Abstract
The relative contributions of genetic and social factors in shaping the living world are a crucial question in ecology. The annual migration of birds to their wintering grounds and back provides significant knowledge in this field of research. Migratory movements are predominantly genetically determined in passerine birds, while in large soaring birds, it is presumed that social (cultural) factors play the largest role. In this study, we show that genetic factors in soaring birds are more important than previously assumed. We used global positioning system (GPS)-telemetry to compare the autumn journeys and wintering ranges of two closely related large raptorial bird species, the greater spotted eagle Clanga clanga and the lesser spotted eagle Clanga pomarina, and hybrids between them. The timing of migration in hybrids was similar to that of one parental species, but the wintering distributions and home range sizes were similar to those of the other. Tracking data were supported by habitat suitability modelling, based on GPS fixes and ring recoveries. These results suggest a strong genetic influence on migration strategy via a trait-dependent dominance effect, although we cannot rule out the contribution of social interactions.
Keywords: genetic dominance, hybridization, spotted eagle, timing, migration route, wintering
1. Background
The relative contributions of genes and the environment in shaping the living world are a crucial question in biology. Among non-genetic factors, the influence of social interactions has been a subject of debate in various research disciplines but has also led to many fruitful studies in ecology [1,2]. Movement ecology has provided an enormous amount of information related to animal behaviour, although most studies have simply measured and described the movement of organisms, without reference to ecological or internal factors [3].
The annual migration of birds to their wintering grounds and back is among the most conspicuous of animal movements. These journeys have provided an opportunity to study the roles of various determinants of behaviour in avian taxa [4,5]. Experimental [6–9] and field studies [8,10] have demonstrated a predominantly genetic basis of migration strategies in passerine birds. The most important innate mechanism influencing the timing, direction and duration of migration is often called the ‘clock-and-compass’ mechanism (reviewed in [8,11,12]). However, the complete migratory syndrome is highly complex and varies among species; it involves physiological adaptations, specific sensors and behavioural mechanisms and is assumed to be governed by the so-called ‘migratory gene package’ [13]. Alternatively, non-genetic mechanisms may be important. Both field studies [14,15] and models [16,17] have suggested that social (cultural) factors are more important than genetic factors in long-lived non-passerines. Social learning is well supported by the experimental translocation of individuals from day-migrating soaring species, such as storks [18] and cranes [19], which are gregarious during migration. However, a similar mechanism has also been suggested to explain the migratory paths of birds of prey, which usually migrate alone [20–23].
Closely related species or even neighbouring populations of the same species can differ dramatically in their migratory behaviour and wintering ranges [24,25]. Such species may also hybridize, which, as a natural cross-breeding experiment, sheds light on the inheritance pattern of migratory traits. Mechanisms underlying migration have been revealed by artificial cross-breeding or field studies of hybrid individuals from populations across zones of migratory divides [7,10,26–28]. Interspecific hybrids may migrate similarly to one of the two parental species, reflecting the dominance of genetic determinants [28–30], with intermediate characteristics revealing an additive effect [7,8,27,31]. Alternatively, hybrids may exhibit variation in traits ranging from those of one parental species to those of another, which may be associated with sex-linked inheritance [10,28]. Migration studies of wild avian hybrids have focused on small passerines, and therefore have been limited by imprecise methods, such as assessments of stable isotope profiles [28,29] or analyses using light-level geolocators [10]. In this study, we applied precise global positioning system (GPS) tracking to analyse the migration of hybrids.
We studied large raptorial birds, i.e. the greater spotted eagle Clanga clanga and the lesser spotted eagle Clanga pomarina, which use mainly soaring-gliding flight during migration. The wintering ranges of these two species differ substantially. Clanga clanga is generally a short-distance migrant, wintering in southern Eurasia and northeast Africa, while the long-distance migrant C. pomarina moves to southern Africa. Clanga clanga population sizes have declined significantly, and a shortage of conspecifics and vanishing habitat barriers [32,33] have forced this globally vulnerable eagle to interbreed with the more numerous C. pomarina across their broad contact zone in eastern Europe [34].
Bird migration is a complex phenomenon characterized by many factors, which often are intercorrelated [5]. We chose four well-established variables (the date of departure breeding territory, longitude, latitude and size of the individual wintering range) to study the timing and routes of migration and the wintering strategy of hybrid spotted eagles, compared with those of the two parental species. We marked 62 C. clanga, C. pomarina and hybrid individuals with GPS tags and determined whether characteristics of migration and wintering behaviour in interspecific hybrids resembled those of parental species. We also evaluated whether the observed patterns are consistent with dominance, additive effects or sex-linked inheritance of genetic determinants.
2. Methods
(a). Study species and marking of birds
Clanga clanga is one of the rarest eagle species in Europe. Within the European Union (EU), only small declining populations remain in Poland (up to 20 birds) and Estonia (fewer than 10 birds); in other EU countries, only a few individuals have recently been found (e.g. in Lithuania) [35]. Clanga pomarina is also protected, but breeds at a much higher density, mostly in central and eastern Europe (16 400–22 100 pairs) [35].
Between 2005 and 2016, 128 autumn journeys of 62 eagles originating from Estonia, Lithuania and Poland were studied (one to three birds per year in 2005–2010, 11–23 birds per year in 2011–2016). The total sample included 27 C. clanga, 21 C. pomarina and 14 hybrids belonging to both sexes and various age groups (electronic supplementary material, table S1), accounting for a substantial proportion of the C. clanga population and hybrids in the western margin of its range.
