Abstract
Males of the bushcricket Metrioptera roeselii bear paired titillators that are spiny genital structures supposedly functioning as copulatory courtship devices. During copulation, the male inserts its titillators into the female's genital chamber, where they rhythmically tap on the sensilla-covered dorsal surface of the genital fold. Here, we investigated the stimulatory function of male titillators during mating in M. roeselii. Tracer backfills of presumptive mechanosensory sensilla at the female genital fold revealed a thick bundle of sensory axons entering the last unfused abdominal ganglion (AG-7). Electrophysiological recordings of abdominal nerves demonstrated that females sense mechanical stimulation at their genital fold. The mechanosensory responses, however, were largely reduced by the insecticide pymetrozine that selectively blocks scolopidia of internal chordotonal organs but not campaniform and hair sensilla on the outer cuticle surface. In mating experiments, the females showed resistance behaviours towards males with asymmetrically shortened titillators, but the resistance was largely reduced when mechanoreceptors at the female's genital fold were either pharmacologically silenced by pymetrozine or mechanically blocked by capping with UV-hardened glue. Our findings support the hypothesis that the male titillators in these bushcrickets may serve as copulatory courtship devices to mechanically stimulate the female genitalia to reduce resistance behaviour.
Keywords: bushcricket, titillators, copulation, mechanosensory stimulation, sensory blinding
1. Introduction
Sexual selection is a strong driving force for evolutionary diversification of sexual organs and reproductive behaviour (reviewed in [1–3]). Male genitalia tend to diverge particularly fast [1,2,4,5] and different selection pressures can fuel this rapid evolution of genital traits: e.g. sperm competition, intersexual conflict, and female choice [3,6–8]. To gain an advantage in sperm competition, the structure and use of male genitalia can adapt to remove or displace rival sperm from the female more effectively [9,10]. Intersexual conflict can arise when male and female interests over fecundity differ, generating an evolutionary arms race between the sexes to maximize their own fitness [7,11]. The resulting sexually antagonistic coevolution can lead to male genital structures that can be used as grasping and anchor devices to restrain the female during copulation and sperm transfer. Females, however, can develop behavioural counter-strategies, such as resistance behaviours, or morphological structures that impede the male's control [7,12–14]. Furthermore, male genitalia can also function as stimulatory courtship devices, when females adjust reproductive processes during or after copulation in favour of males with particular genital traits such as specific stimulation capability [4,15,16]. Most studies on the evolution of behavioural and morphological genital adaptations have focused predominantly on males, despite the fact that male and female genital traits are shaped by complex coevolutionary dynamics [17,18]. Numerous experiments have manipulated male genitalia to evaluate its impact in mating and sperm transfer [3,18]. However, only a few studies have manipulated female genitalia to test the receiver side of potential male genital courtship organs [19–22].
Our study uses ‘sensory blinding’ [8] of female genitalia in the bushcricket Metrioptera roeselii, to investigate whether females can sense and may then react accordingly to the mechanical stimulation by the male titillators during copulation. Bushcrickets (Orthoptera: Tettigoniidae) are an excellent model to test hypotheses on the evolution of reproductive behaviour [23]. Several bushcricket species possess titillators that vary considerably in number, shape, and presence of spines [24–26]. The titillators are concealed inside the male's genital chamber and are only used in mating behaviour [27–29]. For the copulation phase of mating, they are rhythmically inserted into the female's genital chamber, where they persistently tap on the dorsal side of the soft and sensilla-covered flap-like genital fold [29] and finally support the spermatophore transfer at the end of the mating process [28,29]. In previous experiments, M. roeselii females that mated with asymmetrically manipulated males (e.g. one of the two titillators ablated) showed resistance behaviour during the copulation phase, whereas the females were significantly less reluctant when mated with unimpaired males [28].
Cuticle hairs or other mechanosensilla on the dorsal surface of the female genital fold [27], as well as internal scolopidia of chordotonal organs associated with the female genitalia, could play a significant role in the detection of the titillator taps. Here, we used neuroanatomical and electrophysiological methods to explore the mechanosensory pathway by which the females sense mechanical taps on their genital fold and tested whether selective blocking of chordotonal mechanoreceptors by the insecticide pymetrozine [30] reduces the mechanosensory response to these stimulations. We then investigated female resistance behaviour in matings with asymmetrically manipulated males (one titillator shortened) combined with either pharmacological or mechanical treatment, to block mechanoreceptor subsets of the females' genital fold. By reducing the mechanical sensitivity of the females’ genital fold, we expect a suppression of the resistance behaviour towards asymmetric titillator stimulation.
