Abstract
Ocean acidification is a threat to the continued accretion of coral reefs, though some undergo daily fluctuations in pH exceeding declines predicted by 2100. We test whether exposure to greater pH variability enhances resistance to ocean acidification for the coral Goniopora sp. and coralline alga Hydrolithon reinboldii from two sites: one with low pH variability (less than 0.15 units daily; Shell Island) and a site with high pH variability (up to 1.4 pH units daily; Tallon Island). We grew populations of both species for more than 100 days under a combination of differing pH variability (high/low) and means (ambient pH 8.05/ocean acidification pH 7.65). Calcification rates of Goniopora sp. were unaffected by the examined variables. Calcification rates of H. reinboldii were significantly faster in Tallon than in Shell Island individuals, and Tallon Island individuals calcified faster in the high variability pH 8.05 treatment compared with all others. Geochemical proxies for carbonate chemistry within the calcifying fluid (cf) of both species indicated that only mean seawater pH influenced pHcf. pH treatments had no effect on proxies for Ωcf. These limited responses to extreme pH treatments demonstrate that some calcifying taxa may be capable of maintaining constant rates of calcification under ocean acidification by actively modifying Ωcf.
Keywords: environmental variability, biomineralization, ocean acidification, resistance to climate change, corals, coralline algae
1. Introduction
Ocean acidification is a growing threat to marine ecosystems [1,2], particularly those heavily reliant on calcifying taxa, such as coral reefs [3–5]. Coral reefs provide billions of dollars annually to the world's economy through tourism and fisheries [6], and they are a hotspot of biodiversity [7]. Therefore, determining the impacts of future ocean acidification on these ecosystems is of paramount importance. Both the habitat-forming corals and their settlement substrate of preference, coralline algae, are expected to be vulnerable to the effects of ocean acidification [5,8,9]. However, predictions of coral reef futures are often based on open ocean pH regimes (e.g. [4,10]), but the seawater pH on coral reefs encountered by resident taxa can be drastically different from that of the surrounding open ocean [11]. Corals and coralline algae can encounter large daily fluctuations in pH when they reside in habitats with high biomasses of macroalgae or seagrass, and with low seawater exchange with the open ocean [12–14]. This has led to suggestions that organisms living within highly variable pH environments will be less vulnerable to ocean acidification compared to those that encounter more constant seawater pH [15–17].
Despite the dramatic daily pH oscillations that occur on many coral reefs, little is known regarding how pH variability influences the physiology of calcifying taxa, especially when superimposed upon declines in mean seawater pH. It has been proposed that higher pH during the day could offset some of the negative effects of ocean acidification for organisms living in diurnally variable pH regimes [12,15,17]. However, experimental evidence supporting this hypothesis is ambiguous. Roughly half of all coral and coralline algal species tested to date have responded positively to increased pH variability, while half of the coralline algal species respond negatively to greater pH variability [12,18–24]. Thus, while elevated pH during the day could potentially offset the impacts of ocean acidification, it is equally plausible that reductions in pH at night within these habitats could exacerbate night-time dissolution [12,25,26].
Individuals or populations residing in more variable environmental regimes are typically more tolerant to mean changes in these parameters. For example, corals residing in environments with greater thermal variability can be more tolerant to the effects of increasing mean temperature [14,27,28]. Environments with greater variability can also favour populations with greater phenotypic plasticity, while stable environments eliminate the selection pressure for these traits, resulting in their eventual loss from the population [29]. Similarly, individuals exposed to greater extremes in environmental variables throughout their lifetime could also be acclimatized to such effects [30]. The role of prior exposure to greater pH variability to promote resistance to ocean acidification has been observed in some temperate invertebrate species from upwelling systems [16]. However, whether prior exposure to greater pH variability promotes tolerance or complete resistance to ocean acidification in corals and coralline algae is unknown [14]. Coral reef habitats typically do not contain the large biomasses of resident autotrophs required to shift pH significantly, when compared with other ecosystems such as temperate kelp forests in well-flushed reefs [31]. Therefore, it is possible that the coral reef taxa used in studies determining how an organisms' past pH history could affect responses to ocean acidification (e.g. [18–20]) were from conditions that were too constant to elicit detectable responses (though see [20]). To further understand whether greater pH variability or past exposure to pH variability influences responses of calcification to ocean acidification, we need to use larger oscillations in pH and to further explore the mechanisms responsible. One possible mechanism that has been evoked is a greater capacity to maintain elevated pH in the calcifying fluid (pHcf) of corals exposed to natural pH variability under ocean acidification [32].
The capacity to elevate pH in the calcifying fluid (pHcf) has been linked with greater resistance to ocean acidification in both corals and coralline algae [33,34]. How the elevation of pHcf is influenced by natural pH variability is poorly known. When the coral Porites cylindrica was exposed to variable pH regimes both during and prior to a long-term ocean acidification experiment, they demonstrated relatively constant calcification rates and pHcf under ocean acidification treatments [32]. Therefore, exposure of corals to natural pH variability could promote elevated pHcf under ocean acidification [32]. However, the correlation between declines in pHcf and calcification rates under ocean acidification does not occur for all corals under constant pH regimes [35]. Reconstructions of 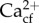 using recently developed geochemical proxies show that the capacity to elevate Ωcf through either increased Ca2+ pumping or elevating pHcf could be two different strategies for maintaining faster calcification rates and higher Ωcf in the face of ocean acidification [36]. If current (or prior) exposure to elevated seawater pH variability promotes pHcf upregulation or increased Ca2+ uptake, and subsequent resistance to ocean acidification, then this will have important ramifications regarding how we predict the impacts of ocean acidification based on the majority of prior laboratory studies where it is unknown how the pH variability employed matches that of the organisms' site of collection [14].
using recently developed geochemical proxies show that the capacity to elevate Ωcf through either increased Ca2+ pumping or elevating pHcf could be two different strategies for maintaining faster calcification rates and higher Ωcf in the face of ocean acidification [36]. If current (or prior) exposure to elevated seawater pH variability promotes pHcf upregulation or increased Ca2+ uptake, and subsequent resistance to ocean acidification, then this will have important ramifications regarding how we predict the impacts of ocean acidification based on the majority of prior laboratory studies where it is unknown how the pH variability employed matches that of the organisms' site of collection [14].
Here, we assess the responses of two populations of corals (Goniopora sp.) and coralline algae (Hydrolithon reinboldii) from two sites with markedly different pH regimes (Shell Island, less than 0.05 units; and Tallon Island, 1.4 units daily pH variability) to four different experimental treatments each. These treatments are the fully factorial interaction between pH variability (low (approx. 0.11) and high (approx. 0.75 unit) daily variability) and mean pH (ambient (approx. 8.05) and ocean acidification (7.65)). In the highly variable pH environment of Tallon Island, oxygen concentrations also covary with pH. Therefore, we also assess the responses of Goniopora sp. to these treatments over both constant and variable oxygen concentrations during two time periods. We hypothesize that (i) both corals and coralline algae from highly variable pH regimes will be more resistant to the effects of ocean acidification than those from more constant pH regimes; and (ii) that both corals and coralline algae will be more resistant to the effects of ocean acidification when grown under pH regimes that vary on diurnal cycles, compared with those grown under constant regimes. We also hypothesize that (iii) any resistance to ocean acidification will be driven by favourable carbonate chemistry in the calcifying fluid (pHcf and Ωcf), which will promote faster calcification rates.