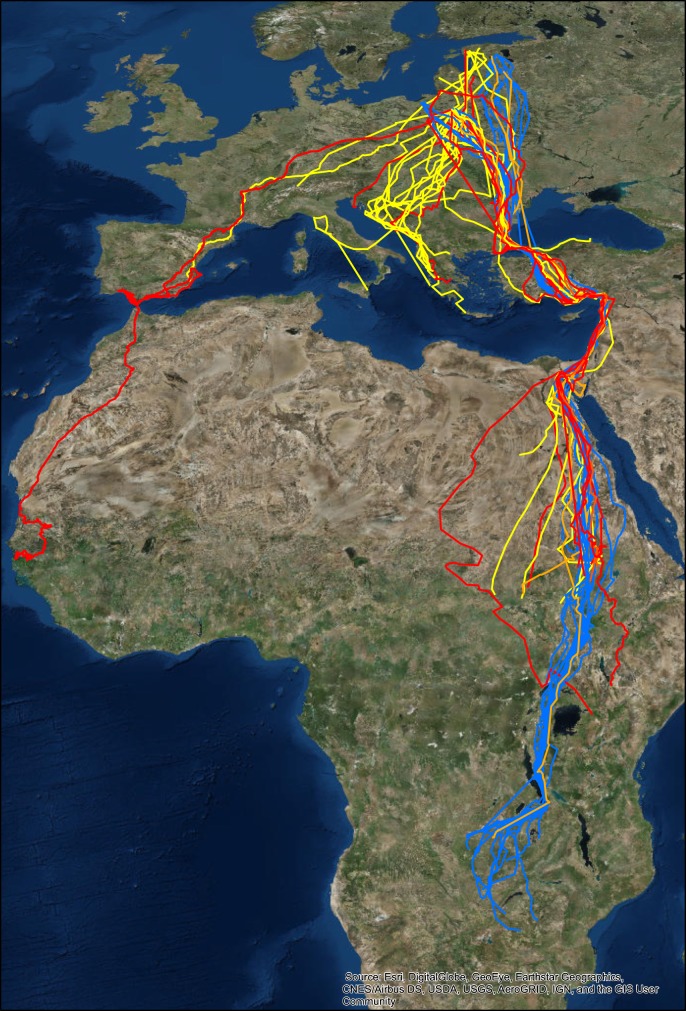
The species identity of the studied individuals was determined by morphological [36,37] and genetic analysis using a combination of neutral microsatellite and single-nucleotide polymorphism markers [34,38]. The genetic method, applied here to approximately 1500 referenced individuals sampled across Europe, separates hybrids efficiently from parental species, and, with a somewhat higher error rate, separates F1 hybrids from backcrosses to the parental species [38]. The migration routes of these groups are presented separately (figure 1), but, to account for the small number of backcrosses (three birds were identified as F1 × C. pomarina) and to eliminate any potential errors, all of the hybrids were pooled for statistical analyses. Birds were sexed based on the chromo-helicase-DNA-binding protein (CHD) gene [39], wing length or behaviour (adult birds only).
Figure 1.
Autumn migration routes of the spotted eagles followed in the current study. Tracks of C. clanga are shown in yellow, C. pomarina in blue, F1 hybrids in red and F1 × C. pomarina backcrosses in orange. Note that all sea crossings, except the flight over the Adriatic Sea to Italy and over the Gibraltar Strait, are artefacts owing to gaps in the dataset.
Birds were equipped with a 30–45 g (less than 2% of the body mass of the birds) solar-powered GPS tag as a backpack. These were either satellite transmitters that registered the location every 2 h during the daytime (Microwave Telemetry Inc., Northstar Inc.) or Global System for Mobile communications (GSM) transmitters, which could be programmed remotely (Aquila IT, Ecotone, Ornitela), enabling us to change programmes according to bird behaviour and location. The types of GPS devices and their mean sampling frequencies for each bird are presented in the electronic supplementary material, table S2. Since various types of devices were used over the study period, the number of locations for each bird differed considerably, and this variation has the potential to influence the results. However, significant correlations between the variables of interest and the number of GPS fixes were not detected (timing, r = –0.12, p = 0.48; wintering range, r = 0.03, p = 0.85).
(b). Migration and wintering data
Spotted eagles have relatively small home ranges, and most of their flights during the breeding season are within a radius of a few kilometres around the nest [40]. Therefore, the onset of migration was easy to distinguish from local flights. The onset of each migratory journey was first determined by expert opinion based on a sudden change in the movement pattern of a tagged individual (a long, straight flight in the main migratory direction). Thereafter, to objectively verify this choice, the daily distance between the two most distant locations in a given full day was checked to verify that it exceeded a threshold of 56 km, which is exactly double the distance from nest registered for spotted eagles in a single day on the breeding grounds. The last locations at the breeding home range and first migration overnight locations (both randomly modified for conservation reasons) are presented in the electronic supplementary material, table S3. An individual was considered to have ended its migration when it stopped this clearly southward directed continuous movement and the same threshold value was not exceeded in the next 5 days (after stopovers, southward migration was continued).
In total, one to eight autumn journeys were followed per bird. As the timing differed substantially among years, the departure and arrival dates for each year were included in the analysis, but year was added as a random variable to the model (see below). In most cases, individual consistency in route and wintering quarter selection was observed; hence to avoid pseudo-replication effects, only one route and wintering site per adult were analysed. However, as migration performance may change according to the age of the bird [41], for juvenile birds, tracks as a second-year bird (10 individuals) or as an adult (one individual), if available, were also evaluated, and individual identity was included as a random factor in the model. As the migration of juveniles as a group may be different from that in other age classes [20,41], all analyses were repeated without juvenile birds, but a significant difference compared with the analysis using the full dataset, presented in Results, was not detected. Our study included related birds (adult–offspring pairs or offspring from consecutive years; electronic supplementary material, table S1). Therefore, identity of breeding territory, a proxy for relatedness, was included as a random factor in the model.
