Abstract
Orally disintegrating tablets and oral lyophilisates are novel attractive dosage forms that disintegrate or dissolve in the buccal cavity within seconds without necessity of drinking. The major limitation in designing of these dosage forms is unpleasant taste of the drug substance. Cetirizine dihydrochloride is a H1-antihistamine substance indicated for the treatment of allergy. It is characterized by extremely bitter taste, therefore in order to deliver cetirizine dihydrochloride using orodispersible formulations, effective taste-masking is required. The aim of this study was to investigate whether microparticles containing cetirizine dihydrochloride could be successfully used to formulate orally disintegrating tablets by direct compression method and oral lyophilisates by freeze-drying process. Taste masking of cetirizine dihydrochloride was achieved by the spray-drying technique using Eudragit® E PO as the drug agent carrier. Based on the preliminary studies, optimal compositions of microparticles, tablets and lyophilisates were chosen. Obtained dosage forms were characterized for drug content, disintegration time and mechanical properties. In order to determine whether the microparticles subjected to direct compression and freeze-drying process effectively mask the bitter taste of cetirizine dihydrochloride, the in vivo and in vitro evaluation was performed. The results showed that designed formulates with microparticles containing cetirizine dihydrochloride were characterized by appropriate mechanical properties, uniformity of weight and thickness, short disintegration time, and the uniform content of the drug substance. Taste-masking assessment performed by three independent methods (e-tongue evaluation, human test panel and the in vitro drug release) revealed that microparticles with Eudragit® E PO are effective taste – masking carriers of cetirizine dihydrochloride and might be used to formulate orally disintegrating tablets and oral lyophilisates.
Keywords: Orally disintegrating tablets, Oral lyophilisates, Cetirizine dihydrochloride, Taste masking, E-tongue
1. Introduction
Unacceptable taste is an important problem encountered in the designing of orodispersible dosage forms which disintegrate or dissolve in the patient’s oral cavity and drug has direct contact with the taste buds. In pharmaceutical applications, a wide variety of techniques is available for taste-masking of bitter drugs. The simplest and most common approach is using flavors or sweeteners (Hannan et al., 2016). However, their efficacy is limited in the case of very bitter or highly water-soluble drugs administered at high doses, and therefore this method is often used with other more advanced taste-masking techniques like: complexation, coating or granulation with hydrophilic polymers, melting and liquid extrusion and ion-exchange resins (Badgujar and Mundada, 2011, Pein et al., 2014, Kaushik and Dureja, 2014, Fu et al., 2004). One of the frequently used and the most effective taste masking method is microencapsulation by the spray-drying process, in which polymer barrier between bitter drug and the taste buds is creating (Szakonyi and Zelkó, 2012, Rogers and Wallick, 2012).
Cetirizine dihydrochloride (CET) is a second-generation antihistaminic drug indicated for the treatment of allergies. CET is an extremely bitter drug requiring effective taste-masking, therefore it was chosen as a model drug (Drug bank Version 5.0, xxxx, Chen, 2008).
In order to develop taste masked CET microparticles, preliminary studies have been done. Microparticles were obtained by the spray drying method with CET and Eudragit® E PO as a barrier coating. Eudragit® E PO is poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate 1:2:1 copolymer (Nikam et al., 2011, Rogers and Wallick, 2012). Effective taste masking barrier was created with CET:polymer ratio (0.5:1) (Amelian et al., 2017) and this formulation was used for designing orally disintegrating dosage forms in this study.
Selection of excipients and their amounts depends on the properties of the active substance, target mass of the formulation and technological process used (Badgujar and Mundada, 2011). In order to develop the composition of orally disintegrating tablets via method of direct compression, placebo tablets with various multifunctional mixtures and various disintegrants were prepared. A number of preliminary studies were performed and formulation characterized by sufficient mechanical properties and short disintegration time was selected to obtain tablets with taste-masked CET microparticles (Amelian and Winnicka, 2012). The excipients used in the formulation of lyophilisates are binders (e.g. gelatin, sodium alginate, hypromellose) constituting the skeleton of the lyophilisate and providing appropriate hardness, and fillers (sugars and sugar alcohols, e.g. mannitol, sorbitol, glucose) (Ohrem et al., 2014). The choice of the optimal composition of oral lyophilisate was preceded by numerous studies in which the effect of various excipients on lyophilisates quality was examined. Lyophilisates were formulated by freeze-drying process using different concentration of gelatin, mannitol and sodium bicarbonate and formulation characterized by the most favorable physicochemical properties was selected.
The purpose of the present study was to prepare orally disintegrating tablets and oral lyophilisates containing taste masked microparticles with CET. Designed formulations were examined in terms of mechanical properties, and uniformity of weight and thickness. Morphology of formulations was determined by scanning electron microscopy. Disintegration time and sensory evaluation of the tablets and lyophilisates were measured in vivo and in vitro. The primary method for the taste measurement is human sensory evaluation. Taste-sensing by human taste panel has some difficulties due to high variability and subjectivity, therefore particularly important aspect of this work was the evaluation of taste masking efficacy by a multichannel taste detector (e-tongue), which is promising and useful tool to evaluate pharmaceutical formulations (Ahn et al., 2016, Nakamura et al., 2015, Latha and Lakshmi, 2012).
