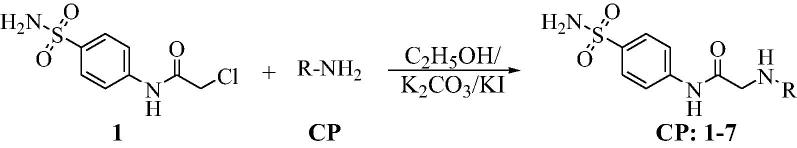Abstract
The Novel target compounds (CP-1-7) were synthesized and tested at doses up to 1000 mg/kg for their entitled activities. They exerted promising results without any behavioral changes and mortality in mice. Therefore, according to the results obtained in our study, it could be categorized as highly safe agents for treating UC since substances possessing LD50 higher than 50 mg/kg are considered nontoxic. They also possessed a potent anti-ulcerogenic activity with different potentials. The most effective compound was CP-4, it produced 97.7% ulcer protection of control followed by CP-3, which produced 90.3% protection, while the standard drug ranitidine (100 mg/kg) produced 49.2% protection. Compound CP-1 showed lowest activity among the current series, it produced 55.5% protection. The target compounds were significantly more effective than the standard in reducing ulcer index. The anti-ulcerative colitis activity was tested using acetic acid induced colitis model. The curative effect of the tested compounds at a dose of 50 mg/kg oral administration on rats showed a potent anti-ulcerative colitis activity with different potentials. They induced a significant decrease in ulcer score, ulcer area, ulcer index and weight/length of the colon specimens.
The percent protection of control colitis ranged from 66.8% for CP-7 to 22.3% for CP-5; however the percent protection for dexamesathone (0.1 mg/kg) was 59.3%. The effect of the tested compounds CP-7 and CP-3 at dose 50 mg/kg were significantly more effective than dexamesathone (0.1 mg/kg) in reducing all parameters.
Liver functions were not affected as there is no effect on the activity of both AST and ALT in animals that received the compounds, so the compounds didn’t reveal hepatotoxic manifestation. Although, the results on kidney functions showed that, CP-1 slightly elevated blood urea concentration and CP-3 & CP-4 slightly elevated serum creatinine; no apparent nephrotoxic manifestations were recorded.
Keywords: Liver functions, Ulcers, Dexamesathone, Amines derivatives, Ulcerative colitis
1. Introduction
Ulcers in the gastrointestinal tract could be divided into two common types according to location; ulcerative colitis (lower) and peptic ulcer (upper). Ulcerative colitis (UC) is an inflammatory bowel disease that primarily affecting the colonic mucosa. In its most limited form it may be restricted to the distal rectum, while in its most extended form, the entire colon is involved (Awaad et al., 2013c, Gower-Rousseau et al., 2017, Schultz et al., 2017). UC can occur in both sexes and in any age group but most often begins in people between 15 and 30 years of age. The exact causes of UC are still not clear but different factors have been postulated as possible etiologic agents. They are genetic factors, infective agents, immunological basis, smoking, medications and pathological factors (Awaad et al., 2013a, Ide et al., 2017, LI et al., 2017).
Medicinal chemistry plays an important role in development of drug for cure; maintain and improved health of human being. It is also equally important to design chemical entities to prevent the growth of micro-organism, which come in contact with human being in day-to-day life (Katke et al., 2011, Alasmary et al., 2015).
Different heterocyclic compounds are made to synthesize by large number of efforts and their derivatives were found to possess antitumor, anti-diabetic, antimicrobial, anticonvulsant and anthelmintic activities. The small and simple benzothiazole nucleus and its derivatives possess various diverse biological properties. These activities are also possessed by its substituted derivatives as well (Pritesh et al., 2009, Patrick et al., 2017, Sharma et al., 2017).
Literature revealed some of the 9-fluorenyl amine derivatives has moderate biological activity on cancer and were reported for fluorenyl derivatives (Venkatesan et al., 2012). A series of 5-substituted (arylmethylene) pyridin-2-amine and 6-chloropyridin-2-yl-amine derivatives were synthesized and screened for antibacterial and antifungal activities (Bhatia et al., 2009, Nagashree et al., 2013, Sharma et al., 2017, Sahu et al., 2016).
