Abstract
Objective
To evaluate the association between the level of vitamin D and glycemic control among patients with diabetes.
Research design and method
We analyzed data collected from NHANES 2003–2006. We included only non-pregnant adult diabetic persons 18 years or older. Participants who had vitamin D level less than 20 ng/ml were considered as having vitamin D deficiency. Participants were considered to have a glucose control if the HbA1c level was less than 7% [53 mmol/L]. We used student’s t test to compare the difference in HbA1c means between people with Diabetes with and without a vitamin D deficiency. We used a multivariate logistic regression model to predict the relationship between glucose control and vitamin D deficiency. We used race/ethnicity, BMI, age, gender, type of diabetic medication used, having health insurance or not, and comorbid conditions (hypertension, anemia, cholesterol, liver disease, and kidney disease) as control variables.
Results
The study population included a total of 929 non-institutionalized, non-pregnant, diabetic adult persons. About 57% of patients with diabetes had a vitamin D deficiency. Blacks (non-Hispanic patients) with diabetes had the highest rate of vitamin D deficiency (79%). The unadjusted means of HbA1c were significantly different between diabetic patients with no vitamin D deficiency and those with a vitamin D deficiency (7.06% [54 mmol/L], 7.56 % [59 mmol/L], respectively, P < 0.0001). Multivariate adjustment showed a small but not significant, increase in odds (11%) of having uncontrolled diabetes in patients with a vitamin D deficiency after adjustment for other factors.
Conclusion
Vitamin D deficiency is very common in patients with diabetes. We found no significant association between vitamin D level and glycemic control in patients with diabetes after adjustment for control variables.
Keywords: Vitamin D deficiency, Diabetes, Glucose control, HbA1c
1. Introduction
Vitamin D has many roles in the regulation of the mineral homeostasis as well as other non-skeletal functions. Of these roles, increasing insulin secretion and insulin sensitivity (Sung et al., 2012). Studies have shown that a low serum level of vitamin D increases the risk of developing diabetes (Afzal et al., 2013, Schöttker et al., 2013, Tsur et al., 2013). Other studies have found that vitamin D deficiency is associated with complications of diabetes such as neuropathy and retinopathy (Patrick et al., 2012). However, little is known about the strength of the association between Vitamin D levels and glucose control.
Only a few studies have examined the association between vitamin D levels and diabetic control. A study conducted in Iran evaluated the effect of vitamin D on insulin resistance in patients with Type 2 diabetes. Researchers found that raising the level of vitamin D improved the fasting plasma glucose and reduced insulin resistance in these patients (Talaei et al., 2013). Another study that was conducted in Saudi Arabia found that vitamin D supplementation significantly improved their insulin resistance and lipid profile (Al-Daghri et al., 2012). A couple of studies used HbA1c as an outcome, which might had a better estimation of diabetes control over fasting blood glucose (FBG) (Jorde and Figenschau, 2009, Ljunghall et al., 1987, Mohamad et al., 2016). However, no studies were found to determine the association between vitamin D and glycemic control in diabetic patients in the U.S.
Based on the Institute of Medicine (IOM), there are four categories of vitamin D status: (1) risk of deficiency: if the level of serum 25-hydroxyvitamin D (25OHD) is less than 12 ng/ml, (2) risk of inadequacy: if the level of 25OHD is between 12 to less than 20 ng/ml, (3) sufficiency: if the level of 25OHD is between 20 to 50 ng/ml, (4) and possibly harmful: if the level of 25OHD is more than 50 ng/ml (Ross et al., 2011). In 2011, a report claimed that about 32% of United States population had a level of vitamin D of less than sufficiency (Looker et al., 2011).
The purpose of this retrospective cross-sectional study was to examine the relationship between levels of vitamin D and diabetes control among patients with diabetes (both Type 1 and 2 but not gestational) drawn from National Health and Nutrition Examination Survey (NHANES) 2003–2006. We also explored the association between HbA1c levels and serum vitamin D status (deficiency or non-deficiency) in patients with diabetes in the U.S.
Patients with diabetes are more susceptible to have serious health complications such as cerebrovascular disease, retinopathy, coronary heart disease, nephropathy and neuropathy. From this study we will be able to know the effect of vitamin D level on the HbA1c that may help to reduce the complications of diabetes. Moreover, the study will describe the vitamin D status among the patients with diabetes.