2. Materials and methods
2.1. Materials
Ludiflash® and crospovidone (Kollidon® CL-SF - superfine) were obtained from BASF, Ludwigshafen, Germany. Magnesium stearate was a product of POCH, Piekary Śląskie, Poland. Gelatin (type B), sodium bicarbonate, mannitol and glycerol were purchased from Sigma Aldrich, Steinheim, Germany. Water was distilled and passed through a reverse osmosis system Milli-Q Reagent Water System (Billerica, MA, USA). Polivinyl chloride (PCV) blisters (diameter of 13 mm and a depth of 5 mm) were obtained from Fagron, Kraków, Poland. Cetirizine dihydrochloride (CET) was a gift from ZF Polfa S.A., Warszawa, Poland. Polyvinyl chloride (PVC), plasticizers, lipophilic salts, ionophores were obtained from Fluka (Sigma-Aldrich, Saint Louis, MO, USA).
2.2. Microparticles
Based on the preliminary studies, microparticles formulation with acceptable taste and appropriate release profile was chosen (Amelian et al., 2017). Microparticles were obtained by the spray drying method using Mini Spray Dryer B-290 (Buchi Labortechnik AG, Flawil, Switzerland). CET/polymer ratio of 0.5:1 and 10% Eudragit® E PO was used for microparticles formulation.
2.3. Preparation of orally disintegrating tablets
Orally disintegrating tablets (composition per tablet: 150.8 mg Ludiflash, 5.4 mg Kollicoat® CL-SF, 1.8 mg magnesium stearate and microparticles with CET in the amount corresponding to 10 mg of CET) were prepared by direct compression method using a single punch tablet machine (Type XP1, Korsch, Berlin, Germany) equipped with 8 mm diameter flat-faced punches. In order to set the appropriate parameters of the direct compression, different tensile force values were tested. The tensile force at which no damage of the surface of microparticles was observed, was applied for the process of tableting.
2.4. Preparation of oral lyophilisates
Oral lyophilisates (composition per lyophilisate: 5.0 mg gelatin, 50.0 mg mannitol, 1.25 mg sodium bicarbonate and microparticles with CET in the amount corresponding to 10 mg of CET) were obtained by the freeze-drying method. Gelatin, mannitol and sodium bicarbonate were dissolved in distilled water at the temperature about 40 °C, then microparticles were suspended in the find solution. Obtained suspensions were dosed into PCV blisters, frozen at −20 °C for 10 min and freeze-dried (Lyophilizer FreeZone System, Labconco, Kansas, MO, USA). After number of preliminary tests, the following conditions were chosen: primary drying for 4 h at −48 °C, pressure of 0.08 mbar with gradually increasing the temperature to 20 °C. In the second drying step, the temperature was set to 20 °C for 1 h and vacuum pressure of 0.08 mbar.
2.5. Uniformity of weight and thickness
Weight and thickness of obtained formulations were evaluated according to the pharmacopoeial requirements (European Pharmacopoeia, 2010). Thickness was measured using calibrated digital caliper (Beta 1651 DGT, Milan, Italy). Each formulation was weighed individually using an electronic balance (XA 60/220, Radwag, Radom, Poland).
2.6. Uniformity of drug content
The amount of CET was examined using the HPLC system Agilent Technologies 1200 and Zorbax Eclipse XDB–C18, 4.6 × 150 mm, 5 μm column (Agilent Technologies, Waldbronn, Germany). Data collection and analysis were conducted with Chemstation 6.0 software. Acetonitrile/water solution (40:60, v/v) with addition of 0.1 mol L−1 triethylamine (pH 3.5) was used as the mobile phase. The flow rate was 1.0 mL min−1 and ultraviolet detection was done at 215 nm (Paw et al., 2002, Jelińska et al., 2000). CET retention time was 3.5 min. Standard calibration curve was linear over the range of 1–100 μg/mL with the correlation coefficient R2 = 0.999. The studies were carried out in triplicate.
2.7. Morphology analysis
The morphology analysis of microparticles (used as a control), orally disintegrating tablets and oral lyophilisates was performed using scanning electron microscopy (SEM) (Hitachi S4200, Tokyo, Japan). The samples were secured using double-faced sticky tape and were sputter-coated with gold under vacuum condition before imaging.
2.8. Mechanical characteristics of tablets
The tablets crushing strength was measured using a hardness tester (5Y, Pharmaton AG, Thun, Switzerland) and friability - by friabiator tester (EF-1 W, Electrolab, Mumbai, Indie) (European Pharmacopoeia, 2010).
