Abstract
The evolution of cooperation and social behaviour is often studied in isolation from the ecology of organisms. Yet, the selective environment under which individuals evolve is much more complex in nature, consisting of ecological and abiotic interactions in addition to social ones. Here, we measured the life-history costs of cooperative chemical defence in a gregarious social herbivore, Diprion pini pine sawfly larvae, and how these costs vary under different ecological conditions. We ran a rearing experiment where we manipulated diet (resin content) and attack intensity by repeatedly harassing larvae to produce a chemical defence. We show that forcing individuals to allocate more to cooperative defence (high attack intensity) incurred a clear cost by decreasing individual survival and potency of chemical defence. Cooperative behaviour and the magnitude of its costs were further shaped by host plant quality. The number of individuals participating in group defence, immune responses and female growth decreased on a high resin diet under high attack intensity. We also found some benefits of cheating: non-defending males had higher growth rates across treatments. Taken together, these results suggest that ecological interactions can shape the adaptive value of cooperative behaviour and maintain variation in the frequency of cooperation and cheating.
Keywords: social behaviour, antipredator defence, hymenoptera, automimicry, life-history costs
1. Background
The problem of cooperation is that it is vulnerable to exploitation by cheats [1]. How can cooperation be an evolutionarily stable strategy if cheats are at a fitness advantage relative to cooperators? A general solution to this problem was proposed by Hamilton [1], known as the inclusive fitness theory (kin selection), and it is summarized in Hamilton's rule: cooperative behaviour will be favoured by selection, when rb − c > 0, where c is the fitness cost to the actor, b is the fitness benefit for the recipient and r is the relatedness of these two individuals. Since its publication, Hamilton's theory has become a focus of attention for much theoretical and empirical research (reviewed in, e.g. [2]). While empirical evidence has focused on the measurement of relatedness and kin selection (i.e. estimation of r), it lags behind in understanding the ecological and evolutionary mechanisms behind cooperative behaviours (i.e. estimation of b and c) [2,3]. Particularly, we lack experimental estimates of how the individual costs of cooperation and investment in cooperative behaviour depend on multiple biological interactions that comprise an individual's selective environment in nature [4,5]. This is crucial to understanding why cooperation and cheating evolve in some environmental conditions but not others [4,6–9].
Chemically defended organisms offer a great opportunity to test these neglected aspects: cooperation in chemical defence against predation is widespread in nature and occurs in both single- and multicellular organisms [10,11]. Many of these species deter predators with external defensive secretions, which can be costly to produce and maintain [10,12,13]. These types of responsive chemical defences are often facultative, with individuals having the possibility to choose whether they deploy a defensive fluid during an attack or not [10]. In general, prey individuals deploying toxic secretions are considered to contribute to the public good by educating predators to avoid prey of similar appearance in future encounters [3,10] or by participating in producing higher quantities of a defensive fluid surrounding and protecting prey aggregations, like in some bacterial cells [12]. These shared costs of predator education and deterrence can be considered a collaborative production of benefits shared by group members, i.e. cooperation [3,10]. A high frequency of defending individuals with greater toxicity of defensive secretions [14–17] should be selected for by predators as each of these characters have been shown to enhance predators' avoidance learning and deterrence. Consequently, if the frequency of cheats increases, it degrades protection as attack rates by predators increase on both defended and undefended prey individuals [12,16,17].
To evaluate how ecological conditions shape the cost of cooperative defence and social interactions within species, we used the sub-social chemically defended Diprion pini pine sawfly as a study system. Diprion pini larvae feed and defend gregariously during the larval stage; when threatened by predators, larvae perform a defensive display in concert by raising their head and regurgitating a resinous droplet of fluid [18–20] (figure 1a). Larvae also try to actively tap the defensive secretion on the predator [18]. A group of defending pine sawfly larvae therefore forms a ‘sticky physical barrier’ that makes it very difficult for the predator to catch an individual without smudging its feathers or cuticle with the resinous fluid. This defensive behaviour makes pine sawfly larvae unprofitable as prey for both avian and arthropod predators [18,19,21–23]. Pine sawfly larval survival is higher in groups in comparison to solitary individuals [19,23,24]. In addition, recent data suggest that when the proportion of undefended individuals in a group is high (greater than 60% compared with less than 30%), individual survival against predators decreased in gregarious Neodiprion sertifer pine sawfly larvae (C. Lindstedt, J. Valkonen, J. Mappes 2016, unpublished data). Thus, chemical defence in groups offers both individual benefits and also cooperative benefits at the group level and should therefore be under positive frequency-dependent selection [17,25].
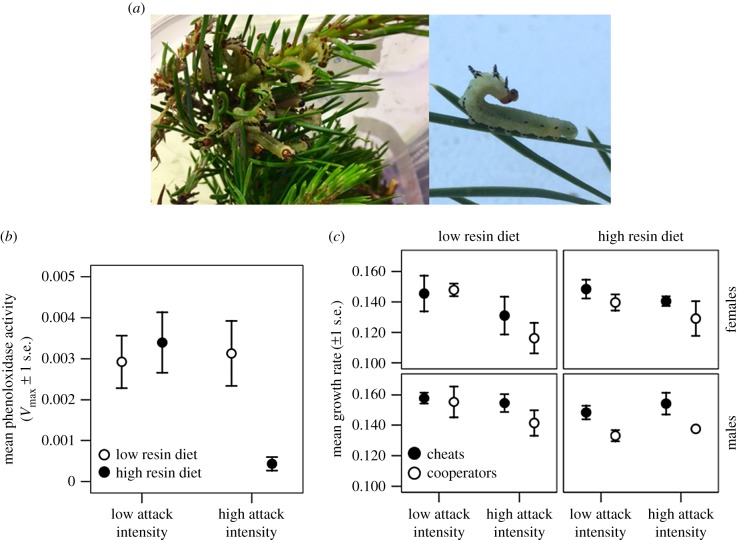
Figure 1.
(a) Defending D. pini larvae. (b) Effect of attack intensity and diet quality on mean PO activity. (c) Mean growth rate of cheat and cooperative female and male larvae on attack intensity and diet treatments. (Online version in colour.)
Pine sawflies are herbivores specialized on pines. They exploit their host plant's secondary compounds, namely monoterpenes and terpenes (i.e. resin acids) in their chemical defence against predators [26,27]. At the same time, pine sawflies need to invest energy and resources in the sequestration of these compounds and their detoxification to protect their own tissues from the compounds' harmful effects [27,28]. Host plant quality varies genetically and phenotypically in terms of its resin content [18,21,22,24], which can result in variation in the expression of chemical defence, survival and life-history traits [18,21,22,24]. This variation enables us to test how investment in common defence evolves under multiple selection pressures from host plants and predators.
