Abstract
In this study, we applied various assays to reveal new activities of phenylcyanomethylenequinone oxime-4-(hydroxyimino) cyclohexa-2,5-dien-1-ylidene](phenyl)ethanenitrile (4-AN) for potential anti-microbial applications. These assays demonstrated (a) the antimicrobial effect on bacterial and fungal cultures, (b) the effect on the in vitro activity of the kinase CK2, (c) toxicity towards human erythrocytes, the Caco-2 cancer cell line, and embryonic development of Zebrafish. We demonstrated the activity of 4-AN against selected bacteria and Candida spp. The MIC ranging from 4 µg/ml to 125 µg/ml proved effective in inhibition of formation of hyphae and cell aggregation in Candida, which was demonstrated at the cytological level. Noteworthy, 4-AN was found to inhibit the CK2 kinase with moderate potency. Moreover, at low concentrations, it did not exert any evident toxic effects on human erythrocytes, Caco-2 cells, or Zebrafish embryos. 4-AN can be a potential candidate as a novel drug against Candida infections.
Keywords: Arylcyanomethylenequinone oximes, Antifungal agents, Candida albicans, Hyphae, Phosphorylation
1. Introduction
In humans, ten genera of fungi, i.e. Aspergillus, Candida, Cryptococcus, Blastomyces, Coccidioides, Histoplasma, Paracoccidioides, Penicillium, Pneumocystis, and Rhizopus, have a high prevalence, and more than 40% of infections are caused by Candida (Bitar et al., 2014, Brown et al., 2012). Candidemias are caused by approximately 20 species of Candida, the most common of which is C. albicans, which together with C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei is the causal agent of over 95% of invasive candidiasis (McCarty and Pappas, 2016). For example, one of the most frequent Candida mycoses is vulvovaginal candidiasis, affecting up to 75% of healthy women, with a tendency to recur (Dorgan et al., 2015, Foxman et al., 2013, Nyirjesy and Sobel, 2003, Sobel, 2002, Sobel, 2006, Sobel et al., 2004). Antifungal resistance is an increasing threat for the effective treatment of invasive mycoses, making their therapy difficult, expensive, or even impossible (WHO, 2014). Unfortunately, the resistance to individual drugs or multiple drug resistance renders the antibiotic therapy partially ineffective (Campoy and Adrio, 2017, Pfaller, 2012). One of the causes of drug resistance in fungi is the intensive prophylactic use of antifungal drugs such as polyene antibiotics (nystatin, amphotericin B), purine antimetabolites (5-fluorocytosine), and azoles (ketoconazole, fluconazole) (Roca et al., 2015). It seems that another cause is the suppression of the immune system. Experiments carried out on Candida strains isolated from HIV patients showed that almost 10% of the fungi were resistant to these antibiotics (Magaldi et al., 2001).
C. albicans fungi are capable of colonizing and persisting in the mucosa of the oral cavity, gastrointestinal system, and genitourinary tract in healthy humans. It has been suggested that 40–50% of each sample population carries Candida in their gastrointestinal tract (San-Blas and Richard A, 2004, Tong and Tang, 2017). C. albicans is known to be a dimorphic microorganism that can exist as typical budding yeast cells (type Y) and as mycelium cells forming hyphae (type M), depending on the environmental conditions (Baillie and Douglas, 1999, Brown and Gow, 1999). Their virulence is promoted by the capacity to form hyphae, i.e. long, apically growing threads greatly favoring implantation in the mucosa (Jacobsen et al., 2012, Sudbery et al., 2004, Sudbery, 2011). Although the formation of C. albicans hyphae has been evidenced as one of the major factors required for infection, the general mechanism of virulence is not clear (De Bernardis et al., 1994, Kumamoto and Vinces, 2005, Sandini et al., 2007, Saville et al., 2003). Some researchers have demonstrated that strains that lack the capacity to undergo dimorphic transition lose pathogenicity (Lo et al., 1997, Peters et al., 2014), while other authors have suggested that both morphological forms are capable to form a biofilm and are therefore pathogenic (Chandra et al., 2001).
Reversible protein phosphorylation catalyzed by protein kinases plays an important role in key cellular processes, including the cell cycle, metabolism, and cell death (Battistutta et al., 2011, Klopffleisch et al., 2012, Pierre et al., 2011). There is ample evidence showing a strict relationship between the activity of protein kinases and the phosphorylation status of many proteins and Candida virulence. The results presented recently by Caballero-Lima and Sudbery have shown that Cdk1-mediated phosphorylation of protein Exo84 in Candida cells is necessary for efficient hyphal extension (Caballero-Lima and Sudbery, 2014). Another protein whose phosphorylation status is crucial for candidiasis development is Nap1, i.e. a protein that is essential for the morphology, invasive growth, and septin ring dynamics in Candida. The authors showed that deletion of NAP1 or mutation in the N-terminal phosphorylation cluster strongly reduced the virulence of C. albicans in a mouse model of systemic infection (Huang et al., 2014). Phosphorylation is also a key process in cell chain formation, which is tightly associated with Candida hyphal growth and virulence. It has been found that phosphorylation of a conserved developmental regulator, Efg1, by cyclin-dependent kinase Cdc28 downregulates Ace2 target genes during hyphal growth in G1 (Wang et al., 2009).