2. Materials and methods
2.1. Design
Secondary database analysis using data collected in NHANES that used a cross-sectional design.
2.2. Data source
NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the United States. It is implemented by the US National Center for Health Statistics, part of the Centers for Disease Control and Prevention. NHANES uses a multistage stratified sampling design to collect data from the non-institutionalized civilian US population. The survey is unique in that it combines interviews, physical examinations, and laboratory tests.
The analysis sample consists of non-pregnant diabetic persons 18 years or older selected from the NHANES 2003–2006 cross-sections. We used the data from 2003 to 2006 because the measurement technique was changed after 2006 in the dataset, and the vitamin D level was not available at the time of analysis. A participant was excluded if HbA1c or serum vitamin D level data was missing. The participants were defined as having diabetes if they answer yes to the question, “they have ever been told by a doctor or health professional that they have diabetes or sugar diabetes” in the NHANES questionnaire.
2.2.1. Dependent variable
2.2.1.1. HbA1c and diabetes control
According to the American Diabetic Association (ADA), the goal for HbA1c for non-pregnant adults is less than 7% [53 mmol/L]. Therefore, participants who had HbA1c less than 7% [53 mmol/L] were considered to have glucose control (American Diabetes Association, 2017).
2.2.2. Independent variable
2.2.2.1. Serum vitamin D deficiency
From the categories of vitamin D levels, we classified the participants into two groups. The first group consisted of participants with serum vitamin D ≥ 20 ng/ml (non-vitamin D deficiency). The second group consisted of participants with serum vitamin D < 20 ng/ml (vitamin D deficiency).
2.2.3. Control variables
The analysis included the following additional covariates: age (young adults, aged 18–44, middle age adults, ages 45–64, and elderly, 65 years or older), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and other), gender, body mass index (BMI), having health insurance or not, type of diabetic medications used, and co-morbid conditions (i.e., hypertension, anemia, cholesterol, liver disease, and kidney disease). Diabetic patients were characterized into four groups based on Body Mass Index (i.e., underweight if the BMI was <18.5, normal weight if the BMI was between 18.5 and 24.9, overweight if the BMI was between 25 and 29.9, and obese if the BMI was ≥30). In regard to the type of diabetic medications, the precipitants were also classified into one of four groups (i.e., not using medications, using insulin only, using oral medication only, or using both insulin and oral medications).
2.3. Statistical analysis
Statistical analysis was performed using STATA® 11.0 statistical package. Data were weighted to represent the U.S. non-pregnant diabetic adults aged ≥18 years. Descriptive analyses were conducted to characterize the participant and to examine demographic differences between patients by Vitamin D deficiency category. Student’s t test was used to compare the difference in HbA1c means between diabetic patients with and without a vitamin D deficiency. A multivariate logistic regression model was used to predict the relationship between glucose control (HbA1c <7% [53 mmol/L]) and vitamin D deficiency. Adjusted odds ratios, 95% confidence intervals were used to present the results, and the significance was set at P < 0.05.
3. Results
The study population included a total of 929 non-institutionalized, non-pregnant, diabetic adult persons that represent a total of 15,233,753 similar persons in the entire United States. Demographic characteristics and medication use and co-morbidity characteristics of the study sample are presented in Table 1, Table 2, respectively. The participants were mainly of middle to older age, overweight or obese, and roughly have equivalent distribution in gender. The majority used only oral medications for diabetes and had health insurance, and most of the participants reported having comorbidities including hypertension and hypercholesterolemia.
Table 1.
Demographic characteristics of diabetic patients, 2003–2006, U.S.
| Characteristic | Number (%) |
|---|---|
| Gender | |
| Male | 465 (50.1) |
| Female | 464 (49.9) |
| Age | |
| Young adults (18–44 year) | 104 (11.2) |
| Middle age adults (45–64 | 368 (39.6) |
| Elderly (≥65 year) | 457 (49.2) |
| Race/ethnicity | |
| Hispanic | 264 (28.4) |
| Non-Hispanic White | 382 (41.1) |
| Non-Hispanic Black | 246 (26.5) |
| Other | 37 (4.0) |
| Body mass index | |
| Under weight | 4 (0.4) |
| Normal weight | 141 (15.2) |
| Over weight | 285 (30.7) |
| Obese | 499 (53.7) |
Table 2.