We can also expect to have natural variation in the relative importance of indirect (i.e. kin) and direct benefits in the maintenance of cooperation in gregarious pine sawflies. For example, in many gregarious pine sawflies, females mate multiple times in the wild in addition to single mating or not mating at all (i.e. virgin females lay haploid male clutches) [29,30]. The relatedness structure in larval groups can also change owing to competition for food at high population densities: pine sawflies are pests of pine and they have regular outbreaks during which they can cause significant damage to pine forests [30]. During an outbreak, the kin structure of the group is likely to change as females tend to lay their eggs in trees with a higher defoliation level [28] and larval groups are likely to merge into larger aggregations around green foliage in highly defoliated trees [30].
Perhaps most interestingly, pine sawfly larvae show extensive individual variation in their probability to deploy the defensive fluid or not (nil defence) (electronic supplementary material, figure S1). This gives us the possibility to test whether individual variation has adaptive significance. That is, whether individuals who contribute less (nil defence) gain any benefits from not contributing to the common defence.
We conducted a two-by-two factorial rearing experiment (figure 1). The experiment consisted of (i) different resin acid content of the pine foliage diet (low and high resin acid content) reflecting natural variation in host plant quality, and (ii) simulated long-lasting non-lethal predation stress at different intensity (low versus high). In the high attack intensity treatment, we provoked individuals to deploy a responsive defence by pressing them gently on the dorsal side, which enabled us to manipulate the costs of allocation to cooperative defence. We used a full-sibling design to control for genetic and phenotypic variation among treatments and to estimate family-level variation in defensive behaviour.
We had four aims. Our first aim was to quantify the cost for individuals of contributing to cooperative chemical defence. This was done by comparing the fitness proxies of individuals between the low and high attack intensity treatments. We hypothesized that the repetitive production of a responsive defence under high attack intensity is costly [31,32], thereby decreasing performance in different life-history traits as well as in the potency of chemical defence in future defensive attempts. Our second aim was to test how these costs are shaped by ecological interactions (here host plant quality): while a resin-rich diet can increase the efficacy of the chemical defence [21,22,26], as well as increase protection against parasites [33,34] in pine sawflies, it can also incur a cost of detoxification [27,28,35,36] decreasing performance in, e.g. growth (but see [21]).
Our third aim was to test how variation in ecological interactions shapes investment in cooperative behaviour. If individuals modify their cooperative behaviour depending on the availability of resources to produce the defence, we expect to see lower numbers of defending individuals under the high attack intensity treatment, where the cumulative costs of repetitive production and loss of defensive fluid are higher [31]. These cumulative costs of defence can be further modified by dietary conditions and may decrease the contribution on the high resin diet owing to potentially higher detoxification costs [27,28,35,36]. On the other hand, a higher concentration of defensive compounds in the diet may facilitate the sequestration of these compounds for an individual's own defence, enabling higher contributions to cooperative defence [21].
Our fourth aim was to determine if individuals who contribute less to common defence (nil defence individuals in electronic supplementary material, figure S1) across treatments gain any life-history benefits and could be defined as ‘cheats’ [37]. Based on the broad-sense heritability reported in this study (see below), non-defending behaviour in D. pini larvae in last larval instar is not solely dependent on the environment, but can also vary between families. We therefore conducted additional statistical analyses where we classified individuals as non-defending or defending (factor called ‘cheating’) based on this trait. We then compared if life-history traits differed depending on cheating or via its interactive effects with diet quality and attack intensity. We assumed that if less defensive individuals are exploiting the common defence and can be defined as ‘cheats’, they should have better performance (i.e. benefit from cheating) than defending individuals. However, if less defensive individuals perform worse than defending individuals, it indicates that they contribute less because they are in a poorer condition. Similarly, if less defensive individuals are cheats, the observed variation in defensive behaviour should be owing to strategic allocation (i.e. individuals do not perform a defensive display even though their defensive glands are filled) and not simply owing to depletion (i.e. individuals do not perform a defensive display because their glands are empty) [10,38]. Because pine sawfly larvae dispose of their defensive glands in the pupa during metamorphosis [18], we were able to exclude the latter option by comparing individual defensive behaviour during the later larval stage to how filled their disposed glands were.
2. Material and methods
(a). Diprion pini colony
The experiment was conducted in Jyväskylä, Finland. All D. pini families used in this experiment descend from a sample of 100 females and 100 males originating from an outbred laboratory population at the Freie University of Berlin, Germany and reared on Scots pine (Pinus sylvestris) (electronic supplementary material, figure S1).
After mating, D. pini females were allowed to lay their eggs on randomly chosen Scots pine branches. After the larvae hatched, they were fed with pine branches ad libitum, and fresh branches were provided twice a week. All the larvae were reared in similar conditions under constant temperature (20 ± 2°C) in a laboratory room that included both natural light coming from the windows (nights are short in the summer in Finland) and some level of electric light at all times.
(b). Experimental design
The experiment follows a full-sibling design (electronic supplementary material, figure S1b). At the age of 11–13 days, 40 larvae from each family (n = 12) were randomly divided into eight treatments. Five larvae per family per treatment were moved to transparent plastic containers with fabric on top for ventilation. We weighed the larvae individually before dividing them among treatments to minimize differences in weights between treatment groups (F7,88 = 0.007, p > 0.99). The distribution of males and females among treatments did not differ significantly (all p > 0.377, electronic supplementary material, appendix S2).
Larvae were first divided into low and high resin content diet consisting of a mixture of Scots pine (P. sylvestris) foliage collected from multiple trees. Scots pine shows significant genetic variation in its secondary metabolites among individuals [36,39]. We used the amount of resin ducts in the needles of host plants as an indirect estimate of the resin acid content (method described in [21,39]; electronic supplementary material, appendix S1). Branches from trees with a mean resin acid duct number below five and above six were chosen as low and high resin acid diets, respectively. Altogether, we had eight low and 12 high resin acid pine trees from which the branches were collected one to three times per tree. The branches were collected during one season (August–October), and the high and low resin acid trees grew near each other in the same location. Thus, we assume that their abiotic conditions and nutritional status potentially affecting their secondary metabolites [39,40] were similar.
Next, the larvae on the low and high resin content diets were further divided into low and high attack intensity treatments. We forced individual larvae in the high attack intensity treatment (simulating long-lasting predation stress) to face an attack and display their defensive behaviour once per day until the prepupal stage (mean 20.5 times ± 7.4 s.d.). Individuals in the low attack intensity treatment were attacked only when their chemical defence efficacy was measured. Each attacking event consisted of pressing the larvae gently from the dorsal side, up to five times, with a capillary without harming or piercing the skin of the larvae. For the majority of individuals, this was enough to provoke them to produce defensive movements and/or regurgitation of a defence fluid, which was then sucked into the capillary. This ensured that the ‘attacked’ larvae were not able to recover the potentially valuable fluid for subsequent use, as is probably the case in attack and defence events in nature [18].