Therefore, new compounds targeting different cellular processes, including phosphorylation, are required to deal with Candida infections.
The aim of the present study was to apply various assays to find, for the first time, the activity of 4-(hydroxyimino) cyclohexa-2,5-dien-1-ylidene](phenyl)ethanenitrile (4-AN) against bacteria and Candida spp. Recently, the compound has been analyzed together with other arylcyanomethylenequinone oximes to reveal its activity against cancer cell lines (Hong et al., 2016). The assays presented here were designed to determine the antimicrobial effect on the growth/cell multiplication/cell aggregation of bacterial and fungal cultures, the effect on the in vitro activity of CK2, i.e. one of the most pleiotropic kinases, the hemolytic activity towards human erythrocytes, and the toxicity against the Caco-2 cancer cell line and Zebrafish embryos.
2. Material and methods
2.1. Chemistry
2.1.1. General
All reagents were purchased from Sigma-Aldrich, Strem, TCI, and Alfa Aesar chemical companies and used without further purification. Analytical thin-layer chromatography (TLC) was performed using silica gel 60 F254 precoated plates (0.25 mm thickness) with a fluorescent indicator. Visualisation of the TLC plates was performed by means of UV light or either KMnO4 or I2 stains. NMR spectra were recorded on Bruker Avance 500 MHz spectrometers; chemical shifts were reported in ppm and calibrated to residual solvent peaks at 7.27 ppm and 77.00 ppm for 1H and 13C in CDCl3 or internal reference compounds. The following abbreviations are used in reporting the NMR data: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and br (broad). Coupling constants (J) are expressed in Hz. Spectra are reported as follows: chemical shift (δ, ppm), multiplicity, integration, and coupling constants (Hz). The products were purified by flash chromatography on silica gel 60 (230–400 mesh) using a BUCHI chromatograph. MS spectra were recorded on a Shimadzu LCMS IT-TOF spectrometer.
2.1.2. 4-(hydroxyimino)cyclohexa-2,5-dien-1-ylidene](phenyl)ethanenitrile (4-AN)
3 g of benzyl cyanide followed by 3 g of nitrobenzene was added to a solution of 6 g of KOH in 30 ml of absolute methanol stirred at 50 °C. The stirring at that temperature was continued for the next 4 h; next, the reaction mixture was cooled down to RT and 40 ml of water was added. The reaction mixture was acidized with 20 ml of 50% of acetic acid, and the rough product was filtered off, washed with a water/methanol mixture and diethyl ether, and then dried under reduced pressure to yield 4 g (74%) of the pure 4-AN compound. The purity of the substance was above 95%. HRMS analysis is presented in the Supplementary material. 1H NMR (RT, DMSO‑d6, 500.13 MHz), δ (ppm): 12.85 (brs, 1H, NOH), 7.56–7.47 (m, 5H, ArH), 7.42–7.19 (m, 2H, ArH), 7.07–6.87 (m, 2H, ArH).
1H NMR (−6 °C, acetone‑d6, 500.13 MHz), δ (ppm): 12.40 (brs), 7.57–7.50 (m, 5H), 7.49 (dd, J = 10.1, 1H), 7.37 (dd, J = 10.1, 1H), 7.05 (dd, J = 9.9, 1H), 6.84 (dd, J = 9.9, 1H), 3.37 (brs).
1H NMR (+22.5 °C, acetone‑d6, 500.13 MHz), δ (ppm): 7.57–7.50 (m, 10H), 7.49 (dd, J = 10.1, 1H), 7.37 (dd, J = 10.1, 1H), 7.34 (dd, J = 10.1, 1H), 7.27 (dd, J = 9.9, 1H), 7.13 (dd, J = 10.1, 1H), 7.05 (dd, J = 9.9, 1H), 6.97 (dd, J = 9.9, 1H), 6.84 (dd, J = 9.9, 1H), 3.03 (brs).
13C NMR (−6 °C, acetone‑d6, 125.75 MHz), δ (ppm): 151.42 (C), 143.03 (C), 133.85 (C), 131.40 (CH), 131.21 (CH), 130.42 (CH), 129.95 (CH), 125.74 (CH), 119.94 (CH), 119.53 (C), 111.88 (C).
HRMS (ESI): m/z = 223.0864 [C14H10N2O+H]+, m/z (teor.) = 223.0866, diff. = 0.90 ppm.
2.2. Microbial strains
Gram-positive bacteria Staphylococcus aureus (ATCC 6538) and Enterococcus faecalis (PCM 2673), gram-negative bacteria Pseudomonas aeruginosa (PCM 2562), Klebsiella pneumoniae (PCM1), Escherichia coli (PCM 2560), Enterobacter cloacae (PCM 2569), Proteus vulgaris (PCM 2668), and Salmonella bongori (PCM 2552) as well as Candida albicans (ATCC 10231), Candida parapsilosis (ATCC 22019), Candida krusei (ATCC 14243), Candida tropicalis (ATCC 13803), Candida kefyir (ATCC 204093), Candida lusitaniae (ATCC 34449), and Candida glabrata (ATCC 15126) were used as reference bacterial and fungal strains.
