro studies has the characteristics summarized in the Table 5.
Table 5.
Physicochemical characterization of the optimized F2 (0.2% Tween 80 and 1.2% Span 80).
| Storage time | Z-Ave (nm) | PdI | ZP |
|---|---|---|---|
| Day 0 | 174,1 | 0.182 | −28.1 |
| Day 1 | 181.4 | 0.229 | n.a. |
| Day 7 | 178.5 | 0.196 | n.a. |
n.a., not analysed.
Usually, particle aggregation is less likely to occur for charged particles with ZP > |20| mV, since there is electrostatic repulsion between particles with the same electrical charge (Souto et al., 2004). The size and PdI of the formulation were analysed during storage time at 25 °C. At naked eye nanocrystals were perfectly homogeneous.
LUMiSizer® has become an instrument of choice for regular analysis of long-term stability of particles in suspension and of demixing phenomena (Hou et al., 2010, Caddeo et al., 2013). LUMiSizer employs the STEP-Technology, which allows the measurement of the transmitted light intensity during centrifugation, as a function of time and position, over the entire sample length. Near infrared light is emitted through the entire sample cell and the transmitted light is detected by sensors arranged linearly across the sample from top to bottom. The shape and progression of the transmission profiles contains information on the kinetics of the separation process and allows particle characterization as well as evaluation of particle-particle interactions. Based on the extinction profiles, demixing processes are quantified regarding clarification velocity, sedimentation and flotation velocity of particles, residual turbidity, separated phase volume (liquid or solid). The centrifugal sedimentation method used with this equipment allows a fast stability ranking and shelf-life estimation of undiluted dispersions at their original concentration, in minutes/hours instead of months/years. The evolution of the transmission profiles of tested samples enables the analysis of their demixing behaviour and stability, as stable colloidal dispersions allow the formation of a flatbed under a centrifugal field, while aggregated particles usually give a step-profile. Indeed, the centrifugal acceleration causes different sedimentation velocities of particles with different size ranges. Instability phenomena is directly related to a migration of particles (sedimentation, flotation) and to changes in particle size distribution (e.g., due to particle interaction) followed by particle migration. The transmission profiles of nanocrystals produced from F1 and F2 are shown in Figs. 5 and 6, respectively.
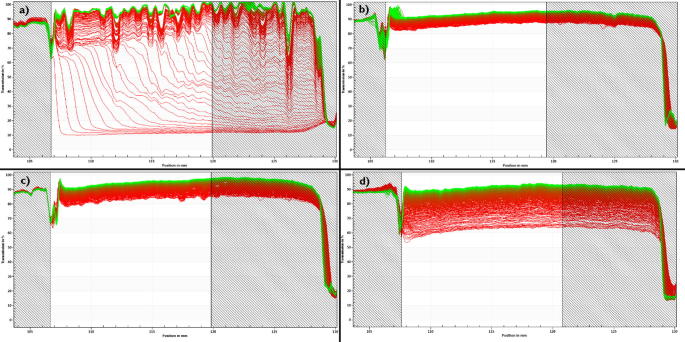
Fig. 5.
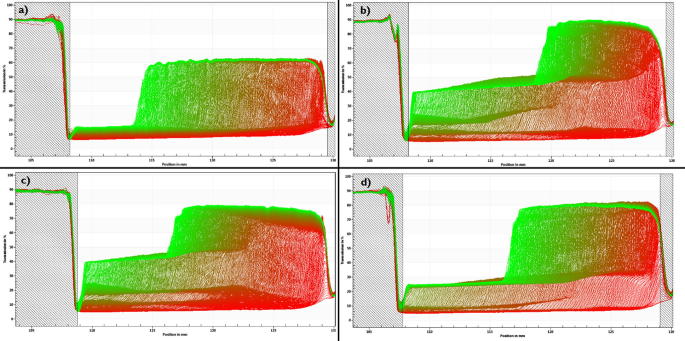
Comparison of the instability profiles between different samples of the same formulation F1: (a) F1, day 0; (b) F1, stored at 4 °C for 48 h; (c) F1, stored at 22 °C for 48 h; (d) F1, stored at 40 °C for 48 h.
Fig. 6.
Comparison of the instability profiles between different samples of the same formulation F2: (a) F2, day 0; (b) F2, stored at 4 °C for 48 h; (c) F2, stored at 22 °C for 48 h; (d) F2, stored at 40 °C for 48 h.
Fig. 5 compares the instability profiles obtained for F1, in which the sedimentation is observed for the entire sample. In the case of Fig. 5(a) a multimodal particle size distribution, i.e. particles with different sizes, is clearly presented, which translates the most unstable formulation in comparison to the other recorded profiles. Samples b) and c) showed a very high level of clarification since the beginning of the assay, which compromised the analysis. The low opacity of the sample limited the detection of migration phenomena of the particles by the equipment. The spacing between the profiles decreased over time (Fig. 5(a)), i.e. did not remain constant as happens with monodispersed populations (Fig. 5(d)). As time progresses, the particles tend to migrate more slowly, which may be the result of some particle aggregation. Profile (d) presents a unimodal sample, i.e. a homogeneous formulation, which translates higher stability. Fig. 6 shows the separation profiles obtained for the different F2 formulations that were tested.