In this regard we thought to synthesize some amines derivatives backbone and to test its antiulcer and anti-ulcerative colitis activities.
2. Experimental
2.1. Synthesis
2.1.1. 2-Chloro-N-(4-aminosulphonylphenyl)acetamide (1)
2-Chloroacetyl chloride (1.12 g, 0.01 mol) was added drop wise with vigorous stirring to a cold suspension of sulfanilamide (1.72 g, 0.01 mol) in 10 ml dichloromethane containing 2 drops triethylamine. Stirring was continued for 1 h and the separated solid was filtered, washed with ether, dried and crystallized from aqueous-ethanol.
2.1.2. 2-(4-Substituted-amino)-N-(4-aminosulphonylphenyl)acetamide, (CP-1-7)
2.1.2.1. General procedures
A mixture of equimolar proportion (0.001 mol) of compound 1, anhydrous potassium carbonate, potassium iodide and the appropriate amine derivative were refluxed in ethanol (20 ml) for 3 h (Fig. 1). The reaction mixture was filtered while hot, cooled and the precipitated solid was cooled and purified by column chromatography to obtain the target compounds in a considerable yield.
Fig. 1.
Scheme for synthesis of compounds CP-1-7.
2-[(4-Cyano-4-phenyl)piperidinyl]-N-(4-aminosulphonylphenyl)acetamide (CP-1). Yield (70%); m.p. 196–198 °C; IR ν 3372–3347 (NH, NH2), 1695 (C O) cm−1; 1H NMR (DSMO-d6) δ 2.20 (t, 4H, J = 3.2 Hz, 2CH2), 2.51 (t, 4H, J = 3.7 Hz, 2CH2), 3.45 (s, 2H, CH2), 7.05 (s, 2H, NH2), 7.21–7.94 (m, 9H, ArH and NH2), 10.27 (s, D2O exchangeable, 1H, NH); 13C NMR (DSMO-d6) δ 35.3 (CH2), 38.3 (CH2), 50.3 (CH2), 61.2 (CH2), 119.1, 122.1, 125.6, 126.5, 128.0, 128.3, 128.7, 129.0, 138.5, 140.2, 141.4 (Ar-C), 168.9 (C O); MS m/z (Rel. Int.) 398 (M+, 100). Anal. (C20H22N4O3S, 398.48) C, 60.28 (60.13); H, 5.56 (5.42); N, 14.06 (13.85); S, 8.05 (8.24).
2-[(4-Hydroxy-4-phenyl)piperidinyl]-N-(4-aminosulphonylphenyl)acetamide (CP-2). Yield (65%); m.p. 221–223 °C; IR ν 3351–3342 (NH, NH2), 1674 (C O) cm−1; 1H NMR (DSMO-d6) δ 2.21 (t, 4H, J = 3.2 Hz, 2CH2), 2.50 (t, 4H, J = 3.7 Hz, 2CH2), 3.34 (s, 2H, CH2), 4.87 (s, 1H, OH), 7.29–7.87 (m, 11H, ArH and NH2), 10.17 (s, D2O exchangeable, 1H, NH); 13C NMR (DSMO-d6) δ 39.1 (CH2), 49.3 (CH2), 62.1 (CH2), 69.2 (CH2), 119.1, 124.8, 126.2, 126.5, 127.8, 128.1, 128.4, 128.7, 138.5, 141.5, 150.0 (Ar-C), 169.4 (C O); MS m/z (Rel. Int.) 389 (M+, 100). Anal. (C19H23N3O4S, 389.47) C, 58.59 (58.71); H, 5.95 (6.20); N, 10.79 (10.92); S, 8.23 (8.47).
2-[4(4-Chlorophenyl)piperidinyl]-N-(4-aminosulphonylphenyl)acetamide (CP-3). Yield (63%); m.p. 270–272 °C; IR ν 3351–3342 (NH, NH2), 1674 (C O) cm−1; 1H NMR (DSMO-d6) δ 1.64–1.90 (m, 4H, 2CH2), 2.22–2.29 (m, 4H, 2CH2), 2.71 (m, 1H, CH), 3.35 (s, 2H, CH2), 7.22–7.88 (m, 10H, Ar-H and NH2), 10.09 (s, D2O exchangeable, 1H, NH); 13C NMR (DSMO-d6) δ 37.7 (CH2), 49.2 (CH2), 62.0 (CH2), 69.1 (CH2), 119.1, 126.5, 126.8, 127.7, 130.8, 138.5, 141.5, 149.0 (Ar-C), 169.3 (C O); MS m/z (Rel. Int.) 409 (M+ + 2, 26), 407 (M+, 87). Anal. (C19H22ClN3O3S, 407.91) C, 55.94 (56.11); H, 5.44 (5.27); N, 10.30 (10.16); S, 7.86 (7.70).