2.3. MIC and MFC determination
The bacterial strains were inoculated into Mueller–Hinton broth (Biocorp, Poland) for 24 h before determination of the minimal inhibitory concentration (MIC) and incubated at 37 °C with vigorous shaking (180 rpm). The MIC was determined with the microbroth dilution method. Bacterial suspensions in Mueller–Hinton liquid medium at initial inoculums of 5 × 105 colony forming units per ml were added to 96-well polystyrene plates and exposed to the investigated compound at adequate concentrations in DMSO (range: 0.001–5 mg/ml) for 20 h at 37 °C. MICs were defined as the lowest drug concentrations at which microbial growth was inhibited. The experiments were performed in triplicate.
All Candida strains were cultured in Sabouraud glucose liquid medium (Biocorp, Poland) for 48 h at a temperature of 25 °C with shaking (130 rpm). The activity of the examined compound against these yeasts was determined with the microbroth dilution method. Microbial cell suspensions at initial inoculums of 3 × 103 colony forming units per ml in Sabouraud glucose broth were exposed to the examined compound at adequate concentrations (range: 0.001–5 mg/ml) for 48 h at 25 °C. The MIC was the lowest concentration of the compound that inhibited the visible growth of the microorganisms after incubation. After MIC readings, 10 µl aliquots were removed from the wells corresponding to MIC, 2xMIC, and 4xMIC and spread on SDA (Sabouraud Dextrose Agar) Petri dishes. The plates were incubated at 30 °C and the fungal colonies were counted after approximately 2 days of incubation. The MFC was defined as the lowest drug concentration at which ≤1 colony was visible on the agar plate. The experiments were performed in triplicate.
Tetracycline (TET), ketoconazole (KET), and amphotericin B (AMP-B) were used as reference compounds.
2.4. Hyphal growth of Candida
The effect of 4-AN on the hyphal growth of the Candida reference strain was evaluated using Spider medium. Candida cells were grown overnight in Sabouraud broth medium (Biocorp, Poland) in a shaker at 180 rpm and 37 °C. At the late exponential growth phase, the yeast cells were harvested using a microcentrifuge (Polygen 1-15PK, Poland) at 2300g for 15 min. The yeast cells were washed twice with phosphate buffered saline, pH 7.2, and resuspended in PBS to reach an optical density (OD600) of 0.38 (107 cells/ml). 100 µl of the suspension containing 107 cells/ml were used for the assays. Candida cells were grown on Spider medium plates containing 10% fetal bovine serum (FBS) with or without the tested compound at the concentration of MIC/2, MIC/4, MIC/8, and MIC/16. The plates were incubated at 37 °C for 36 h. The morphology of Candida colonies was inspected under a light microscope and imaged using a digital camera.
2.5. Growth rate of C. albicans
The starting Candida culture in 20 ml of Sabouraud glucose broth was inoculated on an agar plate and incubated at 30 °C overnight. The next day, the final cultures in 50 ml of Sabouraud medium were inoculated with the overnight culture and treated with an appropriate concentration of the examined compound (0.5; 1; 2; and 4 µg/ml). DMSO was used as a control. The initial OD600 was set to 0.05 and monitored every hour; the values obtained were plotted against time.
2.6. Hemolytic activity
Human blood samples were provided voluntarily by the author of the manuscript. They were collected in sterile tubes containing a citrate dextrose solution as an anticoagulant.
In order to separate erythrocytes from plasma, the samples were centrifuged at 500g for 10 min at 4 °C and the supernatant was discarded. Next, the erythrocytes were resuspended in PBS buffer (10 mM phosphate, pH 7.5; 150 mM NaCl) and centrifuged as previously. The washing procedure was repeated until a transparent supernatant was obtained. After washing, the erythrocytes were finally resuspended in PBS buffer at a final concentration of 2%. Simultaneously, appropriate concentrations (in the range of 1–250 µg/ml) of the examined compound were prepared in a final volume of 50 µl DMSO. The compound prepared in this way was mixed with 450 µl of the 2% erythrocyte suspension and incubated for 1 h at 37 °C. Next, the samples were centrifuged at 5000g for 10 min and absorbance was measured at a wavelength of 415 nm.
2.7. Fluorescence microscopy
In order to visualize the effect of 4-AN on Candida, cells were harvested from cultures containing 2 µg/ml of the compound and from the control. The cells were stained with DAPI (4,6-diamidino-2-phenylindole) and visualized using fluorescence microscopy. Briefly, the Candida liquid culture samples (10 ml each) were centrifuged, the culture medium was removed, and the cells were fixed in 3:1 ethanol – glacial acetic acid and stored at −20 °C until required. The fixed suspensions were centrifuged again and, after removing the fixative, the cells were suspended in 45% acetic acid. A drop of each suspension was gently squashed between the microscope slide and the cover glass. The squeezed preparations were frozen in liquid nitrogen, the cover glasses were removed, and the preparations were air dried and mounted in a drop of a DAPI solution (7 µg DAPI/ml in PBS buffer in 70% glycerol). The preparations were sealed with rubber cement (Marabu) and examined in a fluorescence microscope with UV illumination. Images were taken with a cooled monochrome camera.
2.8. Bioactivity prediction
For prediction of bioactivity, the cheminformatics Molinspiration tool available at http://www.molinspiration.com/ was used following the instructions of the web server developer.
2.9. Kinase assays
The activity of protein kinases was determined as the rate of incorporation of phosphate from [γ-32P]ATP into the protein substrate in conditions described below.