2. Material and methods
(a). Animals
All experiments were performed with M. roeselii (Hagenbach, 1822), an abundant Tettigoniidae species in Germany. Nymphs were caught near Berlin (52°23′14″ N, 13°12′54″ E) in May and June 2015 for behavioural experiments and in 2017 for neurophysiological and neuroanatomical experiments. Nymphs were reared in groups of 20 animals per container (27 × 35 × 27 cm). Before reaching sexual maturity, the adults were separated and individually accommodated in 0.5 l plastic containers covered with gauze. Ambient temperature in the laboratory was 22–25°C, light–dark cycle was 16 L : 8 D and the diet contained fresh grass, oat flakes, and fish food pellets.
(b). Neuronal tracing
For anatomical tracing of mechanosensory afferents, five females were pinned down onto a plasticine block. Cuticle hairs of the female's genital fold were shaved off with a microscalpel and the surface of the soft cushion-like median area was pierced repeatedly with a minute needle to potentially injure sensory neurons of the small sensory pegs [27]. The area was then encircled by a small wall of petroleum jelly to be filled with 2% neurobiotin tracer (Vector Laboratories, Burlingame, CA, USA) dissolved in distilled water to backfill the sensory neurons. After 12 h in a humid chamber at 4°C to allow the tracer to diffuse, the two most posterior abdominal ganglia were excised and processed following a conventional protocol [31] to visualize neurobiotin with avidin-Cy3 (Dianova, Hamburg, Germany) in whole-mount preparations using confocal laser-scanning microscopy (TCS SP5, Leica, Wetzlar, Germany).
(c). Electrophysiology
After removing legs, wings, and cerci, the females were opened by a dorsal incision and pinned onto a plasticine block ventral side down. Ovaries, gut, and overlying fat tissue were carefully removed to access the abdominal nerve cord. Mechanical tap stimuli were delivered using a stiff synthetic bristle attached on a custom-build touch stimulation apparatus [31]. With a micromanipulator (Narishige, Japan), the stimulator was positioned, so that the tip of the bristle was tapping from about 1 mm above on the sensilla-covered soft tissue cushions at the dorsal side of the flap-like genital fold. This procedure mimics the mechanical tap stimulation of male titillators, which also contact the dorsal side of the opened genital fold during copulation [27,29]. The mechanosensory responses in three peripheral nerves of the last unfused abdominal ganglion (a7-N2) and fused terminal abdominal ganglion (TAG) (a8-N2 and a9-N1) were recorded with double-hook electrodes [31,32] and amplified using a differential AC amplifier (Model 1700; A-M Systems, Sequim, WA, USA). Recordings were monitored with an analogue oscilloscope (Tektronix 5110), digitized (PowerLab-4SP, AD Instruments, Spechbach, Germany) with 20 kHz sampling rate per channel, and stored with LabChart v. 8.1.6 (ADInstruments) for offline analysis.
We used the pesticide pymetrozine to pharmacologically block mechanosensory responses from the internal scolopidia of chordotonal organs [30], which are very sensitive to vibration or other mechanical stretch of the cuticle [33]. Chordotonal receptors have been anatomically described in the female genital chamber of crickets [34] and are therefore also very likely to be associated with the female genitalia of bushcrickets. Pymetrozine was dissolved in insect saline (pH 7.4; concentrations in mmol l−1: NaCl 140, KCl 10, CaCl2 7, NaHCO3 8, MgCl2 1, N-trismethyl-2-aminoethanesulfonic acid 5, d-trehalose dihydrate 4) to a concentration of 10−3 mol l−1 and then further diluted with insect saline for pharmacological treatment with increasing concentrations. In two animals, a small piece of paper tissue was soaked with 10−3 mol l−1 pymetrozine solution and placed on the dorsal surface of the female's genital lobe for 5 min. After removal of the tissue, the sensory responses of 50 tap stimulations at 0.5 Hz repetition rate were recorded in the abdominal nerve a9-N1. In three other animals, the same test procedure was done for the abdominal nerves a7-N2, a8-N2, and a9-N1, but with successive bath application of insect saline with increasing pymetrozine concentration to the abdominal body cavity. For quantitative analysis, the extracellular recording signal was full-wave rectified to prevent cancellation of biphasic spike signals [32] before averaging the 50 responses. From these averages, as a measure of the overall afferent response, the integral (signal area over the background activity) was calculated in µVms [35] and compared for each drug concentration relative to the initial response (saline control).