2.9. Evaluation of disintegration time
2.9.1. In vivo
Disintegration time of tablets and lyophilisates in the oral cavity was evaluated by six healthy volunteers. The study protocol was approved by the Research Ethics Committee at the Medical University of Białystok (number R-J-002/262/2014) and complied with the principles of the Declaration of Helsinki. After the mouth was rinsed with purified water, tablet or lyophilisate was hold in the mouth without chewing until the dosage form disintegrated. The time required for the complete disintegration in the oral cavity was noted.
2.9.2. In conventional disintegration apparatus
Disintegration time of formulations was measured using pharmacopoeial apparatus (Erweka ED-2L, Heusenstamm, Germany) (European Pharmacopoeia, 2010). Distilled water was used as a disintegration medium.
2.10. Evaluation of taste-masking effectiveness
2.10.1. E-tongue evaluation
Sensor array of the electronic tongue was composed of 16 ion-selective electrodes (ISEs) of various cross-selectivity (Table 1) (Wesoły et al., 2017a, Wesoły et al., 2017b). All measurements were carried out in cells of the following type: Ag, AgCl; KCl 3 M|CH3COOLi 1 M|sample solution||membrane||internal solution; AgCl, Ag. Potentiometric multiplexer (EMF 16 Interface, Lawson Labs Inc., Malvern, PA, USA) was used for electromotive force (EMF) measurements. All calculations and data analysis was performed in MatLab (The MathWorks, Inc., Natick, MA, USA) and Origin (Microcal Software, Inc., Northampton, MA, USA) software. Measurements were conducted at 37 °C (Wesoły et al., 2016). The release of CET from the formulations was observed as the difference in sensor signals in time. These signals were also subjected to multivariate analysis to visualize release modifications related to the CET microparticles.
Table 1.
Sensor array of the electronic tongue.
| Electrode type | Cross - selectivity | Plasticizer | Lipophilic salt | Ionophore | Internal filling/conditioning solution |
|---|---|---|---|---|---|
| CS-D | Cation -selective | 66 w/wDOS | 1 w/w KTFPB | – | 0.01/0.001 M NaCl |
| CS-N | 66 w/wo-NPOE | 1 w/w KTFPB | – | ||
| AS-D | Anion - selective | 65 w/wDOS | 3 w/w TDMAC | – | |
| AS-N | 65 w/wo-NPOE | 3 w/w TDMAC | – | ||
| PS-D | 65 w/wDOS | 2 w/w TBHDPB | – | ||
| PS-N | 65 w/wo-NPOE | 2 w/w TBHDPB | – | ||
| CARB-B | Carbonate/carboxy – selective | 64 w/wDOA | 1 w/w TDMAC | 2 w/w carbonate ionophore VII | 0.1 M NaH2PO4, 0.1 M Na2HPO4, 0.01 M NaCl/0.01 M NaH2PO4, 0.01 M Na2HPO4, 0.001 M NaCl |
| AM-D | Amine - selective | 68 w/wDOS | – | 5 w/w amine inophore I | 0.01/0.001 M KCl |
DOA – bis(2-ethylhexyl) adipate.
DOS – bis(2-ethylhexyl) sebacate.
KTFPB – potassium tetrakis [3,5-bis-(trifluoromethyl)phenyl]borate.
o-NPOE – 2-nitrophenyl octyl ether.
PVC – polyvinyl chloride.
TBHDPB – tributylhexadecylphosphonium bromide.
TDMAC – tridodecylmethylammonium chloride.
2.10.2. Human taste panel
Sensory evaluation of roughness and taste of formulations was carried out by six healthy volunteers (Research Ethics Committee at Medical University of Białystok approval number R-J-002/262/2014). Before the test, potential probants were tested by the sensitivity of taste. For this reason, basic taste solutions of different substances: sour (tartaric acid), sweet (sucrose), salty (sodium chloride) and bitter (quinine hydrochloride) in distilled water with different concentration were prepared (Wesoły et al., 2017b, Amelian et al., 2017). For further studies, volunteers with the highest taste sensitivity were selected. A numerical scale was used with the following values: 0 - pleasant/not rough; 1 - slightly pleasant/slightly rough; and 2 - unpleasant/rough.
2.10.3. In vitro drug release
To determine CET release profiles, a USP dissolution apparatus type II (Erweka GmbH, Heusenstamm, Germany) was used. In vitro drug release was obtained from orally disintegrating tablets, oral lyophilisates and microparticles (in the amount corresponding to 10 mg of CET). Formulations were suspended in 750 mL of phosphate buffer (pH 6.8) and stirred at 75 rpm at 37 ± 1 °C. As CET is freely soluble in water, the sink conditions were achieved during the dissolution test. At predetermined time intervals, samples were withdrawn and replaced with fresh dissolution medium. Concentration of CET was analyzed using the HPLC method as described in the point HPLC analysis. Each formulation was analyzed in triplicate.