2-[4-(1-Piperidino)piperidinyl]-N-(4-aminosulphonylphenyl)acetamide (CP-4). Yield (62%); m.p. 215–217 °C; IR ν 3361–3339 (NH, NH2), 1677 (C O) cm−1; 1H NMR (DSMO-d6) δ 1.45–1.65 (m, 10H, 5CH2), 2.21–2.29 (m, 8H, 4CH2), 2.72 (m, 1H, CH), 3.35 (s, 2H, CH2), 7.27 (s, 2H, SO2NH2), 7.66 (d, 2H, J = 7.2 Hz, Ar-H),), 7.83 (d, 2H, J = 7.5 Hz, Ar-H), 10.09 (s, D2O exchangeable, 1H, NH); 13C NMR (DSMO-d6) δ 24.6, 26.0, 27.4 (CH2), 49.7, 53.1, 61.5, 61.9 (CH2), 118.9, 126.5, 138.5, 141.5 (Ar-C), 169.2 (C O); MS m/z (Rel. Int.) 380 (M+, 100). Anal. (C18H28N4O3S, 380.50) C, 56.82 (56.69); H, 7.42 (7.64); N, 14.72 (14.82); S, 8.43 (8.23).
2-[4(4-Methoxyphenyl)piperidinyl]-N-(4-aminosulphonylphenyl)acetamide (CP-5). Yield (55%); m.p. 175–177 °C; IR ν 3354–3340 (NH, NH2), 1675 (C O) cm−1; 1H NMR (DSMO-d6) δ 1.65–1.89 (m, 4H, 2CH2), 2.20–2.27 (m, 4H, 2CH2), 2.73 (m, 1H, CH), 3.43 (s, 2H, CH2), 6.97 (s, 2H, SO2NH2), 7.16–7.92 (m, 8H, Ar-H),), 10.38 (s, D2O exchangeable, 1H, NH); 13C NMR (DSMO-d6) δ 20.3, 40.9, 51.6, 53.2, 61.6 (CH2), 118.9, 124.5, 125.7, 126.5, 130.4, 137.2, 141.6, 151.3 (Ar-C), 169.0 (C O); MS m/z (Rel. Int.) 403 (M+, 100). Anal. (C20H25N3O4S, 403.50) C, 59.53 (59.33); H, 6.25 (6.47); N, 10.41 (10.63); S, 7.95 (8.13).
2-[4(2,3-Xylyl)piperazinyl]-N-(4-aminosulphonylphenyl)acetamide (CP-6). Yield (69%); m.p. 241–243 °C; IR ν 3368–3347 (NH, NH2), 1671 (C O) cm−1; 1H NMR (DSMO-d6) δ 2.45 (s, 6H, 2CH3), 2.57 (t, 4H, J = 3.7 Hz, 2CH2), 3.41 (s, 2H, CH2), 3.60 (t, 4H, J = 3.5 Hz, 2CH2), 6.83 (s, 2H, SO2NH2), 7.27–7.84 (m, 7H, Ar-H),), 10.10 (s, D2O exchangeable, 1H, NH); 13C NMR (DSMO-d6) δ 11.0, 18.3, 49.4, 52.8, 55.1 (CH2), 114.2, 117.3, 118.9, 121.3, 126.5, 135.1, 138.4, 141.5, 145.3, 152.9 (Ar-C), 168.9 (C O); MS m/z (Rel. Int.) 403 (M+, 71). Anal. (C20H26N4O3S, 402.51) C, 59.68 (59.62); H, 6.51 (6.75); N, 13.92 (14.16); S, 7.97 (8.16).