Journeys from the breeding to wintering grounds are complex phenomena determined by many intercorrelated factors. To simplify the analysis and interpretation, only the most straightforward and universal variables were used when testing for differences between groups (data presented in the electronic supplementary material, tables S3–S5). Migration timing was defined as the date of departure from the breeding site. The location of the wintering home range was characterized by the geometric mean latitude and longitude. Wintering strategy was revealed by the size of an individual's winter home range. The direction and distance between breeding and wintering sites are also widely used in migration studies; therefore, these distances as loxodromes (rhumb lines) were evaluated using the birdring package v. 1.3 for R [42]. The direction of the wintering site with respect to the breeding site was highly correlated with the longitude (r = –0.89, p < 0.001), and total flight distance during migration was highly correlated with the latitude (r = –0.96, p < 0.001). As the latitude and longitude of the wintering site depend less on the location of the breeding site, only these variables were used to characterize the spatial migration strategy.
(c). Data analysis
To overcome possible bias resulting from limited samples of tracked individuals, habitat suitability models were built (using Maxent v. 3.3.3 k) to check for overlap between the wintering ranges of the target species and their hybrids [43]. The input data were the GPS fixes acquired in the course of the telemetry studies for C. clanga (16 942 fixes), C. pomarina (8790 fixes) and hybrids (7808 fixes; electronic supplementary material, tables S6–S8). The variables used to predict habitat suitability were 19 bioclimatic variables downloaded at a 2.5 min raster resolution from the WorldClim database [44] and 5 min resolution global MODIS land cover [45]. Prior to model execution, using ArcGIS v. 10.3 [46], values for each variable were extracted using pooled GPS locations from the wintering period of all individuals of both species. Then, a Pearson correlation matrix was built to discard the strongly correlated variables (with r > 0.6). The predictors were the remaining, uncorrelated variables, i.e. land cover, annual mean temperature (bio1), annual temperature range (bio7), mean temperature during the driest quarter (bio9), annual precipitation (bio12), precipitation during the warmest quarter (bio18) and precipitation during the coldest quarter (bio19). Each raster variable was cropped to the extent of data (WGS84 extend top: 75.4, left: –18.8, right: 55.0, bottom: –36.4). Land use was upscaled by the nearest neighbour method using ArcGIS.
Models were evaluated first at the individual level and then at the species level. In the first approach, separate models were built for each individual tracked for at least three winter months (n = 18 models for C. clanga, 10 for C. pomarina and six for hybrids). All data were used as training datasets. Final models were obtained by averaging all models for a given species/hybrids using Raster Calculator in ArcGIS. In the second approach, GPS fixes were pooled for species/hybrids groups and assigned to training (75%) and testing (25%) datasets. A jack-knife test was used to determine the variables that yielded the best gain during model training. Finally, models at the individual and species levels were averaged to smooth the biases resulting from the two different approaches.
Models were validated in two ways. First, receiver operating characteristic curves were compared between the training and testing data, and the area under the curve (AUC) was used to evaluate the models. Model validation with AUC values is a standard procedure, although it has been criticized [47]. Therefore, the model was also validated using an external dataset from the recovery of wintering C. clanga (n = 13) and hybrid (n= 5) individuals in Poland and Estonia (electronic supplementary material, table S9); the model for C. pomarina was highly consistent with a number of earlier telemetry studies of this species ([22] and references therein). It was assumed that places suitable for wintering were those in which the modelled habitat suitability was equal to or greater than the threshold for the maximum training sensitivity plus specificity [48]. A binary surface of suitable/non-suitable areas and another with ringing recoveries marked as single pixels of equal resolution to the Maxent models were created. Validation was conducted using Map Comparison Kit v. 3.2.3 [49] with a fuzzy inference system [50] to check if both matched, taking into account a fuzzy binary relation.
The size of winter home ranges was calculated using all available GPS fixes for each tracked bird (except for one individual, in which 1010 points were drawn from an incomparably larger dataset consisting of 21 632 locations). Home ranges were estimated by applying 95% kernel density functions using ArcMET plugin v. 10.2.2.v. 2 [51] within ArcGIS. The reference bandwidth was used, and the output raster resolution was set to 200 m for the small- and medium-range wintering sites and 400 m for large sites. Calculations were performed using the cylindrical equal-area projection.
Interspecific differences in the onset of migration as well as in the location and size of wintering home ranges were analysed using R v. 3.3.3 [52]. As the number of variables was not large and the sample sizes were small and differed between variables, separate models were developed for each variable. The homogeneity of variances was evaluated using the Fligner–Killeen test. Differences between group means were evaluated by linear mixed models (LMMs) using the lme function of the package nlme v. 3.1-131 [53]. The significance of differences between groups was evaluated by assessing the deviance of the model after the removal of the explanatory variable using a likelihood-ratio test. Additionally, the difference between Akaike's information criterion (ΔAIC) values for the models with and without the explanatory variables was calculated. Julian date, longitude, latitude and home range size were the response variables, group (either species or hybrids) was included as an independent fixed factor, and individual identity and breeding territory were included as random factors. In the model of migration timing, ‘year’ was also used as a random factor. To explore the sample size limitation, the analysis was repeated without ‘year’, but nearly identical results were obtained (ΔAIC = 2.0; likelihood ratio (LR) < 0.1, p = 1). To control for detected homoscedasticity, weights were applied to the grouping factor levels using the function VarIdent in nlme. The significance of differences between the groups was tested by Tukey post hoc comparisons using the function glht in the package multcomp v. 1.4-1 [54]. The R-script is available in the electronic supplementary material.
3. Results
In the initial models, we detected no effect of sex on the relationships between the four variables and the migration and wintering properties of birds. Therefore, we pooled data for both sexes for further analyses and focused on detecting differences between hybrids and the two species.