(d). Mating experiments
In previous experiments, M. roeselii females stayed motionless during normal copulations but resisted against males with asymmetrically manipulated titillators [28]. Here, we used these female resistance behaviours during copulation as a marker for the sensory perception of titillator manipulation by females. Each female was therefore mated with a virgin male with the left titillator tip shortened (T-1 males) using fine scissors (No. 15024-10, Fine Science Tools GmbH, Heidelberg, Germany) under a stereomicroscope. To recover from potential handling stress during the procedure, males were allowed to rest for 1 day before the mating experiments. Females were assigned randomly to one of the two treatment groups in a cross-over design: (i) in the first group (n = 16, treatment at remating), females were initially mated as control without prior interference of their genital fold. One day later, the surface of the females' genital fold was covered with UV-hardening glue (UV-Star, Marston-Domsel GmbH, Zülpich, Germany), applied under a stereomicroscope with the tip of a fine long brush-hair and hardened for 30 s with a UV-Lamp (‘UV-Beamer’, Marston-Domsel GmbH, Zülpich, Germany). After hardening, the correct and firm placement of the glue-cover was verified by close inspection under the stereomicroscope, before remating the females with a new virgin male. (ii) In the second group (n = 20, treatment at initial mating), the female's genital fold was locally treated with 10−3 mol l−1 pymetrozine in insect saline. Five microlitres of that solution was applied for 5 min on the female's genital fold and then removed with a tissue. The females were first mated after removal of the pymetrozine solution and remated 1 day after the pymetrozine treatment. It is important to note that the form of blocking differed between the two experiments, which makes our ‘cross-over’ design imperfect. However, sensory blinding by the two methods resulted in similar behavioural responses during mating in females, allowing the assumption that the two approaches are equivalent.
During mating in an arena (30 × 30 × 20 cm), several parameters were observed and registered following previously established protocols [28]. Durations of copulation and spermatophore transfer were measured using an electronic stopwatch. We counted the titillator movements for 2 min and quantified the success of spermatophore transfer as well as the occurrence of female resistance behaviour during mating. Females normally stay motionless during the copulation, prior to spermatophore transfer. The occurrence of female walking or in combination with jumping, kicking, and eventually biting during copulation was therefore classified as mating resistance. To keep the females sexually motivated, immediately after mating the spermatophore was removed from the female's genitalia. Spermatophore and male body mass were registered with an electronic precision balance (Kern EG 300-3 M, 0.001/300 g, Kern & Sohn GmbH, Balingen-Frommern, Germany). Statistical analysis was performed using Excel and SPSS Version 24 (IBM SPSS Statistics 24).
3. Results
(a). Sensilla tracing
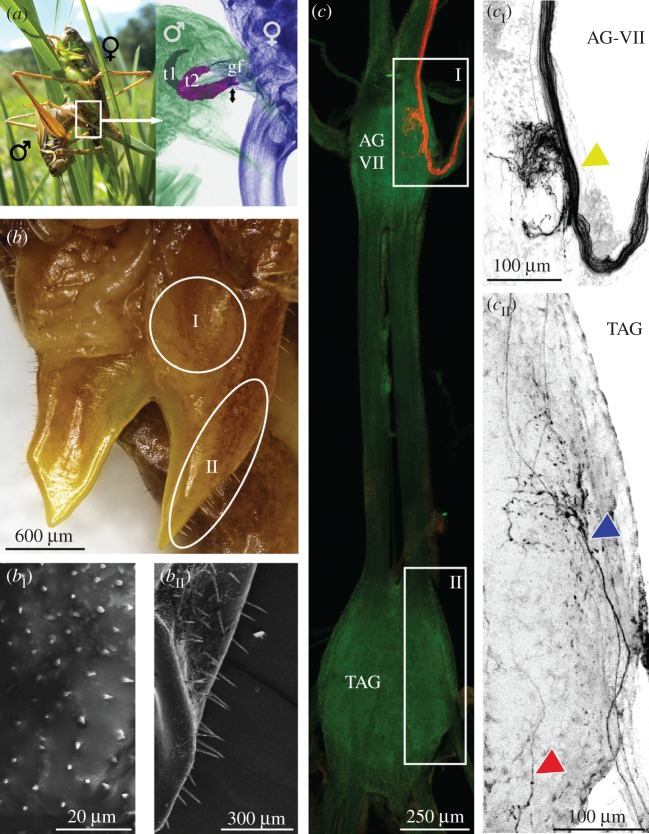
In the bushcricket M. roeselii, the dorsal surface of the female genital lobe, which is the body part that is mechanically stimulated by the male titillator movements during copulation, is covered with short cuticle pegs and at the outer rim area with longer hair sensilla resembling sensilla cheatica (figure 1). By backfilling the sensory neurons of these sensilla with neurobiotin (figure 1b), we identified a thick bundle of 15–20 tracer-labelled axons entering the last unfused abdominal ganglion via nerve a7-N2 (figure 1c, cI). These sensory axons branch out in the ipsilateral hemisphere and send axon collaterals further anterior via the ascending connective. The labelling also revealed a few sensory axons entering the TAG via the peripheral nerves a8-N2 and a9-N1 (figure 1cII). These afferents also show axonal ramifications only in the ipsilateral hemisphere of the ganglion and also seem to have ipsilateral ascending axonal collaterals.