2-(2-Methylquinolin-4-ylamino)-N-(4-aminosulphonylphenyl)acetamide (CP-7). Yield (66%); m.p. >300 °C; IR ν 3375–3346 (NH, NH2), 1677 (C O) cm−1; 1H NMR (DSMO-d6) δ 2.55 (s, 3H, CH3), 4.01 (s, 2H, CH2), 6.95 (s, 2H, SO2NH2), 7.32–8.01 (m, 9H, Ar-H and NH),), 10.44 (s, D2O exchangeable, 1H, NH) 10.89 (s, D2O exchangeable, 1H, NH); 13C NMR (DSMO-d6) δ 24.2 (CH3), 52.7 (CH2), 102.0, 117.0, 118.9, 119.2, 122.1, 123.1, 126.6, 128.7, 132.7, 138.8, 141.3, 152.1, 157.8 (Ar-C), 168.2 (C O); MS m/z (Rel. Int.) 370 (M+, 77). Anal. (C18H18N4O3S, 370.43) C, 58.36 (58.17); H, 4.90 (5.17); N, 15.12 (15.28); S, 8.66 (8.85).
Chemical properties of synthesized amines (CP-1-7) were recorded in Table 1.
Table 1.
Code, chemical structure and molecular weight of the synthesized amides (CP-1-7).
| Code | Chemical Structure | Mol. Wt. |
|---|---|---|
| CP-1 | 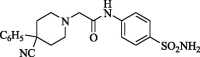 |
398.48 |
| CP-2 | 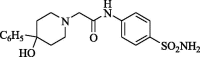 |
389.14 |
| CP-3 | 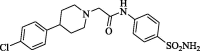 |
407.91 |
| CP-4 | 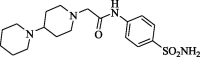 |
380.19 |
| CP-5 | 403.51 | |
| CP-6 | 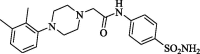 |
402.51 |
| CP-7 | 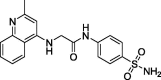 |
370.43 |
2.2. Biological activity
2.2.1. Animals
Swiss albino mice of both sex (26–30 g) and male Wistar rats (150–180 g) were purchased from the animal house of Faculty of Pharmacy, King Saud University, KSA. Animals were housed in standard polypropylene cages with wire mesh top and maintained under standard conditions (temperature 23 ± 1.0 °C, humidity 55 ± 10%, 12 h light/12 h dark cycle). They fed with a standard pellet diet with water ad libitum and were allowed to adapt to the laboratory environment for one week before experimentation.
2.2.1.1. Determination of median lethal dose (LD50)
The oral median lethal dose (LD50) of the target compounds was determined as described by (Lorke, 1983). Swiss albino mice in 10 groups each of six animals, received one of 50, 100, 500, or 1000 mg/kg doses of the target compounds. Control animals were received the vehicle and kept under the same conditions. Signs of acute toxicity and number of deaths per dose within 24 h were recorded.
2.2.1.2. Antiulcerogenic activity
Evaluation of the anti-ulcerogenic activity was carried out using absolute ethanol-induced ulcer model as described by (Bighetti et al., 2005) with some modifications. Male Wistar rats were divided into 9 groups each of 6 rats. Group 1 received the vehicle and served as control ulcer, group 2 received ranitidine (100 mg/kg) and served as standard, and other group received the target compounds at dose 50 mg/kg.
Rats of all groups were fasted for 24 h and received an oral dose of absolute ethanol (1 ml/200 g) and after one hour, all medications were administered orally. One hour later, the animals sacrificed, by ether inhalation, the stomachs were rapidly removed, opened along their greater curvature and gently rinsed under running tap water.
Number of lesions in the glandular part of the stomach were measured under an illuminated magnifying microscope (10×). Long lesions were counted and their lengths were measured. Petechial lesions were counted, and then each five petechial lesions were taken as 1 mm of ulcer.
The lesion scores the mucosal lesions were quantified by the scoring system (0 −5) 0 = no damage, 1 = Local edema and inflammation without ulcers; 2 = One ulcer without inflammation; 3 = one to two ulcers with inflammation & lesion diameter < 1 cm; 4 = More than two ulcers with lesion diameter 1–2 cm; 5 = Sever ulceration with lesion diameter > 2 cm (Morris et al., 1989).