2. Material and methods
(a). Sample collection, site characterization and physiological measurements
pH, temperature, oxygen concentration and light data were all collected from two focal sites in the Buccaneer Archipelago of the Kimberley region, Western Australia, Australia from April 2016 until October 2016 as part of several long-term monitoring projects. This region is macrotidal and possesses an approximately 10 m tidal range that creates a highly dynamic environmental regime at many of the exposed tidal pools [37]. The two locations were Tallon/Jalan Island (16°40S, 123°14E) and Shell Island (16°48S, 123°04E). These in situ data will be explored in a complementary study [38]; see electronic supplementary material, SI 1 for more details on data collection.
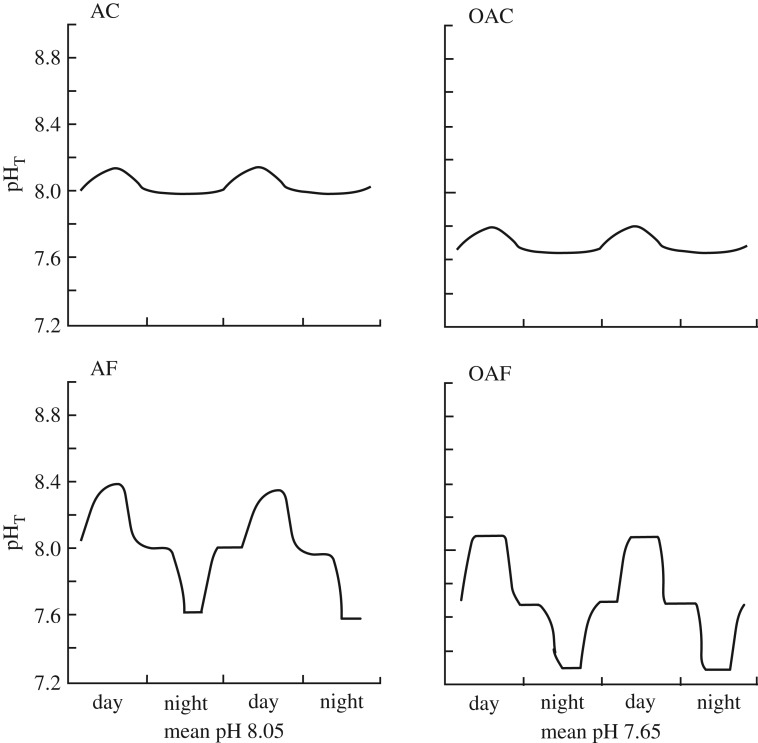
Seventy Goniopora sp. and H. reinboldii individuals were collected from each site and transported to the Indian Ocean Marine Research Centre at Watermans Bay, Perth. Six individuals per treatment and site combination were placed into the four pH treatments in this experiment (figure 1), two low variability and two high variability treatments for each of the two mean pH treatment levels (8.05 and 7.65). These four treatments were: (i) ambient mean and constant pH (hereafter ‘AC’, mean pH 8.05 < ±0.06 units daily variability), similar to Shell Island today; (ii) ambient mean and fluctuating pH (‘AF’, pH 8.05 ± 0.31 units), similar to Tallon Island today; (iii) an ocean acidification scenario with constant pH (‘OAC’, mean pH 7.65 < ±0.06 units), similar to an end of century scenario at Shell Island; and (iv) an ocean acidification scenario with fluctuating pH (‘OAF’, mean pH 7.65 ± 0.40 units), similar to an end of century Tallon Island scenario. For more experimental design details, see electronic supplementary material, S1. Calcification, photosynthetic and respiration rates were all measured. These are also detailed in electronic supplementary material, S1. See electronic supplementary material, S2 for the full range of seawater carbonate chemistry in each treatment during this experiment.
Figure 1.
Schematic of pH treatments achieved within the experimental tanks in this study. AC, ambient mean and constant pH; AF, ambient mean and fluctuating pH; OAC, ocean acidification scenario with constant pH; OAF, ocean acidification scenario with fluctuating pH.
(b). pHcf, dissolved inorganic carbon (DICcf) and Raman spectroscopy
Calcifying fluid pH (pHcf) for all organisms and dissolved inorganic carbon (DICcf) for corals were calculated using the δ11B proxy method for pHcf [39] and the δ11B and B/Ca method for DICcf [40,41]. Further details beyond these references are presented in electronic supplementary material, S1. We also used confocal Raman spectroscopy to determine sample mineralogy and as a proxy of calcifying fluid Ω [42], where Ωcf decreases as full width at half peak maximum (FWHM) decreases. These methods are detailed in DeCarlo et al., but for specific details also see electronic supplementary material, SI 1.
(c). Statistical analysis
We used linear mixed models to detect differences caused by the interaction between the mean and variability of pH treatments and the site of origin on the measured metrics. For Goniopora sp. under low oxygen concentration variability, these were: calcification rates, pHcf, DICcf, B/Ca, 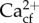 and Ωcf. For H. reinboldii under high oxygen concentration variability, these were calcification rates, photosynthetic rates, respiration rates, pHcf, B/Ca, and Raman-derived residual FWHM. Goniopora sp. calcification, photosynthetic and respiration rates under low oxygen concentration variability were also analysed in this way. The site of origin (Tallon or Shell Islands), seawater pH variability (constant or fluctuating) and seawater mean pH (8.05 and 7.65) were fixed factors in a fully factorial model, where header tank identity was also included as a random factor. The mean of responses for experimental tanks containing more than one individual was used as the raw data in the model. All data conformed to assumptions of normality and homogeneity of variance. Turkey's post hoc tests were conducted when significant differences were detected in linear mixed models that were not due to main effects.
and Ωcf. For H. reinboldii under high oxygen concentration variability, these were calcification rates, photosynthetic rates, respiration rates, pHcf, B/Ca, and Raman-derived residual FWHM. Goniopora sp. calcification, photosynthetic and respiration rates under low oxygen concentration variability were also analysed in this way. The site of origin (Tallon or Shell Islands), seawater pH variability (constant or fluctuating) and seawater mean pH (8.05 and 7.65) were fixed factors in a fully factorial model, where header tank identity was also included as a random factor. The mean of responses for experimental tanks containing more than one individual was used as the raw data in the model. All data conformed to assumptions of normality and homogeneity of variance. Turkey's post hoc tests were conducted when significant differences were detected in linear mixed models that were not due to main effects.
3. Results
(a). In situ seawater carbonate chemistry
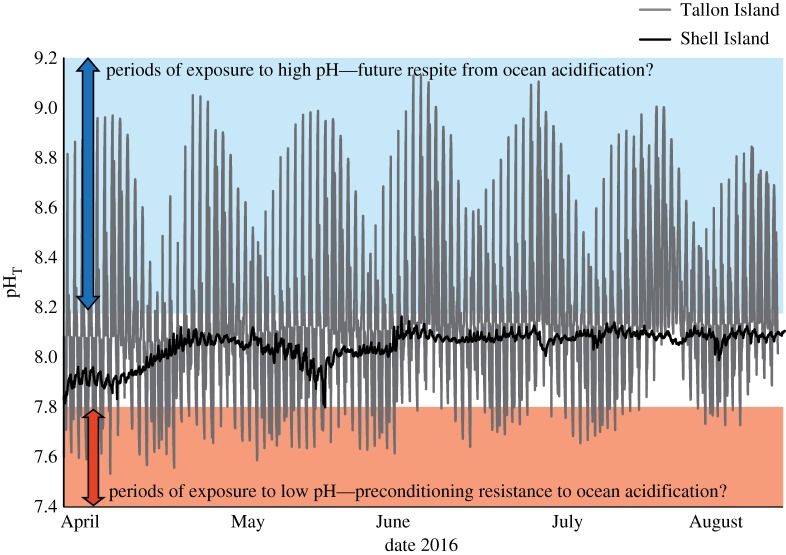
Mean pH was 8.08 at Tallon Island and 7.96 at Shell Island. The variability in seawater pH was orders of magnitude greater at Tallon Island compared with Shell Island (figure 2 and electronic supplementary material, figure S1). pH ranged from 7.8 to 8.2 at Shell Island, and the greatest variability in any given day was 0.14 units. On all other days pH varied by less than 0.1 units. By contrast, at Tallon Island, pH varied between 7.51 and 9.15, with the greatest pH variability being 1.4 units on any given day. The ranges between the lowest and highest standard deviations of seawater pH at any given hour of the day, e.g. 95.45% of all pH values encountered at either site, were 0.10 at Shell Island and 1.06 pH units at Tallon Island (electronic supplementary material, figure S1).