Finally, larvae were divided into two groups: one group where individuals experienced immunological assays and the other serving as a control group (no assays were performed). At the start of the experiment, we reared larvae in family groups of five individuals for 6 days. To be able to get individual data on sex, life-history, immune and defence traits, we weighed the larvae at the age of 17–19 days and moved them (all the individuals per group) to Petri dishes to be reared individually until pupation or death (electronic supplementary material, figure S1c).
(c). Aims 1 and 2: costs of cooperative behaviour under varying ecological conditions
(i). Immune responses
Immune responses were measured from individuals at the age of 19–20 days, after the larvae were moved from groups to be reared individually. Immune responses were measured from the larvae (mean length 3.96 cm, s.d. ±0.31 cm) using phenoloxidase (PO) activity and encapsulation response. Both of them were measured from the same individual. PO activity was measured following a modified protocol from Nerg et al. [41] (electronic supplementary material, appendix S1). Examining the activity of the PO enzyme [42,43] gives a measure of insect defence against several pathogens (e.g. [43]). Higher values correspond to higher activity (i.e. better response). Encapsulation response was measured similar to the study of Lindstedt et al. [21] (electronic supplementary material, appendix S1). Insects respond primarily to foreign intrusions in their body by an encapsulation reaction, in which a capsule-like layer composed of cells forms around the foreign object and hardens [44]. The layer contains melanin and the darker the implant, the stronger the response [45].
(ii). Performance
To study the costs of antipredator defence and the effects of diet quality on larval performance, we recorded the development time of larvae to reach pupation (in days), pupa weight and survival to pupation. After weighing individuals within 3–4 days of their pupation, growth rate could be calculated as the natural logarithm of pupal mass (mg) divided by larval development time to pupal stage in days. Because immunological assays can interfere with life-history and antipredator defence traits [21], only individuals not used in the immunological assay (figure 1) were used in the statistical analyses of life-history and antipredator defence traits.
Diprion pini is sexually dimorphic in the adult stage [30]. Sawfly larvae usually have five to six larval instars and males usually have one instar less (i.e. shorter development time) than females [30]. During the pupal stage, sexes were distinguished by pupal weight [21,30]: individuals weighing over 100 mg were classified as females (mean pupal mass for eclosed females 127.71 ± 12.595 mg) and below 100 mg as males (mean pupal mass for eclosed males 57.59 ± 9.368 mg). Owing to differences in defensive strategy (see below) and life history, growth rates of females and males were analysed separately.
(iii). Potency of chemical defence
We analysed the volume of defensive fluid that a larva regurgitated when it was ‘attacked’ individually; age 14–16 days for group rearing larvae and age 21–23 days for last larval instar on individual rearing. In these measurements, the defence droplet was sucked into a capillary and its quantity was measured with an electronic ruler. We measured the body length of larvae with a ruler at the same time to control for the effect of body size on the amount of defence fluid produced.
In all statistical analyses of chemical defence and defensive behaviour, we only used one value per individual at the relevant measurement point. In the final measurement, body length and defensive behaviour were also remeasured for individuals that had moulted their skin. Thus, for all individuals, the last measurement of chemical defence traits is from the last instar before larvae reached their final prepupal instar.
We also gathered individual data on the quality of chemical defence. A random subset of defence fluid samples collected from last instar larvae (n = 79 individuals, 11 families) were stored in 1.5 ml Eppendorf tubes with 500 µl of n-hexane and kept in a −20°C freezer. Concentrations of mono- and other terpene compounds in the samples were then analysed with a gas chromatograph coupled with the analyses with a mass spectrometer for the identification of defensive compounds (electronic supplementary material, appendix S1).
(d). Aims 3 and 4: contribution to common defence under varying ecological conditions and life-history benefits of cheating
We recorded the defensive behaviour (individual produces fluid or not) at the same time with the measurements of the quality and quantity of chemical defence during the group rearing stage (age 14–16 days) and last instar larvae during the individual rearing.
To assess the benefits of cheating, we classified individuals as either cheats or ‘cooperators’ based on the last measurement point of defensive behaviour in last instar. This was because at this age we could relate behavioural responses to chemical defence, growth and survival at the individual, rather than group level. Similar to analyses of life-history and antipredator traits, we only included control individuals in these analyses.
To estimate whether an individual's probability to deploy a defensive secretion was more likely to be owing to strategic allocation rather than depletion of defensive fluid, we recorded if the disposed defensive glands were filled or empty: after the adults had eclosed, we opened their pupae with preparation scissors and removed the defensive gland with forceps as in [18]. We weighed the detached glands to estimate the amount of fluid inside them. Since we were not able to collect individual data during the group rearing phase, we compared the state of the glands to the last measurements of the control individuals' defensive behaviour.
(e). Statistical analyses
We used generalized linear mixed models to estimate the treatment effects and family variance on larval traits. For all analyses, predation intensity and diet quality, as well as their interaction, were included as fixed factors in the models. Family was included as a random factor in all models assuming a certain degree of within family genetic similarity ought to exist for all traits (model comparisons for family-by-treatment interactions reported in the electronic supplementary material, appendix S2).
Data for the quantity of defensive fluid, PO activity, toxicity (terpene content) of the defensive fluid and mass of defensive glands were positively skewed and thus not normally distributed. We instead modelled them as gamma distributed with a log link. Because gamma distributions do not allow for zero values, one decimal more than the precision of the data was added to the values referring to the quantity of defensive fluid (0.01) and PO activity (0.00000001). Growth rate and encapsulation rate were assumed normal. Production of defensive fluid (yes/no), survival to pupation and the condition of defensive glands (glands are either filled with defensive fluid or empty) were treated as dichotomous variables modelled as binomial response variables with a logit link function. Levene's test was used to confirm the homoscedasticity of variables and the Satterthwaite approximation for degrees of freedom was applied when using function ‘lmer’.
We used variance components from the final model for defensive behaviour in last larval instar (electronic supplementary material, table S2) associated with family (VG) and residual variance (VR) to estimate broad-sense heritability (H2), where H2 = VG/(VG + VR). Because defensive behaviour is the binary variable, we calculated residual variance as π2/3 [46]. The significance of family-level variation was tested by comparing the model with the family as a random factor to the null model without the family.
To test the effect of cheating on individual's performance and state of defensive glands, we ran additional analyses for the growth rate, survival and defensive gland measurements where we included cheating as a fixed factor in the final models together with the treatments and their interactions. Owing to low sample size for defensive gland measurements, we included individuals both from the immune and control treatments for analyses to increase the sample size and only included the main effect of cheating into the statistical models. All correlations were analysed with Pearson correlation coefficient. The volume and toxicity of defensive fluid in later instars were log-transformed in order for them to meet the test's assumption of a linear relationship between variables.
All statistical analyses were performed in R Studio (v. 1.1.419, 2009–2018 R Studio and packages ‘lmer’ and ‘car’), except for correlations which were analysed using IBM SPSS Statistics 20 (IBM Corporation, NY, USA). Datasets of the experiment can be found in [47].