2.11. Statistical analysis
All data are presented as mean ± standard deviation. A probability level of p < 0.05 was considered to be statistically significant.
3. Results and discussion
The physical evaluation of designed orally disintegrating dosage forms revealed a uniform thickness and weight for all the formulations. Thickness of lyophilisates and orally disintegrating tablets were about 2 mm and 4.0 mm, respectively. Thickness uniformity is extremely important as it directly influences drug dosage accuracy. CET loading was uniform in all formulations (for orally disintegrating tablets it ranged between 9.7 and 10.4 mg, and for lyophilisates - between 8.78 and 10.65 mg).
The short disintegration time and appropriate mechanical strength are crucial factors in orodispersible formulations. Friability and hardness tests indicate if tablets possess suitable mechanical resistance to avoid crumbling or breaking during the manufacturing process or subsequent packing. Mechanical properties of tablets mainly depend on the set parameters during compression (Hannan et al., 2016), therefore the influence of tensile strength and lower punch ejection force on the tablets quality was evaluated (Table 2). Disintegration time, hardness and friability are directly connected with tensile force values (Ishino et al., 1990). Friability below 1%, sufficient hardness and disintegration time about 30 s were achieved using upper punch tensile force of 3.7 kN. Interestingly, tablets prepared using higher tensile forces (6.5 and 7.2 kN) showed higher friability than tablets prepared with tensile forces between 3.7 and 6.0 kN. Properly selected value of tensile force is especially significant in case of microparticles compression. High tensile force is the cause of cracking of microparticles. Obtained data have shown that microparticles were cracked using 7.2 kN tensile force for upper punch (Fig. 1). Formulations obtained using tensile forces in the range 4.2–6.5 kN possessed appropriate hardness (61.0–75.1 kN) and friability (0.38–0.80%), but were characterized by longer disintegration times. Tablets formulation T6 with appropriate mechanical properties (hardness 56.9 N, friability 0.59%) and with short disintegration time (about 30 s) was obtained using 3.7 kN tensile force for upper punch and 1.9 kN for lower punch. Disintegration time of oral lyophilisates was about 10 s.
Table 2.
The influence of tensile force on characteristics of obtained orally disintegrating tablets.
| Formulation | Tensile force (kN) |
Hardness (N) | Friability (%) | Disintegration time (s) |
||
|---|---|---|---|---|---|---|
| Upper punch | Lower punch | In vivo | In vitro | |||
| T1 | 1.2 | 1.0 | 21.0 ± 1.2 | 3.23 | 10 | 9 |
| T2 | 1.5 | 1.1 | 25.2 ± 1.3 | 2.41 | 11 | 9 |
| T3 | 2.0 | 1.2 | 30.3 ± 2.3 | 1.52 | 18 | 15 |
| T4 | 2.5 | 1.5 | 35.4 ± 1.5 | 1.81 | 25 | 20 |
| T5 | 3.0 | 1.7 | 40.0 ± 1.3 | 1.12 | 34 | 28 |
| T6 | 3.7 | 1.9 | 56.9 ± 2.5 | 0.59 | 30 | 31 |
| T7 | 4.2 | 2.4 | 61.0 ± 1.5 | 0.38 | 40 | 36 |
| T8 | 6.0 | 5.5 | 60.0 ± 1.5 | 0.48 | 45 | 41 |
| T9 | 6.5 | 5.7 | 75.1 ± 2.4 | 0.80 | 67 | 64 |
| T10 | 7.2 | 6.8 | 79.0 ± 4.6 | 0.92 | 78 | 60 |
Fig. 1.
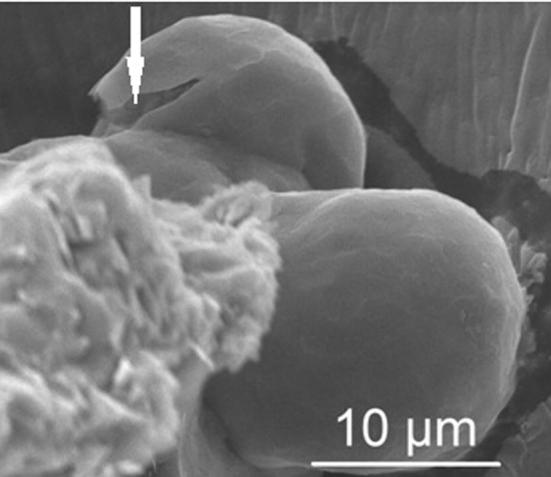
SEM image of tablet obtained by direct compression using 7.2 kN tensile force (x 10 000; arrow indicates cracked surface of microparticle).