Figure 2.
Example of pH variability measured at the collection sites. Tallon Island (in grey) being a coralline algal platform and Shell Island (in black) being a large coral-dominated intertidal pool. Note that early April corresponds to the coral bleaching even described in Le Nohaïc et al. [37]. Blue denotes pH range above those encountered at Shell Island, and red indicates those encountered below those at Shell Island. (Online version in colour.)
(b). Experimental seawater carbonate chemistry
Experimental treatments were achieved within 0.01–0.09 pH units of the target for all treatments at any given time phase over 24 h cycles, except for during the ‘day’ time period for the AF treatment, where pH was on average 0.18 pH units lower than initially desired (electronic supplementary material, table S2). This treatment reached a mean of 8.37 by the end of its time phase, but was much slower than the other treatments to reach its ‘day’ time phase target, hence the lower mean during this time phase. As this is a variable treatment, the impact of differences in the AF treatment is not likely to be as notable as if this were a stable pH treatment.
(c). Calcification rates
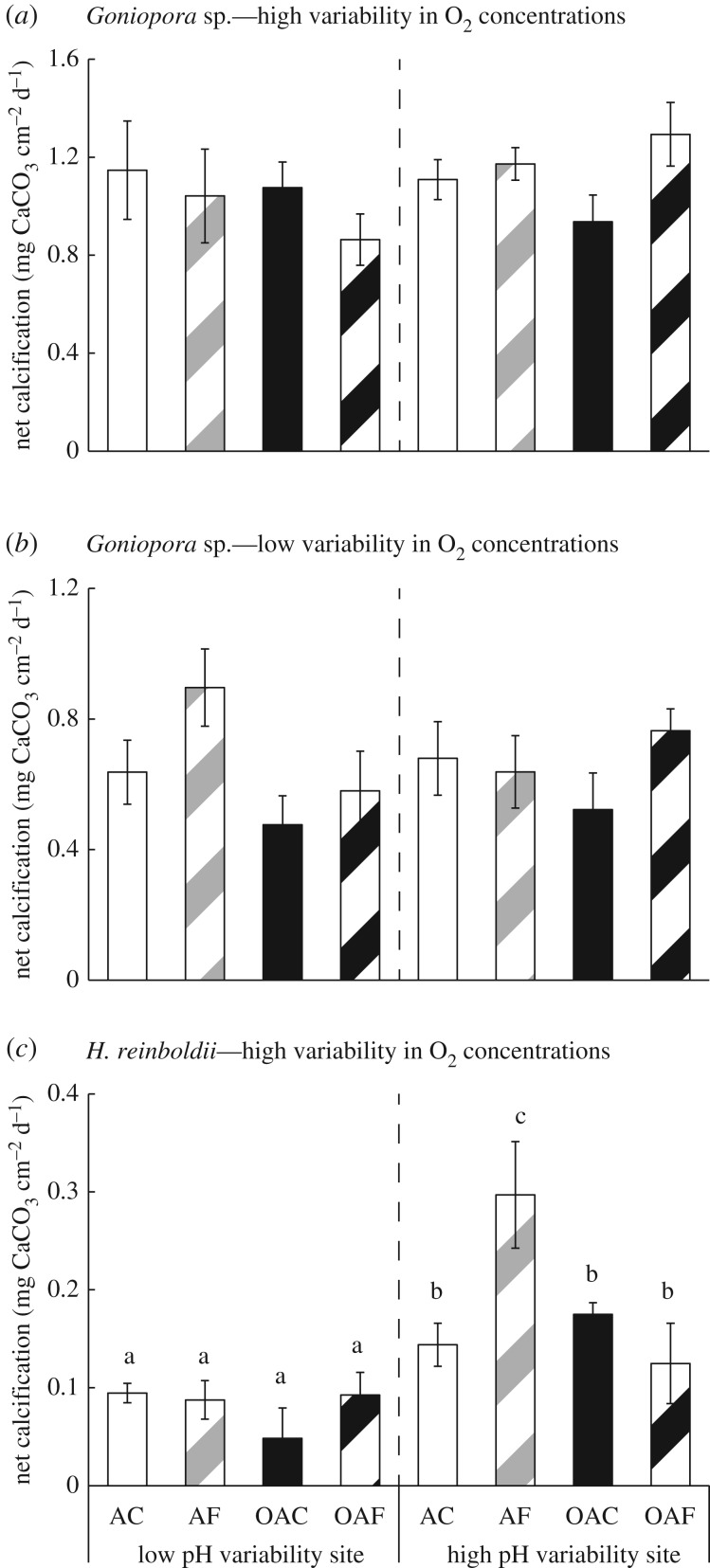
Calcification rates did not vary by the treatment or site of origin for Goniopora sp. under both high and low oxygen concentration variability (figure 3a,b; electronic supplementary material, S2 and table S2). However, calcification rates of H. reinboldii from Tallon Island were significantly faster than those from Shell Island under high oxygen concentration variability. Calcification rates of H. reinboldii from Tallon Island were also significantly faster in the AF treatment than in any other treatment (figure 3c; electronic supplementary material, SI 2 and table S3).
Figure 3.
Calcification rates of coral (a,b) (Goniopora sp.) and (c) crustose coralline algae (H. reinboldii) during the laboratory experiment under the four treatments from two different populations. The following details apply to this figure and all subsequent figures: the treatments are: AC, ambient mean and constant pH; AF, ambient mean and fluctuating pH; OAC, ocean acidification scenario with constant pH; OAF, ocean acidification scenario with fluctuating pH. Collected from a low pH variability site, Shell Island and a high pH variability site, Tallon Island in the presence of high variability in oxygen concentrations (a,c) and under low oxygen concentration variability (b). Means ± s.e., n = 4–6 per treatment and site combination. Treatments with different letters above the error bars are statistically different from one another.
(d). Photosynthetic and respiration rates
Goniopora sp. and H. reinboldii photosynthetic and respiration rates were unaffected by pH treatments (electronic supplementary material SI 3, figure S2, S2 materials and tables S2 and S3). However, Goniopora sp. from Shell Island had significantly higher gross photosynthetic rates compared with those from Tallon Island, and respiration rates of Tallon Island H. reinboldii were significantly higher than those from Shell Island. Respiration rates of Goniopora sp. within constant treatments were nearly significantly higher (p = 0.053) than those within fluctuating treatments when an α-value of 0.05 was employed.