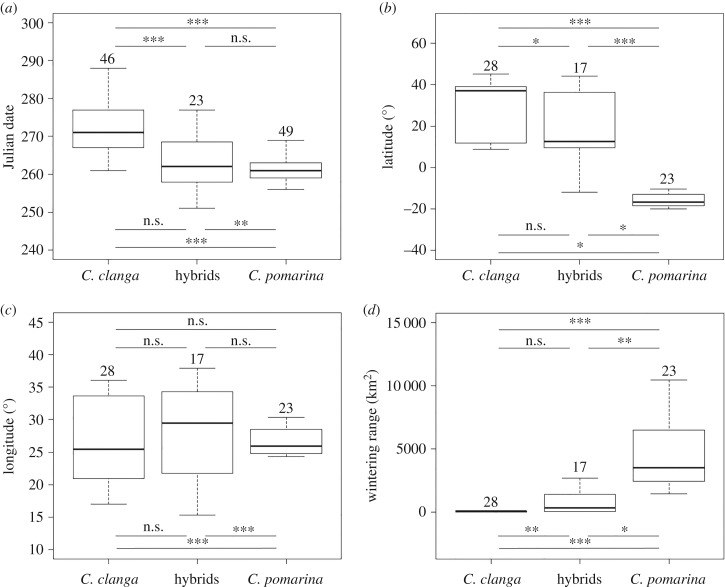
The tracked C. clanga individuals left their breeding sites significantly later than C. pomarina or hybrids, and we did not detect a difference between the latter two groups (figure 2a and table 1; electronic supplementary material, video V1). However, we observed significantly less variation among departure times in C. pomarina than in C. clanga or hybrids (figure 2a).
Figure 2.
Differences between the two species and hybrids with respect to various migration characteristics. Start of the autumn migration (a), latitude (b), longitude (c), and size of the individual home range (d). The bold line indicates the median, the box shows quartiles and the whiskers indicate the extreme data points that are not beyond 1.5× the interquartile range from the quartile boundaries. The adjusted level of significance of the differences between group means (GLMM post hoc Tukey comparisons) is indicated above and the adjusted level of significance of the differences between variances (Fligner–Killeen test) is shown below the boxes: *p < 0.05, **p < 0.01, ***p < 0.001, n.s., not significant. Above each group, sample sizes are presented.
Table 1.
LMMs of between-group differences in variables characterizing migration. (Individual identity was controlled for by including it as a random effect in the model of migration timing.)
| dependent variable | group | parameter estimate ± s.e. | N | LR test | p-value | AIC | ΔAIC |
|---|---|---|---|---|---|---|---|
| mean start date of autumn migration | intercept (C. clanga) | 271.9 ± 1.1 | 118 | 44.6 | <0.001 | 746.3 | 40.6 |
| hybrids | −8.6 ± 1.9 | ||||||
| C. pomarina | −10.9 ± 1.4 | ||||||
| mean latitude of individual wintering ranges | intercept (C. clanga) | 29.3 ± 2.6 | 57 | 60.0 | <0.001 | 446.5 | 56.0 |
| hybrids | −13.1 ± 4.9 | ||||||
| C. pomarina | −45.0 ± 2.8 | ||||||
| mean longitude of individual wintering ranges | intercept (C. clanga) | 25.2 ± 1.8 | 57 | 1.0 | 0.597 | 413.0 | −3.0 |
| hybrids | −1.0 ± 4.3 | ||||||
| C. pomarina | 1.5 ± 1.9 | ||||||
| mean size of individual wintering ranges | intercept (C. clanga) | 67.6 ± 32.8 | 38 | 13.6 | 0.001 | 604.9 | 9.6 |
| hybrids | 773.5 ± 376.0 | ||||||
| C. pomarina | 5303.8 ± 1395.2 |
Clanga clanga migrated in a wide range of directions and wintered in southern Europe, Anatolia and eastern Africa (southeastern Sahel; figure 1; electronic supplementary material, V1). All of the tracked C. pomarina individuals used a narrow migration corridor to southern Africa (figure 1; electronic supplementary material, V1), although a few ring recoveries also indicated the use of another route east of the Black Sea. We detected significant interspecific differences in wintering ranges with respect to latitude but not longitude, and observed significantly greater variation in wintering range selection in C. clanga than in C. pomarina (figures 2b,c and 3 and table 1).
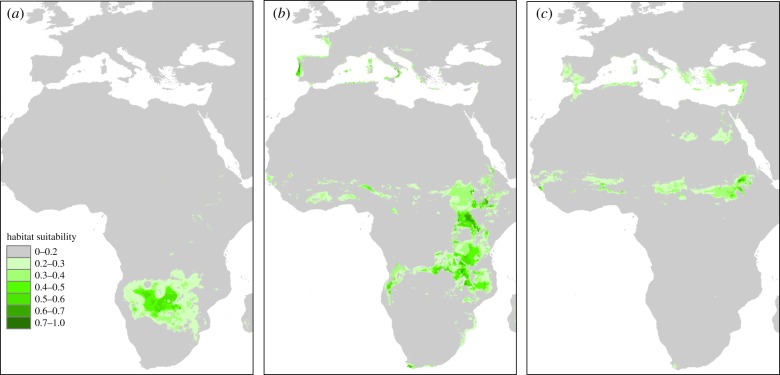
Figure 3.
Distribution of the suitable wintering ranges of Clanga pomarina (a), hybrids (b), and C. clanga (c) according to habitat suitability modelling. The models showed high accuracy as the AUC value for the test data reached 0.930 in the case of C. clanga, 0.898 for C. pomarina, and 0.935 for hybrids. Global matching with ringing recoveries (fuzzy inference system) reached 0.819 for C. clanga and 0.806 for hybrids, confirming the reliability of models. (Online version in colour.)
