Abstract
The impact of a pathogen on the fitness and behaviour of its natural host depends upon the host–parasite relationship in a given set of environmental conditions. Here, we experimentally investigated the effects of Borrelia afzelii, one of the aetiological agents of Lyme disease in humans, on the fitness of its natural rodent host, the bank vole (Myodes glareolus), in semi-natural conditions with two contrasting host population densities. Our results show that B. afzelii can modify the reproductive success and spacing behaviour of its rodent host, whereas host survival was not affected. Infection impaired the breeding probability of large bank voles. Reproduction was hastened in infected females without alteration of the offspring size at birth. At low density, infected males produced fewer offspring, fertilized fewer females and had lower mobility than uninfected individuals. Meanwhile, the infection did not affect the proportion of offspring produced or the proportion of mating partner in female bank voles. Our study is the first to show that B. afzelii infection alters the reproductive success of the natural host. The effects observed could reflect the sickness behaviour due to the infection or they could be a consequence of a manipulation of the host behaviour by the bacteria.
Keywords: Borrelia afzelii, fitness, host–pathogen interaction, Myodes glareolus, zoonosis, natural host
1. Introduction
The impact of pathogens on the physiology, behaviour and fitness of their natural hosts is a key determinant for the coevolution between the pathogen and the host [1–4]. Identifying the effect of a pathogen on all components of host fitness is also essential for predicting the population dynamics of a host–pathogen association and is fundamental for anticipating zoonotic outbreaks [5–8]. However, the study of the impact of parasites on their natural hosts often focuses on host survival [3,9–11], despite the recognition that host reproduction is an important component of host fitness [12–14]. Indeed, subtle effects of an endemic pathogen on the reproduction of its natural host can influence the population dynamics of the wild host [15,16] and ultimately the population dynamics of the pathogen [17].
Numerous studies have shown that pathogen virulence depends on ecological factors such as temperature and nutrition [18–21]. Another important ecological factor is host population density because it generates intra-specific competition for limited resources such as space, food and mating partners [22–24]. High host density is, therefore, expected to exacerbate pathogen virulence. Fluctuations in population density are typical in many small mammal species such as rodents [25]. However, experimental studies on density-dependent costs of infection in rodents are rare because it is often difficult to manipulate host density in an ecologically relevant way (but see [10,26,27]).
Spirochaete bacteria belonging to the Borrelia burgdorferi sensu lato (s.l.) complex cause Lyme borreliosis in humans, which is the most common vector-borne disease in the Northern Hemisphere [28,29]. Borrelia afzelii, which is transmitted by Ixodes ticks and hosted by rodents, is the most common aetiological agent of human Lyme borreliosis in Europe [28,30]. While Lyme borreliosis causes serious morbidity in humans [31,32], there is currently no clear evidence that B. burgdorferi s.l. reduces the fitness of the rodent or avian reservoir hosts [6,9,33–36]. However, most studies that have investigated the virulence of B. burgdorferi s.l. pathogens were correlational and focused on host survival and, to date, the potential effects on host reproductive success have been ignored (but see [37,38] for physiological cost and effect on host behaviour, respectively).
We conducted a field experiment to test whether B. afzelii reduces the survival and reproductive success of its rodent host, the bank vole (Myodes glareolus). Rodent populations are often strongly influenced by density-dependent effects [25]. We, therefore, hypothesized that the detrimental effects of B. afzelii infection on the fitness of bank voles would be more pronounced at high population density. Here, we show that while B. afzelii did not affect the host survival, the infection impaired the reproduction of large bank voles, and unexpectedly, that male bank voles had lower reproductive performances at low population density.
2. Material and methods
(a). Experimental design
The schedule of the experimental procedure is shown in electronic supplementary material, figure S1, and all methods are detailed in the electronic supplementary material. Male and female bank voles (M. glareolus) from the laboratory colony at the University of Jyväskylä were measured and assigned to either the B. afzelii infection group (injected with a local strain of B. afzelii) or the uninfected control group (injected with phosphate-buffered saline (PBS)). All infected and uninfected voles (total of 136 individuals, 68 females and 68 males) were released in 12 large outdoor vegetated enclosures (each 0.2 ha) that were assigned to ‘high’ density (16 individuals per enclosure, 8 females and 8 males, half of each sex infected, 5 enclosures) and ‘low’ density (8 individuals per enclosure, 4 females and 4 males, half of each sex infected, 7 enclosures) treatments. In the enclosures, the bank voles could move and reproduce freely for 18 days, which is the minimum gestation length in females. During this period, spacing behaviour was monitored using live trapping. At the end of this period, all trapped individuals were taken to the laboratory for measurements and monitoring of parturition. Male reproductive success was determined by paternity analyses conducted on the offspring born in the laboratory.
(b). Measurements
Before the enclosure period, individuals were weighed, and the head width was measured with a calliper ruler (Electronic Digital Caliper, Scala). These measurements were taken into account when experimental animals were assigned to treatments and enclosures. An ear tissue sample was taken for paternity analysis. A blood sample was taken for an ELISA (enzyme-linked immunosorbent assay) targeting B. burgdorferi s.l.-specific IgG antibodies [39] (electronic supplementary material).
After the enclosure period, the body measurements and blood sampling were carried out as described above. Males were processed shortly after the trapping day, gravid females were processed after parturition, and females that were not gravid were processed at the end of the experiment. Pups were measured (body mass and head width) within 24 h of parturition. All measurements were performed blind regarding the infection treatment and density treatment.
(c). Statistical analysis
All statistical analyses were carried out using the statistical software R v. 3.1.1 [40]. Survival of bank voles in the enclosures and individual breeding probability are binary variables. For survival, individuals were assigned 1 or 0 depending on whether they were trapped at the end of the experiment or not. For the assessment of the breeding probability, individuals were assigned 0 or 1 depending on whether their number of produced offspring was zero or at least one. Moreover, two response variables of reproductive success were calculated: (1) ‘relative number of offspring’ is the proportion of offspring produced in an enclosure by a given individual, (2) ‘relative number of partners' is the proportion of partners with which a given individual produced offspring. Eventually, space trapping data allowed calculation of two different home range variables: home range perimeter (m) and home range surface (m2) (electronic supplementary material, table S1).