Medication use, and co-morbidity characteristics of diabetic patients, 2003–2006, U.S.
| Characteristic | Number (%) |
|---|---|
| Medication use | *(927 responses) |
| Oral medication only | 549 (59.22) |
| Oral medication and insulin | 110 (11.87) |
| Insulin | 132 (14.24) |
| No medication | 136 (14.67) |
| Having health insurance | *(928 Responses) |
| Yes | 825 (88.90) |
| No | 103 (11.10) |
| Having anemia | *(927 Responses) |
| Yes | 58 (6.26) |
| No | 869 (93.74) |
| Having hypertension | *(925 Responses) |
| Yes | 621 (67.14) |
| No | 304 (32.86) |
| Having hypercholesterolemia | *(813 Responses) |
| Yes | 502 (61.75) |
| No | 311 (38.25) |
| Having liver disease | *(923 Responses) |
| Yes | 62 (6.72) |
| No | 861 (93.28) |
| Having kidney disease | *(923 Responses) |
| Yes | 85 (9.2) |
| No | 839 (90.8) |
Some patients did not answer all the questions in the survey.
3.1. Vitamin D deficiency
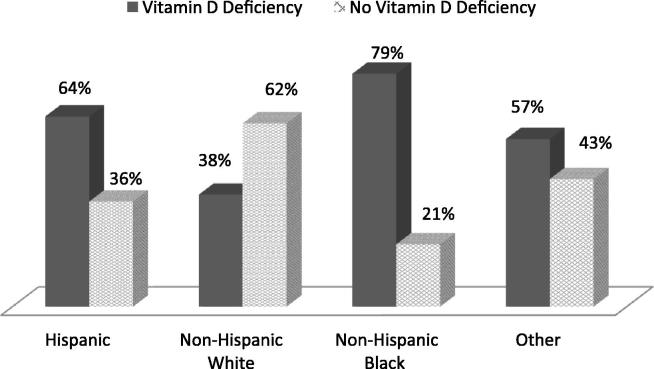
In the U.S. in 2003–2006, we found that more than half (57%) of non-institutionalized, non-pregnant, adults with diabetes had a vitamin D deficiency. The Somewhat more females (279/464 [60%]) than males (251/465 [54%]) with diabetes were deficient in vitamin D. In regard to age, young adults with diabetes (18–44 years) had the highest rate of deficiency (72/104 [69%]), and the vitamin D deficiency in the middle age adults with diabetes (45–64 years) were (217/368 [59%]), while it was 52% (241/457) in elderly patients. Black patients with diabetes had an extremely high rate of vitamin D deficiency at (194/246[79%]). Hispanics with diabetes also had an above average rate of deficiency (170/264[64%]). Whites with diabetes were much less likely than the other groups to be deficient (Fig. 1). In terms of BMI, underweight patients with diabetes had a high rate of deficiency (3/4 [75%]). The vitamin D deficiency in normal weight patients with diabetes were (61/141 [43.26%], while the vitamin D deficiency in overweight patients with diabetes were (145/285 [51%]), and obese patients with diabetes were (321/499 [64.33]).
Fig. 1.
Vitamin D status in diabetic patients in U.S., 2003–2006, by race/ethnicity.
3.2. Association of HbA1c and vitamin D deficiency
The result of student’s t test that was done for 929 patients showed that the means of the HbA1c were significantly different between diabetic patients with no vitamin D deficiency and those with a vitamin D deficiency (7.06% [54 mmol/L], 7.56% [59 mmol/L], respectively, P < 0.0001. Results from multiple regression modeling after adjustment for age, race/ethnicity, gender, BMI, having health insurance or not, type of diabetic medications used, and co-morbid conditions (hypertension, anemia, cholesterol, liver disease, kidney disease) are presented in Table 3. The analysis sample consists of 802 respondents without missing values for all the variables. The result shows that a small but insignificant, increase in odds (11%) of having uncontrolled diabetes exists in patients with a vitamin D deficiency after adjustment for control variables.
Table 3.