3. Results
(a). Aims 1 and 2: costs of cooperative behaviour under varying ecological conditions
(i). Immune responses
Repetitive production of defensive display in the high attack intensity treatment on high resin diet reduced PO activity significantly (table 1 and figure 1b). No significant main effects of diet or attack intensity treatment (table 1) interactions were observed. Encapsulation response, however, was not significantly affected by attack intensity, diet quality or their interaction (table 1).
Table 1.
Effects of attack intensity, diet quality and their interaction on the different components of fitness and defensive behaviour. (Traits with significant effects of fixed factors are noted with asterisks. *p < 0.05.)
| traits | fixed factors |
||
|---|---|---|---|
| diet quality | attack intensity | diet × predation | |
| immunological costs | |||
| PO activity (n = 147, 12 families) |
0.088 ± 0.275, t = 0.320, p = 0.749 |
0.022 ± 0.310, t = 0.071, p = 0.944 |
−1.256 ± 0.426, t = −2.946, p = 0.003* |
| encapsulation rate (n = 130, 12 families) |
F1,117.23 = 0.8246, p = 0.366 |
F1,121.20 = 0.056, p = 0.809 |
F1,119.73 = 0.280, p = 0.598 |
| costs in performance | |||
| female growth rate (n = 66, nine families) |
F1,57.220 = 0.198, p = 0.658 |
F1,58.65 = 3.441, p = 0.069 |
F1,56.79 = 6.171, p = 0.016* |
| male growth rate (n = 89, 12 families) |
F1,75.78 = 5.5685, p = 0.021* |
F1,76.66 = 0.387, p = 0.536 |
F1,75.26 = 5.102, p = 0.027* |
| survival to pupal stage (n = 213, 12 families) | 0.588 ± 0.593, Z = 0.991, p = 0.322 |
−1.608 ± 0.643, Z =−2.502, p = 0.013* |
0.052 ± 0.762, Z = 0.069, p = 0.945 |
| contribution to cooperative defence behaviour | |||
| probability to defend in groups at the age of 14–16 days (n = 451, 12 families) | −0.844 ± 0.425, Z = −1.98, p = 0.047* |
−2.159 ± 0.401, Z = −5.379, p < 0.001* |
1.509 ± 0.524, Z = 2.881, p = 0.004* |
| potency of chemical defence | |||
| volume of defensive fluid at the age of 14–16 days (n = 452, 12 families) |
−0.308 ± 0.214, t = −1.438, p = 0.150 |
−1.486 ± 0.214, t = −6.942, p < 0.001* |
0.834 ± 0.307, t = 2.716, p = 0.007* |
| volume of defensive fluid at the age of 21–23 days (n = 201, 12 families) |
−0.242 ± 0.295, t = −0.823, p = 0.411 |
−1.272 ± 0.305, t = −4.165, p < 0.001* |
0.310 ± 0.434, t = −4.165, p < 0.001* |
| monoterpene concentration at the age of 21–23 days (n = 79, 11 families) |
−0.032 ± 0.298, t = −0.106, p = 0.916 |
−0.586 ± 0.280, t = −2.097, p = 0.036* |
−0.373 ± 0.410, t = −0.911, p = 0.362 |
| other terpene concentration at the age of 21–23 days (n = 79, 11 families) |
0.121 ± 0.302, t = 0.400, p = 0.689 |
−0.210 ± 0.288, t = −0.729, p = 0.466 |
−0.378 ± 0.427, t = −0.884, p = 0.377 |
(ii). Performance
Investment in cooperative defence in the high attack intensity treatment reduced survival to the pupal stage; however, diet quality or an interaction between attack intensity and diet quality had no effect (table 1).
In females, investment in cooperative defence in the high attack intensity treatment decreased the growth rate on the low resin diet (table 1 and figure 1c). Attack intensity or diet quality did not have a significant effect (table 1). Opposite to females, males grew faster on the low resin acid diet in the low attack intensity treatment (table 1 and figure 1c).
(iii). Potency of chemical defence
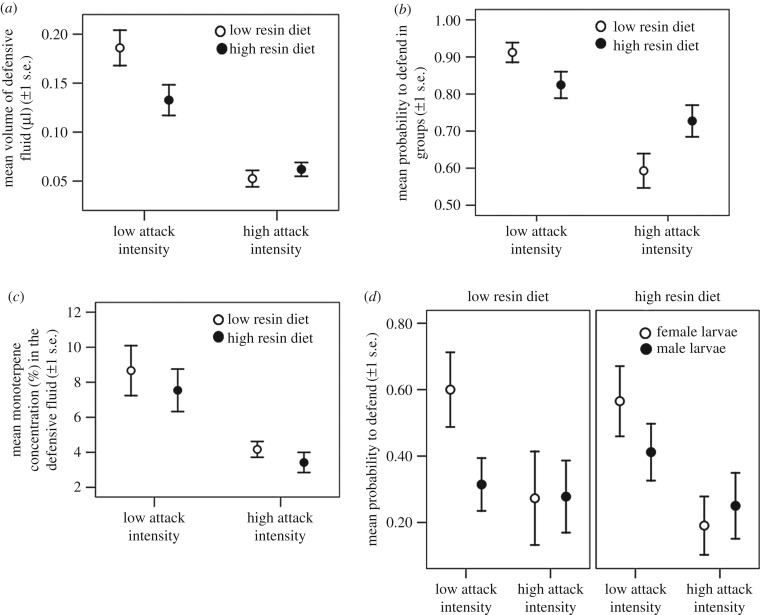
Larvae in the low attack intensity treatment produced higher volumes of defence fluid than larvae under high attack intensity during both group rearing and individual rearing stages (table 1 and figure 2a). In addition, during the group rearing stage, larvae produced higher volumes on low than on high resin acid diet under low attack intensity treatment (table 1 and figure 2a). Diet quality had no main effect on the quantity of defensive fluid produced in group or individual rearing stages (table 1). In general, larger larvae produced higher volumes of fluid (early instars: 0.762 ± 0.199, T = 3.839, p < 0.001; last larval instars: −0.063 ± 0.024, T = −2.666, p = 0.008).
Figure 2.
(a) Change in the mean volume of defensive fluid produced during the group rearing. (b) Mean probability to defend (1) or not (0) during the group rearing. (c) Effect of attack intensity and diet quality on monoterpene concentration (i.e. toxicity) in defensive fluid during the individual rearing in last instar larvae. (d) Mean probability to defend in female and male D. pini last instar larvae under attack intensity and diet treatments.
The samples of defensive fluid contained altogether eight different monoterpenes and 22 other terpenes listed in the electronic supplementary material, appendix S4. The defence fluid became more diluted when produced repeatedly: high attack intensity significantly decreased the concentration of monoterpenes in the defensive fluid of last instar larvae (table 1 and figure 2c). There were no significant differences in the concentration of other terpenes (table 1). Diet quality did not affect terpene concentrations independently (table 1). In general, the concentration of monoterpenes (r = −0.819, n = 79, p < 0.001) and other terpenes (r = −0.881, n = 79, p < 0.001) decreased with higher volumes of defence fluid.