In the statistical analyses, the injection (B. afzelii versus PBS) was used to define ‘infection’ treatment (infected versus uninfected), and the population density in the enclosure defined the ‘density’ treatment (‘low’ versus ‘high’). The explanatory variables of the full models always included the two experimentally manipulated factors, i.e. the infection treatment and the population density in the enclosure, sex, body mass before injection (BM) and relevant two- and three-way interactions. Enclosure ID was included as a random effect in all models. Three-way interactions involving vole sex were expected in models assessing bank vole reproductive success because the drivers of reproductive success differ between male and female bank voles [41–44]. When these three-way interactions were significant in the full model (see electronic supplementary material, table S1), separate analyses were conducted for males and females to ease the interpretation of the interactions. Otherwise, reductions of the full models were carried out starting from the non-significant interactions (see electronic supplementary material).
For gravid females, the parturition delay was calculated as the difference in the number of days between the date the first litter was observed and the parturition date for the other pregnant females. This variable was modelled as a function of infection, density, BM and the interaction infection × density. Moreover, offspring body mass at birth and head width at birth were modelled as a function of the infection status of the mother and father, density and all their two- and three-way interactions. Offspring sex and litter size were included as covariates. Enclosure ID, mother ID and father ID were included as random effects.
To analyse the data, we used generalized linear mixed models (GLMMs) with an error distribution that was either normal (home range perimeter, home range surface, body mass and head width of offspring), binomial (survival, breeding probability and variables describing reproductive success: relative number of offspring and relative number of partners) or negative binomial (female parturition delay).
3. Results
Out of the 68 female and 68 male bank voles released into the enclosures at the beginning of the experiment, 48 females and 30 males (one of which was found dead in the trap) were recovered, and the remaining 58 individuals were considered as dead. Of these 58 individuals, 56 were never observed during the 14 trapping occasions and 2 were not observed during the six last trapping occasions. As we did not observe any introduction of unmarked wild bank voles in the enclosures, and all trapped animals were found in their original enclosure, we assume that missing animals died, rather than escaped. Of the 78 captured individuals, 39 were from the B. afzelii infection group (24 females, 15 males), and 39 were from the control group (24 females, 15 males, including the individual found dead in the trap). There was no effect of B. afzelii infection or population density on the survival of bank voles (GLMM: p > 0.35, electronic supplementary material, table S4), but females survived better than male bank voles (GLMM: p < 0.01, electronic supplementary material, table S4).
(a). Borrelia afzelii infection reduces the breeding probability of large bank voles
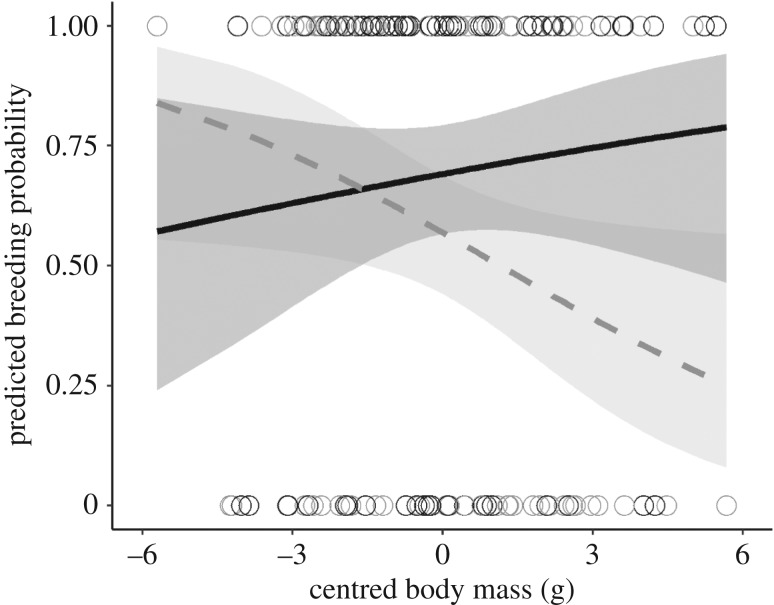
Based on the paternity test, 39 of 68 males reproduced during the study (18 of the 30 males that were trapped and 21 of the 38 males that were not trapped). For the analysis of reproductive success, all males were included, regardless of whether they were trapped or not at the end of the study. Out of the 48 captured females, 45 gave birth in the laboratory. We found that the effect of B. afzelii infection on bank vole breeding probability was dependent on body size: among small individuals, there was no difference in the breeding probability between infected and uninfected animals. However, uninfected individuals had significantly higher breeding probability than B. afzelii-infected individuals among large bank voles (GLMM: body mass × infection, p = 0.05, table 1 and figure 1; electronic supplementary material, table S4 and figure S4).
Table 1.
Selected final models for reproductive success and spacing behaviour in bank voles. BM, centred value of body mass before injection; HW, centred value of head width before injection; low, low population density; inf., infected bank voles; σ2 is the variance attributable to random effect; s.d., standard deviation; s.e. standard error. Significant effects are shown in bold.
| response variable | predictors | estimates (s.e.) | t-value | z-value | p-value | random effect (enclosure) | |
|---|---|---|---|---|---|---|---|
| males and females | breeding probability | intercept infection (inf.) density (low) sex (male) BM infection: BM sex: BM |
1.40 (0.39) −0.53 (0.38) −0.93 (0.38) −0.42 (0.38) −0.11 (0.15) −0.33 (0.17) 0.40 (0.17) |
3.60 −1.39 −2.42 −1.10 −0.71 −2.00 2.27 |
<0.01
0.16 0.02 0.27 0.48 0.05 0.02 |
σ2: 0.00 s.d.: 0.00 |
|
| males | relative number of offspring | intercept infection (inf.) density (low) BM infection: density |
−2.08 (0.31) 0.20 (0.42) 1.76 (0.47) 0.16 (0.07) −2.72 (0.89) |
−6.80 0.493.79 2.31 −3.06 |
<0.01
0.63 <0.01 0.03 <0.01 |
σ2: 1.19 × 10−9
s.d.: 3.46 × 10−5 |
|
| relative number of partners | intercept infection (inf.) density (low) BM infection: density |
−1.52 (0.28) 0.07 (0.39) 1.24 (0.48) 0.15 (0.06) −1.95 (0.82) |
−5.51 0.18 2.59 2.31 −2.38 |
<0.01
0.86 0.03 0.03 0.02 |
σ2: 1.11 × 10−9
s.d.: 3.33 × 10−5 |
||
| home range surface | intercept infection (inf.) density (low) infection: density |
378.18 (78.24) 146.38 (107.54) 429.71 (126.16) −594.50 (176.51) |
4.83 1.36 3.41 3.37 |
<0.01
0.19 <0.01 <0.01 |
σ2: 0.00 s.d.: 0.00 |
||
| females | parturition delay | intercept infection (inf.) density (low) |
1.70 (0.18) −0.75 (0.25) −0.27 (0.26) |
9.49 −3.00 −1.04 |
<0.01
<0.01 0.30 |
σ2: 0.00 s.d.: 0.00 |
Figure 1.