Figure 1.
Sensory sensilla at the female's genital fold. (a) A mating couple (left) and X-ray micro-CT close-up view (right) of male and female genitalia during copulation (details: [27,28]). During mating, the male's paired titillators (t1, t2; highlighted in purple) rhythmically tap (black arrows) against the female's genital fold (gf). (b) Dorsal top view of the female's genital fold. Electron microscopy scans show median area of the genital fold (bI) covered with short cuticle pegs and outer rim area (bII) with longer sensilla (details: [27]). (c) Confocal scan of TAG and last unfused abdominal ganglion (AG-VII) with fluorescence labelling (red) of sensilla from the genital fold. (cI) Close-up of afferent axon bundle entering AG-VII (yellow arrowhead). (cII) Close-up of the labelled afferent axons in the TAG (blue and red arrowheads).
(b). Nerve recordings
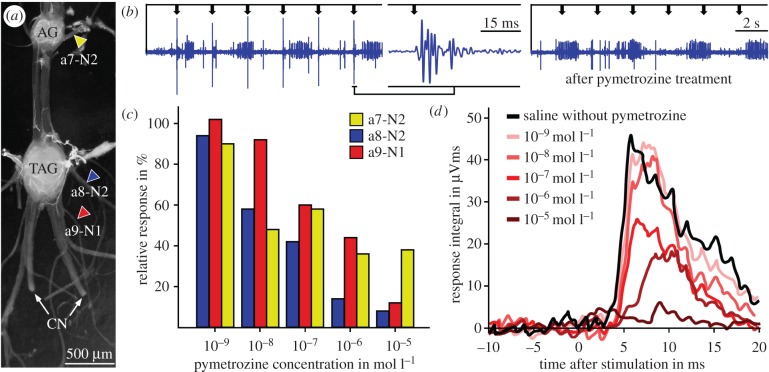
Extracellular nerve recordings demonstrated mechanosensory responses of afferent axons to mechanical tap stimulation at the female's genital fold in different peripheral nerves of the last unfused abdominal ganglion and the TAG (figure 2). This includes the nerve a7-N2 that contains the thick axon bundle of sensory neurons that we anatomically traced from the genital fold (figures 1c and 2a). Pymetrozine (5 µl, 10−3 mol l−1 in insect saline) directly applied for 5 min on the dorsal surface of the females' genital fold, selectively abolished the mechanosensory response in the nerve a8-N2 of the TAG, while the rhythmic spike activity of centrally generated motor bursts in this nerve was not notably affected (figure 2b). Bath application of increasing pymetrozine concentrations into the abdominal haemocoel reduced the mechanosensory responses in a dose-dependent fashion (figure 2c). The lowest effective pymetrozine concentration was found at 10−8–10−7 mol l−1 and the full effect was seen with 10−6–10−5 mol l−1 pymetrozine in insect saline that was filled into the abdominal haemocoel. In the nerves a8-N2 and a9-N1 of the TAG (figure 2a: blue and red arrowheads), where only few individual axons from external sensilla of the genital lobe were labelled (figure 1cII), the mechanosensory response vanished almost completely with 10−5 mol l−1 pymetrozine in the bath solution (figure 2c,d). By contrast, in the peripheral nerve a7-N2 of the last unfused abdominal ganglion (figure 2a: yellow arrowhead) with the thick bundle of labelled sensory axons from external sensilla of the genital lobe (figure 1cI), all pymetrozine concentrations from (10−8 to 10−5 mol l−1) only reduced the mechanosensory response to about 60–40% of the initial response (figure 2c: yellow).
Figure 2.
Pymetrozine reduces responses to mechanical stimulation at the female's genital fold. (a) Microphotograph of dissected last unfused abdominal ganglion (AG-VII) and TAG (fused neuromers of abdominal segments IIX–XI) with the major peripheral nerves. Corresponding to the colour coding in figure 1c, the recorded nerves are marked with yellow (a7-N2), blue (a8-N2), and red (a9-N1) arrowheads. Cercal nerves had been severed by removing the cerci before the experiment. (b) Extracellular nerve recording of the sternal nerve of the eighth abdominal neuromere (a8-N2) during 0.5 Hz tap stimulation at the genital fold (taps indicated by arrows in the top trace) before (left) and 5 min after external treatment with 10−3 mol l−1 pymetrozine at the genital fold (right). (c) Dose-dependent reduction in the mechanosensory response in recordings from three different abdominal nerves (red: a9-N1, blue: a8-N2, and yellow: a7-N2; each nerve recorded in a different animal). For each concentration, the mean response of 50 stimulations is given as percentage relative to the initial saline control. (d) Dose-dependent reduction of mechanosensory response in the tergal nerve of the ninth abdominal neuromere (a9-N1) by pymetrozine upon tap stimulation at the genital fold (mean response of 50 stimulations for each concentration). (Online version in colour.)