The median range of wintering hybrid eagles was located at intermediate latitudes, although the ranges of C. clanga and the hybrids overlapped to a great extent (figures 2b and 3). Upon the application of a suitability threshold, the overlap of the modelled suitable wintering range reached 7.6% between C. clanga and hybrids, 1.1% between hybrids and C. pomarina and only 0.02% between C. clanga and C. pomarina. There was no difference in the longitudes of the wintering sites between hybrids and the other groups, but, similar to C. clanga, we detected significantly greater variation in longitudes for the hybrids than for C. pomarina (figure 2c and table 1).
Habitat suitability modelling indicated that the wintering sites of C. clanga were defined by precipitation during the coldest quarter and the mean annual precipitation, while the greatest gain in the C. pomarina model was attributed to the land cover type and the mean temperature of the driest quarter of the year. The most suitable wintering sites of hybrids were determined by the annual temperature range and precipitation. The wintering home ranges of C. pomarina individuals were significantly larger than those of C. clanga or hybrids, and we did not detect any significant difference between the two latter groups (figure 2d and table 1).
4. Discussion
It has been suggested that, in large birds, which typically use soaring flight during migration, the expression of an individual's migratory journey is predominately determined by learning and the social transmission of migratory behaviour, rather than by an inherited migration programme [5]. One may argue that this applies only to gregarious species, which form large migration flocks and whose families migrate together, such as storks and cranes [18,19]. Eagles have prolonged parental care, but families usually migrate separately [55], which reduces the opportunity to learn migration routes from parents. However, a recent translocation experiment suggested the importance of learning for the selection of migration routes in C. pomarina [22]. Moreover, small flocks with other conspecifics could form during various stages of migration, and high concentrations of C. pomarina individuals are found at migration bottlenecks [56–58], creating an opportunity for observational learning.
However, the importance of learning was not supported by our results, although we acknowledge the limitations of our data. If social influence predominated in spotted eagles, one would expect hybrids to follow C. pomarina to their wintering range because hybrids started their migration as early as C. pomarina, while most C. clanga individuals departed their breeding grounds significantly later. This was not the case, as the wintering ranges of most hybrids were similar to those of C. clanga, and the median latitude was intermediate between those of the two species. Additionally, there was substantial variation among wintering longitudes in hybrids, similar to observations in C. clanga. Finally, the wintering strategy of hybrids also resembled the strategy of C. clanga in that they often used a restricted home range instead of travelling long distances over the course of winter, which is a typical behaviour in C. pomarina.
These observations suggest genetic influences on the selection of a migration strategy. Our results corroborate the results of previous displacement experiments using wild-caught migrants and orientation tests illustrating that genetic factors in birds determine the direction and approximate distance of migration [8]. Unlike the authors, we argue that the recent translocation experiment of naive C. pomarina from Latvia to Germany [22] also suggests that migration has a genetic basis. Differently from native German birds, most eagles translocated from Latvia headed south. This direction would have led them from their original location directly to the Bosporus land-bridge; however, after translocation, these individuals perished in the Mediterranean Sea. This is direct evidence for the elimination of non-adaptive genes by natural selection. The experiment also supports the genetic determination of timing because translocated birds started their migration significantly earlier than local birds. This is expected because they originated from higher latitudes where earlier autumnal weather conditions become less suitable for soaring flight.
It has been argued that, if the migration route is largely genetically determined, then individual birds would follow roughly the same route each year [59], and learning may result in the slight adjustment of paths over the years [41]. This was the case for the spotted eagles in the present study, which virtually always used the same wintering site and often followed the same route over the years. In only a few cases, we noted differences in routes and destinations between the first 2 years of life, suggesting the importance of learning. Genetic determination is further supported by two examples of studied families verified by genetic analyses. First, tracking data and ring recoveries indicated that four studied brothers of C. clanga from four different years migrated to southwestern Europe; second, a tracked hybrid female wintered in Sicily, and her brother was identified by a colour ring in Sardinia. However, a tracked hybrid juvenile migrated to east Africa, but offspring from the same nest migrated at least twice to west Africa; parental identity was not known in this case.
Several hypotheses have been proposed to explain the genetic determination of migration and the selection of wintering grounds in hybrids. We found only weak support for an additive genetic effect, which would result in an intermediate wintering strategy in hybrids, and no support for sex-linked inheritance. It is important to note that hybridization between spotted eagles, at least in the western part of the range, is strongly asymmetric, predominantly between female C. clanga and male C. pomarina [34], which sets limits for the separation of sex- and species-specific effects. This does not influence Z-chromosomal linkage, which is dependent only on the sex of hybrids, but leaves open the potential for maternal W-linked or cytoplasmic inheritance. However, in this study, the mother of the abovementioned four C. clanga males used a completely different flyway and wintered in Anatolia, more than 3000 km from her sons. The most parsimonious explanation for our data is the genetic dominance mechanism, which predicts the clustering of hybrids with one parental species. However, the dominance effect differed between the determination of migration routes and migration timing. This is somewhat surprising, because timing and wintering destination are interactive and are crucial traits from an evolutionary perspective, i.e. birds need to leave in a very specific time interval to arrive at their destination at the appropriate time. Accordingly, these variables should ideally be orchestrated together, but our data suggest that they are under different selective pressures.