Ulcer index To calculate the ulcer index (mm), the sum of the total length of long ulcers and petechial lesions in each group of rats was divided by its number. The curative ratio was determined according to the formula:
% Protection of control ulcer = Control UI − Test UI/Control UI × 100
2.2.1.3. Anti-ulcerative colitis activity
Groups of animals each of 6 rats were used. The 1st group received dexamesathone at dose 0.1 mg/kg orally to serve as standard; two other groups received water orally and served as normal control and control colitis. Other groups received the tested compounds at dose 50 mg/kg. Ulcerative colitis was induced by slowly infusion (20 drops/min.) of 2 mL (4%, v/v) acetic acid in saline into the colon through the catheter. Two hours after the induction of colitis, animals received the 1st dose of all medication, then all groups received medication for 5 consecutive days, two hours after the last dose, animals were sacrificed by ether anesthesia, colonic segments (8 cm in length and 3 cm proximal to the anus) were excised, opened and were used for macroscopic scoring (Awaad et al., 2013b).
Assessment of colonic lesions: The colon specimens were weighted and wet weight/length ratio was calculated for all the rats. The specimens were examined under a dissecting microscope and the mucosal lesions were quantified by the scoring system (0–5) given by (Awaad et al., 2013b) after some modifications.
Ulcer area was measured using plane glass square. Each cell on the glass square was 1 mm2 in area and the number of cells was counted and the ulcer area was determined for each colon. Ulcer index was measured by summing the lesion score and the ulcer area for each colon specimen (Awaad et al., 2013b).
2.2.1.4. Effect on Liver and kidney functions
Male Wister rats were divided into 8 groups each of 10 rats. The 1st group was left as a control and administrated the vehicle orally, while the other groups were orally administrated the synthesized compounds in a dose of 50 mg/kg for 15 days. After the examination period, 6 h after the last dose blood samples were collected from the orbital plexus of rats. Samples were left to clot at room temperature for 30 min then centrifuged at 1000 rpm for 20 min.
The collected sera were used for determination of the activity of both (AST) aspirate aminotransferase and (ALT) alanine aminotransferase as liver markers. In addition, levels of blood urea, serum creatinine were also estimated as kidney markers (Awaad et al., 2013b).
Statistical analysis: All values were expressed as mean ± S.D. Statistical analysis was done by using SPSS 10. Statistical significance of differences between two means was assessed by unpaired Student’s ‘t’ test. Differences at p < 0.05, 0.01, and 0.001 were considered statistically significant.
2.3. Results and discussion
The target compounds were obtained from the key starting material 2-chloro-N-(4-aminosulphonylphenyl)-acetamide, 1 with the appropriate amine derivative by nucleophilic substitution reaction. The reaction was activated by using potassium carbonate as acid acceptor in presence of potassium iodide to make it easier to release the chlorine atom. The structure of the target compounds was confirmed by IR, NMR and mass spectra. Generally, the obtained data are in accordance with the proposed structures. IR spectra showed the presence of NH and NH2 at around 3350 cm−1, and the carbonyl group at around 1670 cm−1. The 1H NMR spectra showed the presence of NH and SO2NH2 in addition to methylene and carbonyl in the 13C spectra. The mass spectra showed the molecular ion peak with relative intensity ranging from 100 to 77%.
2.3.1. Determination of median lethal dose (LD50)
The target compounds in doses up to 1000 mg/kg did not produce any behavioral changes and mortality in mice. Therefore, it can be categorized as highly safe since substances possessing LD50 higher than 50 mg/kg are nontoxic (Soliman et al., 2012).
2.3.2. Anti-ulcerogenic activity
The present results showed that the target compounds possessed a potent anti-ulcerogenic activity with different potentials. The most effective compound was CP-4, it produced percent protection of control ulcer 97.7% followed by CP-3 which produced 90.3% protection, while the standard drug ranitidine (100 mg/kg) produced 49.2% (Table 2). The tested compound cp1 showed lowest activity amount the target compounds, it produced 55.5% protection. The target compounds were significantly more effective than the standard in reducing ulcer index.
Table 2.