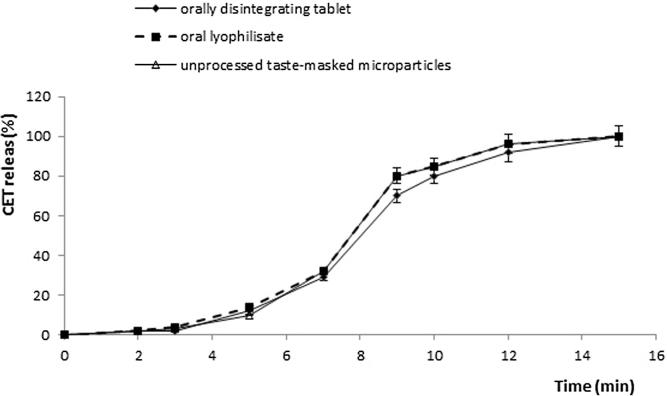
To design orodispersible formulations, spherical microparticles with smooth surface were used (Fig. 2a and b). In the SEM analysis of tablets and lyophilisates, not distorted microparticles and their aggregates were observed (Fig. 2c and d). Intact structure of microparticles was also confirmed by the CET release profiles obtained from unprocessed microparticles (used as a control) and from designed tablets and lyophilisates (Fig. 3).
Fig. 2.
SEM images of unprocessed microparticles with CET (a) x 10000, (b) x 20000, orally disintegrating tablet (formulation T6) obtained by direct compression method using 3.7 kN tensile force (c) and oral lyophilisate (d) x 20000 (arrows indicate microparticles in the tablet or lyophilisate matrix).
Fig. 3.
In vitro CET release from unprocessed taste-masked microparticles used as a control, orally disintegrating tablets (formulation T6) and oral lyophilisates in phosphate buffer (pH 6.8).
In orodispersible dosage forms, extremely important parameter is acceptable taste of the drug. For taste-masking of CET, Preis et al. (2015) and Labib (2015) successfully applied β-cyclodextrin and cherry/sucralose flavor or mannitol addition. Also Stojanov et al., 2011, Ono et al., 2011 and Mahesh et al. (2010) reported that bitter taste of CET was masked using complexion with cyclodextrin. In this study, effectiveness of CET taste masking by using Eudragit® E PO microparticles was measured using e-tongue, human taste panel and the in vitro release profile test.
For the acquisition of the sensors signals in the e-tongue, pharmaceutical formulations containing CET: microparticles, lyophilisates with pure CET, lyophilisates containing microparticles with CET as well as orally disintegrating tablets with unmodified CET and with microparticles containing CET were used. Pure CET and respective placebos were also analyzed as control samples (Table 3). Chemical images of the investigated samples were obtained using the differences of sensors responses observed after 60 s of the release process. Each sample was analyzed in triplicate and each was characterized by 16 variables (16 ISEs outputs). The data were processed with the use of PCA (Principal Component Analysis), prior to which autoscaling was applied. Chemical images of the studied samples were presented in two dimensional PCA plots in Fig. 4. Chemical images of the pure CET, microparticles with CET and placebo (microparticles without CET) are shown in Fig. 4a. Additionally, another models were build for the liophilisates and orally disintegrating tablets formulations - obtained results are presented in Fig. 4b and c, respectively. Chemical image of M-CET exhibits intermediate PC1 value in comparison to pure CET and placebo, which can be interpreted as possessing intermediate properties comparing to pure cetirizine and pure coating agent (Fig. 4a). However, calculated Euclidean distance in PCA space between chemical images of M-CET and M-placebo was significantly smaller than distance between M-CET and pure CET (4.4 and 6.6 in PC arbitrary units, respectively). Therefore, chemical images of M-CET were closer to M-placebo than to pure CET, especially in terms of PC1 (the first principal component captures more than 80% of the variance of the whole dataset). It must be underlined, that after 60 s of the release process, chemical image of M-CET is more similar to placebo than to pure CET, which proves appropriate taste masking obtained in the case of this formulation. In the next step, the pattern of sensor responses for lyophilisates samples was analyzed. Separable and distinct clusters are visible on PCA plot (Fig. 4b). The largest difference between chemical images obtained by the electronic tongue can be observed between pure CET and both placebos M-placebo and L-placebo, which are placed close to each other on PCA plot and exhibit the highest value of PC1. This shows that PC1 reveals mainly the presence of the drug in analyzed solution. Both formulations: M-CET and L-M-CET exhibit chemical images more similar to respective placebos than to pure CET – they are closer to placebos than to CET, in terms of PCA distance and PC1 value. This may indicate that decreased release of CET from such formulations was observed due to taste masking by using Eudragit® E PO microparticles. The distance between chemical images of L-CET and pure CET was 5.8 (Euclidean distance in PC1-PC2 space), which is similar to distance between microparticles and pure CET. However, L-CET samples are placed on the opposite direction than placebo, therefore they can be regarded as the most similar to pure CET and the most distinguishable from placebo. The similarity of L-M-CET to its placebo is higher than similarity of M-CET to its placebo (smaller differences in PC1 values), therefore lyophilisation process influences releasing of CET from the microparticles. Moreover, for lyophilisates with pure CET, taste masking is poorly observed (the highest similarity of L-CET to pure CET). The last step was the investigation of sensor responses for orally disintegrating tablets. Similar arrangement of the clusters on PCA plot obtained for orally disintegrating tablets samples and respective microparticles was noticed (see Fig. 4c). Chemical images of microparticles with CET samples (M-CET) and orally disintegrating tablets containing microparticles with CET (O-M-CET) are closer to respective placebos than to pure CET, both in terms of PCA distance and PC1 value. Therefore, after 60 s of releasing process, both formulations with microparticles reveal higher similarity to placebo than to pure CET, which indicates efficient taste masking. Moreover, since chemical images of O-CET have the largest distance to both: CET and O-placebo, tabletting courses insufficient taste masking of pure CET - similarly to lyophilisates case discussed above.