The estimated probability of reproduction for a bank vole (±95% CI) depends on their B. afzelii infection treatment (uninfected individuals in solid black, N = 68, infected individuals in dashed grey, N = 68) and their body size (measured as the body mass before injection). In small bank voles, there is no effect of B. afzelii infection on breeding probability. In large bank voles, by contrast, uninfected individuals have higher breeding probability than infected individuals. The observed values are shown with open circles.
(b). Borrelia afzelii infection reduces male reproductive success at low density
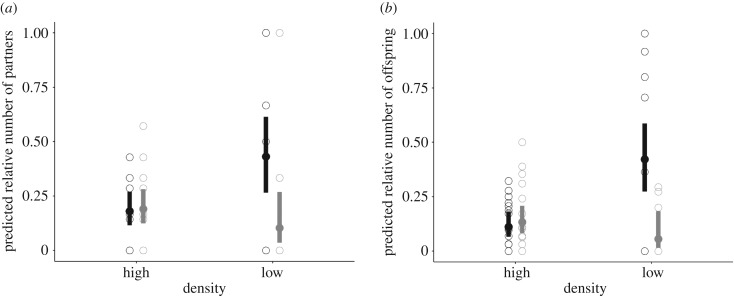
Reproductive success was further explored as the analysis of the relative number of produced offspring and the relative number of partners. The three-way interaction infection × density × sex was significant for the relative number of offspring (GLMM: p = 0.02, electronic supplementary material, table S1) and the relative number of partners (GLMM: p = 0.03, electronic supplementary material, table S1), providing evidence that infection and breeding density affected these components of reproductive success differently in males and females. In male bank voles, the relative numbers of offspring and partners were associated with B. afzelii infection status, but the effect differed between the population density treatments (table 1 and figure 2; electronic supplementary material, table S2). At low density, uninfected control males sired a higher relative number of offspring (0.42) and fertilized a higher relative number of females (0.43) than B. afzelii-infected males (0.05 offspring sired and 0.10 female fertilized). Conversely, in high density there was no effect of the infection treatment: the relative numbers of offspring sired by uninfected and infected males were 0.13 and 0.11, and the relative numbers of females fertilized by uninfected and infected males were 0.18 and 0.19, respectively (GLMM: p = 0.004 and p = 0.02, table 1 and figure 2). For female bank voles, the relative numbers of offspring and partners were not affected by the infection (the proportion of offspring produced by uninfected and infected females was 0.27 and 0.26, respectively; GLMM: p = 0.79, electronic supplementary material, table S3). As expected, population density influenced the relative number of offspring produced by a female bank vole (relative number of offspring produced by females from low- and high-density enclosures was 0.41 and 0.18, respectively; GLMM: estimate on the logit scale (s.e.): density = 0.85 (0.25), p < 0.001, electronic supplementary material, table S3).
Figure 2.
The estimated reproductive success of male bank voles depends on the interaction between B. afzelii infection (uninfected individuals in black, N = 34, infected individuals in grey, N = 34) and population density. (a) Predicted proportion of females successfully fertilized by a male bank vole (±95% CI) as a function of infection and density. (b) Predicted proportion of offspring sired by a male bank vole (±95% CI) as a function of infection and density (electronic supplementary material, table S2). The observed values are shown with open circles.
(c). Borrelia afzelii infection reduces male home range at low density
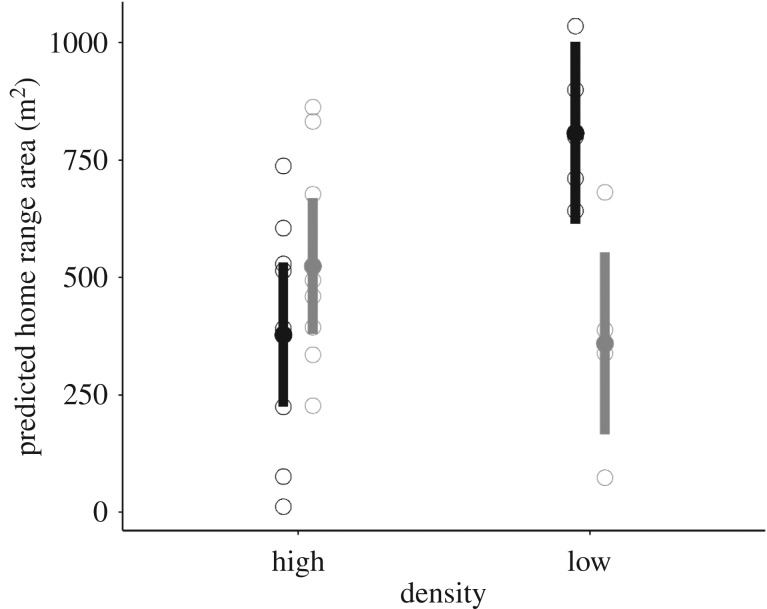
We found evidence that male and female bank voles differ in their spacing behaviour as the three-way interaction infection × density × sex was significant for home range surface and home range perimeter (GLMM: p < 0.01, p = 0.03, respectively, electronic supplementary material, table S1). For the uninfected male bank voles, the home range surface was significantly larger in the low-density enclosures (808 m2) compared with the high-density enclosures (378 m2) (LMM: p = 0.003, table 1 and figure 3). By contrast, the home range surface of the B. afzelii-infected male bank voles was not significantly different between the low-density (360 m2) and high-density (524 m2) enclosures (table 1 and figure 3). Female home range surface and perimeter were not affected by the infection or the density treatments (electronic supplementary material, table S3).
Figure 3.
The estimated home range (in m2) of male bank voles in the enclosures (±95% CI) depends on the interaction between B. afzelii infection (uninfected individuals in black, N = 13, infected individuals in grey, N = 14) and population density. At low population density, uninfected males have much larger home ranges than B. afzelii-infected males. At high population density, infection with B. afzelii does not affect the home range of male bank voles (electronic supplementary material, table S2). The observed values are shown with open circles.