Results of the adjusted logistic regression model, for the effect of vitamin D deficiency in diabetic control (n = 802).
| Independent variable | Adjusted odds ratio (95%) | P value |
|---|---|---|
| Vitamin D | ||
| Non-deficient | – | Reference |
| Deficient | 0.891 [CI: 0.58–1.38] | 0.605 |
| Medication | ||
| Oral medication only | – | Reference |
| Oral medication and Insulin | 0.31 [CI: 0.17–0.57] | 0.000* |
| Insulin | 0.30 [CI: 0.17–0.55] | 0.000* |
| No medication | 4.05 [CI: 2.15–7.66] | 0.000* |
| Gender | ||
| Female | – | Reference |
| Male | 0.76 [CI: 0.51–1.14] | 0.184 |
| Race/ethnicity | ||
| Non-Hispanic White | – | Reference |
| Hispanic | 0.43 [CI: 0.24–0.78] | 0.006* |
| Non-Hispanic Black | 0.47 [CI: 0.29–0.74] | 0.001* |
| Other | 0.86 [CI: 0.36–2.04] | 0.735 |
| Age | ||
| Middle age adult (45–64 year) | – | Reference |
| Young adult (18–44 year) | 0.95 [CI: 0.47–1.89] | 0.884 |
| Elderly (≥65 year) | 1.79 [CI: 1.16–2.79] | 0.009* |
| BMI | ||
| Normal weight | – | Reference |
| Under weight | 7.57 [CI: 0.35–162.31] | 0.196 |
| Over weight | 1.51 [CI: 0.80–2.85] | 0.201 |
| Obese | 1.89 [CI: 1.02–3.52] | 0.043 |
| Having health insurance | ||
| No | – | Reference |
| Yes | 1.6 [CI: 0.79–3.26] | 0.192 |
| Having anemia | ||
| No | – | Reference |
| Yes | 1.62 [CI: 0.77–3.40] | 0.203 |
| Having hypertension | ||
| No | – | Reference |
| Yes | 1.26 [CI: 0.79–2.02] | 0.335 |
| Having hypercholesterolemia | ||
| No | – | Reference |
| Yes | 0.87 [CI: 0.58–1.32] | 0.512 |
| Having liver disease | ||
| No | – | Reference |
| Yes | 0.91 [CI: 0.41–2.05] | 0.826 |
| Having kidney disease | ||
| No | – | Reference |
| Yes | 0.65 [CI: 0.34–1.27] | 0.209 |
Statistical significant.
The model showed that the patients with diabetes who do not use medication were more likely to have controlled diabetes than those who were using oral medication (adjusted OR: 4.05 [95% CI 2.15–7.66]), while the patients who were using insulin or insulin and oral medication were less likely to have controlled diabetes compared to the patients who were using oral medication only (adjusted OR: 0.30 [95% CI 0.17–0.55]), (adjusted OR: 0.30 [95% CI 0.17–0.57] respectively).
In regard to race/ethnicity, the results showed that Hispanic and non-Hispanic Black patients with diabetes were less likely to have controlled diabetes than non-Hispanic White patients with diabetes (adjusted OR: 0.43 [95% CI 0.24–0.78]), (adjusted OR: 0.47 [95% CI 0.29–0.74]) respectively. The results also showed that obese patients with diabetes were more likely to have controlled diabetes than those with normal weight (adjusted OR: 1.89 [95% CI 1.02–3.52]). Lastly, the models showed that elderly patients with diabetes were more likely to have controlled diabetes than middle age adult patients with diabetes (adjusted OR: 1.79 [95% CI 1.16–2.79]).
Furthermore, we conducted sub-analysis in Hispanic, non-Hispanic White, and non-Hispanic Black groups to assess the relationship between vitamin D and glycemic control. The regression model showed that vitamin D deficiency was not a significant factor when stratified by subgroup. (Adjusted OR for Hispanic, non-Hispanic White, and non Hispanic Black: 0.64 [95% CI 0.25–1.64], 0.95 [95% CI 0.54–1.67], and 0.97 [95% CI 0.36–2.06], respectively).