(e). Geochemistry
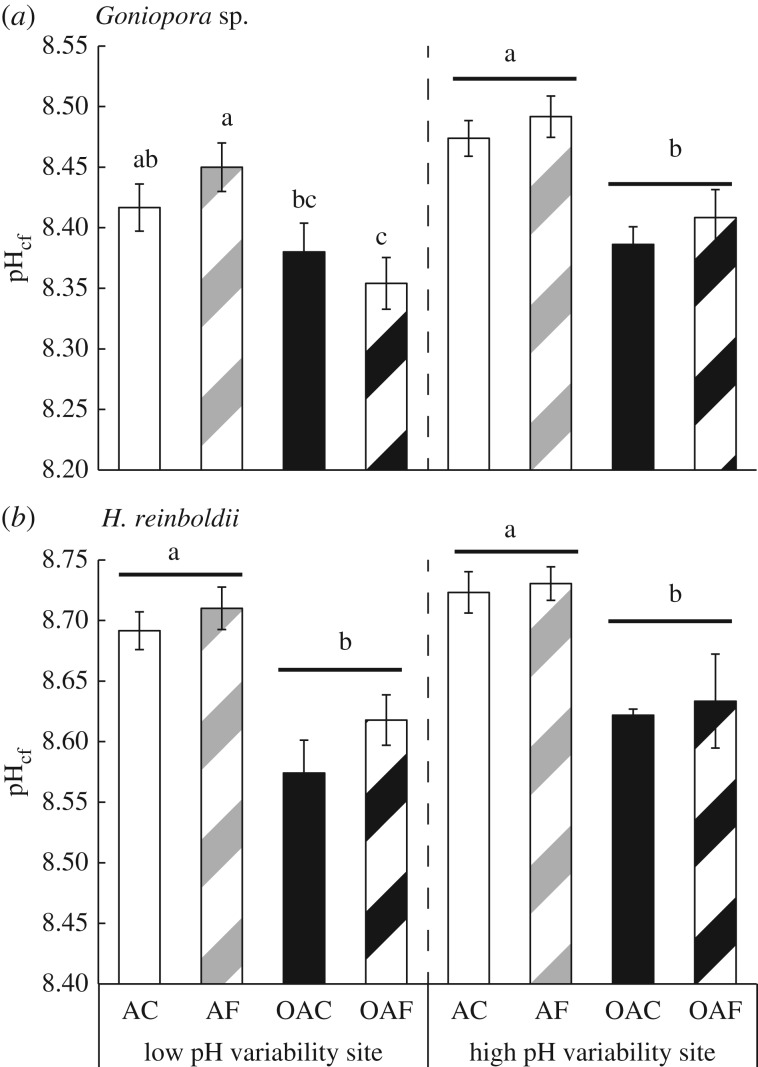
pHcf was significantly lower in both taxa when grown in the ocean acidification treatments compared with the treatments with ambient mean pH, irrespective of pH variability or site of origin (figure 4; electronic supplementary material, SI 2 and tables S4 and S5). This equated to a mean 0.07 unit decrease in Goniopora sp. (8.45–8.38) and a 0.10 unit decrease in pHcf for H. reinboldii (8.71–8.61). This equates to slopes of 0.21 and 0.30 unit declines in pHcf per unit pH of seawater for Goniopora sp. and H. reinboldii, respectively. There was no significant effect of variability in pH or site of origin on pHcf for either taxa.
Figure 4.
pHcf of (a) coral (Goniopora sp.) and (b) crustose coralline algae (H. reinboldii) grown under the four treatments from two different populations.
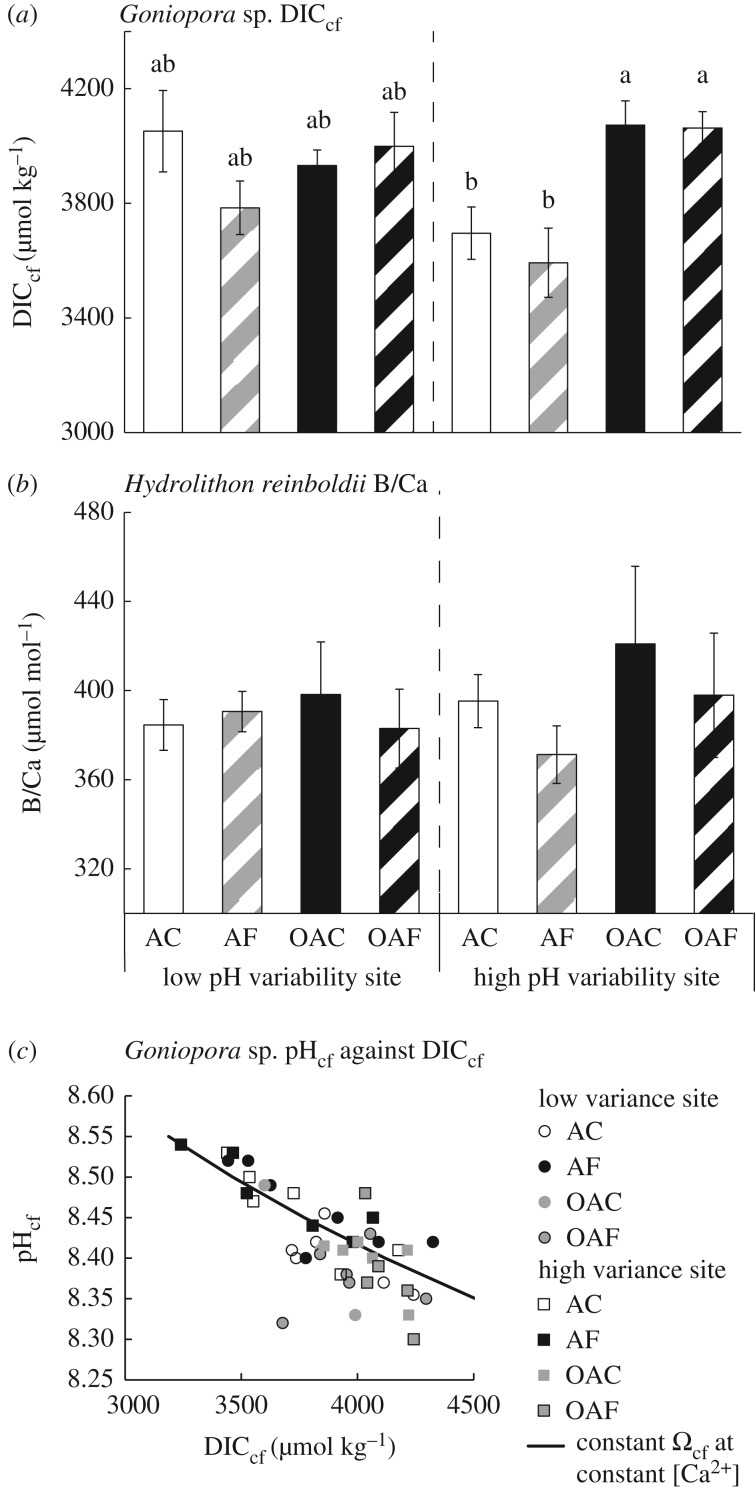
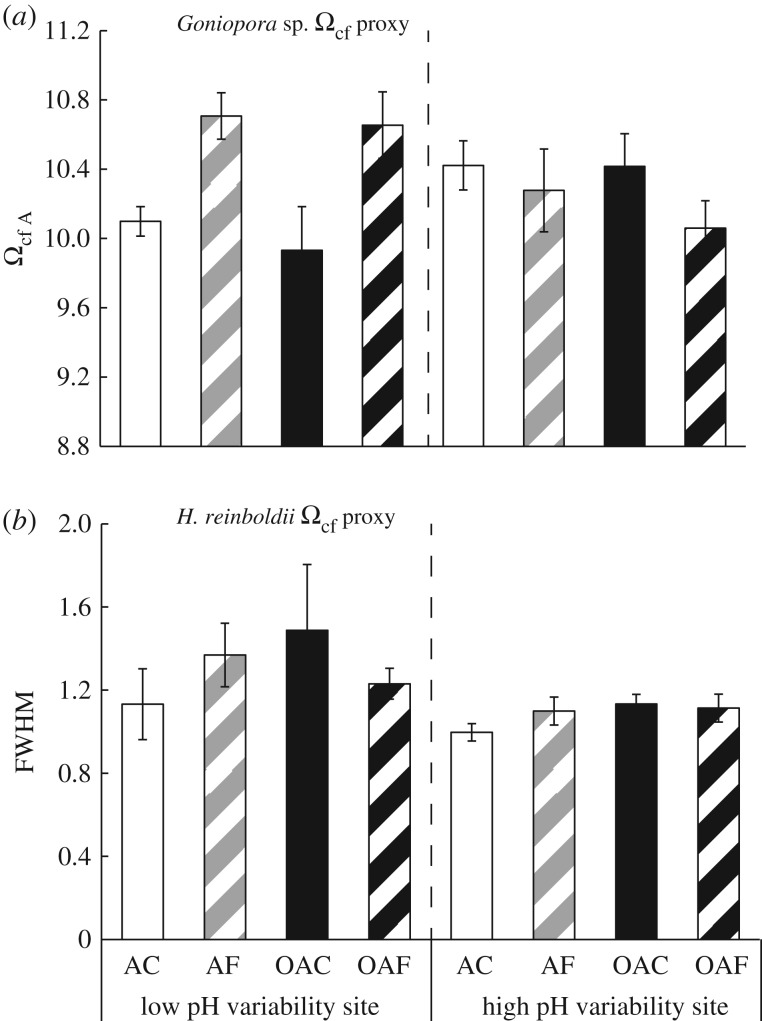
DICcf was significantly lower for Goniopora sp. individuals in the AF treatments compared with the OAF treatments (figure 5a; electronic supplementary material, S2 table S5, Tukey HSD p = 0.03), equating to a mean reduction of 342 µmol kg−1. DICcf was also significantly lower in Goniopora sp. from Tallon Island when grown under both ambient mean pH treatments (AF and AC) in general compared with ocean acidification treatments (OAC and OAF; figure 5a; electronic supplementary material, S2 table S5, Tukey HSD p < 0.01). There was no significant effect of treatments or site of origin on the B/Ca of H. reinboldii (figure 5b), nor on Gonopora sp. Ωcf or 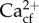 (figure 6a; electronic supplementary material, SI 3 and figure S3). H. reinboldii residual FWHM, a potential proxy for Ωcf, was significantly lower in individuals from Tallon Island, but did not vary by pH treatment (figure 6b).
(figure 6a; electronic supplementary material, SI 3 and figure S3). H. reinboldii residual FWHM, a potential proxy for Ωcf, was significantly lower in individuals from Tallon Island, but did not vary by pH treatment (figure 6b).
Figure 5.
DICcf of (a) coral (Goniopora sp.) and (b) B/Ca of crustose coralline algae (H. reinboldii) grown under the four treatments from two different populations. (c) Relationship between Goniopora sp. pHcf and DICcf, demonstrating how modifications of both under different treatments result in near constant Ωcf.
Figure 6.
Raman spectroscopy-derived Ωcf A of (a) coral (Goniopora sp.) and (b) FWHM intensity of crustose coralline algae (H. reinboldii) grown under the four treatments from two different populations. Note: as Ωcf decreases as FWHM decreases.
4. Discussion
Here, we demonstrate that corals and coralline algae from locations with greater variability in pH are not more tolerant to the impacts of ocean acidification than those from sites with lower pH variability. In addition, experimental exposure to greater pH variability does not provide resistance against the impacts of ocean acidification for the taxa examined. This applies to both the calcification rates and chemistry within the calcifying fluid. Here, physiological control over calcification rates in these two species from different biological kingdoms limited the capacity to detect an effect of pH variability promoting tolerance or providing respite against the impacts of ocean acidification. This was irrespective of high or low variability in oxygen concentrations for Goniopora sp. Thus, while pH variability still had some effects, species-specific physiological control over calcification appears more important than pH variability in determining responses to ocean acidification in these two key coral reef taxa with vastly different calcification mechanisms.