Table 3.
Pharmaceutical formulations of CET used in e-tongue evaluation.
| Type of pharmaceutical formulation | Presence of CET | Abbreviation |
|---|---|---|
| Powder | Pure CET | CET |
| Microparticles | CET with Eudragit® E PO | M-CET |
| Placebo (microparticles of Eudragit® E PO without CET) | M-placebo | |
| Lyophilisates | Lyophilisates with unmodified CET | L-CET |
| Lyophilisates with microparticles containing CET | L-M-CET | |
| Placebo (lyophilisates without CET) | L-placebo | |
| Orally disintegrating tablets | Orally disintegrating tablets with unmodified CET | O-CET |
| Orally disintegrating tablets with microparticles containing CET | O-M-CET | |
| Placebo (orally disintegrating tablets without CET) | O-placebo |
Fig. 4.
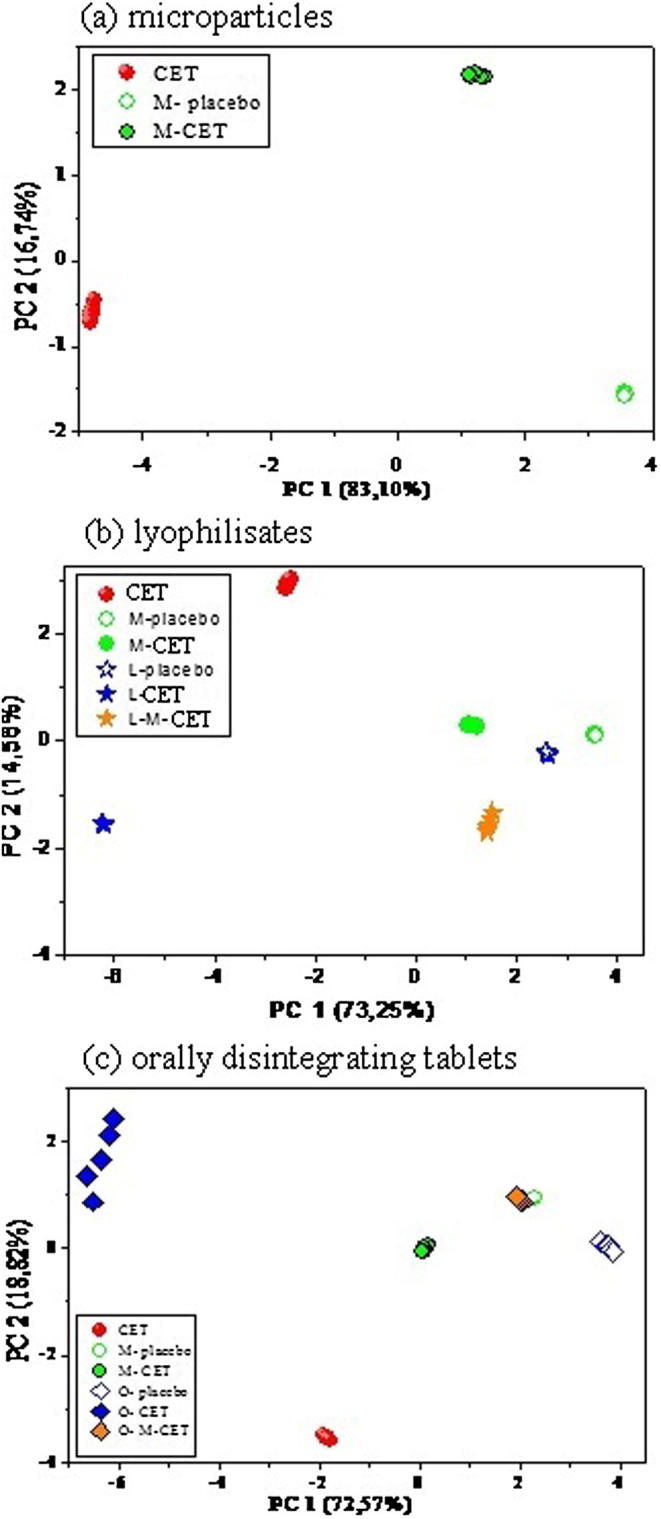
Two dimensional principal component analysis score plots of chemical images of CET formulations (description of samples according to Table 3).