In case of F2, a creaming process in all profiles was recorded, i.e. the migration of the dispersed phase of an emulsion. Fig. 6(a) shows a sample with a more homogeneous particle size distribution, suggested by the symmetrical spacing observed for the majority of the profiles. In the remaining samples, different sections in the overall profile were recorded, suggesting the existence of different particle size populations. Each section represents a different particle size population. Table 6 shows how temperature and storage conditions influence the nanocrystals stability and their sedimentation velocity. The experiments performed with the LUMiSizer® allowed to calculate the samples sedimentation velocity (Table 6).
Table 6.
Instability index, sedimentation velocity and their interval for each sample of F1 and F2.
| Sample Name | Instability Index | Velocity in µm/s | Correlation Coefficient | Standard deviation in µm/s |
|---|---|---|---|---|
| F1 – day 0 | 0.883 | 33.16 | 0.9788 | 1.543 |
| F1 – stored at 4 °C for 48 h | 0.285 | 2.90 | 0.9590 | 0.121 |
| F1 – stored at 22 °C for 48 h | 0.285 | 3.96 | 0.9890 | 0.087 |
| F1 – stored at 40 °C for 48 h | 0.391 | 12.47 | 0.9679 | 1.599 |
| F2 – day 0 | 0.400 | 0.8811 | 0.9998 | 0.0045 |
| F2 – stored at 4 °C for 48 h | 0.593 | 0.7679 | 0.9982 | 0.0100 |
| F2 – stored at 22 °C for 48 h | 0.579 | 0.9183 | 0.9996 | 0.0026 |
| F2 – stored at 40 °C for 48 h | 0.516 | 0.5162 | 0.9997 | 0.0029 |
To confirm the results shown in Fig. 5, Fig. 6, instability index was measured. The instability index is calculated based on the clarification (increase in transmission due to phase separation by sedimentation) at a given separation time, divided by the maximum clarification. The instability index is a dimensionless number and ranges from 0 (more stable) to 1 (more unstable). This means that, for the same total clarification, samples with high clarification rates tend to be more unstable. Regarding formulation F1, results show that the sample (F1- day 0) has the highest instability index, confirming the previous information obtained with the separation profile observed in Fig. 5(a). Samples (b) and (c) appear to be the most stable ones and sample d) has an intermediate instability index. However, samples (b) and (c) present a high level of transparency from the first profile to the last, indicating that there is a very low concentration of particles in the suspension, which may compromise the analysis.
Each sample of this formulation 2 was also analysed with the LUMiSizer®. The formulation F2 – day 0 was shown to be the most stable, although its sedimentation velocity was not the lowest among all samples, but the total clarification obtained for this sample was the lowest, which explains the best instability index of this group of samples. In case of F2 stored at 40 °C, despite having the lowest sedimentation velocity, it reached a higher level of total clarification when compared to sample (a), resulting in a higher level of instability. However, in the case of formulation F2 there was no significant difference in the instability index observed between samples (b), (c) and (d), suggesting that in this case the temperature and storage conditions are not critical for the overall stability of the formulation.
Thus, based in the instability index, F1 formulations stored at 4 °C and at 22 °C for 48 h are the most stable compared to the results obtained for the freshly prepared sample. In case of F2 the freshly prepared sample (F2 - day 0) is more stable than those of the same composition and there is no significant difference between the samples stored at 4 °C, 22 °C and 40 °C for 48 h.
4. Conclusions
Production techniques, especially the high-pressure homogenization, have been employed in large-scale production of nanocrystals. This study reports an approach to use a 2-level 2-factor factorial design in the optimization of nanocrystal formulations of poorly water soluble drugs (i.e. ibuprofen) produced with different surfactants. The desired mean particle size, PdI and ZP were obtained using 0.20% Tween 80 and 1.20% PVP K30 for F1 and 0.20% Tween 80 and 1.20% Span 80 for F2. The decrease of the concentration of Tween 80 contributed for the decrease of the mean particle size in both formulations. In the stability analysis, F1 and F2 formulations have different separation process, i.e. in the case of F1 sedimentation occurs when the samples are submitted to a strong acceleration, while in the case of F2 creaming is the observed. Also, calculated instability index suggests that the storage of F1 at 4 °C and 22 °C enhances the stability of the formulation. In the case of F2, this formulation appears to be more stable when freshly produced.
Acknowledgements
The financial support was received from Portuguese Science and Technology Foundation (FCT/MCT) and from European Funds (PRODER/COMPETE) under the projects M-ERA-NET-0004/2015-PAIRED, UID/AGR/04033/2013 and UID/QUI/50006/2013, co-financed by FEDER, under the Partnership Agreement PT2020. FCT is also acknowledged for the individual fellowship granted to ACS (SFRH/BD/109261/2015).