4. Discussion
To our knowledge, this the first study in the United States that has evaluated the vitamin D status in patients with diabetes and studied the association between HbA1c levels and vitamin D status among patients with diabetes using national representative sample (NHANES). The findings of our study showed that there is a significant unadjusted difference in HbA1c between the diabetic patients with no vitamin D deficiency group and diabetic patients with a vitamin D deficiency group. However, after we added the other variables to the model and ran the logistic regression, the findings indicated that there was only a small association that lacked statistical significance between vitamin D deficiency and glucose control in patients with diabetes.
The other findings of our study that displayed in Table 3 indicated that patients with diabetes who use combined insulin and oral medications or insulin only were less likely to be controlled compared to patients who used oral medication only. Also, the finding shows that patients who did not use any medication for diabetes were more likely to be controlled than patients who used oral medication. This might be explained by the natural history of type-2 diabetes, which is characterized by continuous declining in β-cells function and worsening of insulin resistance as the disease progress. Patients with no medication use might be in the first stage of the disease progression with healthier β-cells than patients on diabetic medications and they could be maintaining a healthy diet, exercising, and other lifestyle modifications. Likewise, β-cell function and insulin resistance of patients on combined insulin and oral medications or insulin only might be worse than patients on oral medication only and resulting in uncontrolled glycemic levels. Moreover, many clinical trials showed an increasing loss of glycemic control over time as type-2 diabetes progresses, which manifested clinically by deterioration in A1C levels and thus require more aggressive treatment. Further evaluation and explanations to the study findings were limited due to the cross-sectional design of the survey (Kahn et al., 2006, UKPDS., 1998a, UKPDS., 1998b).
Also, our findings show that Hispanic and non-Hispanic Black patients were less likely to be controlled compared to non-Hispanic White patients. These were similar to results of previous work that compared the difference in HbA1c between African American and non-Hispanic White (Kirk et al., 2006). These results could be attributed to the level of the access to quality care or the different lifestyles of the race/ethnicity groups (Looker et al., 2011).
One of our findings in this study indicated that vitamin D deficiency is more common in patients with diabetes. Patients with diabetes are almost twice as likely to have vitamin D deficiency (57%) as compared to general population (32%) (Looker et al., 2011). This could have consequences beyond glycemic control because low vitamin D levels are associated with many other health risks including bone disease, cancer, cognitive impairment, and death from cardiovascular disease (Feldman et al., 2014, Giovannucci et al., 2008, Gocek and Studzinski, 2009, Holick et al., 2011, Wang et al., 2008, Wilkins et al., 2006).
The results showed that non Hispanic Black patients with diabetes had the highest rate of vitamin D deficiency. This finding could be due to the fact that dark skin produces less vitamin D (Harris, 2006). So, we did a subgroup analysis to determine if there is a difference between each race/ethnicity in regards to glycemic control and vitamin D deficiency. However, no association was found when the data was stratified by race/ethnicity.
The strength of our study is that we used a nationally representative sample of the U.S. population. NHANES oversamples minority groups, which help to give better estimates of population trends. One limitation of our study is that the analysis is based on a cross-sectional survey, which means that measurements of vitamin D levels and HbA1c were taken only once and are subject to measurement error. Also, we did not separate diabetes into Type 1/Type 2 because there is no question in the NHANES questionnaire allows separating between the two types accurately. Separation between the types of diabetes in a future study may give different results.
5. Conclusion
In conclusion, vitamin D deficiency is more common in patients with diabetes. Therefore, monitoring of serum vitamin D level in diabetics is advised. Although we found that correcting the level of vitamin D is not likely to improve glycemic control, other studies suggested that vitamin D supplementation may help to reduce the development of other health risks such as bone diseases, cognitive impairment, and cardiovascular diseases.