Our results contrast with the general ecological theory that exposure to greater envelopes of variability in an environmental parameter will provide greater tolerance to shifts in means of that factor [30], a point that has been questioned previously in respect to pH where conflicting results have been observed [14]. While we did observe some effects—mainly in the form of faster calcification and respiration in the coralline alga H. reinboldii from Tallon Island (particularly under the AF treatment) and faster photosynthetic rates in Goniopora sp. from Shell Island, we observed limited interactive effects between changes in mean pH and either site of origin or pH variability. Both the corals and coralline algae displayed resistance to the effects of ocean acidification through the regulation of calcifying fluid carbonate chemistry.
The lack of strong responses to pH variability here supports the overall trend that the interplay between pH variability and ocean acidification is complex, often yielding contrasting responses in these two calcifying coral reef taxa [14]. The calcification of three coral [19,21,22] and three coralline algal species [20,23] have responded positively to increased pH variability under ocean acidification. By contrast, experimental pH variability did not influence the other half of coral species examined [18,21] and negatively impacted both the adults and F1 recruits of a temperate coralline algal species [12,24]. However, strong effects of pH variability were not observed here, except for its impacts on Tallon Island H. reinboldii at mean pH 8.05, i.e. once out of a possible eight treatment combinations. This is surprising, particularly for the coralline algae whose sites of calcification are likely more ‘open’ to seawater than corals. Coralline algae calcify in the top three cell layers [33], whereas the calcifying fluid of corals is separated from seawater by four layers of tissues [43]. This indicates that both taxa are capable of physiological control over calcification, regardless of their very distinct calcification mechanisms.
Our findings therefore add to the understanding that there are multiple considerations that must be taken into account when determining how pH variability will affect corals and coralline algae. All species have differential susceptibilities to changes in seawater carbonate chemistry [44], which seem to be more related to species-specific controls over carbonate chemistry than to their kingdom of origin when corals and coralline algae are compared [33,35,45,46]. Species with limited physiological control over their calcification physiology would be expected to respond more dramatically to changes in seawater pH, while species with greater physiological control would respond more robustly [33,35,45], as shown here.
It is possible that a proportion of the inconsistencies between the responses of different species to the effects of greater pH variability could also be elicited by methodological differences between laboratory studies. Only by employing fully factorial assessments of pH variability and ocean acidification will their isolated and combined effects be known. For example, if the response of calcification to declines in mean seawater pH is parabolic in a particular population (i.e. increases first before eventually decreasing) [47], then treatments with higher pH variability and lower mean pH than the ‘control’ low pH variability treatments (e.g. [20,21]) could elicit faster calcification rates that are in response to mean seawater pH. Similarly, differences in seawater carbonate chemistry between the collection site of organisms could elicit vastly different responses to the same treatments (e.g. [48]). This could explain why many organisms from diurnally variable sites (high pH variability and higher mean pH) do not respond as consistently robust to the effects of ocean acidification as those from upwelling locations (high pH variability, but lower mean pH) [14,16].