(d). Infection caused early reproduction in female bank voles
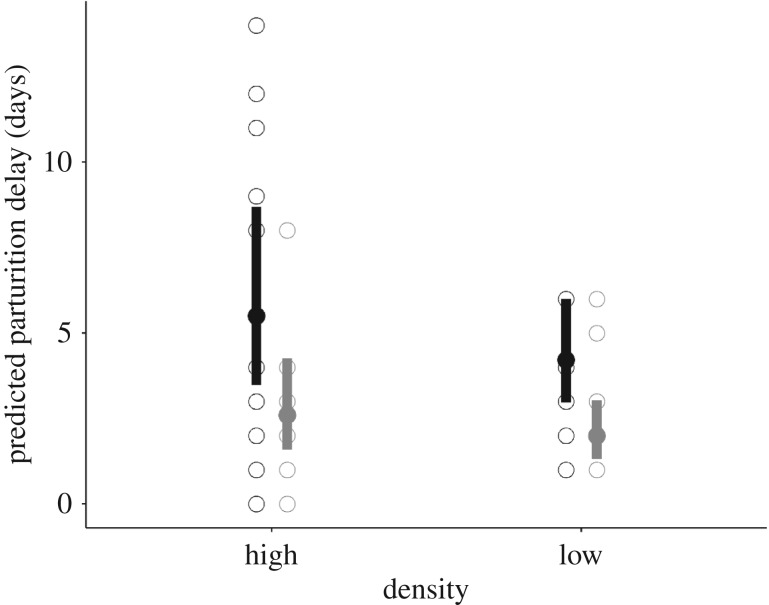
Of the 48 females captured from the enclosures, 45 were gravid and produced a total of 226 pups, with a mean number of 5 pups per female (range: 1–7). Borrelia afzelii-infected females reproduced on average 3 days earlier than uninfected control females (GLMM: p = 0.003, figure 4 and table 1) and this effect was independent of the population density (GLMM: p = 0.30, table 1). The size of the offspring at birth was not affected by the infection treatment of the mother or father or population density (LMM for all variables: p > 0.05, electronic supplementary material, table S6).
Figure 4.
Estimated parturition delay in female bank voles (±95% CI) depends on B. afzelii infection (uninfected individuals in black, N = 23; infected individuals in grey, N = 22) and population density (table 1 and electronic supplementary material S4). The observed values are shown with open circles.
4. Discussion
We examined the hypothesis that B. afzelii infection reduces the reproductive success of the rodent host and we tested the density-dependence of this effect. We found that B. afzelii infection had density-dependent and statistically differing effects on the relative numbers of partners and offspring of male and female bank voles. In males, infected individuals kept at low population density sired a lower proportion of offspring, fertilized a lower proportion of females and displayed smaller home range surface than uninfected males (figures 2 and 3). In females, by contrast, B. afzelii infection did not affect the relative offspring number, relative number of partners and home range surface, but infected individuals gave birth approximately 3 days earlier than uninfected individuals. The offspring size (head width and body mass) was not affected by the mother's infection status (figure 4; electronic supplementary material, table S6). Finally, in both sexes, infection reduced the breeding probability of large individuals but did not affect their survival (figure 1; electronic supplementary material, table S4 and table S5).
Previous studies found no evidence that infection with B. burgdorferi s.l. reduces the fitness of natural hosts; however, most of them were correlational or focused on another genospecies than B. afzelii. For instance, capture–mark–recapture (CMR) studies on wild populations of the white-footed mouse (Peromyscus leucopus) or the black-legged kittiwake found no effect of infection with B. burgdorferi s.l. on the survival of these hosts [9,34,35]. Similarly, we found that infection with B. afzelii did not impair survival of the bank vole. Another study on white-footed mice found no effect of B. burgdorferi sensu stricto on the wheel-running behaviour over the six weeks following experimental infection [6]. In our study, by contrast, the effect of infection on home range size may result from altered running behaviour. A recent study reports a trend in increased foraging behaviour in white-footed mice treated with an anti-B. burgdorferi vaccine compared with sham-treated individuals, suggesting similarly to our finding, a wider ranging behaviour in individuals with low or with no infection burden [38]. To our knowledge, our study is the first to address the effect of B. afzelii infection on host reproduction experimentally under field conditions. Studying the effects of infections on host reproduction is challenging in wild rodent populations, and reproduction is often a latent variable inferred from observed variables. Our experimental setting allows controlling for several sources of variation and confounding factors (e.g. age of the host), and we were able to estimate the reproductive success reliably.
The experimental infection was performed by peritoneal injection of the bacteria rather than the natural infection route, which involves Ixodes ticks. The infection dose and route were based on the literature [33,36,45–47]. The intraperitoneal route was chosen as it has been shown to give more widely disseminated infection than the subcutaneous route [48]. The use of injection instead of the natural transmission route can be debatable, e.g. due to the lack of tick salivary compounds that enhance the infectivity of B. burgdorferi s.l. [49,50]. Molecules present in tick saliva promote the infection by manipulating or depressing the immune system (e.g. salps) [51]. The injection of B. burgdorferi s.l. with tick salivary gland extract led to higher infection success with higher bacterial dissemination, so-called saliva-assisted transmission [51,52]. The lack of these molecules could lead to misestimation of the effects of the infection on the host. However, the injection allows the experimenter to control the bacterial dose, and it eliminates the variation linked to the tick vectorial capacity [53], hence ensuring a controlled exposure of the study animals to the bacteria. We acknowledge that needle inoculation mimics only grossly the infection via tick bite. However, we can expect any observed effect to be caused by the B. afzelii infection given our controlled experimental conditions.
The demonstration of fitness-related costs caused by B. burgdorferi s.l. infection is important for understanding the evolution of resistance in natural hosts. Recent field studies on the bank vole suggested that polymorphism at the Toll-like receptor 2 (TLR2) gene, a pathogen recognition receptor of the innate immune system, was associated with variation in susceptibility to B. afzelii [54,55]. The prevalence of B. afzelii infection in bank voles that were homozygous for the C2 resistance allele was half that of the bank voles that were homozygous for the C1 susceptibility allele [54]. A study of the TLR2 polymorphism in bank vole populations across Europe found that the resistance allele against B. afzelii (C2) was more common in countries with a high incidence of human Lyme disease [56]. This result led Tschirren to suggest that B. afzelii was driving the evolution of the resistance allele at the TLR2 gene in European bank vole populations. However, without clear evidence of reduced fitness in infected rodents, the mechanism of selection was unclear. Our demonstration that infection with B. afzelii reduces male reproductive success supports the hypothesis that this pathogen could be driving selection on the TLR2 gene in bank vole populations.