(c). Mating experiments
In both experimental groups, each female was mated twice to virgin males with asymmetrically manipulated titillators (one titillator shortened), once with mechanosensory blinding of the genital fold and once without. The sensory blinding treatments, either with UV-hardening glue or pymetrozine, did not change copula or spermatophore transfer duration, the number of titillator movements, or the success of spermatophore transfer between first and second mating in both groups (electronic supplementary material, table S1). Male body mass and spermatophore mass did not significantly differ between the first and second mating with males mated either in experimental group 1 or group 2 (electronic supplementary material, table S1). Female mating resistance, however, was strongly influenced by both sensory blinding treatments (figure 3). In the group of unmanipulated females, 37.5% struggled during copulation against their asymmetrically manipulated partners by walking or walking in combination with jumping, kicking, or biting. The mating resistance of the females completely vanished (0%) when the dorsal surface of their genital fold was mechanically capped with UV-hardening glue for their second mating (untreated genital fold versus glue-capped: Fisher's exact test p = 0.018). Pymetrozine treatment on the female's genital fold had a similar effect by temporarily reducing the resistance behaviour to 5%. In rematings 1 day after pymetrozine treatment, 35% of the females reacted again with resistance behaviour towards males with asymmetrically manipulated titillators (pymetrozine treated versus next day without treatment: Fisher's exact test p = 0.044). The 14 females showing resistance in the two experiments always walked (100%) in combination with kicking (14.3%), jumping (21.4%) or both, kicking and jumping (14.3%), whereas biting occurred in just one case. Jumping was the most effective resistance behaviour as it led in three out of five cases to a separation of the couples. Two of the couples re-engaged in mating afterwards, but in one case, copulation was terminated for good by female resistance.
Figure 3.
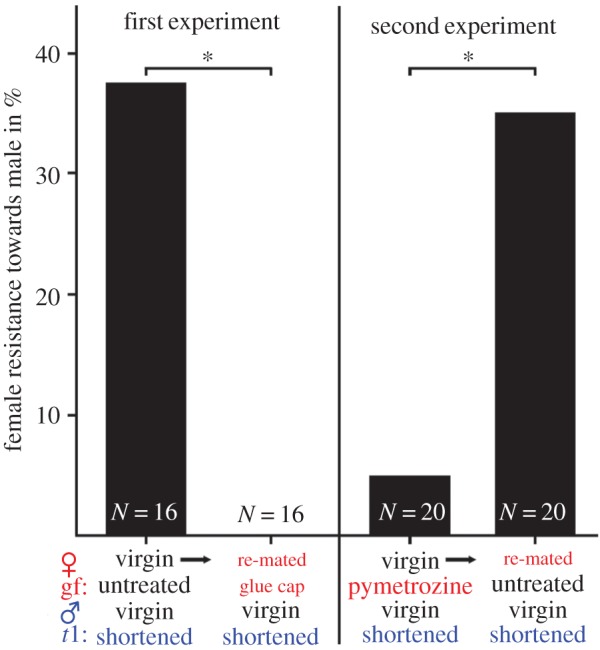
In mating experiments, sensory blinding of the genital fold in females reduces their resistance behaviour towards males with asymmetrically manipulated titillators. Data presented as a percentage bar. In both experiments, the females were mated with virgin males that had one titillator (t1) shortened. First experiment (treatment at remating): females were tested before and after mechanical blocking of mechanoreceptors of the genital fold with a UV-hardening glue cap. Second experiment (treatment at initial mating): females were tested directly after application of pymetrozine (5 µl, 10−3 mol l−1) for 5 min on their genital fold and then again 24 h after the pymetrozine treatment. Fisher's exact tests: *p < 0.05. (Online version in colour.)