Phosphorylation reactions were conducted at 37 °C for 5 min. We used 50-μl samples, each containing human recombinant CK2α, ribosomal P2B protein as a substrate, an appropriate concentration of the tested compound (1–200 µM), 20 μM [γ-32P]ATP (specific radioactivity 300–1000 cpm/pmol), 15 mM Mg2+, 20 mM Tris–HCl pH 7.5, and 6 mM 2-mercaptoethanol.
Phosphorylation was terminated by addition of 2X SDS/PAGE sample buffer and proteins were resolved by electrophoresis and stained with Coomassie Brilliant Blue. Dried gels were exposed to a Kodak X-Omat film. 32P-labeled bands of P2B were excised from the gel and radioactivity was determined with the Cerenkov counting technique in a scintillation counter (MicroBeta, Perkin Elmer).
2.10. Chemicals and cells
Minimum Essential Medium (MEM) with Earl’s Balanced Salts (MEM/EBSS) and 2 mM l-Glutamine was obtained from HyClone Thermo Scientific (South Logan, Utah, USA). Non-essential amino acids (NEAA), fetal bovine serum (FBS), penicillin/streptomycin solution (P/S), 0.25% (w/v) trypsin – 0.53 mM EDTA, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The Caco-2 cell line was obtained from the European Collection of Cell Cultures. The cells were cultured in MEM supplemented with 1% NEAA, 100 IU, 0.1 mg/ml P/S, and 10% FBS – complete MEM (cMEM) at 37 °C, 5% CO2.
The 4-AN compound was dissolved in DMSO to obtain a stock solution. Working solutions (40, 4, 0.4 µg/ml) were prepared with the use of cMEM. The highest concentration of DMSO in the working solution did not exceed 0.1%.
2.11. Cell viability and proliferation
After exposure to the compound, cell viability was determined with the use of a Nucleocounter (ChemoMetec A/S, Allerød, Denmark). The cells were seeded at 2 × 205 cells per ml of the culture medium in a 96-well clear microplate and left to adhere overnight. Next, the medium was replaced with 100 µl of fresh cMEM without the compound (control) or with 40, 4, and 0.4 µg/ml of the compound in triplicate for each concentration. After 24, 48, and 72 h, the medium was removed from the wells and the cells were trypsinized. Next, cMEM was added to each well with detached cells and the cells from every three wells were pooled. The cell suspension was aspirated to a propidium iodide (PI)-containing Nucleocounter cassette and dead cells (DC) were counted. Next, the cell suspension was mixed with lysis and stabilizing buffers and the total cell (TC) number was determined. Each determination was carried out in duplicate. Cell viability was counted using the following formula: TC-DC/TC × 100%.
2.12. ATP level determination
The cytotoxic potential of the 4-AN compound towards Caco-2 cells was assessed with a Cell Titer-Glo cell viability assay obtained from Promega (Madison, WI, USA). It is a luminometric assay determining the number of metabolically active cells in a culture based on quantitation of ATP. The cells were seeded at 4 × 104 cell/ml in 96-well white microplates and left overnight to adhere. Next, the medium was replaced with 100 µl of fresh cMEM without (control) or with the compound at a concentration of 40, 4, and 0.4 µg/ml. Each determination was conducted in three replicates. The assay was performed according to the manufacturer’s protocol. After 24, 48, and 72 h of exposure, the assay was terminated by cell lysis and the generated luminescent signal was measured using a multi-mode plate reader FLUOstar Omega (BMG Labtech GmbH, Ortenberg, Germany).
2.13. Influence of 4-AN on Zebrafish embryo development
The collected embryos were transferred to a Petri dish with E3 medium (5 mM NaCl, 0.33 mM MgCl2, 0.33 mM CaCl2, 0.17 mM KCl; pH 7.2); next, 10 embryos per well were placed in 6-well plates, The stock solutions of 4-AN were prepared in DMSO. In the experiments, we used 0.2, 10, and 20 µg/ml solutions of the compound; they were freshly prepared by dissolving the stock solutions in the E3 solution each time directly before addition to the wells. The solutions were changed once a day and the embryos were maintained in the incubator at 28.5 °C. Zebrafish embryo development and viability were evaluated for up to 5 days using bright-field microscopy (Zeiss Axio Vert, ZEISS, Germany). Image analysis was performed using Zeiss software to determine the percentage of dead and malformed embryos over time. The same embryos (n = 10) were analyzed throughout the whole study.
2.14. Statistical analysis
The tests of the cell viability, proliferation, and ATP level were conducted in duplicate. Statistical analysis was performed with the use of Statistica 10.0 software (StatSoft Inc., Tulsa, OK, USA). Differences between the control and experimental groups were compared using Tukey’s test and were considered statistically significant at P < .05.