(b). Aim 3: contribution to common defence under varying ecological conditions
Diet quality altered defensive behaviour in groups: in the low attack intensity treatment, there was a larger number of defending individuals on low than on high resin diet. In the high attack intensity treatment, there was a larger number of defending individuals on high than on low resin diet (figure 2b and table 1). Overall, larvae were more likely to regurgitate defensive fluid in the low attack treatment than in the high attack treatment (table 1).
We found similar results when assessing defensive behaviour during individual rearing in last instars, there was a higher number of defending individuals under the low attack intensity treatment than under the high attack intensity treatment (−2.111 ± 1.018, Z = −2.074, p = 0.038) (figure 2d) and diet quality did not affect defensive behaviour overall (−0.893 ± 0.784, Z = −1.139, p = 0.255). However, diet quality no longer interacted with attack intensity treatment (1.110 ± 1.291, Z = 0.860, p = 0.390) and there were now differences among the sexes, as females were more likely to defend than males (−2.117 ± 0.813, Z = −2.603, p = 0.009). Diet quality did not affect the sexes differently (0.817 ± 1.028, Z = 0.794, p = 0.427), and there was no interaction between diet, attack intensity and sex (−1.306 ± 1.744, Z = −0.749, p = 0.454). Females and males did not differ significantly in their responses to attack intensity (2.385 ± 1.307, Z = 1.825, p = 0.068) (figure 2d).
Overall, cheating in later instars (whether the individual defends or not at the age of 21–23 days) showed moderate broad-sense heritability (VG (s.d.) = 1.66 (1.29), VR = 3.29, H2 = 0.33 and the model with the family effect included was significantly better than the model without the family (χ2 = 11.272, p < 0.001).
(c). Aim 4: life-history benefits of cheating
Neither attack intensity, diet quality nor their interactions significantly affected the mass of the defensive glands (attack intensity: −0.204 ± 0.509, Z = −0.400, p = 0.689; diet: −0.565 ± 0.383, Z = −1.475, p = 0.140; attack intensity × diet: 0.226 ± 0.605, Z = 0.373, p = 0.709) or their emptiness (attack intensity: 0.0911 ± 0.845, Z = 0.108, p = 0.914; diet: 0.328 ± 0.738, Z = 0.444, p = 0.657; attack intensity × diet: −0.886 ± 1.176, Z = −0.754, p = 0.451). Also, the mass (0.157 ± 0.406, Z = 0.385, p = 0.700) or emptiness of the defensive glands (−0.198 ± 0.669, Z = −0.296, p = 0.767) did not differ significantly between cheats and cooperators.
When cheating was added as an additional fixed factor to the final models of growth rate, we found that non-defending males grew faster than defending males (F1,72.092 = 6.255, p = 0.015) (figure 1c). There were no significant interactive effects with diet or attack intensity on male growth rate (diet × cheating: F1,66.572 = 0.226, p = 0.636; attack intensity × cheating: F1,65.847 = 2.315, p = 0.133; attack intensity × cheating × diet: F1,67.476 = 0.353, p = 0.555).
In contrast to males, the growth rates of females did not differ significantly between non-defending and defending individuals (F1,53.66 = 1.2856, p = 0.262). Nor were there any significant interactive effects among diet, attack intensity and cheating (diet × cheating: F1,50.296 = 0.790, p = 0.378; attack intensity × cheating: F1,52.28 = 0.201, p = 0.656; attack intensity × cheating × diet: F1,51.66 = 0.055, p = 0.815).
Finally, survival until the pupal stage did not differ significantly between non-defending and defending individuals (0.762 ± 0.918, Z = 0.830, p = 0.406), and there were no significant interactions among diet quality, attack intensity and cheating (diet × cheating: −0.212 ± 1.561, Z = −0.136, p = 0.892; attack intensity × cheating: 0.952 ± 1.485, Z = 0.641, p = 0.521; attack intensity × diet × cheating: −2.158 ± 2.088, Z = −1.033, p = 0.301).
4. Discussion
Our results support the idea that variation in ecological conditions can affect the evolution of social interactions within species [4,6,7] by modifying the costs of cooperation for individuals and therefore individuals' contribution to cooperative acts. We first show that contribution to cooperative defence incurs life-history costs as individuals that contribute less have a higher performance. Second, we show that ecological interactions play an important part in defining the cost : benefit ratio between cooperation and cheating [4,6]. Third, we show that contribution to cooperation can vary depending on these ecological conditions, but also among sexes and larval families. Together, these results suggest that ecological interactions can alter the adaptive value of cooperation and maintain genetic and phenotypic diversity in its expression.
We found several lines of evidence for a cost of cooperation. First, pine sawfly larvae experiencing higher attack intensity, i.e. forced allocation to cooperative antipredator defence, showed a decline in survival. Repeated production of defensive fluid under high attack intensity also reduced the potency of chemical defence in future encounters with predators, by decreasing the toxicity and volume of defensive fluid [48]. Second, the decision to defend or not was not explained simply by the emptiness of defensive glands; non-defending larvae retained the fluid into pupation. Less defensive individuals also gained benefits for contributing less: cheating males grew faster than defending males. Altogether, these lower life-history costs for cheats compared to defending individuals could facilitate the evolution of cheating in a cooperative antipredator defence [10,37,49].
We also found that diet quality played an important role in modifying cooperation. Larvae showed a trade-off between allocating resources to cooperative defence at the expense of their immune responses, and this was more pronounced under the high resin diet. High PO activity is usually correlated with higher immunocompetence [41], but it also yields high autoimmune costs, owing to the release of free radicals (a side product of the PO activation pathway). These need to be neutralized to reduce tissue and DNA/RNA damage [50]. The herbivore must also protect its tissues from the direct harmful costs of consuming a high resin diet [27,28,35,36]. As a result, high attack intensity and high resin diet are likely to be physiologically and energetically demanding environments and therefore individuals might have fewer resources to allocate to their immune responses. Another pine sawfly species, N. sertifer, have been suggested to benefit from a high resin acid diet under high risk of predation and virus infection [33,35]. Our results would appear to disagree with these arguments. However, resin acids have been shown to have antimicrobial properties [34], and during an infection, they may increase survival directly [33] without having any effects on the innate immunological responses we measured in this study.