Taste masking efficiency was also studied in the in vitro release test carried out in phosphate buffer pH 6.8 (corresponding to saliva pH). CET release profiles from microparticles formulation, tablets and lyophilisates are shown in Fig. 3. In all formulations, no CET release was observed up to 3 min and 100% of CET was released after about 15 min. The next study, to which all formulations were subjected was the in vivo sensory analysis by six volunteers. The characteristic component of orally disintegrating tablets and lyophilisates is mannitol, which in addition to sweetening properties, upon dissolution in the oral cavity, provides cooling effect which beneficially affects the sensory sensation (Ohrem et al., 2014, Al-khattawi and Mohammed, 2013). Obtained results from human taste panel have shown that excipients used to formulating tablets and lyophilisates improved palatability. All volunteers assessed designed formulations as pleasant or slightly pleasant tasting, and no roughness was reported.
The present study suggests that microparticles with Eudragit® E PO obtained by the spray-drying technique are effective CET taste – masking carriers and might be successfully used to formulate orally disintegrating tablets by direct compression and oral lyophilisates by freeze drying method. E-tongue as the multifunctional taste sensing device can provide useful information for the development of orodispersible formulations with acceptable palatability. Obtained data confirmed effective masking of bitter CET taste by microencapsulation and the possibility of formulating designed microparticles into orodispersible dosage forms characterized by very short disintegration time and good physico-chemical properties.
Acknowledgment
This work has been supported from the financial resources for science in years 2013 – 2017 as a research project within a framework of “Diamond Grant” programme (DI 2012 0019 42).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Aleksandra Amelian, Email: aleksandra.amelian@umb.edu.pl.
Katarzyna Wasilewska, Email: katarzyna.wasilewska@umb.edu.pl.
Małgorzata Wesoły, Email: mwesoly@ch.pw.edu.pl.
Patrycja Ciosek-Skibińska, Email: pciosek@ch.pw.edu.pl.
Katarzyna Winnicka, Email: kwin@umb.edu.pl.
References
- Ahn S.R., An J.H., Song H.S., Park J.W., Lee S.H., Kim J.H., Jang J., Park T.H. Duplex bioelectronic tongue for sensing umami and sweet tastes based on human taste receptor nanovesicles. ACS Nano. 2016;8:7287–7296. doi: 10.1021/acsnano.6b02547. [DOI] [PubMed] [Google Scholar]
- Al-khattawi A., Mohammed A.R. Compressed orally disintegrating tablets: excipients evolution and formulation strategies. Expert Opin. Drug Deliv. 2013;5:651–663. doi: 10.1517/17425247.2013.769955. [DOI] [PubMed] [Google Scholar]
- Amelian A., Szekalska M., Ciosek P., Basa A., Winnicka K. Characterization and taste masking evaluation of microparticles with cetirizine dihydrochloride and methacrylate-based copolymer obtained by spray drying. Acta Pharm. 2017;67 doi: 10.1515/acph-2017-0002. [DOI] [PubMed] [Google Scholar]
- Amelian A., Winnicka K. Effect of the type of disintegrant on the characteristics of orally disintegrating tablets manufactured using new ready-to-use excipients (Ludiflash® or Parteck®) by direct compression method. African J. Pharmacy Pharmacol. 2012;31:2359–2367. [Google Scholar]
- Badgujar B.P., Mundada A.S. The technologies used for developing orally disintegrating tablets: a review. Acta Pharm. 2011;2:117–139. doi: 10.2478/v10007-011-0020-8. [DOI] [PubMed] [Google Scholar]
- Chen C. Physicochemical, pharmacological and pharmacokinetic properties of the zwitterionic antihistamines cetirizine and levocetirizine. Curr. Med. Chem. 2008;21:2173–2191. doi: 10.2174/092986708785747625. [DOI] [PubMed] [Google Scholar]
- Drug bank Version 5.0, http://www.drugbank.ca/drugs/DB00341 (accessed 10.02.17).
- European Phamacopoeia, 10th edition, Council of Europe, Strasbourg, 2010.