3. Results and discussion
3.1. Chemistry
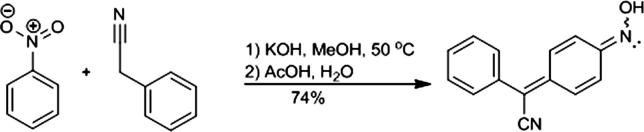
The classical reactivity of nitrobenzene towards the active methylene compounds in strong basic conditions was employed for the synthesis of 4-(hydroxyimino)cyclohexa-2,5-dien-1-ylidene](phenyl)ethanenitrile (4-AN) as presented in Scheme 1 (Davis et al., 1961). The NMR spectra of the product recorded in DMSO at RT were consistent with those reported previously (Hong et al., 2016) and indicated that the solution of 4-AN contained a mixture of syn- and anty-isomers in an almost equal ratio. To obtain a single isomer, the reaction product was recrystallized from benzene. The 1H NMR spectra recorded in DMSO‑d6 immediately after the sample preparation contained signals of 2 isomers in a 1:9 ratio, while the spectrum of the same solution recorded in a few hours later contained signals of isomers in a 1:1 ratio. We found that 4-(hydroxyimino)cyclohexa-2,5-dien-1-ylidene](phenyl)ethanenitrile was stable in the acetone‑d6 solution at a temperature below −6 °C. The spectra of a single isomer were therefore recorded at low temperature. In the HPLC-HRMS chromatogram (Supplementary data), we found a single compound with an accurate mass corresponding to 4-AN, which may indicate rapid interconversion of isomers on the HPLC column eluted by the acidic aqueous acetonitrile mobile phase.
Scheme 1.
Synthesis of 4-(hydroxyimino)cyclohexa-2,5-dien-1-ylidene](phenyl)ethanenitrile (4-AN).
3.2. 4-AN influences microbial growth
The analyzed compound was screened against several gram-positive and gram-negative bacteria and one representative of the Candida genus (Table 1). Preliminary screening revealed that 4-AN inhibited the growth of all the tested microorganisms. In the case of the bacteria, the MIC values were between 125 and 250 µg/ml, depending on the species.
Table 1.
Antimicrobial screening of the 4-AN compound. The results are the MIC values.
| Microorganism | 4-AN [µg/ml] | TET [µg/ml] | Microorganism | 4-AN [µg/ml] | TET [µg/ml] |
|---|---|---|---|---|---|
| C. albicans | 4 | – | S. aureus | 125 | 7.5 |
| P. aeruginosa | 250 | 15 | E. cloacae | 250 | 7.5 |
| K. pneumoniae | 250 | 15 | P. vulgaris | 250 | 7.5 |
| E. coli | 125 | 0.5 | S. bongori | 125 | 3.2 |
In contrast to the low susceptibility of the bacteria to 4-AN, the only representative of fungi, i.e. C. albicans, appeared to be remarkably more sensitive to the action of the compound. Given the preliminary results, we tested the other Candida species. The results showed great potency of the agent towards the selected Candida species, which was at the level of the reference antifungals or even lower. The MIC values determined for the tested Candida species were between 4 and 125 µg/ml (Table 2). Interestingly, 4-AN affected the growth of C. kefyir, which was found to acquire antibiotic resistance to common antifungals (e.g. caspofungin) very rapidly (Fekkar et al., 2013).
Table 2.
Screening of the 4-AN against Candida spp.
| Strain | 4-AN | KET | AMP-B |
|---|---|---|---|
| MIC/MFC [µg/ml] | |||
| C. albicans | 4/4 | 8/- | 1.5/– |
| C. krusei | 31/31 | 8/– | 1.5/– |
| C. parapsilosis | 31/62 | 8/– | 31/– |
| C. tropicalis | 125/250 | 8/– | 31/– |
| C. kefyir | 8/31 | 1.5/– | 8/– |
| C. lusitaniae | 62/62 | 16/– | – |
| C. glabrata | 31/31 | 8/– | 16/– |
Promising is also the fact that, besides the high fungistatic activity, the compound exerts a potent fungicidal effect against six of the seven tested Candida species with MFC values of 4–62 µg/ml.
Based on the highest susceptibility to 4-AN, C. albicans was found as the most attractive target for the compound; thus, the further investigations were focused on the influence of 4-AN on this species.
3.3. Influence of 4-AN on C. albicans growth
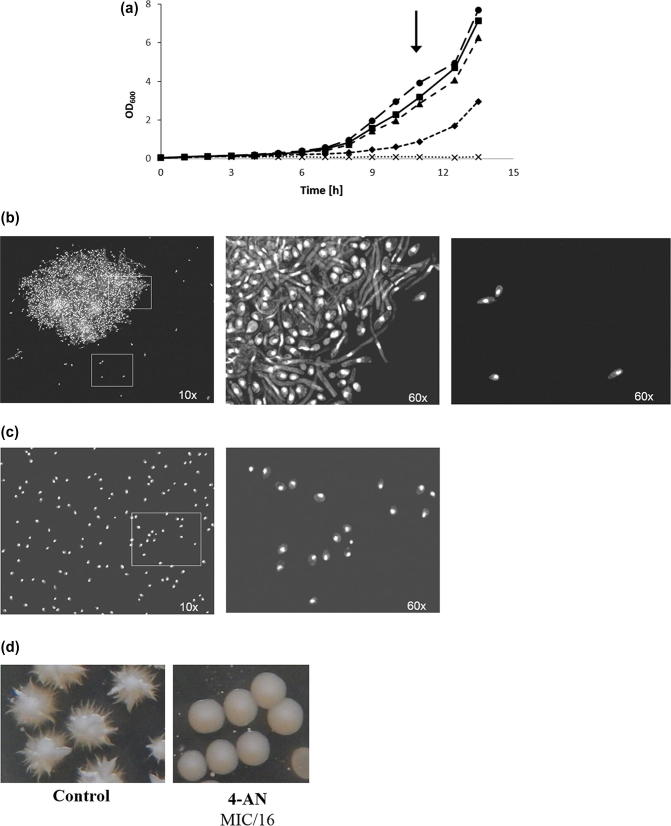
In order to verify the effect of the analyzed compound on Candida cells, the fungi were cultured in the presence of 4-AN at different concentrations as a function of time (Fig. 1A). The growth of C. albicans was totally inhibited at the concentration of 4 µg/ml (MIC value) and significantly reduced at 2 µg/ml. 0.5 and 1 µg/ml did not influence the growth of the fungi.