The potency of chemical defence was shaped by diet quality via interactive effects with attack intensity: overall, the volumes of defensive fluid were lower under high attack intensity, but those individuals who fed on a low resin diet were able to upregulate their defensive fluid production and regurgitate larger volumes than individuals on a high resin diet. Again, this could be explained by higher detoxification costs associated with higher resin levels in the diet which could constrain an individual's ability to produce higher volumes. Pine sawfly larvae were also able to compensate for the lower quantities of defensive fluid to some extent as the concentration of terpenes was higher in lower volumes across all of the treatments. The significance of variation in defence quality and quantity is very poorly understood, and we need more behavioural studies that test how the concentration of defensive toxins affects defence efficacy against predators. If defence fluids with low concentrations of defensive toxins are already enough to deter a predator, then only the volume of the produced fluid is likely to be critical [51].
Ecological conditions also partly defined an individual's contribution to cooperation in groups. Individuals defended less under high attack intensity, and this reduction was more pronounced on the low resin compared with the high resin diet. The higher concentration of resin compounds under high attack intensity may have facilitated a more effective sequestration of defensive compounds, enabling a higher proportion of defending individuals. However, under low attack intensity where individuals do not need to refill the glands so frequently, the higher detoxification costs of high resin diet could have decreased the proportion of defending individuals. The effect of diet became marginal for the last instar individuals, who were defending less in the high attack treatment.
One possibility may be that our larvae simply became habituated to the constant attacks and stopped responding. However, this is unlikely because the group defence measurements were done relatively early on the fourth day of the experiment, and results with last instar larvae were similar when we used different types of the stimuli (electronic supplementary material, appendix S5). Our manipulation may have also overestimated the costs of defence (and therefore its effect on individual's cooperative behaviour), because individuals always lost the produced defensive fluid. In the wild, there is the possibility that the defending individual can reabsorb part of the excreted fluid that does not reach the predator. This is, however, unlikely as previous observations indicate that the excreted fluid is rarely recovered under a defence event [18]. Irrespective of how precisely accurate our manipulation was to natural conditions, the results are consistent with a theoretical model by Higginson & Ruxton [31], which predicts that defensive responses will become smaller when cumulative costs of defence and predation risk are high. In other words, in environments where production of cooperative defence is more costly, individuals are more likely to cheat and are less likely to contribute to a common defence [32].
Interestingly, our results suggest that the strength of selection for cooperation and cheating can differ for females and males depending on the ecological context. In females, the high attack intensity treatment reduced growth rate more on the low resin diet than on the high resin diet. However, for the males, diet quality had stronger effect on growth rates than attack intensity (figure 1c). One possible reason for these sex differences is sexual dimorphism in size and development times. Female larvae grew larger and developed for longer than male larvae, and therefore they experienced the experimental environments for longer than males. Females also contributed more to chemical defence in the last instar than males. If males had defended less throughout the experiment, it could explain why attack treatment had a stronger effect on females. Owing to haplodiploidy in pine sawflies, indirect benefits of cooperation are expected to be higher for females in kin groups because sisters are more related to each other than sisters and brothers [1]. These relatedness asymmetries may favour different cooperative and life-history strategies between sexes in this species, similarly to eusocial haplodiploids [1,52,53], and offer a fascinating avenue for future studies.
As Hamilton's rule suggests, to fully understand how environmentally induced variation in costs of cooperation affects its maintenance, we need to consider the costs together with the benefits of cooperative acts and levels of relatedness within cooperative units [1,11]. Based on other studies with chemically defended organisms, we can expect predators' responses to an increase in the frequency of cheats in chemically defended prey group to be nonlinear [16,17,54]. Therefore, positive frequency-dependent selection on defending individuals in a chemically defended prey could allow for a low frequency of non-defending individuals without affecting the per capita survival of prey individuals in a group [17]. In the light of our results, we can predict cooperation to be most favoured under a low resin diet and low attack rates because under both of these conditions some costs of cooperation were low [31], and we observed the highest frequency of defending individuals, which should ensure better protection against predators [16,17]. In future research, we need information on the relatedness structure of sawfly larval groups in the wild and how it may change at different stages of the outbreaks. Multiple mating by pine sawfly females [29] and large larval aggregations during outbreak peaks [28] are likely to reduce the impact of relatedness on the maintenance of cooperation in pine sawflies.
To conclude, our results highlight that detailed knowledge of the ecological interactions under which cooperation occurs, as well as species’ ecology, is necessary to understand the evolution of social behaviours and mechanisms that maintain variation in cooperative interactions [7,8]. One of the challenges for future work, therefore, is to shift focus from two-way interaction between the cooperative actor and the recipient towards more complex biological interactions both in theoretical and empirical research [6,8,9,55–57]. Separating all components of cooperation (c, b and r) individually and testing their effects experimentally should also help to resolve how these multiple biological interactions alter both benefits and especially costs of cooperative behaviour.
Supplementary Material
Acknowledgements
We thank Arne Jungwirth and two anonymous reviewers for the valuable comments. Jimi Kirvesoja, Emma-Liina Marjakangas, Timo Mikkonen, Henna-Riikka Mäenpää and Anna-Lotta Hiillos helped in the maintenance of the labstock and sample analyses. We thank Prof. Monika Hilker and Ute Braun for providing the pine sawflies, and Johanna Mappes for general and intellectual support. The Plantaginis Journal Club, Johanna Mappes, Emily Knott, Rose Thorogood, Lutz Fromhage and Swanne Gordon kindly commented on the manuscript.
Data accessibility
Data have been deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.3885sv0 [47].
Authors' contributions
C.L. conceived the ideas. C.L., A.M. and T.K. designed the methodology. A.M. and C.L. collected the data. H.P. analysed the chemical data. D.F. and A.M. analysed the immunological data. E.M. reared and provided the parental generation of the experimental individuals. A.M., A.L.-S. and C.L. analysed the data and samples. C.L. and A.M. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication. The authors are listed in alphabetical order after C.L. and A.M.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the Academy of Finland via projects no. 257581 (C.L.), 278751 (T.K.), the Centre of Excellence in Biological Interactions (A.M. and D.F.) and the Olvi and Niemi foundations (A.M.).