In long-lived birds, it is likely that the innate genetic programme acts in concert with other determinants of migration. For example, daylength and weather are important triggers of avian migration [60,61], and a suite of different compasses may be cross-calibrated or integrated for direction finding, depending on the geographical and ecological situation [11]. Social interactions strongly affect migratory schedules [62], and the departure of spotted eagles from breeding grounds may be influenced by encounters with conspecifics [22]. Hence, the onset of migration of hybrids may be additionally induced by early-departing C. pomarina. The importance of learning and social cues in finding the optimal migration routes and wintering sites cannot be neglected [22,23]; the importance of social cues is, for example, supported by the change in routes over the first years of life [41], seen also in our data. We did not analyse the impact of weather on migration (but see [22]), but our results indicated relationships among various climatic factors and the wintering ranges of spotted eagles. Interestingly, the ranges of both C. clanga and hybrids seemed to be determined by precipitation, but this factor was not included in the distribution model for C. pomarina, which indicated a lack of overlap not only with the wintering range of C. clanga, but also with that of hybrids. In fact, a similar tendency has been detected at a local scale on breeding grounds, where interspecific pairs seem to form predominantly in habitats typical for C. clanga [31]. Given the inheritance of habitat selection [63–65] and the similarity of breeding and wintering habitats among spotted eagles [31,32,50], hybrid individuals may prefer wintering habitats similar to those of C. clanga. This, accompanied by the adjustment to different prey resources [66,67], may be further reflected by differences in the size of the wintering home range from that of C. pomarina. Hence, wintering strategy seems to be additionally determined by the ecological niche, which is influenced by genetic factors [63–65].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank all the people who assisted us in the field. Ringing data were obtained from national ringing centres in Estonia and Poland. Comments from Kamran Safi and anonymous reviewers greatly improved the manuscript.
Data accessibility
The datasets supporting this article have been uploaded in the electronic supplementary material.
Authors' contributions
All authors conducted the fieldwork, collated the telemetry data and participated in the preparation of the final manuscript; Ü.V. conducted the genetic testing and data analyses, P.M. conducted the habitat suitability modelling and spatial analyses, and developed the supplementary video, Ü.V. and P.M. prepared the first draft of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The study was financed by the projects LIFE04NAT/EE/000072 and LIFE08NAT/PL/000511 and by the Estonian Environmental Board. Ü.V. was supported by the grant no. IUT21-1 from the Estonian Ministry of Education and Research.
References
- 1.Barlow GW. 1991. Nature–nurture and the debates surrounding ethology and sociobiology. Am. Zool. 31, 286–296. ( 10.1093/icb/31.2.286) [DOI] [Google Scholar]
- 2.Krebs JR, Davies NB. 2009. Behavioural ecology: an evolutionary approach. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 3.Holyoak M, Casagrandi R, Nathan R, Revilla E, Spiegel O. 2008. Trends and missing parts in the study of movement ecology. Proc. Natl Acad. Sci. USA 105, 19 060–19 065. ( 10.1073/pnas.0800483105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alerstam T, Hedenström A, Åkesson S. 2003. Long-distance migration: evolution and determinants. Oikos 103, 247–260. ( 10.1034/j.1600-0706.2003.12559.x) [DOI] [Google Scholar]
- 5.Pulido F. 2007. The genetics and evolution of avian migration. Bioscience 57, 165–174. ( 10.1641/B570211) [DOI] [Google Scholar]
- 6.Berthold P, Wiltschko W, Miltenberger H, Querner U. 1990. Genetic transmission of migratory behavior into a nonmigratory bird population. Experimentia 46, 107–108. ( 10.1007/BF01955432) [DOI] [Google Scholar]
- 7.Helbig AJ. 1991. Inheritance of migratory direction in a bird species: a cross-breeding experiment with SE-and SW-migrating blackcaps (Sylvia atricapilla). Behav. Ecol. Sociobiol. 28, 9–12. ( 10.1007/BF00172133) [DOI] [Google Scholar]
- 8.Helbig A. 1996. Genetic basis, mode of inheritance and evolutionary changes of migratory directions in palaearctic warblers (Aves: Sylviidae). J. Exp. Biol. 199, 49–55. [DOI] [PubMed] [Google Scholar]
- 9.Pulido F, Berthold P, Mohr G, Querner U. 2001. Heritability of the timing of autumn migration in a natural bird population. Proc. R. Soc. Lond. B 268, 953–959. ( 10.1098/rspb.2001.1602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delmore KE, Irwin DE. 2014. Hybrid songbirds employ intermediate routes in a migratory divide. Ecol. Lett. 17, 1211–1218. ( 10.1111/ele.12326) [DOI] [PubMed] [Google Scholar]
- 11.Åkesson S, Hedenström A. 2007. How migrants get there: migratory performance and orientation. BioScience 57, 123–133. ( 10.1641/B570207) [DOI] [Google Scholar]