4. Discussion
Bushcricket species with titillators have been shown to copulate longer prior to spermatophore transfer than species without titillators [36]. Moreover, the female's refractory period lasted longer in species with titillators, but was shortened with higher titillator complexity [26]. However, practically nothing is known about the female's ability to perceive the stimulations from those male organs. Our electrophysiology results in M. roeselii demonstrate for the first time that female bushcrickets can sense the mechanical titillator tapping on the inside of their genital fold. Application of the insecticide pymetrozine on the female's genitalia reduced the mechanosensory responses to tap stimulation. Pymetrozine is a pesticide that interferes with a subgroup of insect transient receptor potential channels [37,38], selectively blocking chordotonal receptors [30]. We therefore assume that besides mechanosensilla on the surface the titillator taps are also perceived by proposed internal chordotonal receptors of the female's genital fold [27]. As pymetrozine is not primarily affecting the response of mechanosensory hair sensilla and campaniform sensilla [30], the remaining mechanosensory response in nerve a7-N2 after pymetrozine treatment is probably due to the mechanosensory afferents from external sensilla of the genital fold. We therefore speculate that internal scolopidia, which can be selectively blocked by pymetrozine, may play a significant role in the sensory perception of mechanical stimulation at the female's genital fold. Scolopidia of chordotonal organs associated with the female genital chamber have been anatomically identified in field crickets [34], but not in bushcrickets yet. Our approach by repeatedly piercing the median section of the genital fold leaves the possibility open that the three nerves (a7-N2, a8-N2, and a9-N1) recorded contain only a subset of sensory axons involved, and that additional mechanoreceptors may be involved in female titillator sensing. In typical matings, a female cooperates by climbing on a male's back and stays motionless, showing in less than 5% resistance behaviour [28]. By contrast, abnormal titillator stimulation from asymmetrically manipulated males (T-1), resulted in 38% of females resisting during copulation [28], which was repeated in our current study, with 37.5% of control and 35% females 1-day post-pymetrozine treatment opposing the copulation with asymmetrically manipulated males. ‘Sensory blinding' of the females' genital fold by application of the insecticide pymetrozine or by glue-capping reversed the proportion of resisting females back to less than 5%. By forming a rigid surface cover on the dorsal (inner) side of the female's genital fold, the UV-hardening glue treatment not only immobilized the external sensilla [27], but also impeded the impact transfer of the mechanical stimulation to internal chordotonal receptors [33,34]. Despite unchanged mating parameters in the manipulated males [28], female resistance behaviour against the mating efforts of males with asymmetric titillators may also be argued to be the response to subtle changes of the males' behaviour due to the titillator manipulation [8]. Male behaviour (e.g. the titillator movements as well as the copula duration and the spermatophore transfer duration) was unmodified by our treatment of the female's genital fold (see electronic supplementary material, table S1). Moreover, any other possible behavioural changes of the male should have been detectable to both the manipulated and unmanipulated females. However, only manipulated females stopped resisting against mating with manipulated males. We therefore conclude that female resistance behaviour is caused by the asymmetrical titillator stimulations performed by the manipulated males. The bushcricket titillators appear thus to be a genital courtship device. Their stimulatory potential is supported by the tapping movements on the female's genital fold, visible while employing synchrotron-based in vivo X-ray cineradiography of mating couples [29]. As the females do not change their behaviour when their genital fold is sensory blinded and even stop resisting asymmetric mating partners, the symmetry or the intensity of the titillator stimulations might be a major clue of a male's quality as a mate towards females. Natural fluctuations in titillator symmetry occur (e.g. the number of spines on the left and right titillator of one individual are not always equal; GUC Lehmann 2014, unpublished data), but it is still unknown to what extent titillator asymmetry is tolerated by females. Moreover, female mating resistance might not always be an attempt to physically remove the male to end the copulation. It could also serve females to assess the males [4] or even communicate with them [39,40]. Walking as the main form of female resistance during copulations lasted on average less than 1 min (see electronic supplementary material, table S1) and in one case, the female's walking and jumping terminated the copulation for good. This is in line with our previous data [28], where no separations occurred, only two males were injured, and only the removal of both titillators resulted in a significant reduction in the success of spermatophore transfer. The male fitness costs of titillator asymmetry remain unclear, as two-thirds of the females accept asymmetric males without obvious resistance and almost all resisting females eventually accepted the spermatophore transfer.
The male's titillator taps during copulation could therefore be used by the females to evaluate the partner and (if the stimulations are not satisfying) female resistance could be a (negative) signal to the male, showing her discontent. Such results were reported in tsetse flies, where sensory blinding of the females had similar effects on female post-mating behaviour as manipulation of the males' courtship structures [20,21]. Further studies on the post-mating effects of titillator manipulation in this bushcricket species are required for a better understanding of the actual fitness costs for males. It appears that genital evolution of the male titillators in M. roeselii may be driven by female choice imposing sexual selection pressure on the titillators to function as copulatory courtship devices. As females actively resist males with unfavourable titillation capability, the rhythmic titillator movements during copulation may mechanically stimulate the female genital fold in a way that suppresses resistance behaviours [28,29].
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Nicole Naumann (Leipzig University) for technical assistance with the histological processing and confocal scans and thank Arne Lehmann and William Eberhard for their helpful comments on previous versions of the manuscript.
Ethics
All experiments complied with the principles of Laboratory Animal Care.
Data accessibility
The datasets supporting this article are accessible via the electronic supplementary material.
Authors' contributions
N.C.W. collected and reared the specimens. N.C.W. and S.S. performed the experiments, analysed the data, and prepared the figures. N.C.W. drafted the manuscript. G.U.C.L. initiated and supervised the study. All authors interpreted the results, revised the manuscript, and approved the final version before submission.