Understanding the impacts of variability in seawater carbonate chemistry could be more complex than the effects of other parameters like temperature. This is due to the covarying nature of different components of seawater carbonate chemistry, all of which could have differing physiological effects on marine species. Treatments with differing variability in pH and identical means will not have the same mean in pCO2 or Ω and vice versa. This is because of the nonlinear relationship between these parameters. For example, the mean pCO2 (in µatm) in our AC treatments was 385, AF = 557, OAC = 1095 and OAF = 1454. This nonlinearity is especially problematic given the poor understanding of drivers of calcification, particularly regarding the causal mechanism and exact component of carbonate chemistry responsible for decreases in calcification under ocean acidification/reduced seawater pH [49]. Therefore, treatments that manipulate one component of variability in carbonate chemistry could find different results if means in another aspect of carbonate chemistry drives changes calcification. Furthermore, predicting the combined impacts of changes in variability in these parameters will be even more difficult unless larger multi-treatment experimental designs are employed if more than one parameter of seawater carbonate chemistry influences calcification [25,50] and/or other physiological processes such as photosynthesis [51,52]. These same problems exist when comparing the past exposure of marine species with different ranges of seawater carbonate chemistry.
Exposure to greater pH variability did not promote a greater capacity to maintain elevated pHcf or Ωcf within the calcifying fluid in either corals or coralline algae examined here. This is in contrast to prior observations that the coral P. cylindrica from a highly dynamic pH environment was capable of maintaining constant pHcf when exposed simultaneously to low/variable pH and ambient/variable pH [32]. Here, we tested the two hypotheses that could be responsible: (i) that the corals used by Georgiou et al. [32] were pre-conditioned to low pH events, having come from the highly dynamic Heron Island reef flat (e.g. the red in our figure 2) or (ii) that the benefit of elevated daytime pH equalled or outweighed any detrimental effects of low night-time pH (e.g. the blue in our figure 2). However, we found neither such response in our experiment, ruling out the possibility that this is a ubiquitous response to pH variability among coral species. Similar to the findings of Georgiou et al. [32], is the fact that numerous species of corals have also displayed limited declines in pHcf when reductions in seawater pH are not extreme (i.e. not more than 0.3–0.4 units [35,48,53]). This points to the more likely possibility that declines in pHcf do not occur in every coral species under ocean acidification, or that these declines are not always linked to decreased calcification rates under ocean acidification [35].
It was interesting that mean seawater pH in our study, and not variability in pH, was the driver of mean pHcf. This indicates that when changes in mean seawater pH are larger, such as here and in some other studies [33,35,53], mean conditions are more important in driving mean pHcf in both corals and coralline algae. pHcf calculated using δ11B records both day- and night-time precipitation (when night-time precipitation does occur). Here, the contrasting effects of day and night seawater pH on pHcf were averaged over a 24 h cycle in our fluctuating treatments. This is likely because of the similar seawater pH means in both the constant and variable pH treatments. This might not occur for all species, such as in those with limited precipitation during the night. However, further work in either the field or the laboratory should aim to use methods that allow high frequency or real-time determination of changes in seawater carbonate chemistry within the calcifying fluid, perhaps by using Raman microscopy, pH-sensitive dye or even well-replicated microsensor measurements, to determine changes in calcifying fluid carbonate chemistry over day- and night-time scales under diurnally fluctuating pH conditions.
Reduced mean seawater pH and not pH variability increased Goniopora sp.'s DICcf, particularly those from Tallon Island. This higher DICcf was not related to elevated photosynthetic rates. It has been previously proposed that the maintenance of higher overall Ωcf relative to seawater in coral, and not simply pHcf, could promote resistance to the effects of ocean acidification [35]. It is possible that increases in Goniopora sp. DICcf partially offset its declining pHcf in the ocean acidification treatments, resulting in constant Ωcf (figure 5c). This increase in DICcf and decline in pHcf likely resulted in the constant calcification rates, as seen previously in Porites compressa from Kāne'ohe Bay in Hawaii [48], which maintained constant 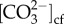 and calcification rates when grown in reduced seawater pH.
and calcification rates when grown in reduced seawater pH.
Constant Ωcf under the ocean acidification treatments in Goniopora sp. could have led to its resistance to ocean acidification here. Pocillopora damicornis from Rottnest Island, Australia had lower 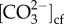 when grown under conditions simulating ocean acidification, but Ωcf was maintained at a constant value by increased [Ca2+]cf [35,36,46]. Here, we did not find increased [Ca2+]cf in Goniopora sp. under ocean acidification, and their [Ca2+]cf was lower than that of seawater (10.64 mmol kg−1) in all treatments. However, constant Ωcf and calcification rates of Goniopora sp. with declining mean pH support the overall hypothesis that the capacity to maintain constant and elevated Ωcf could be linked to resistance to the effects of ocean acidification. While we cannot as yet quantify Ωcf in high magnesium calcite, no changes in H. reinboldii FWHM also suggest overall constant Ωcf. Combined with the pHcf sensitivity, this indicates a likely increase in DICcf and/or
when grown under conditions simulating ocean acidification, but Ωcf was maintained at a constant value by increased [Ca2+]cf [35,36,46]. Here, we did not find increased [Ca2+]cf in Goniopora sp. under ocean acidification, and their [Ca2+]cf was lower than that of seawater (10.64 mmol kg−1) in all treatments. However, constant Ωcf and calcification rates of Goniopora sp. with declining mean pH support the overall hypothesis that the capacity to maintain constant and elevated Ωcf could be linked to resistance to the effects of ocean acidification. While we cannot as yet quantify Ωcf in high magnesium calcite, no changes in H. reinboldii FWHM also suggest overall constant Ωcf. Combined with the pHcf sensitivity, this indicates a likely increase in DICcf and/or 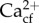 in H. reinboldii.
in H. reinboldii.
Together, these recent results indicate that resistance of these two key coral reef taxa to the impacts of ocean acidification is related to the maintenance of constant Ωcf., which can occur via three possibly complementary mechanisms under ocean acidification: (i) maintenance of constant pHcf; (ii) elevation of DICcf; and (iii) elevation of 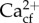 , for which the capacity of all processes differs between species [32,35,36,46,48]. Additionally, these processes were not strongly influenced by variability in seawater pH or past exposure to greater pH variability, with the only effects caused by pH variability being higher DICcf for Goniopora sp. under the OAF treatments compared to AF treatments. While past environmental variability and exposure to greater pH variability may be important in driving the resistance of temperate upwelling species to ocean acidification [16], this is not ubiquitous in the two calcifying coral reef taxa examined here. Our two focal calcifying species were the only two common at both study sites. Therefore, further research should explore whether the trends observed here hold for other locations, and other coral and coralline algal species. We demonstrate that physiological control over calcification physiology can be more important in predicting the responses of coral reef taxa to ocean acidification than either past or current exposure to pH variability. This study has revealed that many pertinent questions regarding the calcification physiology of corals and coralline algae remain unanswered, while at the same time supporting the conclusion that physiological controls on calcification drive resistance to ocean acidification in corals and coralline algae.
, for which the capacity of all processes differs between species [32,35,36,46,48]. Additionally, these processes were not strongly influenced by variability in seawater pH or past exposure to greater pH variability, with the only effects caused by pH variability being higher DICcf for Goniopora sp. under the OAF treatments compared to AF treatments. While past environmental variability and exposure to greater pH variability may be important in driving the resistance of temperate upwelling species to ocean acidification [16], this is not ubiquitous in the two calcifying coral reef taxa examined here. Our two focal calcifying species were the only two common at both study sites. Therefore, further research should explore whether the trends observed here hold for other locations, and other coral and coralline algal species. We demonstrate that physiological control over calcification physiology can be more important in predicting the responses of coral reef taxa to ocean acidification than either past or current exposure to pH variability. This study has revealed that many pertinent questions regarding the calcification physiology of corals and coralline algae remain unanswered, while at the same time supporting the conclusion that physiological controls on calcification drive resistance to ocean acidification in corals and coralline algae.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Bardi Jawi people who enabled this research through their advice, consent to access their traditional lands and assistance in the field. We also thank the staff at Cygnet Bay Pearl Farm and the Kimberley Marine Research Centre, and V. Schoepf for their support in the field, and A.-M. Nisumaa-Comeau and Kai Rankenburg for assistance in the laboratory.