Acknowledgment
We would like to acknowledge the Deanship of Research Chairs and Medication Safety Research Chair, King Saud University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afzal S., Bojesen S.E., Nordestgaard B.G. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin. Chem. 2013;59(2):381–391. doi: 10.1373/clinchem.2012.193003. [DOI] [PubMed] [Google Scholar]
- Al-Daghri N.M., Alkharfy K.M., Al-Othman A., El-Kholie E., Moharram O., Alokail M.S., Al-Saleh Y., Sabico S., Kumar S., Chrousos G.P. Vitamin D supplementation as an adjuvant therapy for patients with T2DM: an 18-month prospective interventional study. Cardiovasc. Diabetol. 2012;11:85. doi: 10.1186/1475-2840-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Standard of medical care in diaetes-2017. Diabetes Care. 2017;40(Suppl. 1):1–142. [Google Scholar]
- Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14(5):342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Liu Y., Hollis B.W., Rimm E.B. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch. Intern. Med. 2008;168(11):1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocek E., Studzinski G.P. Vitamin D and differentiation in cancer. Crit. Rev. Clin. Lab. Sci. 2009;46(4):190–209. doi: 10.1080/10408360902982128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S.S. Vitamin D and African Americans. J. Nutr. 2006;136(4):1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Hassan Murad M., Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- Jorde R., Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;48(6):349–354. doi: 10.1007/s00394-009-0020-3. [DOI] [PubMed] [Google Scholar]
- Kahn S.E., Haffner S.M., Heise M.A., Herman W.H., Holman R.R., Jones N.P., Kravitz B.G., Lachin J.M., O'neill M.C., Zinman B., Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- Kirk J.K., D'Agostino R.B., Jr., Bell R.A., Passmore L.V., Bonds D.E., Karter A.J., Narayan K.M.V. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care. 2006;29(9):2130–2136. doi: 10.2337/dc05-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunghall S., Lind L., Lithell H., Skarfors E., Selinus I., Sorensen O.H., Wide L. Treatment with one-alpha-hydroxycholecalciferol in middle-aged men with impaired glucose tolerance – a prospective randomized double-blind study. Acta Med. Scand. 1987;222(4):361–367. doi: 10.1111/j.0954-6820.1987.tb10684.x. [DOI] [PubMed] [Google Scholar]
- Looker, A.C., Johnson, C.L., Lacher, D.A., Pfeiffer, C.M., Schleicher, R.L., Sempos, C. T., 2011. Vitamin D status: United States, 2001-2006. NCHS Data Brief (59), 1–8. [PubMed]
- Mohamad M.I., El-Sherbeny E.E., Bekhet M.M. The effect of vitamin D supplementation on glycemic control and lipid profile in patients with type 2 diabetes mellitus. J. Am. Coll. Nutr. 2016;35(5):399–404. doi: 10.1080/07315724.2015.1026427. [DOI] [PubMed] [Google Scholar]
- Patrick P.A., Visintainer P.F., Shi Q., Weiss I.A., Brand D.A. Vitamin d and retinopathy in adults with diabetes mellitus. Arch. Ophthalmol. 2012;130(6):756–760. doi: 10.1001/archophthalmol.2011.2749. [DOI] [PubMed] [Google Scholar]
- Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., 2011. Dietary Reference Intakes for Calcium and Vitamin D. [PubMed]
- Schöttker B., Herder C., Rothenbacher D., Perna L., Müller H., Brenner H. Serum 25-hydroxyvitamin D levels and incident diabetes mellitus type 2: a competing risk analysis in a large population-based cohort of older adults. Eur. J. Epidemiol. 2013;28(3):267–275. doi: 10.1007/s10654-013-9769-z. [DOI] [PubMed] [Google Scholar]
- Sung C.C., Liao M.T., Lu K.C., Wu C.C. Role of vitamin D in insulin resistance. J. Biomed. Biotechnol. 2012;2012:634195. doi: 10.1155/2012/634195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaei A., Mohamadi M., Adgi Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol. Metab. Synd. 2013;5(1):8. doi: 10.1186/1758-5996-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsur A., Feldman B.S., Feldhammer I., Hoshen M.B., Leibowitz G., Balicer R.D. Decreased serum concentrations of 25-hydroxycholecalciferol are associated with increased risk of progression to impaired fasting glucose and diabetes. Diabetes Care. 2013;36(5):1361–1367. doi: 10.2337/dc12-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKPDS, 1998a. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet, 352(9131), 854–865. [PubMed]
- UKPDS, 1998b. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet, 352(9131), 837–853. [PubMed]
- Wang T.J., Pencina M.J., Booth S.L., Jacques P.F., Ingelsson E., Lanier K., Vasan R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins C.H., Sheline Y.I., Roe C.M., Birge S.J., Morris J.C. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am. J. Geriat. Psychiatr. 2006;14(12):1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]