Fig. 1.
(A) C. albicans growth curve as a function of time. Cells were cultured in the presence of 4-AN at concentrations: 0.5 µg/ml (-■-), 1 µg/ml (-▲-), 2 µg/ml (-♦-), and 4 µg/ml (-×-), DMSO was used as a control (-●-). The black arrow indicates the time of cell harvesting for fluorescence microscopy visualization. (B and C) DAPI staining of C. albicans treated with DMSO (control) (B) and 2 µg/ml of 4-AN (C): Pictures with 25 μm scale bars are magnifications of the white line rectangle areas. The relative position of the cells is however not always exactly the same between the magnifications since prolonged high-energy UV illumination causes cell movement in the mounting medium.
The fluorescent images revealed that the 2 µg/ml concentration of 4-AN (MIC/2) totally inhibited hyphal formation (Fig. 1C). Only numerous well-separated cells of the budding yeast type were present. In contrast, the control culture, which was not treated with the drug, exhibited the presence of numerous hyphae entangled in huge dense cell aggregates together with some fraction of entrapped budding yeast-type cells (Fig. 1B). Single budding yeast-type cells outside the cellular aggregates were relatively scarce. The organization of DAPI-stained nuclei was quite regular, without any obvious structural abnormalities. All this indicates that 4-AN is an effective inhibitor of both hyphal growth and cellular aggregation in Candida. This result was confirmed with the use of Spider medium supplemented with a hypha-inducing factor – fetal calf serum. The presence of the 4-AN compound at the concentration of MIC/16 (0.25 µg/ml) totally abrogated the hyphal growth of the fungi (Fig. 1D). Although there is still no formal proof, Candida hyphae tend to be viewed as necessary for virulence. Indeed, hyphal forms are invasive, as they invade the agar substratum when grown in the laboratory. Although filamentous growth of fungi is not obligatorily coupled with tissue invasion, it is likely to promote tissue penetration and help in organ colonization in the case of C. albicans. Undoubtedly, the hyphal tip is an effective drill-bit enabling the fungus to burrow into a tissue. As a site of apical secretion of a number of enzymes, the hyphal tip facilitates infiltration into solid substrates and tissues. Furthermore, fungal hyphae also exhibit contact guidance or thigmotropism, which enables them to navigate according to surface topography and get access to weak surface sites that are susceptible to invasion. A relationship between pathogenicity, drug resistance, filamentous hyphal forms, and cell aggregation can be seen when considering the ability of C. albicans to form a biofilm on biotic and abiotic surfaces, especially on implanted medical devices. Biofilms are organized cell aggregates with an upper layer made up of hyphal cells. They render the fungus more resistant to drugs and contribute to approximately half of all nosocomial infections (Gow et al., 2002, Kabir et al., 2012, Sudbery et al., 2004).
To date, some agents inhibiting hyphal formation have been developed. High-throughput screening performed by Heintz-Bushart and others have revealed that compounds from methyl aryl-oxazoline carboxylate, dihydrobenzo[d]isoxazolone, and thiazolo[4,5-e]benzoisoxazole families can effectively suppress production of hyphae (Heintz-Buschart et al., 2013). Messier and coworkers have also found that a natural isopentenyloxychalcone, i.e. 4-hydroxycordoin, inhibits C. albicans hyphal formation at a concentration ranging from 50 to 200 µg/ml (Messier et al., 2011). Moreover, gymnemic acids were found to inhibit this process as well (Vediyappan et al., 2013). Other authors described natural extracts from Alium sativum (Low et al., 2008) and Solidago virgaurea (Chevalier et al., 2012) as hyphal production suppressors. None of these authors have used 4-AN as an antifungal agent. Thus, our study provides an interesting alternative to the already existing potential antimicrobial strategies.