Competing interests
The authors declare that there are no competing interests.
Funding
N.C.W. received financial support by the Friedrich-Ebert-Stiftung (Friedrich Ebert academic foundation). The funders did not participate in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Eberhard WG. 2010. Genitalic evolution: theories and data updated. In Evolution of primary sexual characters in animals (eds Leonard J, Cordoba-Aguilar A), pp. 40–78. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Eberhard WG. 2010. Evolution of genitalia: theories, evidence, and new directions. Genetica 138, 5–18. ( 10.1007/s10709-009-9358-y) [DOI] [PubMed] [Google Scholar]
- 3.Simmons LW. 2014. Sexual selection and genital evolution. Austral. Entomol. 53, 1–17. ( 10.1111/aen.12053) [DOI] [Google Scholar]
- 4.Eberhard WG. 1985. Sexual selection and animal genitalia. Cambridge, MA: Harvard University Press. [Google Scholar]
- 5.Rowe L, Arnqvist G. 2012. Sexual selection and the evolution of genital shape and complexity in water striders. Evolution 66, 40–54. ( 10.1111/j.1558-5646.2011.01411.x) [DOI] [PubMed] [Google Scholar]
- 6.Hosken DJ, Stockley P. 2004. Sexual selection and genital evolution. Trends Ecol. Evol. 19, 87–93. ( 10.1016/j.tree.2003.11.012) [DOI] [PubMed] [Google Scholar]
- 7.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 8.Eberhard WG. 2011. Experiments with genitalia: a commentary. Trends Ecol. Evol. 26, 17–21. ( 10.1016/j.tree.2010.10.009) [DOI] [PubMed] [Google Scholar]
- 9.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 10.Waage JK. 1979. Dual function of the damselfly penis: sperm removal and transfer. Science 203, 916–918. ( 10.1126/science.203.4383.916) [DOI] [PubMed] [Google Scholar]
- 11.Parker GA. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum MS, Blum NA), pp. 123–166. New York, NY: Academic. [Google Scholar]
- 12.Arnqvist G, Rowe L. 2002. Antagonistic coevolution between the sexes in a group of insects. Nature 415, 787–789. [DOI] [PubMed] [Google Scholar]
- 13.Polak M, Rashed A. 2010. Microscale laser surgery reveals adaptive function of male intromittent genitalia. Proc. R. Soc. B 277, 1371–1376. ( 10.1098/rspb.2009.1720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamimura Y, Polak M. 2011. Does surgical manipulation of Drosophila intromittent organs affect insemination success? Proc. R. Soc. B 278, 815–816. ( 10.1098/rspb.2010.2431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornhill R. 1983. Cryptic female choice and its implications in the scorpionfly Hylobittacus nigriceps. Am. Nat. 122, 765–788. ( 10.1086/284170) [DOI] [Google Scholar]
- 16.Eberhard WG. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 17.Ah-King M, Barron AB, Herberstein ME. 2014. Genital evolution: why are females still understudied? PLoS Biol. 12, e1001851 ( 10.1371/journal.pbio.1001851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan PL, Prum RO. 2015. Mechanisms and evidence of genital coevolution: the roles of natural selection, mate choice, and sexual conflict. Cold Spring Harb. Perspect. Biol. 7, a017749 (doi.10.1101/cshperspect.a017749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberhard WG. 2002. Physical restraint or stimulation? The function(s) of the modified front legs of male Archisepsis diversiformis (Diptera, Sepsidae). J. Insect. Behav. 15, 831–850. ( 10.1023/A:1021161915227) [DOI] [Google Scholar]
- 20.Briceño RD, Eberhard WG. 2009. Experimental modifications imply a stimulatory function for male tsetse fly genitalia, supporting cryptic female choice theory. J. Evol. Biol. 22, 1516–1525. ( 10.1111/j.1420-9101.2009.01761.x) [DOI] [PubMed] [Google Scholar]
- 21.Briceño RD, Eberhard WG. 2009. Experimental demonstration of possible cryptic female choice on male tsetse fly genitalia. J. Insect Physiol. 55, 989–996. ( 10.1016/j.jinsphys.2009.07.001) [DOI] [PubMed] [Google Scholar]