References
- 1.Hamilton W. 1964. The genetical evolution of social behaviour. J. Theor. Biol . 7, 17–52. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.West SA, Griffin AS, Gardner A. 2007. Evolutionary explanations for cooperation. Curr. Biol . 17, R661–R672. ( 10.1016/j.cub.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 3.Barker JL, Bronstein JL, Friesen ML, Jones EI, Reeve HK, Zink AG, Frederickson ME. 2017. Synthesizing perspectives on the evolution of cooperation within and between species. Evolution 71, 814–825. ( 10.1111/evo.13174) [DOI] [PubMed] [Google Scholar]
- 4.Morgan AD, Quigley BJZ, Brown SP, Buckling A. 2012. Selection on non-social traits limits the invasion of social cheats. Ecol. Lett . 15, 841–846. ( 10.1111/j.1461-0248.2012.01805.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokko H, Chaturvedi A, Croll D, Fischer MC, Guillaume F, Karrenberg S, Kerr B, Rolshausen G, Stapley J. 2017. Can evolution supply what ecology demands? Trends Ecol. Evol . 32, 187–197. ( 10.1016/j.tree.2016.12.005) [DOI] [PubMed] [Google Scholar]
- 6.Friman V-P, Diggle SP, Buckling A. 2013. Protist predation can favour cooperation within bacterial species. Biol. Lett . 9, 20130548 ( 10.1098/rsbl.2013.0548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornwallis CK, Botero CA, Rubenstein DR, Downing PA, West SA, Griffin AS. 2017. Cooperation facilitates the colonization of harsh environments. Nat. Ecol. Evol . 1, 57 ( 10.1038/s41559-016-0057) [DOI] [PubMed] [Google Scholar]
- 8.Avila P, Fromhage L. 2015. No synergy needed: ecological constraints favor the evolution of eusociality. Am. Nat . 186, 31–40. ( 10.1086/681637) [DOI] [PubMed] [Google Scholar]
- 9.Groenewoud F, Frommen JG, Josi D, Tanaka H, Jungwirth A, Taborsky M. 2016. Predation risk drives social complexity in cooperative breeders. Proc. Natl Acad. Sci . USA 113, 4104–4109. ( 10.1073/PNAS.1524178113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speed MP, Ruxton GD, Mappes J, Sherratt TN. 2012. Why are defensive toxins so variable? An evolutionary perspective. Biol. Rev . 87, 874–884. ( 10.1111/j.1469-185X.2012.00228.x) [DOI] [PubMed] [Google Scholar]
- 11.Best R, Ruxton GD, Gardner A. 2018. Intragroup and intragenomic conflict over chemical defense against predators. Ecol. Evol . 8, 3322–3329. ( 10.1002/ece3.3926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jousset A, Rochat L, Péchy-Tarr M, Keel C, Scheu S, Bonkowski M. 2009. Predators promote defence of rhizosphere bacterial populations by selective feeding on non-toxic cheaters. ISME J. 3, 666 ( 10.1038/ismej.2009.26) [DOI] [PubMed] [Google Scholar]
- 13.Diggle SP, Griffin AS, Campbell GS, West SA. 2007. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450, 411–414. ( 10.1038/nature06279) [DOI] [PubMed] [Google Scholar]
- 14.Leimar O, Enquist M, Sillen-Tullberg B. 1986. Evolutionary stability of aposematic coloration and prey unprofitability: a theoretical analysis. Am. Nat . 128, 469–490. ( 10.1086/284581) [DOI] [Google Scholar]
- 15.Rowland HM, Ihalainen E, Lindström L, Mappes J, Speed MP. 2007. Co-mimics have a mutualistic relationship despite unequal defences. Nature 448, 64–67. ( 10.1038/nature05899) [DOI] [PubMed] [Google Scholar]
- 16.Lindström L, Alatalo RV, Mappes J. 1997. Imperfect Batesian mimicry: the effects of the frequency and the distastefulness of the model. Proc. R. Soc. Lond. B 264, 149–153. ( 10.1098/rspb.1997.0022) [DOI] [Google Scholar]
- 17.Jones SR, Davis SC, Speed MP. 2013. Defence cheats can degrade protection of chemically defended prey. Ethology 119, 52–57. ( 10.1111/eth.12036) [DOI] [Google Scholar]
- 18.Eisner T, Johnessee JS, Carrel J, Hendry LB, Meinwald J. 1974. Defensive use by an insect of a plant resin. Science 184, 996–999. ( 10.1126/science.184.4140.996) [DOI] [PubMed] [Google Scholar]
- 19.Codella SG Jr, Raffa KF. 1996. Individual and social components of wood ant response to conifer sawfly defence (Hymenoptera: Formicidae, Diprionidae). Anim. Behav . 52, 801–811. ( 10.1006/anbe.1996.0225) [DOI] [Google Scholar]
- 20.Costa J. 2006. Other insect societies. Cambridge, MA: The Belknap Press of Harvard University Press. [Google Scholar]
- 21.Lindstedt C, Huttunen H, Kakko M, Mappes J. 2011. Disengtangling the evolution of weak warning signals: high detection risk and low production costs of chemical defences in gregarious pine sawfly larvae. Evol. Ecol . 25, 1029–1046. ( 10.1007/s10682-010-9456-4) [DOI] [Google Scholar]
- 22.Lindstedt C, Mappes J, Päivinen J, Varama M. 2006. Effects of group size and pine defence chemicals on Diprionid sawfly survival against ant predation. Oecologia 150, 519–526. ( 10.1007/s00442-006-0518-9) [DOI] [PubMed] [Google Scholar]
- 23.Sillén-Tullberg B. 1990. Do predators avoid groups of aposematic prey? An experimental test. Anim. Behav . 40, 856–860. ( 10.1016/S0003-3472(05)80986-8) [DOI] [Google Scholar]
- 24.Codella SG, Raffa KF. 1995. Contributions of female oviposition patterns and larval behavior to group defense in conifer sawflies (hymenoptera: diprionidae). Oecologia 103, 24–33. ( 10.1007/BF00328421) [DOI] [PubMed] [Google Scholar]
- 25.Riipi M, Alatalo RV, Lindström L, Mappes J. 2001. Multiple benefits of gregariousness cover detectability costs in aposematic aggregations. Nature 413, 512–514. ( 10.1038/35097061) [DOI] [PubMed] [Google Scholar]
- 26.Codella SG, Raffa KF. 1995. Host plant influence on chemical defense in conifer sawflies (Hymenoptera: Diprionidae). Oecologia 104, 1–11. ( 10.1007/BF00365555) [DOI] [PubMed] [Google Scholar]
- 27.Björkman C, Larsson S. 1991. Pine sawfly defence and variation in host plant resin acids: a trade-off with growth. Ecol. Entomol . 16, 283–289. ( 10.1111/j.1365-2311.1991.tb00219.x) [DOI] [Google Scholar]
- 28.Björkman C, Larsson S, Bommarco R. 1997. Oviposition preferences in pine sawflies: a trade-off between larval growth and defence against natural enemies. Oikos 79, 45–52. ( 10.2307/3546088) [DOI] [Google Scholar]
- 29.Östrand F, Anderbrant O. 2001. Mating duration and frequency in a pine sawfly. J. Insect Behav . 14, 595–606. ( 10.1023/A:1012271100412) [DOI] [Google Scholar]
- 30.Wagner M, Raffa KF. 1993. Sawfly life history adaptations to woody plants. New York, NY: Academic Press. [Google Scholar]