3.4. Impact of 4-AN on human cells and Zebrafish embryos
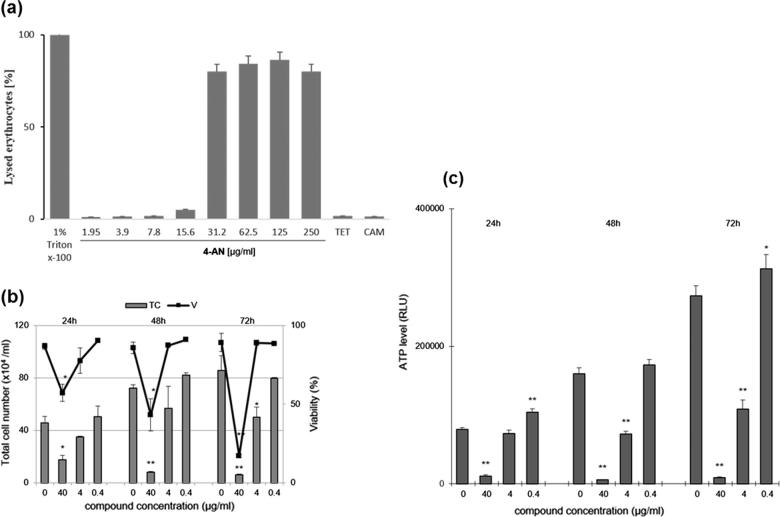
In order to verify the effect of 4-AN on human cells, induction of erythrocyte lysis was assessed. To this end, human erythrocytes were incubated in the presence of different concentrations of 4-AN as described in Section 2.6. The results revealed that only the high concentrations of the compound (31–250 µg/ml) significantly induced cell lysis. In turn, at a concentration corresponding to the MIC value, 4-AN destroyed no more than 2% of erythrocytes (Fig. 2A), which suggests that 4-AN is safe to human blood cells at low concentrations. An additional experiment was carried out in order to check whether 4-AN is toxic to epithelial colorectal adenocarcinoma Caco-2 cancer cells. Upon the treatment with 4-AN, the viability of Caco-2 cells was significantly reduced in the presence of 40 µg/ml of compound (Fig. 2B). At the concentration of 4 µg/ml corresponding to the MIC value, the viability was only slightly reduced (by 9%).
Fig. 2.
(A) Effect of 4-AN on human erythrocytes. Triton X-100 and antibiotics (tetracycline, chloramphenicol) were used as positive and negative controls, respectively. (B) The influence of 4-AN on proliferation (TC) and viability (V) of human cancer cells Caco-2.(C) The level of ATP in Caco-2 cells treated with different concentrations of 4-AN after 24, 48, and 72 h (mean ± SD, ** significantly different from the control at P < .01, * at P < .05).
Additionally, the cellular ATP level was measured in the presence of the increasing 4-AN concentrations. Interestingly, the lowest amount of the compound (0.4 µg/ml) caused an increase in ATP (by 31%), in comparison to the control. In turn, the higher concentrations of 4-AN resulted in 60% and 97% reduction of this nucleoside in the case of 4 and 40 µg/ml of 4-AN, respectively (Fig. 2C). Noteworthy, the concentration of 0.4 µg/ml does not disturb the time-dependent synthesis of ATP, whereas 4-AN at concentrations of 4 µg/ml and 40 µg/ml can interfere with the processes of oxidative phosphorylation and glycolysis, as major sources of cellular ATP, similarly to anticancer drugs (Goldin et al., 2007). Taking the above into consideration, 4-AN affects Caco-2 cells by inducing disorders in cell proliferation and ATP content only at a high concentration but is safe when used at a low concentration.
Our data show relatively low potency of 4-AN towards Caco-2 cancer cells. In turn, Hong and coworkers showed slightly higher antitumor activity of 4-AN and other arylcyanomethylenequinone oximes against renal cell carcinoma Caki-1 and renal cell adenocarcinoma 769-P cells with IC50 values between 4.5 and 66.4 µM (Hong et al., 2016).
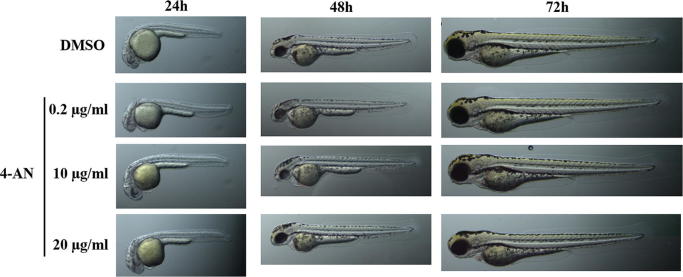
Since Zebrafish is a promising model for whole-organism toxicology screening due to its rapid development, we evaluated the morphological changes in the zebrafish embryos induced by 4-AN. The experiment revealed neither morphological changes in the zebrafish embryos nor toxic effects of the compound used at the doses of 0.2–20 µg/ml during 5 days of observation (Fig. 3).
Fig. 3.
Influence of 4-AN on the embryonic development of Danio rerio.