The effect of the infection on the relative number of offspring sired and the relative number of females fertilized by a given male bank vole was density-dependent. In the low-density populations, uninfected control males fertilized more females and fathered more offspring compared with the infected males and males kept in high population density (figure 2). This result was counterintuitive, as we predicted that the negative effects of high population density, such as reduced per capita food availability, more aggressive interactions and potentially higher stress levels, would exacerbate the cost of B. afzelii infection [10,43,57–59]. Three hypotheses can explain this result. First, several studies have shown that the strength of male–male competition can vary with population density in a nonlinear fashion [see, for instance, 60–62]. For example, males can modify their reproductive strategy in high population density leading to lower rates of aggression and lower reproductive success [60,63]. Second, as estimates of the relative number of partners and the relative number of offspring were based on paternity tests, cryptic female choice (i.e. a female choice that occurs in the reproductive tract of the female, leading to fertilization bias in favour of specific males [64,65]) might have occurred. Thus, a density-dependent female cryptic choice favouring healthy males in low-density populations cannot be excluded. Finally, a spurious effect linked to the length of our experiment, which covers only one reproductive episode, cannot be ruled out [66].
In the low-density populations, uninfected control males had larger home range sizes than infected males whereas, in the high-density enclosures, there was no significant difference in the home range size between uninfected and infected male bank voles (figure 3). One possible explanation for this density-dependent home range reduction is that at high density, males may reduce their exploratory behaviour to avoid encountering other males and having to engage in aggressive male–male interactions. Moreover, at high density, with eight females available in the enclosure, the chance for a male to encounter a receptive female might be higher than in the low-density enclosure where only four females are available. Indeed, female bank voles are territorial and hyperdispersed [42,67]. Consequently, at low density, male bank voles may need to explore a larger home range to search for receptive females than at high density. As expected, the uninfected males had a larger home range in low population density, whereas the infected males presumably allocated resources to their immune response instead of explorative behaviour. In contrast, female bank voles had a smaller home range size than males, which was not affected by population density, reflecting the territorial behaviour of females especially, during late gestation when the space trapping took place [42,67,68].
We found that the cost of infection was more important in large bank voles, which are the most frequently infested with ticks and B. burgdorferi s.l. in nature [9,69,70]. Large infected individuals showed reduced reproductive success compared with large healthy individuals. Food resource is generally known to constrain reproduction and food addition has been shown to enhance reproductive success in similar outdoor enclosure setups [43,71,72]. These food constraints might have a more negative effect on the large individuals, which have greater energetic needs [73]. Infected large voles showed altered breeding probability regardless of the population density.
Infected females plastically modified their life history and reproduced approximately 3 days earlier than uninfected females without alteration of the size of the offspring at birth, i.e. without signs of premature birth (figure 4 and table 1; electronic supplementary material, table S3). In nature, reproducing females give birth to 1 or 2 litters per reproductive season [74], and most individuals live only one season. The biological importance of giving birth 3 days earlier is not clear, as concerns population dynamics. At the individual level, early reproduction can be a compensatory strategy if parasites reduce the reproductive success of the adult host later in life via morbidity, mortality or castration [75–77]. According to the terminal investment theory, individuals maximize their fitness by allocating resources to immediate reproduction when the prospects for future reproduction are reduced, for example by chronic infection [27,78–80]. It remains to be estimated whether B. afzelii impairs reproduction of female bank vole during the late stage of infection.
In summary, our study shows, for the first time, that the zoonotic pathogen B. afzelii can influence the reproductive success of its rodent host. The effect of the infection on the relative numbers of offspring and partners differed between male and female bank voles. Although large body size favoured reproduction in uninfected individuals, this size benefit disappeared if the individual was infected with B. afzelii. In males, infected individuals kept at low population density displayed smaller home range surface than uninfected males. Lower mobility can be a consequence of sickness behaviour due to the infection. On the other hand, predation risk by small carnivores generally increases with vole mobility [81]. By reducing home range size, infection with B. afzelii could lower the predation risk of male bank voles by small carnivores, enhancing at the same time its own fitness [82]. The hypothesis of manipulation of the rodent host by B. afzelii is yet to be explored.
Supplementary Material
Acknowledgements
We thank Anja Siukkola, Susanne Varjola and Joannes Van Cann and the Konnevesi research station for their assistance in the field. We thank Sami Kyröläinen, Elina Virtanen, Annukka Pietikäinen, Julia Honkasalo, Anouk Sarr and Olivier Rais for their help in the laboratory. Swanne Gordon gave constructive feedback at an early stage of this manuscript. We thank Maarten Voordouw's and Johanna Mappes's laboratory meetings for their comments. We thank two anonymous referees for valuable comments on the manuscript.
Ethics
The Finnish Animal Experiment Board approved the trapping and handling methods used in this study under the authorizations ESAVI/3834/04.10.03/2011, ESAVI/7256/04.10.07/2014 and ESAVI/3457/04.10.07/2015.
Data accessibility
The dataset analysed during the current study is available in the JYX repository, http://urn.fi/URN:NBN:fi:jyu-201806133148 [83].
Authors' contributions
C.C., E.K., T.M., E.R.K. conceived the study. The fieldwork and laboratory work were carried out by all authors, with the helpers listed in the Acknowledgements. C.C., E.K., T.M., E.R.K., M.J.V. analysed the results, with input from the other authors. All authors contributed to the interpretation and critical revision. C.C. led the writing of the paper. All authors gave final approval for publication.
Funding
This project was supported by the Kone Foundation, the University of Jyväskylä and the Academy of Finland (250524, 310104 (E.R.K.), 257340 (E.K.) and 132190, 268670 (T.M.)).
Competing interests
We declare we have no competing interests.