Curative anti-ulcerogenic effect of synthesized amides (CP-1-7) on absolute alcohol-induced ulcer in rats.
| Gp | Dose mg/kg | Score | No of ulcers | Ulcer index | % Protection |
|---|---|---|---|---|---|
| Control | 4.00 | 13.20 ± 1.30 | 95.20 ± 1.10 | 0 | |
| Ranitidine | 100 | 2.20 | 8.60* ± 3.05 | 48.40*±1.24 | 49.2 |
| CP-1 | 50 | 3.20 | 7.20*±0.84 | 42.40*±1.14 | 55.5 |
| CP-2 | 50 | 3.20 | 7.60*±1.14 | 26.80*@ ± 1.64 | 71.9 |
| CP-3 | 50 | 2.20 | 2.80*@ ± 0.84 | 8.80*@ ± 1.30 | 90.8 |
| CP-4 | 50 | 2.00 | 1.40*@ ± 0.55 | 2.20*@ ± 0.84 | 97.7 |
| CP-5 | 50 | 2.40 | 3.40*@ ± 0.89 | 15.60*@ ± 1.14 | 83.6 |
| CP-6 | 50 | 3.00 | 6.80*±0.84 | 11.00*@ ± 0.71 | 88.5 |
| CP-7 | 50 | 3.60 | 8.00*±0.71 | 34.20*@ ± 1.10 | 64.1 |
Data are expressed as mean ± SD, n = 6.
Significantly different from control ulcer at p < 0.05.
Significantly different from ranitidine at p < 0.05.
2.3.3. Anti-ulcerative colitis
The model of acetic acid induced colitis shares many of the histologic features of ulcerative colitis in human beings including mucosal edema and sub-mucosal ulceration. In rats of control group, no abnormal changes were observed suggesting that handling procedure had no interference with the experimental outputs. Macroscopic damage parameters of the colon of control colitis rats after rectal infusion of acetic acid revealed dark brown lesions, mucosal hyperemia, edema, erosion, and ulceration. The inflammatory changes of the intestinal tract were associated with a significant increase of wet weight/length of the colon specimens as an indicator of inflammation.
The curative effect of the tested compounds (CP-1-7) at dose 50 mg/kg on acetic acid-induced colitis in rats is shown in Table 2. The tested compounds administrated orally to rats showed a potent anti-ulcerative colitis activity with different potentials. They induced a significant decrease in ulcer score, ulcer area, ulcer index and weight/length of the colon specimens.
The percent protection of control colitis ranged (Table 3) from 66.8% for CP-7 to 22.3% for CP-5.; however the percent protection for dexamesathone (0.1 mg/kg) was 59.3%.
Table 3.
Effect of synthesized amides (CP-1-7) on acetic acid induced colitis in rats.
| GP | Score | Ulcer area (mm2) | Ulcer index | Wt/l | % Protection |
|---|---|---|---|---|---|
| Normal control | 0 | 0 | 0 | 0.38 ± 0.05 | 0 |
| Control colitis | 4.00 ± 0.89 | 40.20 ± 0.75 | 44.20 ± 1.33 | 0.95 ± 0.07 | 0 |
| Dexamethasone | 2.00* ± 0.63 | 16.00* ± 0.63 | 18.00*±1.10 | 0.52 ± 0.07 | 59.3 |
| CP-1 | 2.67* ± 0.52 | 27.50*@ ± 1.38 | 30.17*@ ± 1.60 | 0.79 ± 0.08 | 31.7 |
| CP-2 | 2.33* ± 0.52 | 20.17*@ ± 1.17 | 22.50*@ ± 1.05 | 0.74 ± 0.02 | 49.1 |
| CP-3 | 1.67*@ ± 0.52 | 14.17*@ ± 0.98 | 15.83*@ ± 1.17 | 0.64 ± 0.05 | 64.2 |
| CP-4 | 3.00 ± 0.63 | 30.00*@ ± 1.41 | 33.00*@ ± 1.67 | 0.84 ± 0.02 | 25.3 |
| CP-5 | 2.67* ± 0.52 | 31.67*@ ± 1.37 | 34.33*@ ± 1.63 | 0.75 ± 0.02 | 22.3 |
| CP-6 | 2.67* ± 0.52 | 28.00*@ ± 1.41 | 30.67*@ ± 1.21 | 0.71 ± 0.05 | 30.6 |
| CP-7 | 1.50*@ ± 0.55 | 13.17*@ ± 1.17 | 14.67*@ ± 0.82 | 0.49 ± 0.02 | 66.8 |
Significantly different from control colitis at p < 0.05.