Footnotes
Peer review under responsibility of King Saud University.
References
- Bleumink G.S. Fatal combination of moclobemide overdose and whisky. Neth. J. Med. 2003;61(3):88–90. [PubMed] [Google Scholar]
- Brigo L. Mesoporous silica sub-micron spheres as drug dissolution enhancers: Influence of drug and matrix chemistry on functionality and stability. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;59:585–593. doi: 10.1016/j.msec.2015.10.039. [DOI] [PubMed] [Google Scholar]
- Caddeo C. Nanocarriers for antioxidant resveratrol: formulation approach, vesicle self-assembly and stability evaluation. Colloids Surf., B. 2013;111:327–332. doi: 10.1016/j.colsurfb.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Castillo M. Disposition and covalent binding of ibuprofen and its acyl glucuronide in the elderly. Clin. Pharmacol. Ther. 1995;57(6):636–644. doi: 10.1016/0009-9236(95)90226-0. [DOI] [PubMed] [Google Scholar]
- Feng S., Huang G. Effects of emulsifiers on the controlled release of paclitaxel (Taxol) from nanospheres of biodegradable polymers. J. Control Release. 2001;71(1):53–69. doi: 10.1016/s0168-3659(00)00364-3. [DOI] [PubMed] [Google Scholar]
- Garzón L.C., Martínez F. Temperature dependence of solubility for ibuprofen in some organic and aqueous solvents. J. Solution Chem. 2004;33(11):1379–1395. [Google Scholar]
- Hou Z. Investigation into the physicochemical stability and rheological properties of β-carotene emulsion stabilized by soybean soluble polysaccharides and chitosan. J. Agric. Food Chem. 2010;58(15):8604–8611. doi: 10.1021/jf1015686. [DOI] [PubMed] [Google Scholar]
- Jarosz M. Nanoporous anodic titanium dioxide layers as potential drug delivery systems: drug release kinetics and mechanism. Colloids Surf. B Biointerf. 2016;143:447–454. doi: 10.1016/j.colsurfb.2016.03.073. [DOI] [PubMed] [Google Scholar]
- Krasniqi V. How polymorphisms of the cytochrome P450 genes affect ibuprofen and diclofenac metabolism and toxicity. Arh. Hig Rada Toksikol. 2016;67(1):1–8. doi: 10.1515/aiht-2016-67-2754. [DOI] [PubMed] [Google Scholar]
- Loh Z.H., Samanta A.K., Sia Heng P.W. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015;10(4):255–274. [Google Scholar]
- Nerurkar J. Solubility of (+/-)-ibuprofen and S (+)-ibuprofen in the presence of cosolvents and cyclodextrins. Pharm. Dev. Technol. 2005;10(3):413–421. doi: 10.1081/pdt-54446. [DOI] [PubMed] [Google Scholar]
- Neunzig I. Production and NMR analysis of the human ibuprofen metabolite 3-hydroxyibuprofen. J. Biotechnol. 2012;157(3):417–420. doi: 10.1016/j.jbiotec.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Oncel M.Y., Erdeve O. Oral medications regarding their safety and efficacy in the management of patent ductus arteriosus. World J. Clin. Pediatr. 2016;5(1):75–81. doi: 10.5409/wjcp.v5.i1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oner M., Uysal U. Synthesis of hydroxyapatite crystals using carboxymethyl inulin for use as a delivery of ibuprofen. Mater. Sci. Eng. C Mater. Biol. Appl. 2013;33(1):482–489. doi: 10.1016/j.msec.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Rashid A. Effect of solvent composition and temperature on the solubility of ibuprofen in aqueous ethanol. J. Chem. Eng. Data. 2014;59(9):2699–2703. [Google Scholar]
- Rudy A.C. Stereoselective metabolism of ibuprofen in humans: administration of R-, S- and racemic ibuprofen. J. Pharmacol. Exp. Ther. 1991;259(3):1133–1139. [PubMed] [Google Scholar]
- Shaw L.R. The effect of selected water-soluble excipients on the dissolution of paracetamol and Ibuprofen. Drug Dev. Ind. Pharm. 2005;31(6):515–525. doi: 10.1080/03639040500215784. [DOI] [PubMed] [Google Scholar]
- Souto E.B. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur. J. Pharm. Biopharm. 2004;58(1):83–90. doi: 10.1016/j.ejpb.2004.02.015. [DOI] [PubMed] [Google Scholar]
- States, P.C.U., 2000. The United States Pharmacopoeia, ed. G.N. 24th rev. ed. The United States Pharmacopoeia Conventional Inc., Rockville, 8.
- Woodman T.J. Chiral inversion of 2-arylpropionyl-CoA esters by human alpha-methylacyl-CoA racemase 1 A (P504S) – a potential mechanism for the anti-cancer effects of ibuprofen. Chem. Commun. (Camb) 2011;47(26):7332–7334. doi: 10.1039/c1cc10763a. [DOI] [PubMed] [Google Scholar]