References
- 1.Anderson RM, May RM. 1979. Population biology of infectious diseases: part I. Nature 280, 361–367. ( 10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 2.Scott ME, Dobson A. 1989. The role of parasites in regulating host abundance. Parasitol. Today 5, 176–183. ( 10.1016/0169-4758(89)90140-3) [DOI] [PubMed] [Google Scholar]
- 3.Cattadori IM, Haydon DT, Hudson PJ. 2005. Parasites and climate synchronize red grouse populations. Nature 433, 737–741. ( 10.1038/nature03276) [DOI] [PubMed] [Google Scholar]
- 4.Smith MJ, White A, Sherratt JA, Telfer S, Begon M, Lambin X. 2008. Disease effects on reproduction can cause population cycles in seasonal environments. J. Anim. Ecol. 77, 378–389. ( 10.1111/j.1365-2656.2007.01328.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson RM, May RM. 1978. Regulation and stability of host-parasite population interactions. I. Regulatory processes. J. Anim. Ecol. 47, 219–247. ( 10.2307/3933) [DOI] [Google Scholar]
- 6.Schwanz LE, Voordouw MJ, Brisson D, Ostfeld RS. 2011. Borrelia burgdorferi has minimal impact on the Lyme disease reservoir host Peromyscus leucopus. Vector Borne Zoonotic Dis. 11, 117–124. ( 10.1089/vbz.2009.0215) [DOI] [PubMed] [Google Scholar]
- 7.Feore SM, Bennett M, Chantrey J, Jones T, Baxby D, Begon M. 1997. The effect of cowpox virus infection on fecundity in bank voles and wood mice. Proc. R. Soc. Lond. B 264, 1457–1461. ( 10.1098/rspb.1997.0202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karesh WB, et al. 2012. Ecology of zoonoses: natural and unnatural histories. Lancet 380, 1936–1945. ( 10.1016/S0140-6736(12)61678-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voordouw MJ, Lachish S, Dolan MC. 2015. The Lyme disease pathogen has no effect on the survival of its rodent reservoir host. PLoS ONE 10, e0118265 ( 10.1371/journal.pone.0118265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallio ER, Voutilainen L, Vapalahti O, Vaheri A, Henttonen H, Koskela E, Mappes T. 2007. Endemic hantavirus infection impairs the winter survival of its rodent host. Ecology 88, 1911–1916. ( 10.1890/06-1620.1) [DOI] [PubMed] [Google Scholar]
- 11.Dobson AP, Hudson PJ. 1992. Regulation and stability of a free-living host-parasite system: Trichostrongylus tenuis in red grouse. II. Population models. J. Anim. Ecol. 61, 487–498. ( 10.2307/5339) [DOI] [Google Scholar]
- 12.Ehrlich PR, Roughgarden J, Roughgarden J. 1987. The science of ecology. London, UK: Collier-Macmillan.
- 13.Begon M, Harper JL, Townsend CR. 1996. Ecology: individuals, populations and communities. London, UK: Blackwell Science. [Google Scholar]
- 14.Cockburn A. 1991. An introduction to evolutionary ecology. London, UK: Wiley. [Google Scholar]
- 15.Lafferty KD. 1993. Effects of parasitic castration on growth, reproduction and population dynamics of the marine snail Cerithidea californica. Mar. Ecol. Prog. Ser . 96, 229–237. ( 10.3354/meps096229) [DOI] [Google Scholar]
- 16.Telfer S, Bennett M, Bown K, Carslake D, Cavanagh R, Hazel S, Jones T, Begon M. 2005. Infection with cowpox virus decreases female maturation rates in wild populations of woodland rodents. Oikos 109, 317–322. ( 10.1111/j.0030-1299.2005.13734.x) [DOI] [Google Scholar]
- 17.Begon M, Telfer S, Smith MJ, Burthe S, Paterson S, Lambin X. 2009. Seasonal host dynamics drive the timing of recurrent epidemics in a wildlife population. Proc. R. Soc. B. 276, 1603–1610. ( 10.1098/rspb.2008.1732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanford S, Thomas MB, Pugh C, Pell JK. 2003. Temperature checks the Red Queen? Resistance and virulence in a fluctuating environment. Ecol. Lett. 6, 2–5. ( 10.1046/j.1461-0248.2003.00387.x) [DOI] [Google Scholar]
- 19.Mitchell SE, Rogers ES, Little TJ, Read AF. 2005. Host-parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution 59, 70–80. ( 10.1111/j.0014-3820.2005.tb00895.x) [DOI] [PubMed] [Google Scholar]
- 20.Scholthof KB. 2007. The disease triangle: pathogens, the environment and society. Nat. Rev. Microbiol. 5, 152–156. ( 10.1038/nrmicro1596) [DOI] [PubMed] [Google Scholar]
- 21.Ilmonen P, Hakkarainen H, Koivunen V, Korpimäki E, Mullie A, Ilrnonen P, Korpimaki E, Shutler D. 1999. Parental effort and blood parasitism in Tengmalm's owl: effects of natural and experimental variation in food abundance. Oikos 86, 79–86. ( 10.2307/3546571) [DOI] [Google Scholar]
- 22.Begon M, Mortimer M, Thompson DJ. 2009. Population ecology: a unified study of animals and plants. Oxford, UK: Wiley. [Google Scholar]
- 23.Krebs CJ. 1970. Microtus population biology behavioral changes associated with the population cycle in M. ochrogaster and M. pennsylvanicus. Ecology 51, 34–52. ( 10.2307/1933598) [DOI] [Google Scholar]
- 24.Ostfeld RS, Canham CD, Pugh SR. 1993. Intrinsic density-dependent regulation of vole populations. Nature 366, 259–261. ( 10.1038/366259a0) [DOI] [PubMed] [Google Scholar]
- 25.Krebs CJ, Myers JH. 1978. Population cycles in small mammals. Adv. Ecol. Res. 8, 267–399. ( 10.1016/S0065-2504(08)60280-9) [DOI] [Google Scholar]
- 26.Burthe S, Telfer S, Begon M, Bennett M, Smith A, Lambin X. 2008. Cowpox virus infection in natural field vole Microtus agrestis populations: significant negative impacts on survival. J. Anim. Ecol. 77, 110–119. ( 10.1111/j.1365-2656.2007.01302.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kallio ER, Helle H, Koskela E, Mappes T, Vapalahti O. 2015. Age-related effects of chronic hantavirus infection on female host fecundity. J. Anim. Ecol . 84, 1264–1272. ( 10.1111/1365-2656.12387) [DOI] [PubMed] [Google Scholar]
- 28.Kurtenbach K, Hanincová K, Tsao JI, Margos G, Fish D, Ogden NH. 2006. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol . 4, 660–669. ( 10.1038/nrmicro1475) [DOI] [PubMed] [Google Scholar]
- 29.Rizzoli A, Hauffe HC, Carpi G, Vourc'h GI, Neteler M, Rosà R. 2011. Lyme borreliosis in Europe. Euro Surveill. 16 ( 10.2807/ese.16.27.19906-en) [DOI] [PubMed] [Google Scholar]
- 30.Hanincová K, Schäfer SM, Etti S, Sewell HS, Taragelová V, Ziak D, Labuda M, Kurtenbach K. 2003. Association of Borrelia afzelii with rodents in Europe. Parasitology 126, 11–20. ( 10.1017/S0031182002002548) [DOI] [PubMed] [Google Scholar]