- 22.Friesen CR, Uhrig EJ, Squire MK, Mason RT, Brennan PL. 2014. Sexual conflict over mating in red-sided garter snakes (Thamnophis sirtalis) as indicated by experimental manipulation of genitalia. Proc. R. Soc. B 281, 20132694 ( 10.1098/rspb.2013.2694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann GUC. 2012. Weighing costs and benefits of mating in bushcrickets (Insecta: Orthoptera: Tettigoniidae), with an emphasis on nuptial gifts, protandry and mate density. Front. Zool. 9, 19 (doi:1186/1742-9994-9-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vahed K, Gilbert JDJ, Weissman DB, Barrientos-Lozano L. 2014. Functional equivalence of grasping cerci and nuptial food gifts in promoting ejaculate transfer in katydids. Evolution 68, 2052–2065. ( 10.1111/evo.12421) [DOI] [PubMed] [Google Scholar]
- 25.Chamorro-Rengifo J, Lopes-Andrade C. 2014. The phallus in Tettigoniidae (Insecta: Orthoptera: Ensifera): revision of morphology and terminology, and discussion on its taxonomic importance and evolution. Zootaxa 3815, 151–199. (doi:10.11646/zootaxa.3815.2.1) [DOI] [PubMed] [Google Scholar]
- 26.Lehmann GUC, Gilbert JDJ, Vahed K, Lehmann AW. 2017. Male genital titillators and the intensity of post-copulatory sexual selection across bushcrickets. Behav. Ecol. 28, 1198–1205. ( 10.1093/beheco/arx094) [DOI] [Google Scholar]
- 27.Wulff NC, Lehmann AW, Hipsley CA, Lehmann GUC. 2015. Copulatory courtship by bushcricket genital titillators revealed by functional morphology, μCT scanning for 3D reconstruction and female sense structures. Arthropod. Struct. Dev. 44, 388–397. ( 10.1016/j.asd.2015.05.001) [DOI] [PubMed] [Google Scholar]
- 28.Wulff NC, Lehmann GUC. 2016. Function of male genital titillators in mating and spermatophore transfer in the tettigoniid bushcricket Metrioptera roeselii. Biol. J. Linn. Soc. 117, 206–216. ( 10.1111/bij.12661) [DOI] [Google Scholar]
- 29.Wulff NC, van de Kamp T, dos Santos Rolo T, Baumbach T, Lehmann GUC. 2017. Copulatory courtship by internal genitalia in bushcrickets. Sci. Rep. 7, 42345 ( 10.1038/srep42345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ausborn J, Wolf H, Mader W, Kayser H. 2005. The insecticide pymetrozine selectively affects chordotonal mechanoreceptors. J. Exp. Biol. 208, 4451–4466. ( 10.1242/jeb.01917) [DOI] [PubMed] [Google Scholar]
- 31.Schöneich S, Schildberger K, Stevenson PA. 2011. Neuronal organization of a fast-mediating cephalothoracic pathway for antennal-tactile information in the cricket (Gryllus bimaculatus DeGeer). J. Comp. Neurol. 519, 1677–1690. ( 10.1002/cne.22594) [DOI] [PubMed] [Google Scholar]
- 32.Schöneich S, Hedwig B. 2015. Corollary discharge inhibition of wind-sensitive cercal giant interneurons in the singing field cricket. J. Neurophysiol. 113, 390–399. ( 10.1152/jn.00520.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Field LH, Matheson T. 1998. Chordotonal organs of insects. Adv. Insect Physiol. 27, 1–228. [Google Scholar]
- 34.Sugawara T. 1996. Chordotonal sensilla embedded in the epidermis of the soft integument of the cricket, Teleogryllus commodus. Cell Tissue Res. 284, 125–142. ( 10.1007/s004410050573) [DOI] [PubMed] [Google Scholar]
- 35.Schöneich S, Hedwig B. 2010. Hyperacute directional hearing and phonotactic steering in the cricket (Gryllus bimaculatus deGeer). PLoS ONE 5, e15141 ( 10.1371/journal.pone.0015141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vahed K, Lehmann AW, Gilbert JD, Lehmann GUC. 2011. Increased copulation duration before ejaculate transfer is associated with larger spermatophores, and male genital titillators, across bushcricket taxa. J. Evol. Biol. 24, 1960–1968. ( 10.1111/j.1420-9101.2011.02325.x) [DOI] [PubMed] [Google Scholar]
- 37.Nesterov A, et al. 2015. TRP channels in insect stretch receptors as insecticide targets. Neuron 86, 665–671. ( 10.1016/j.neuron.2015.04.00) [DOI] [PubMed] [Google Scholar]
- 38.Salgado VL. 2017. Insect TRP channels as targets for insecticides and repellents. J. Pestic. Sci. 42, 1–6. ( 10.1584/jpestics.D16-104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez RL. 2015. Mating is a give-and-take of influence and communication between the sexes. In Cryptic female choice in arthropods (eds Peretti A, Aisenberg A), pp. 479–496. New York, NY: Springer. [Google Scholar]
- 40.Briceño RD, Eberhard WG. 2017. Copulatory dialogues between male and female Tsetse flies (Diptera: Muscidae: Glossina pallidipes). J. Insect. Behav. 30, 394–408. ( 10.1007/s10905-017-9625-1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are accessible via the electronic supplementary material.