Significantly different from Dexamethasone at p < 0.05.
The effect of the tested compounds CP-7 and CP-3 at dose 50 mg/kg were significantly more effective than dexamesathone (0.1 mg/kg) in reducing all parameters.
2.3.4. Effect on Liver and kidney functions
Liver functions were not affected as there is no effect on the activity of both AST and ALT in animals received the tested compounds (CP-1-7) were recorded in Table 4, from it the compounds didn’t reveal hepatotoxic manifestation. These results on kidney functions showed that, only cp1 can slightly elevate blood urea concentration, and both compounds CP-3 and CP-4 slightly elevated serum creatinine. In addition, no apparent nephrotoxic manifestations were recorded.
Table 4.
Effect of synthesized amides (CP-1-7) on liver and kidney functions of rats.
| GP | ALT(U/l) | AST(U/l) | Blood urea (mg/dl) | Serum creatinine (mg/dl) |
|---|---|---|---|---|
| Normal control | 37.25 ± 0.26 | 47.31 ± 0.51 | 53.67 ± 0.84 | 0.89 ± 0.02 |
| CP-1 | 35.77 ± 1.60 | 44.43 ± 1.57 | 70.51*±0.89 | 0.88 ± 0.03 |
| CP-2 | 39.70 ± 0.22 | 66.28 ± 0.40 | 52.55 ± 1.24 | 0.86 ± 0.03 |
| CP-3 | 36.87 ± 1.28 | 44.16 ± 1.57 | 46.02 ± 1.10 | 01.01* ± 0.09 |
| CP-4 | 36.87 ± 1.28 | 44.16 ± 1.57 | 47.96 ± 0.84 | 0.94* ± 0.07 |
| CP-5 | 36.35 ± 1.26 | 44.09 ± 1.25 | 55.92 ± 0.55 | 0.86 ± 0.07 |
| CP-6 | 36.02 ± 1.58 | 44.7 ± 1.66 | 51.00 ± 0.89 | 0.83 ± 0.08 |
| CP-7 | 36.33 ± 1.35 | 46.16 ± 1.48 | 53.00 ± 0.14 | 0.85 ± 0.09 |
Data are expressed as mean ± SD, n = 10.
Acknowledgment
This project was supported by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University under the research group number (2016/01/6235).
Footnotes
Peer review under responsibility of King Saud University.
References
- Alasmary F.A.S., Snelling A.M., Zain M.E., Alafeefy A.M., Awaad A.S., Karodia N. Synthesis and evaluation of selected benzimidazole derivatives as potential antimicrobial agents. Molecules. 2015;20:15206–15223. doi: 10.3390/molecules200815206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awaad A.S., Al-Jaber N.A., Moses J.E., El-Meligy R.M., Zain M.E. Antiulcerogenic activities of the extracts and isolated flavonoids of euphorbia cuneata vahl. Phytother. Res. 2013;27:126–130. doi: 10.1002/ptr.4872. [DOI] [PubMed] [Google Scholar]
- Awaad A.S., El-Meligy R.M., Al-Jaber N.A., Al-Muteeri H.S., Zain M.E., Alqasoumi S.I., Alafeefy A.M., Donia A.E.R.M. Anti-ulcerative colitis activity of compounds from euphorbia granuleta forssk. Phytother. Res. 2013;27:1729–1734. doi: 10.1002/ptr.4985. [DOI] [PubMed] [Google Scholar]
- Awaad A.S., El-Meligy R.M., Soliman G.A. Natural products in treatment of ulcerative colitis and peptic ulcer. J Saudi Chem. Soc. 2013;17:101–124. [Google Scholar]
- Bhatia M.S., Mulani A.K., Choudhari P.B., Ingale K.B., Bhatia N.M. Synthesis and QSAR analysis of 5-substituted (arylmethylene) pyridin-2-amine derivatives as potential antibacterials. Int. J. Drug Disc. 2009;1:1–9. [Google Scholar]