- 31.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379, 461–473. ( 10.1016/S0140-6736(11)60103-7) [DOI] [PubMed] [Google Scholar]
- 32.Wang G, van Dam AP, Schwartz I, Dankert J. 1999. Molecular typing of Borrelia burgdorferi sensu lato: taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev . 12, 633–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bey RF, Loken KI, Wu CC, Lin TL. 1995. Experimental infection of the red-backed vole (Clethrionomys gapperi) with Borrelia burgdorferi. J. Wildl. Dis . 31, 428–431. ( 10.7589/0090-3558-31.3.428) [DOI] [PubMed] [Google Scholar]
- 34.Chambert T, Staszewski V, Lobato E, Choquet R, Carrie C, Mccoy KD, Tveraa T, Boulinier T. 2012. Exposure of black-legged kittiwakes to Lyme disease spirochetes: dynamics of the immune status of adult hosts and effects on their survival. J. Anim. Ecol. 81, 986–995. ( 10.1111/j.1365-2656.2012.01979.x) [DOI] [PubMed] [Google Scholar]
- 35.Hofmeister EK, Ellis BA, Glass GE, Childs JE. 1999. Longitudinal study of infection with Borrelia burgdorferi in a population of Peromyscus leucopus at a Lyme disease-enzootic site in Maryland. Am. J. Trop. Med. Hyg. 60, 598–609. ( 10.4269/ajtmh.1999.60.598) [DOI] [PubMed] [Google Scholar]
- 36.Moody KD, Terwilliger GA, Hansen GM, Barthold SW. 1994. Experimental Borrelia burgdorferi infection in Peromyscus leucopus. J. Wildl. Dis. 30, 155–161. ( 10.7589/0090-3558-30.2.155) [DOI] [PubMed] [Google Scholar]
- 37.Norte AC, Costantini D, Araújo PM, Eens M, Ramos JA, Heylen D. 2018. Experimental infection by microparasites affects the oxidative balance in their avian reservoir host the blackbird Turdus merula. TicksTickborne. Dis. 9, 720–729. ( 10.1016/j.ttbdis.2018.02.009) [DOI] [PubMed] [Google Scholar]
- 38.Ostfeld RS, Brisson D, Oggenfuss K, Devine J, Levy MZ, Keesing F. 2018. Effects of a zoonotic pathogen, Borrelia burgdorferi, on the behavior of a key reservoir host. Ecol. Evol. 8, 4074–4083. ( 10.1002/ece3.3961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salo J, Jaatinen A, Soderstrom M, Viljanen MK, Hytonen J. 2015. Decorin binding proteins of Borrelia burgdorferi promote arthritis development and joint specific post-treatment DNA persistence in mice. PLoS ONE 10, 0121512 ( 10.1371/journal.pone.0121512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team. 2017. R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 41.Sikorski MD, Wójcik AM. 1990. Mating system and reproductive success in a free-living population of the bank vole, Clethrionomys glareolus. In Social systems and population cycles in voles (eds RH Tamarin, RS Ostfeld, SR Pugh, G Bujalska, pp. 193–202. Basel, Switzerland: Springer. [Google Scholar]
- 42.Koskela E, Mappes T, Ylonen H. 1997. Territorial behaviour and reproductive success of bank vole Clethrionomys glareolus females. J. Anim. Ecol . 66, 341–349. ( 10.2307/5980) [DOI] [Google Scholar]
- 43.Koskela E, Jonsson P, Hartikainen T, Mappes T. 1998. Limitation of reproductive success by food availability and litter size in the bank vole, Clethrionomys glareolus. Proc. R. Soc. Lond. B 165, 1129–1134. ( 10.1098/rspb.1998.0408) [DOI] [Google Scholar]
- 44.Mills S, Grapputo A, Jokinen I, Koskela E, Mappes T, Oksanen TA, Poikonen T. 2009. Testosterone-mediated effects on fitness related phenotypic traits and fitness. Am. Nat. 173, 475–487. ( 10.1086/597222) [DOI] [PubMed] [Google Scholar]
- 45.McLean RG, Ubico SR, Cooksey LM. 1993. Experimental infection of the eastern chipmunk (Tamias striatus) with the Lyme disease spirochete (Borrelia burgdorferi). J Wildl. Dis. 29, 527–532. ( 10.7589/0090-3558-29.4.527) [DOI] [PubMed] [Google Scholar]
- 46.Baum E, Hue F, Barbour AG. 2012. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. MBio 3, e00434-12. ( 10.1128/mBio.00434-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson RC, Kodner CL, Russell ME. 1986. Vaccination of hamsters against experimental infection with Borrelia burgdorferi. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 263, 45–48. ( 10.1016/S0176-6724(86)80101-8) [DOI] [PubMed] [Google Scholar]
- 48.Schwan TG, Burgdorfer W, Schrumpf ME, Karstens RH. 1988. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J. Clin. Microbiol. 26, 893–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gern L, Schaible UE, Simon MM. 1993. Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J. Infect. Dis . 167, 971–975. ( 10.1093/infdis/167.4.971) [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro JMC. 1995. How ticks make a living. Parasitol. Today 11, 91–93. ( 10.1016/0169-4758(95)80162-6) [DOI] [PubMed] [Google Scholar]
- 51.Ramamoorthi N, et al. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436, 573–577. ( 10.1038/nature03812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nuttall P, Labuda M, Bowman A. 2008. Saliva-assisted transmission of tick-borne pathogens. In Ticks: biology, disease and control, pp. 205–219. Cambridge, UK: Cambridge University Press; (doi:10.1017/CBO9780511551802.011) [Google Scholar]
- 53.de la Fuente J, et al. 2017. Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 7, 114 ( 10.3389/fcimb.2017.00114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tschirren B, Andersson M, Scherman K, Westerdahl H, Mittl PR, Raberg L. 2013. Polymorphisms at the innate immune receptor TLR2 are associated with Borrelia infection in a wild rodent population. Proc. R. Soc. B 280, 20130364 ( 10.1098/rspb.2013.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tschirren B, Råberg L, Westerdahl H. 2011. Signatures of selection acting on the innate immunity gene Toll-like receptor 2 (TLR2) during the evolutionary history of rodents. J. Evol. Biol . 24, 1232–1240. ( 10.1111/j.1420-9101.2011.02254.x) [DOI] [PubMed] [Google Scholar]
- 56.Tschirren B. 2015. Borrelia burgdorferi sensu lato infection pressure shapes innate immune gene evolution in natural rodent populations across Europe. Biol. Lett . 11, 20150263 ( 10.1098/rsbl.2015.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartolomucci A. 2007. Social stress, immune functions and disease in rodents. Front. Neuroendocrinol . 28, 28–49. ( 10.1016/j.yfrne.2007.02.001) [DOI] [PubMed] [Google Scholar]