- Fu Y., Yang S., Jeong S.H., Kimura S., Park K. Orally fast disintegrating tablets: developments, technologies, taste - masking and clinical studies. Crit. Rev. Ther. Drug Carrier Syst. 2004;6:433–476. doi: 10.1615/critrevtherdrugcarriersyst.v21.i6.10. [DOI] [PubMed] [Google Scholar]
- Hannan P.A., Khan J.A., Khan A., Safiullah S. Oral dispersible system: a new approach in drug delivery system. Indian J. Pharm. Sci. 2016;1:2–7. doi: 10.4103/0250-474x.180244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino R., Yoshino H., Hirakawa Y., Noda K. Influence of tabletting speed on compactibility and compressibility of two direct compressible powders under high speed compression. Chem. Pharm. Bull. 1990;7:1987–1992. doi: 10.1248/cpb.38.1987. [DOI] [PubMed] [Google Scholar]
- Jelińska A., Stanisz B., Zając M., Musiał W., Ostrowicz A. Determination of cetirizine dichloride in tablets by HPLC method. Acta Pol. Pharm. 2000;3:171–173. [PubMed] [Google Scholar]
- Kaushik D., Dureja H. Recent patents and patented technology platforms for pharmaceutical taste masking. Recent Pat. Drug Deliv. Formul. 2014;1:37–45. doi: 10.2174/1872211308666140206150840. [DOI] [PubMed] [Google Scholar]
- Labib G.S. Novel levocetirizine HCl tablets with enhanced palatability: synergistic effect of combining taste modifiers and effervescence technique. Drug Des. Devel. Ther. 2015;9:5135–5146. doi: 10.2147/DDDT.S92245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latha R.S., Lakshmi P.K. Electronic tongue: an analytical gustatory tool. J. Adv. Pharm. Technol. Res. 2012;1:3–8. doi: 10.4103/2231-4040.93556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahesh A., Shastri N., Sadanandam M. Development of taste masked fast disintegrating films of levocetirizine dihydrochloride for oral use. Curr. Drug Deliv. 2010;1:21–27. doi: 10.2174/156720110790396454. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Uchida S., Sugiura T., Namiki N. The prediction of the palatability of orally disintegrating tablets by an electronic gustatory system. Int. J. Pharm. 2015;1–2:305–312. doi: 10.1016/j.ijpharm.2015.07.056. [DOI] [PubMed] [Google Scholar]
- Nikam V.K., Kotade K.B., Gaware V.M., Dolas R.T. Eudragit a versatile polymer: A review. Pharmacol Online. 2011;1:152–164. [Google Scholar]
- Ohrem H.L., Schornick E., Kalivoda A., Ognibene R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm. Dev. Technol. 2014;3:257–262. doi: 10.3109/10837450.2013.775154. [DOI] [PubMed] [Google Scholar]
- Ono N., Miyamoto Y., Ishiguro T., Motoyama K., Hirayama F., Iohara D., Seo H., Tsuruta S., Arima H., Uekama K. Reduction of bitterness of antihistaminic drugs by complexation with β-cyclodextrins. J. Pharm. Sci. 2011;5:1935–1943. doi: 10.1002/jps.22417. [DOI] [PubMed] [Google Scholar]
- Paw B., Misztal G., Hopkała H., Drozd J. Development and validation of a HPLC method for the determination of cetirizine in pharmaceutical dosage forms. Pharmazie. 2002;5:313–315. [PubMed] [Google Scholar]
- Pein M., Preis M., Eckert C., Kiene F.E. Taste-masking assessment of solid oral dosage forms a critical review. Int J. Pharm. 2014;1–2:239–254. doi: 10.1016/j.ijpharm.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Preis M., Grother L., Axe P., Breitkreutz J. In-vitro and in-vivo evaluation of taste-masked cetirizine hydrochloride formulated in oral lyophilisates. Int. J. Pharm. 2015;1–2:8–16. doi: 10.1016/j.ijpharm.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Rogers T.L., Wallick D. Reviewing the use of ethylcellulose, methylcellulose and hypromellose in microencapsulation. Part 3: Applications for microcapsules. Drug Dev. Ind. Pharm. 2012;5:521–539. doi: 10.3109/03639045.2011.616512. [DOI] [PubMed] [Google Scholar]
- Stojanov M., Wimmer R., Larsen K.L. Study of the inclusion complexes formed between cetirizine and α-, β-, and γ-cyclodextrin and evaluation on their taste-masking properties. J. Pharm. Sci. 2011;8:3177–3185. doi: 10.1002/jps.22539. [DOI] [PubMed] [Google Scholar]
- Szakonyi G., Zelkó R. Taste-masking possibilities in solid dosage forms. Acta. Pharm. Hung. 2012;2:81–90. [PubMed] [Google Scholar]
- Wesoły M., Cal K., Ciosek P., Wróblewski W. Influence of dissolution-modifying excipients in various pharmaceutical formulations on electronic tongue results. Talanta. 2017;162:203–209. doi: 10.1016/j.talanta.2016.10.018. [DOI] [PubMed] [Google Scholar]
- Wesoły M., Kluk A., Sznitowska M., Ciosek P., Wróblewski W. Influence of experimental conditions on electronic tongue results - case of valsartan minitablets dissolution. Sensors. 2016;9:E1353. doi: 10.3390/s16091353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesoły M., Zabadaj M., Amelian A., Winnicka K., Wróblewski W., Ciosek P. Tasting cetirizine-based microspheres with an electronic tongue. Sens. Actuators B: Chem. 2017;238:1190–1198. [Google Scholar]