Ethics
All local regulations and permit requirements were followed during this study.
Data accessibility
All geochemical data are within the electronic supplementary material; all else are accessible in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.250q1g7 [54].
Authors' contributions
C.E.C. and S.C. conceived the research and wrote the paper. C.E.C., S.C., B.M. and Q.D. ran the experiment. C.E.C., S.C. and B.M. conducted fieldwork. C.E.C. and S.C. conducted boron isotope and trace element analysis. T.M.D. conducted the Raman spectroscopy analysis. M.T.M. provided essential laboratory equipment, facilities and analyses. C.E.C. conducted the statistical analyses. All authors edited the manuscript, or provided intellectual input, and agreed to its submission.
Competing interests
The authors declare they have no competing interests.
Funding
This study was funded by an ARC Laureate Fellowship (FL120100049) awarded to M.T.M. and the ARC Centre of Excellence for Coral Reef Studies (CE140100020), and S.C. was supported by an ARC DECRA (DE160100668). The authors acknowledge the facilities, and the scientific and technical assistance of the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy, Characterization and Analysis, The University of Western Australia, a facility funded by the University, State and Commonwealth Governments.
References
- 1.Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99. ( 10.1038/nature07051) [DOI] [PubMed] [Google Scholar]
- 2.Kroeker KJ, Kordas RL, Crim RN, Hendriks IE, Ramajo L, Singh GG, Duarte CM, Gattuso JP. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896. ( 10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleypas JA, Buddemeier RW, Archer D, Gattuso J-P, Langdon C, Opdyke BN. 1999. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284, 118–120. ( 10.1126/science.284.5411.118) [DOI] [PubMed] [Google Scholar]
- 4.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 5.Fabricius K, et al. 2011. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Climate Change 1, 165–169. ( 10.1038/nclimate1122) [DOI] [Google Scholar]
- 6.O'Mahony J, Simes R, Redhill D, Heaton K, Atkinson C, Hayward E, Nguyen M.2017. At what price? The economic, social and icon value of the Great Barrier Reef. See https://www2.deloitte.com/content/dam/Deloitte/au/Documents/Economics/deloitte-au-economics-great-barrier-reef-230617.pdf .
- 7.Mouillot D, et al. 2014. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc. Natl Acad. Sci. USA 111, 13 757–13 762. ( 10.1073/pnas.1317625111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabricius K, Kluibenschedl A, Harrington L, Noonan S, De'Ath G. 2015. In situ changes of tropical crustose coralline algae along carbon dioxide gradients. Sci. Rep. 5, 9537 ( 10.1038/srep09537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2013. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr. 58, 388–398. ( 10.4319/lo.2013.58.1.0388) [DOI] [Google Scholar]
- 10.van Hooidonk R, Maynard JA, Manzello D, Planes S. 2014. Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Glob. Change Biol. 20, 103–112. ( 10.1111/gcb.12394) [DOI] [PubMed] [Google Scholar]
- 11.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 ( 10.1371/journal.pone.0028983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornwall CE, Hepburn CD, McGraw CM, Currie KI, Pilditch CA, Hunter KA, Boyd PW, Hurd CL. 2013. Diurnal fluctuations in seawater pH influence the response of a calcifying macroalga to ocean acidification. Proc. R. Soc. B 280, 20132201 ( 10.1098/rspb.2013.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos IR, Glud RN, Maher D, Erler D, Eyre BD. 2011. Diel coral reef acidification driven by porewater advection in permeable carbonate sands, Heron Island, Great Barrier Reef. Geophys. Res. Lett. 38, L03604 ( 10.1029/2010GL046053) [DOI] [Google Scholar]
- 14.Rivest EB, Comeau S, Cornwall CE. 2017. The role of natural variability in shaping the response of coral reef organisms to climate change. Curr. Clim. Change Rep. 3, 271–281. ( 10.1007/s40641-017-0082-x) [DOI] [Google Scholar]
- 15.Duarte CM, Hendriks IE, Moore TS, Olsen YS, Steckbauer A, Ramajo L, Carstensen J, Trotter JA, McCulloch M. 2013. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts 36, 221–236. ( 10.1007/s12237-013-9594-3) [DOI] [Google Scholar]
- 16.Vargas CA, Lagos NA, Lardies MA, Duarte C, Manríquez PH, Aguilera VM, Broitman B, Widdicombe S, Dupont S. 2017. Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity. Nat. Ecol. Evol. 1, 0084 ( 10.1038/s41559-017-0084) [DOI] [PubMed] [Google Scholar]
- 17.Anthony KRN, Kleypas JA, Gattuso JP. 2011. Coral reefs modify their seawater carbon chemistry— implications for impacts of ocean acidification. Glob. Change Biol. 17, 3655–3666. ( 10.1111/j.1365-2486.2011.02510.x) [DOI] [Google Scholar]
- 18.Camp EF, Smith DJ, EvenhuisC EI, Manzello D, Woodcock S, Suggett DJ. 2016. Acclimatization to high-variance habitats does not enhance physiological tolerance of two key Caribbean corals to future temperature and pH. Proc. R. Soc. B 283, 20160442 ( 10.1098/rspb.2016.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2014. Diel pCO2 oscillations modulate the response of the coral Acropora hyacinthus to ocean acidification. Mar. Ecol. Progr. Ser. 501, 99–111. ( 10.3354/meps10690) [DOI] [Google Scholar]
- 20.Johnson MD, Moriarty VW, Carpenter RC. 2014. Acclimation of crustose coralline alga Porolithon onkodes to variable pCO2. PLoS ONE 9, e87678 ( 10.1371/journal.pone.0087678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dufault AM, Cumbo VR, Fan TY, Edmunds PJ. 2012. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc. R. Soc. B 279, 2951–2958. ( 10.1098/rspb.2011.2545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan WY, Eggins SM. 2017. Calcification responses to diurnal variation in seawater carbonate chemistry by the coral Acropora formosa. Coral Reefs 36, 763–772. ( 10.1007/s00338-017-1567-8) [DOI] [Google Scholar]
- 23.Semesi IS, Beer S, Björk M. 2009. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Mar. Ecol. Prog. Ser. 382, 41–47. ( 10.3354/meps07973) [DOI] [Google Scholar]
- 24.Roleda MY, Cornwall CE, Feng Y, McGraw CM, Smith AM, Hurd CL. 2015. Effect of ocean acidification and pH fluctuations on the growth and development of coralline algal recruits, and an associated benthic algal assemblage. PLoS ONE 10, e0140394 ( 10.1371/journal.pone.0140394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comeau S, Carpenter RC, Edmunds PJ. 2013. Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc. R. Soc. B 280, 20122374 ( 10.1098/rspb.2012.2374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamenos NA, Burdett HL, Aloisio E, Findlay HS, Martin S, Longbone C, Dunn J, Widdicombe S, Calosi P. 2013. Coralline algal structure is more sensitive to rate, rather than the magnitude, of ocean acidification. Glob. Change Biol. 19, 3621–3628. ( 10.1111/gcb.12351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoepf V, Stat M, Falter JL, McCulloch MT. 2015. Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci. Rep. 5, 17 639–17 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA. 2014. Mechanisms of reef coral resistance to future climate change. Science 344, 895–898. ( 10.1126/science.1251336) [DOI] [PubMed] [Google Scholar]