- 31.Higginson AD, Ruxton GD. 2009. Dynamic state-dependent modelling predicts optimal usage patterns of responsive defences. Oecologia 160, 399–410. ( 10.1007/s00442-009-1296-y) [DOI] [PubMed] [Google Scholar]
- 32.Higginson AD, Delf J, Ruxton GD, Speed MP. 2011. Growth and reproductive costs of larval defence in the aposematic lepidopteran Pieris brassicae. J. Anim. Ecol . 80, 384–392. ( 10.1111/j.1365-2656.2010.01786.x) [DOI] [PubMed] [Google Scholar]
- 33.Kollberg I, Bylund H, Schmidt A, Gershenzon J, Björkman C. 2013. Multiple effects of temperature, photoperiod and food quality on the performance of a pine sawfly. Ecol. Entomol . 38, 201–208. ( 10.1111/een.12005) [DOI] [Google Scholar]
- 34.de Roode JC, Lefevre T, Hunter MD. 2013. Self-medication in animals. Science 340, 150–151. ( 10.1126/science.1235824) [DOI] [PubMed] [Google Scholar]
- 35.Larsson S, Ekbom B, Bjorkman C. 2000. Influence of plant quality on pine sawfly population dynamics. Oikos 89, 440–450. ( 10.1034/j.1600-0706.2000.890303.x) [DOI] [Google Scholar]
- 36.Larsson S, Björkman C, Gref R. 1986. Responses of Neodiprion sertifer (Hym., Diprionidae) larvae to variation in needle resin acid concentration in Scots pine. Oecologia 70, 77–84. ( 10.1007/BF00377113) [DOI] [PubMed] [Google Scholar]
- 37.Ghoul M, Griffin AS, West SA. 2014. Toward an evolutionary definition of cheating. Evolution 68, 318–331. ( 10.1111/evo.12266) [DOI] [PubMed] [Google Scholar]
- 38.Frank SA. 2010. A general model of the public goods dilemma. J. Evol. Biol . 23, 1245–1250. ( 10.1111/j.1420-9101.2010.01986.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Björkman C, Kytö M, Larsson S, Niemelä P. 1998. Different responses of two carbon-based defences in Scots pine needles to nitrogen fertilization. Écoscience 5, 502–507. ( 10.1080/11956860.1998.11682484) [DOI] [Google Scholar]
- 40.Nerg A, Kainulainen P, Vuorinen M, Hanso M, Holopainen JK, Kurkela T. 1994. Seasonal and geographical variation of terpenes, resin acids and total phenolics in nursery grown seedlings of Scots pine (Pinus sylvestris L.). New Phytol. 128, 703–713. ( 10.1111/j.1469-8137.1994.tb04034.x) [DOI] [Google Scholar]
- 41.Freitak D, Heckel DG, Vogel H. 2009. Dietary-dependent trans-generational immune priming in an insect herbivore. Proc. R. Soc. B 276, 2617–2624. ( 10.1098/rspb.2009.0323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson K, Cotter SC, Reeson AF, Pell JK. 2001. Melanism and disease resistance in insects. Ecol. Lett . 4, 637–649. ( 10.1046/j.1461-0248.2001.00279.x) [DOI] [Google Scholar]
- 43.Cerenius L, Soderhall K. 2004. The prophenoloxidase-activating system in invertebrates. Immunol. Rev . 198, 116–126. ( 10.1111/j.0105-2896.2004.00116.x) [DOI] [PubMed] [Google Scholar]
- 44.Strand MR. 2008. The insect cellular immune response. Insect Sci . 15, 1–14. ( 10.1111/j.1744-7917.2008.00183.x) [DOI] [Google Scholar]
- 45.Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol . 50, 529–551. ( 10.1146/annurev.ento.50.071803.130420) [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa S, Schielzeth H. 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol. Rev . 85, 935–956. ( 10.1111/j.1469-185X.2010.00141.x) [DOI] [PubMed] [Google Scholar]
- 47.Lindstedt C, Miettinen A, Freitak D, Ketola T, López-Sepulcre A, Mäntylä E, Pakkanen H. 2018. Data from: Ecological conditions alter cooperative behaviour and its costs in a chemically defended sawfly Dryad Digital Repository. ( 10.5061/dryad.3885sv0) [DOI] [PMC free article] [PubMed]
- 48.de Jong PW, Holloway GJ, Brakefield PM, de Vos H. 1991. Chemical defence in ladybird beetles (Coccinellidae). II. Amount of reflex fluid, the alkaloid adaline and individual variation in defence in 2-spot ladybirds (Adalia bipunctata). Chemoecology 2, 15–19. ( 10.1007/BF01240661) [DOI] [Google Scholar]
- 49.Daly D, Higginson AD, Chen D, Ruxton GD, Speed MP. 2012. Density-dependent investment in costly anti-predator defences: an explanation for the weak survival benefit of group living. Ecol. Lett . 15, 576–583. ( 10.1111/j.1461-0248.2012.01770.x) [DOI] [PubMed] [Google Scholar]
- 50.Sadd BM, Siva-Jothy MT. 2006. Self-harm caused by an insect's innate immunity. Proc. R. Soc. B 273, 2571–2574. ( 10.1098/rspb.2006.3574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindstedt C, Boncoraglio G, Cotter S, Gilbert J, Kilner R. 2017. Aposematism in the burying beetle? Dual function of anal fluid in parental care and chemical defence. Behav. Ecol . 28, 1414–1422. ( 10.1093/beheco/arx100) [DOI] [Google Scholar]
- 52.Boomsma JJ, Franks NR. 2006. Social insects: from selfish genes to self organisation and beyond. Trends Ecol. Evol . 21, 303–308. ( 10.1016/j.tree.2006.04.001) [DOI] [PubMed] [Google Scholar]
- 53.Trivers RL, Hare H. 2009. Haplodiploidy and the evolution of the social insects. Science 191, 249–263. ( 10.1126/science.1108197) [DOI] [PubMed] [Google Scholar]
- 54.Lindström L, Alatalo RV, Lyytinen A, Mappes J. 2001. Strong antiapostatic selection against novel rare aposematic prey. Proc. Natl Acad. Sci. USA 98, 9181–9184. ( 10.1073/pnas.161071598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubenstein DR. 2011. Spatiotemporal environmental variation, risk aversion, and the evolution of cooperative breeding as a bet-hedging strategy. Proc. Natl Acad. Sci. USA 108(Suppl. 2), 10 816–10 822. ( 10.1073/pnas.1100303108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jungwirth A, Josi D, Walker J, Taborsky M.. 2015. Benefits of coloniality: communal defence saves anti-predator effort in cooperative breeders. Funct. Ecol . 29, 1218–1224. ( 10.1111/1365-2435.12430) [DOI] [Google Scholar]
- 57.Whitehouse MEA, Lubin Y. 2005. The functions of societies and the evolution of group living: spider societies as a test case. Biol. Rev . 80, 347–361. ( 10.1017/S1464793104006694) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lindstedt C, Miettinen A, Freitak D, Ketola T, López-Sepulcre A, Mäntylä E, Pakkanen H. 2018. Data from: Ecological conditions alter cooperative behaviour and its costs in a chemically defended sawfly Dryad Digital Repository. ( 10.5061/dryad.3885sv0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data have been deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.3885sv0 [47].