- 58.Bian JH, Du SY, Wu Y, Cao YF, Nie XH, He H, You ZB. 2015. Maternal effects and population regulation: maternal density-induced reproduction suppression impairs offspring capacity in response to immediate environment in root voles Microtus oeconomus. J. Anim. Ecol . 84, 326–336. ( 10.1111/1365-2656.12307) [DOI] [PubMed] [Google Scholar]
- 59.Forbes KM, Mappes T, Sironen T, Strandin T, Stuart P, Meri S, Vapalahti O, Henttonen H, Huitu O. 2016. Food limitation constrains host immune responses to nematode infections. Biol. Lett . 12, 20160471 ( 10.1098/rsbl.2016.0471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jirotkul M. 1999. Population density influences male–male competition in guppies. Anim. Behav . 58, 1169–1175. ( 10.1006/anbe.1999.1248) [DOI] [PubMed] [Google Scholar]
- 61.Hughes NK, Banks PB. 2016. Olfactory contacts mediate plasticity in male aggression with variable male density. J. Mammal . 97, 444–454. ( 10.1093/jmammal/gyv188) [DOI] [Google Scholar]
- 62.Milner RNC, Jennions MD, Backwell PRY. 2012. Keeping up appearances: male fiddler crabs wave faster in a crowd. Biol. Lett . 8, 176–178. ( 10.1098/rsbl.2011.0926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Judge PG, De Waal FBM. 1993. Conflict avoidance among rhesus monkeys: coping with short-term crowding. Anim. Behav . 46, 221–232. ( 10.1006/anbe.1993.1184) [DOI] [PubMed] [Google Scholar]
- 64.Kokko H, Brooks R, Jennions MD, Morley J. 2003. The evolution of mate choice and mating biases. Proc. R. Soc. Lond. B 270, 653–664. ( 10.1098/rspb.2002.2235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol. Evol . 21, 296–302. ( 10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- 66.Oksanen TA, Koivula M, Koskela E, Mappes T. 2007. The cost of reproduction induced by body size at birth and breeding density. Evolution 61, 2822–2831. ( 10.1111/j.1558-5646.2007.00245.x) [DOI] [PubMed] [Google Scholar]
- 67.Ostfeld RS. 1985. Limiting resources and territoriality in microtine rodents. Am. Nat. 126, 1–15. ( 10.1086/660279) [DOI] [Google Scholar]
- 68.Wolff JO. 1993. Why are female small mammals territorial? Oikos 68, 364–370. ( 10.2307/3544853) [DOI] [Google Scholar]
- 69.Cayol C, Koskela E, Mappes T, Siukkola A, Kallio ER. 2017. Temporal dynamics of the tick Ixodes ricinus in northern Europe: epidemiological implications. Parasites Vectors 10, 166 ( 10.1186/s13071-017-2112-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bunikis J, Tsao J, Luke CJ, Luna MG, Fish D, Barbour AG. 2004. Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: a longitudinal study in an area where Lyme borreliosis is highly endemic. J. Infect. Dis. 189, 1515–1523. ( 10.1086/382594) [DOI] [PubMed] [Google Scholar]
- 71.Jonsson P, Hartikainen T, Koskela ESA, Mappes T. 2002. Determinants of reproductive success in voles: space use in relation to food and litter size manipulation. Evol. Ecol. 16, 455–467. ( 10.1023/A:1020854525220) [DOI] [Google Scholar]
- 72.Desy EA, Batzli GO, Liu J. 1990. Effects of food and predation on behaviour of prairie voles: a field experiment. Oikos 58, 159–168. ( 10.2307/3545423) [DOI] [Google Scholar]
- 73.White CR, Seymour RS. 2003. Mammalian basal metabolic rate is proportional to body mass2/3. Proc. Natl Acad. Sci. 100, 4046–4049. ( 10.1073/pnas.0436428100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koivula M, Koskela E, Mappes T, Oksanen TA. 2003. Cost of reproduction in the wild: manipulation of reproductive effort in the bank vole. Ecology 84, 398–405. ( 10.1890/0012-9658(2003)084%5B0398:CORITW%5D2.0.CO;2) [DOI] [Google Scholar]
- 75.Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, Martin M, Kaplan H, Gurven M. 2015. Helminth infection, fecundity, and age of first pregnancy in women. Science 350, 6–9. ( 10.1126/science.aac7902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minchella DJ, Loverde PT. 1981. A cost of increased early reproductive effort in the snail Biomphalaria glabrata. Am. Nat . 118, 876–881. ( 10.1086/283879) [DOI] [Google Scholar]
- 77.Weil ZM, Martin LB, Workman JL, Nelson RJ. 2006. Immune challenge retards seasonal reproductive regression in rodents: evidence for terminal investment. Biol. Lett . 2, 393–396. ( 10.1098/rsbl.2006.0475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clutton-Brock TH. 1984. Reproductive effort and terminal investment in iteroparous animals. Am. Nat . 123, 212–229. ( 10.1086/284198) [DOI] [Google Scholar]
- 79.Tersago K, Crespin L, Verhagen R, Leirs H. 2012. Impact of Puumala virus infection on maturation and survival in bank voles: a capture-mark-recapture analysis. J. Wildl. Dis . 48, 148–156. ( 10.7589/0090-3558-48.1.148) [DOI] [PubMed] [Google Scholar]
- 80.Velando A, Drummond H, Torres R. 2006. Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc. R. Soc. B 273, 1443–1448. ( 10.1098/rspb.2006.3480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Norrdahl K, Korpimäki E. 1998. Does mobility or sex of voles affect risk of predation by mammalian predators? Ecology 79, 226–232. ( 10.2307/176877) [DOI] [Google Scholar]
- 82.Hofmeester TR, Jansen PA, Wijnen HJ, Coipan EC, Fonville M, Prins HHT, Sprong H, van Wieren SE. 2017. Cascading effects of predator activity on tick-borne disease risk. Proc. R. Soc. B 284, 20170453 ( 10.1098/rspb.2017.0453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cayol C, Giermek A, Gomez-Chamorro A, Hytönen J, Kallio ER, Mappes T, Salo J, Voordouw MJ, Koskela E.. 2018. Data from: Borrelia afzelii alters reproductive success in a rodent host JYX. See http://urn.fi/URN:NBN:fi:jyu-201806133148. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cayol C, Giermek A, Gomez-Chamorro A, Hytönen J, Kallio ER, Mappes T, Salo J, Voordouw MJ, Koskela E.. 2018. Data from: Borrelia afzelii alters reproductive success in a rodent host JYX. See http://urn.fi/URN:NBN:fi:jyu-201806133148. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The dataset analysed during the current study is available in the JYX repository, http://urn.fi/URN:NBN:fi:jyu-201806133148 [83].