- 12.Chernetsov NS. 2016. Orientation and navigation of migrating birds. Biol. Bull. 43, 788–803. ( 10.1134/S1062359016080069) [DOI] [Google Scholar]
- 13.Liedvogel M, Åkesson S, Bensch S. 2011. The genetics of migration on the move. Trends Ecol. Evol. 26, 561–569. ( 10.1016/j.tree.2011.07.009) [DOI] [PubMed] [Google Scholar]
- 14.Sutherland WJ. 1998. Evidence for flexibility and constraint in migration systems. J. Avian Biol. 29, 441–446. ( 10.2307/3677163) [DOI] [Google Scholar]
- 15.Pettit B, Flack A, Freeman R, Guilford T, Biro D. 2013. Not just passengers: pigeons, Columba livia, can learn homing routes while flying with a more experienced conspecific. Proc. R. Soc. B 280, 20122160 ( 10.1098/rspb.2012.2160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couzin ID, Krause J, Franks NR, Levin SA. 2005. Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516. ( 10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- 17.Bode NWF, Franks DW, Wood AJ, Piercy JJB, Croft DP, Codling EA. 2012. Distinguishing social from nonsocial navigation in moving animal groups. Am. Nat. 179, 621–632. ( 10.1086/665005) [DOI] [PubMed] [Google Scholar]
- 18.Chernetsov N, Berthold P, Querner U. 2004. Migratory orientation of first-year white storks (Ciconia ciconia): inherited information and social interactions. J. Exp. Biol. 207, 937–943. ( 10.1242/jeb.00853) [DOI] [PubMed] [Google Scholar]
- 19.Mueller T, O'Hara RB, Converse SJ, Urbanek RP, Fagan WF. 2013. Social learning of migratory performance. Science 341, 999–1002. ( 10.1126/science.1237139) [DOI] [PubMed] [Google Scholar]
- 20.Hake M, Kjellén N, Alerstam T. 2003. Age-dependent migration strategy in honey buzzards Pernis apivorus tracked by satellite. Oikos 103, 385–396. ( 10.1034/j.1600-0706.2003.12145.x) [DOI] [Google Scholar]
- 21.Mellone U, Lucia G, Mallìa E, Urios V. 2016. Individual variation in orientation promotes a 3000-km latitudinal change in wintering grounds in a long-distance migratory raptor. Ibis 158, 887–893. ( 10.1111/ibi.12401) [DOI] [Google Scholar]
- 22.Meyburg BU, et al. 2017. Orientation of native versus translocated juvenile lesser spotted eagles (Clanga pomarina) on the first autumn migration. J. Exp. Biol. 220, 2765–2776. ( 10.1242/jeb.148932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oppel S, et al. 2015. High juvenile mortality during migration in a declining population of a long-distance migratory raptor. Ibis 157, 545–557. ( 10.1111/ibi.12258) [DOI] [Google Scholar]
- 24.Irwin DE, Irwin JH. 2005. Siberian migratory divides. In Birds of two worlds: the ecology and evolution of migration (eds Greenberg R, Marra PP), pp. 27–40. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 25.Price T. 2008. Speciation in birds. Greenwood Village, CO: Roberts and Co. [Google Scholar]
- 26.Berthold P, Querner U. 1981. Genetic basis of migratory behavior in European warblers. Science 212, 77–79. ( 10.1126/science.212.4490.77) [DOI] [PubMed] [Google Scholar]
- 27.Bensch S, Andersson T, Åkesson S. 1999. Morphological and molecular variation across a migratory divide in willow warblers, Phylloscopus trochilus. Evolution 53, 1925–1935. ( 10.1111/j.1558-5646.1999.tb04573.x) [DOI] [PubMed] [Google Scholar]
- 28.Veen T, et al. 2007. Does migration of hybrids contribute to post-zygotic isolation in flycatchers? Proc. R. Soc. B 274 707–712. ( 10.1098/rspb.2006.0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yohannes E, Lee RW, Jochimsen MC, Hansson B. 2011. Stable isotope ratios in winter-grown feathers of great reed warblers Acrocephalus arundinaceus, clamorous reed warblers A. stentoreus and their hybrids in a sympatric breeding population in Kazakhstan. Ibis 153, 502–508. ( 10.1111/j.1474-919X.2011.01139.x) [DOI] [Google Scholar]
- 30.Toews DP, Mandic M, Richards JG, Irwin DE. 2014. Migration, mitochondria, and the yellow-rumped warbler. Evolution 68, 241–255. ( 10.1111/evo.12260) [DOI] [PubMed] [Google Scholar]
- 31.Ruegg KC, Smith TB. 2002. Not as the crow flies: a historical explanation for circuitous migration in Swainson's thrush (Catharus ustulatus). Proc. R. Soc. Lond. B 269, 1375–1381. ( 10.1098/rspb.2002.2032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lõhmus A, Väli Ü. 2005. Habitat use by the vulnerable greater spotted eagle Aquila clanga interbreeding with the lesser spotted eagle Aquila pomarina in Estonia. Oryx 39, 170–177. ( 10.1017/S0030605305000414) [DOI] [Google Scholar]
- 33.Maciorowski G, Mirski P. 2014. Habitat alteration enables hybridisation between lesser spotted and greater spotted eagles in north-east Poland. Bird Conserv. Int. 24, 152–161. ( 10.1017/S0959270913000348) [DOI] [Google Scholar]
- 34.Väli Ü, et al. 2010. Widespread hybridization between the greater spotted eagle Aquila clanga and the lesser spotted eagle Aquila pomarina (Aves: Accipitriformes) in Europe. Biol. J. Linn. Soc. 100, 725–736. ( 10.1111/j.1095-8312.2010.01455.x) [DOI] [Google Scholar]
- 35.BirdLife International. 2016. Species factsheets: Clanga clanga, C. pomarina. See http://www.birdlife.org (accessed 10 September 2017).