- Bighetti A.E., Antonio M.A., Kohn L.K., Rehder V.L., Foglio M.A., Possenti A., Vilela L., Carvalho J.E. Antiulcerogenic activity of a crude hydroalcoholic extract and coumarin isolated from Mikania laevigata Schultz Bip. Phytomedicine. 2005;12:72–77. doi: 10.1016/j.phymed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Gower-Rousseau C., Sarter H., Savoye G., Tavernier N., Fumery M., Sandborn W.J., Feagan B.G., Duhamel A., Guillon-Dellac N., Colombel J.F., Peyrin-Biroulet L., INT PROGRAMME DEVELOP NEW, I. I. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut. 2017;66:588–596. doi: 10.1136/gutjnl-2015-310151. [DOI] [PubMed] [Google Scholar]
- Ide S., Araki T., Okita Y., Kawamura M., Toiyama Y., Kobayashi M., Ohi M., Tanaka K., Inoue Y., Uchida K., Mohri Y., Kusunoki M. Outcome and functional prognosis of pelvic sepsis after ileal pouch-anal anastomosis in patients with ulcerative colitis. Surgery Today. 2017;47:301–306. doi: 10.1007/s00595-016-1430-5. [DOI] [PubMed] [Google Scholar]
- Katke S.A., Amrutkar S.V., Bhor R.J., Khairnar M.V. Synthesis of biologically active 2-chloro-N-alkyl/aryl acetamide derivatives. Int. J. Pharma. Sci. Res. 2011;2:148–156. [Google Scholar]
- Li, J.Q., Chen, H.Q., Wang, B., Cai, C.X., Yang, X., Chai, Z.F., Feng, W.Y., 2017. ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Scientific Reports, 7. [DOI] [PMC free article] [PubMed]
- Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G., Mellits E.D., Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nagashree S., Mallesha L., Mallu P. Synthesis and in vitro biological activity of 6-chloro-pyridin-2-yl-amine derivatives. Der Pharma Chemica. 2013;5:50–55. [Google Scholar]
- Patrick D.A., Gillespie J.R., McQueen J., Hulverson M.A., Ranade R.M., Creason S.A., Herbst Z.M., Gelb M.H., Buckner F.S., Tidwell R.R. Urea derivatives of 2-aryl-benzothiazol-5-amines: a new class of potential drugs for human African trypanosomiasis. J. Med. Chem. 2017;60:957–971. doi: 10.1021/acs.jmedchem.6b01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritesh P., Pillai J., Darji N., PATEL B. Recent advance in anti inflammatory activity of benzothiazole derivatives. Int. J. Drug Res. Technol. 2009;2:170–176. [Google Scholar]
- Sahu P.K., Sahu P.K., Samadhiya P., Sahu P.L., Agarwal D.D. POM analyses and evaluation of in vitro antimicrobial, antitumor activity of 4H-pyrimido 2,1-b benzothiazole derivatives. Med. Chem. Res. 2016;25:1551–1563. [Google Scholar]
- Schultz, B.M., Paduro, C.A., Salazar, G.A., Salazar-Echegarai, F.J., Sebastian, V.P., Riedel, C.A., Kalergis, A.M., Alvarez-Lobos, M., Bueno, S.M., 2017. A potential role of salmonella infection in the onset of inflammatory bowel diseases. Front. Immunol. 8. [DOI] [PMC free article] [PubMed]
- Sharma P.C., Bansal K.K., Deep A., Pathak M. Benzothiazole derivatives as potential anti-infective agents. Curr. Topics Med. Chem. 2017;17:208–237. doi: 10.2174/1568026616666160530152546. [DOI] [PubMed] [Google Scholar]
- Soliman G.A., Donia Ael R., Awaad A., Alqasoumi S.I., Yusufoglu H. Effect of Emex spinosa, Leptadenia pyrotechnica, Haloxylon salicornicum and Ochradenus baccatus extracts on the reproductive organs of adult male rats. Pharm. Biol. 2012;50:105–112. doi: 10.3109/13880209.2011.601465. [DOI] [PubMed] [Google Scholar]
- Venkatesan K., Dhivya S., Narasimhan S. Synthesis and characterization of N-9-fluorenyl amines and its molecular docking for reversal of multidrug resistance in cancer. Drug Invent. Today. 2012;4:494–496. [Google Scholar]