- 29.Schaum CE, Collins S. 2014. Plasticity predicts evolution in a marine alga. Proc. R. Soc. B 281, 20141486 ( 10.1098/rspb.2014.1486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd PW, Cornwall CE, Davison A, Doney SC, Fourquez M, Hurd CL, Lima ID, McMinn A. 2016. Biological responses to environmental heterogeneity under future ocean conditions. Glob. Change Biol. 22, 2633– 2650 ( 10.1111/gcb.13287) [DOI] [PubMed] [Google Scholar]
- 31.Comeau S, Cornwall CE. 2016. Contrasting effects of ocean acidification on coral reef ‘Animal Forests’ versus seaweed ‘Kelp Forests’. In Marine animal forests (ed. Rosi S.), pp. 1–25. Switzerland: Springer. [Google Scholar]
- 32.Georgiou L, Falter J, Trotter J, Kline DI, Holcomb M, Dove SG, Hoegh-Guldberg O, McCulloch M. 2015. pH homeostasis during coral calcification in a free ocean CO2 enrichment (FOCE) experiment, Heron Island reef flat, Great Barrier Reef. Proc. Natl Acad. Sci. USA 112, 13 219–13 224. ( 10.1073/pnas.1505586112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornwall CE, Comeau S, McCulloch MT. 2017. Coralline algae elevate pH at the site of calcification under ocean acidification. Glob. Change Biol. 23, 4245–4256. ( 10.1111/gcb.13673) [DOI] [PubMed] [Google Scholar]
- 34.Holcomb M, Venn AA, Tambutté S, Allemand D, Trotter J, McCulloch M. 2014. Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 4, 5207 ( 10.1038/srep05207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comeau S, Cornwall CE, McCulloch MT. 2017. Decoupling between the response of coral calcifying fluid pH and calcification under ocean acidification. Sci. Rep. 7, 7573 ( 10.1038/s41598-017-08003-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeCarlo TM, Comeau S, Cornwall CE, McCulloch MT. 2018. Coral resistance to ocean acidification linked to increased calcium at the site of calcification. Proc. R. Soc. B 285, 20180564 ( 10.1098/rspb.2018.0564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Nohaïc M, Ross CL, Cornwall CE, Comeau S, Lowe R, McCulloch MT, Schoepf V. 2017. Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia. Sci. Rep. 7, 14999 (doi:0.1038/s41598-017-14794-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comeau S, Cornwall CE, Gruber R, McCulloch MT. In preparation. Net ecosystem calcification of a tidal reef dominated by coralline algae is not negatively affected by a marine heatwave.
- 39.Trotter J, et al. 2011. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: validation of the boron seawater pH proxy. Earth Planet. Sci. Let. 303, 163–173. ( 10.1016/j.epsl.2011.01.030) [DOI] [Google Scholar]
- 40.McCulloch MT, D'Olivo JP, Falter J, Holcomb M, Trotter JA. 2017. Coral calcification in a changing world and the interactive dynamics of pH and DIC upregulation. Nat. Commun. 8, 15686 ( 10.1038/ncomms15686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holcomb M, DeCarlo T, Gaetani G, McCulloch M. 2016. Factors affecting B/Ca ratios in synthetic aragonite. Chem. Geol. 437, 67–76. ( 10.1016/j.chemgeo.2016.05.007) [DOI] [Google Scholar]
- 42.DeCarlo TM, D'Olivo JP, Foster T, Holcomb M, Becker T, McCulloch MT. 2017. Coral calcifying fluid aragonite saturation states derived from Raman spectroscopy. Biogeosciences 14, 5253–5269. ( 10.5194/bg-14-5253-2017) [DOI] [Google Scholar]
- 43.Allemand D, Ferrier-Pagés C, Furla P, Houlbréque F, Puverel S, Reynaud S, Tambutte E, Tambutte S, Zoccola D. 2004. Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. C. R. Palevol. 3, 453–467. ( 10.1016/j.crpv.2004.07.011) [DOI] [Google Scholar]
- 44.Comeau S, Carpenter R, Edmunds PJ. 2013. Response to coral reef calcification: carbonate, bicarbonate and proton flux under conditions of increasing ocean acidification. Proc. R. Soc. B 280, 20131153 ( 10.1098/rspb.2013.1153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCulloch MT, Falter J, Trotter J, Montagna P. 2012. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Climate Change 2, 623–627. ( 10.1038/nclimate1473) [DOI] [Google Scholar]
- 46.Comeau S, Cornwall CE, DeCarlo TM, Krieger E, McCulloch MT. 2018. Similar controls on calcification under ocean acidification across unrelated coral reef taxa. Glob. Change Biol. ( 10.1111/gcb.14379) [DOI] [PubMed] [Google Scholar]
- 47.Ries JB, Cohen AL, McCorkle DC. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134. ( 10.1130/G30210A.1) [DOI] [Google Scholar]
- 48.Schoepf V, Jury CP, Toonen RJ, McCulloch MT. 2017. Coral calcification mechanisms facilitate adaptive responses to ocean acidification. Proc. R. Soc. B 284, 20172117 ( 10.1098/rspb.2017.2117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jokiel PL. 2013. Coral reef calcification: carbonate, bicarbonate and proton flux under conditions of increasing ocean acidification. Proc. R. Soc. B 280, 20130031 ( 10.1098/rspb.2013.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herfort L, Thake B, Taubner I. 2008. Bicarbonate stimulation of calcification and photosynthesis in two hermatypic corals. J. Phycol. 44, 91–98. ( 10.1111/j.1529-8817.2007.00445.x) [DOI] [PubMed] [Google Scholar]
- 51.Cornwall CE, Hepburn CD, Pritchard DW, McGraw CM, Currie KI, Hunter KA, Hurd CL. 2012. Carbon-use strategies in macroalgae: differential responses to lowered pH and implications for ocean acidification. J. Phycol. 48, 137–144. ( 10.1111/j.1529-8817.2011.01085.x) [DOI] [PubMed] [Google Scholar]
- 52.Britton D, Cornwall CE, Revill AT, Hurd CL, Johnson CR. 2016. Ocean acidification reverses the positive effects of seawater pH fluctuations on growth and photosynthesis of the habitat-forming kelp, Ecklonia radiata. Sci. Rep. 6, 26036 ( 10.1038/srep26036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wall M, Fietzke J, Schmidt GM, Fink A, Hofmann LC, de Beer D, Fabricius KE. 2016. Internal pH regulation facilitates in situ long-term acclimation of massive corals to end-of-century carbon dioxide conditions. Sci. Rep. 6, 30688 ( 10.1038/srep30688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornwall CE, Comeau S, DeCarlo TM, Moore B, D'Alexis Q, McCulloch MT. 2018. Data from: Resistance of corals and coralline algae to ocean acidification: physiological control of calcification under natural pH variability Dryad Digital Repository. ( 10.5061/dryad.250q1g7) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Cornwall CE, Comeau S, DeCarlo TM, Moore B, D'Alexis Q, McCulloch MT. 2018. Data from: Resistance of corals and coralline algae to ocean acidification: physiological control of calcification under natural pH variability Dryad Digital Repository. ( 10.5061/dryad.250q1g7) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All geochemical data are within the electronic supplementary material; all else are accessible in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.250q1g7 [54].