- 36.Väli Ü, Lõhmus A. 2004. Nestling characteristics and identification of the lesser spotted eagle Aquila pomarina, greater spotted eagle A. clanga, and their hybrids. J. Ornithol. 145, 256–263. ( 10.1007/s10336-004-0028-7) [DOI] [Google Scholar]
- 37.Lontkowski J, Maciorowski G. 2010. Identification of juvenile greater spotted eagle, lesser spotted eagle and hybrids. Dutch Birding 32, 384–397. [Google Scholar]
- 38.Väli Ü, et al. 2010. Microsatellites and single nucleotide polymorphisms in avian hybrid identification: a comparative case study. J. Avian Biol. 41, 34–49. ( 10.1111/j.1600-048X.2009.04730.x) [DOI] [Google Scholar]
- 39.Fridolfsson AK, Ellegren H. 1999. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30, 116–121. ( 10.2307/3677252) [DOI] [Google Scholar]
- 40.Scheller W, Bergmanis U, Meyburg BU, Furkert B, Knack A, Röper S. 2001. Raum-Zeit-Verhalten des Schreiadlers (Aquila pomarina). Acta Ornithoecol. 4, 75–236. [Google Scholar]
- 41.Sergio F, et al. 2014. Individual improvements and selective mortality shape lifelong migratory performance. Nature 515, 410–413. ( 10.1038/nature13696) [DOI] [PubMed] [Google Scholar]
- 42.Korner-Nievergelt F, Robinson RA. 2014. Introducing the R-package ‘birdring’. Ring. Migr. 29, 51–61. ( 10.1080/03078698.2014.933053) [DOI] [Google Scholar]
- 43.Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modelling of species geographical distributions. Ecol. Model. 190, 231–259. ( 10.1016/j.ecolmodel.2005.03.026) [DOI] [Google Scholar]
- 44.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 45.Friedl MA, et al. 2010. MODIS collection 5 global land cover: algorithm refinements and characterization of new datasets, 2001–2012, collection 5.1 IGBP land cover. Boston, MA: Boston University. [Google Scholar]
- 46.ESRI. 2011. ArcGIS desktop: release 10 . Redlands, CA: Environmental Systems Research Institute. [Google Scholar]
- 47.Lobo JM, Jimenez-Valverde A, Real R. 2008. AUC: a misleading measure of the performance of predictive distribution models. Global Ecol. Biogeogr. 17, 145–171. ( 10.1111/j.1466-8238.2007.00358.x) [DOI] [Google Scholar]
- 48.Liu C, Newell G, White M. 2016. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 6, 337–348. ( 10.1002/ece3.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visser H, de Nijs T. 2006. The map comparison kit. Environ. Model. Softw. 21, 346–358. ( 10.1016/j.envsoft.2004.11.013) [DOI] [Google Scholar]
- 50.Power C, Simms A, White R. 2001. Hierarchical fuzzy pattern matching for the regional comparison of land use maps. Int. J. Geogr. Inf. Sci. 15, 77–100. ( 10.1080/136588100750058715) [DOI] [Google Scholar]
- 51.Wall J.2014. Movement ecology tools for ArcGIS (ArcMET) version 10.2.2.v2. See http://www.movementecology.net/ .
- 52.R Development Core Team. 2017. R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 53.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2017. nlme: linear and nonlinear mixed effects models. R package version 3.1-131. See https://cran.r-project.org/package=nlme.
- 54.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 55.Meyburg BU, Meyburg C, Mizera T, Maciorowski G, Kowalski J. 2005. Family break up, departure, and autumn migration in Europe of a family of greater spotted eagles (Aquila clanga) as reported by satellite telemetry. J. Raptor Res. 39, 462–466. [Google Scholar]
- 56.Leshem Y, Yom-Tov Y. 1996. The magnitude and timing of migration by soaring raptors, pelicans and storks over Israel. Ibis 138, 188–203. ( 10.1111/j.1474-919X.1996.tb04328.x) [DOI] [Google Scholar]
- 57.Newton I. 2008. The migration ecology of birds. London, UK: Academic Press. [Google Scholar]
- 58.Verhelst B, Jansen J, Vansteelant W. 2011. South West Georgia: an important bottleneck for raptor migration during autumn. Ardea 99, 137–146. ( 10.5253/078.099.0203) [DOI] [Google Scholar]
- 59.Stanley CQ, MacPherson M, Fraser KC, McKinnon EA, Stutchbury BJM. 2012. Repeat tracking of individual songbirds reveals consistent migration timing but flexibility in route. PLoS ONE 7, e40688 ( 10.1371/journal.pone.0040688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gwinner E, Helm B. 2003. Circannual and circadian contributions to the timing of avian migration. In Avian migration (eds Berthold P, Gwinner E, Sonnenschein E), pp. 81–95. Berlin, Germany: Springer. [Google Scholar]
- 61.Shamoun-Baranes J, Van Loon E, Alon D, Alpert P, Yom-Tov Y, Leshem Y. 2006. Is there a connection between weather at departure sites, onset of migration and timing of soaring-bird autumn migration in Israel? Global Ecol. Biogeogr. 15, 541–552. ( 10.1111/j.1466-8238.2006.00261.x) [DOI] [Google Scholar]
- 62.Helm B, Piersma T, Van der Jeugd H. 2006. Sociable schedules: interplay between avian seasonal and social behaviour. Anim. Behav. 72, 245–262. ( 10.1016/j.anbehav.2005.12.007) [DOI] [Google Scholar]
- 63.Tauber CA, Tauber MJ. 1977. A genetic model for sympatric speciation through habitat diversification and seasonal isolation. Nature 268, 702–705. ( 10.1038/268702a0) [DOI] [PubMed] [Google Scholar]
- 64.Rice WR. 1987. Speciation via habitat specialization: the evolution of reproductive isolation as a correlated character. Evol. Ecol. 1, 301–314. ( 10.1007/BF02071555) [DOI] [Google Scholar]
- 65.Schluter D, Conte GL. 2009. Genetics and ecological speciation. Proc. Natl Acad. Sci. USA 106, 9955–9962. ( 10.1073/pnas.0901264106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerkmann B, Meyburg BU. 2009. Habitats used by lesser spotted eagles (Aquila pomarina) during migration and wintering as revealed by satellite tracking and remote sensing. Popul. Ecol. Raptors Owls 6, 87–102. [Google Scholar]
- 67.Maciorowski G, Galanaki A, Kominos T, Dretakis M, Mirski P. In press The importance of wetlands for the greater spotted eagle Clanga clanga wintering in the Mediterranean Basin. Bird Conserv. Int. ( 10.1017/S0959270918000047) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded in the electronic supplementary material.