3.5. Compound 4-AN inhibits the activity of protein kinase CK2
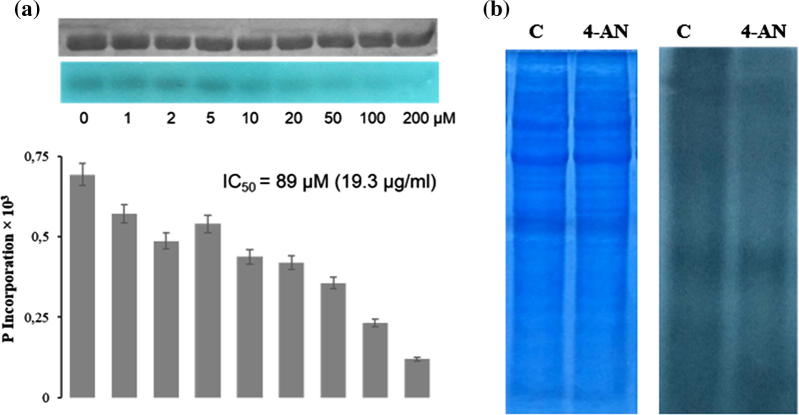
In order to predict a possible cellular target for 4-AN, a cheminformatics tool (Molinspiration) was applied for prediction of the bioactivity of the compound. As revealed by the calculations, 4-AN may probably act as an enzyme inhibitor and/or a kinase inhibitor (data not shown). This prediction together with the role of protein phosphorylation in Candida virulence were intriguing indications to verify whether 4-AN can act as a kinase inhibitor. The preliminary studies included protein kinase CK2, i.e. a constitutively active, pleiotropic, universal enzyme in the cell that phosphorylates more than 300 substrates. It is a key molecular player in many cellular processes, and its role in Candida virulence has been reported. Chiang and coworkers have shown that CK2α‘, i.e. the catalytic subunit of CK2, governs the interactions of C. albicans with endothelial and oral epithelial cells in vitro and virulence during oropharyngeal candidiasis (Kaplancikli et al., 2004). Our results have revealed that 4-AN inhibited CK2 with IC50 of 19.3 µg/ml (Fig. 4A). This preliminary result suggests that protein kinase CK2 may act as one of the molecular targets of the 4-AN action. In future, the activity of the chemical should also be tested towards different molecular forms of CK2 that can exist in yeast cells and may differ from one another in the susceptibility to 4-AN (Denny et al., 1990, Domańska et al., 2005). This is particularly significant in light of the findings reported by Konstantinidou and coworkers, who indicated that CK2α‘ maintained the proper structure of the C. albicans biofilm (Konstantinidou and Morrissey, 2015).
Fig. 4.
Effect of 4-AN on the activity of protein kinases: (A) Protein kinase CK2.The uUpper bars indicate phosphorylated protein substrates resolved with SDS-PAGE. The bottom bars are autoradiograms showing the level of phosphate incorporation into protein substrates. The graphs are graphical representations of autoradiograms. (B) The effect of 4-AN on the level of phosphorylation of lysate proteins from C. albicans. The lysate (50 µg) was incubated in the presence or absence (c – control) of 21.6 µg/ml (100 µM) of 4-AN and next SDS-PAGE (loading control – on the left) and autoradiography were performed.
In order to verify the putative influence of 4-AN on phosphorylation of proteins in Candida cells, the level of total protein phosphorylation in the cell lysate was determined (Fig. 4B). Candida albicans were cultured in standard conditions, the cells were homogenized with glass beads on ice, and 4-AN was added to the lysate at the concentration of 21.6 µg/ml (100 µM); this was followed by incubation for 15 mins. The results indicate that the total amount of phosphate incorporated by cellular kinases into proteins in the presence of the chemical decreased by 20% in comparison with the control (Fig. 4B). Although the authors realize that these preliminary studies need to be continued to include a panel of additional protein kinases, these results clearly show that 4-AN has an incontestable influence on protein phosphorylation at least in in vitro conditions.
4. Conclusions
4-(hydroxyimino)cyclohexa-2,5-dien-1-ylidene](phenyl)ethanenitrile (4-AN) effectively inhibits Candida albicans growth and suppresses hyphal production and cellular aggregation. Simultaneously, the compound is safe to human blood cells at a concentration corresponding to the MIC value and does not influence Zebrafish embryos. The tested drug reduces total protein phosphorylation in Candida lysate by 20% and inhibits the activity of protein kinase CK2 in vitro. Regarding the anti-Candida properties of the representative of arylcyanomethylenequinone oximes, other derivatives should be tested in the future as 4-AN can be a promising starting point for derivatization. In conclusion, 4-AN can be a potential candidate as a novel drug against Candida infections.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jsps.2017.12.004.
Appendix A. Supplementary material
References
- Baillie G.S., Douglas L.J. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 1999;48:671–679. doi: 10.1099/00222615-48-7-671. [DOI] [PubMed] [Google Scholar]
- Battistutta R., Cozza G., Pierre F., Papinutto E., Lolli G., Sarno S., O'Brien S.E., Siddiqui-Jain A., Haddach M., Anderes K., Ryckman D.M., Meggio F., Pinna L.A. Unprecedented selectivity and structural determinants of a new class of protein kinase CK2 inhibitors in clinical trials for the treatment of cancer. Biochemistry. 2011;50:8478–8488. doi: 10.1021/bi2008382. [DOI] [PubMed] [Google Scholar]
- Bitar D., Lortholary O., Le Strat Y., Nicolau J., Coignard B., Tattevin P., Che D., Dromer F. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg. Infect. Dis. 2014;20:1149–1155. doi: 10.3201/eid2007.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.J., Gow N.A. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7:333–338. doi: 10.1016/s0966-842x(99)01556-5. [DOI] [PubMed] [Google Scholar]
- Brown G.D., Denning D.W., Gow N.A., Levitz S.M., Netea M.G., White T.C. Hidden killers: human fungal infections. Sci. Transl. Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- Caballero-Lima D., Sudbery P.E. In Candida albicans, phosphorylation of Exo84 by Cdk1-Hgc1 is necessary for efficient hyphal extension. Mol. Biol. Cell. 2014;25:1097–1110. doi: 10.1091/mbc.E13-11-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoy S., Adrio J.L. Antifungals. Biochem. Pharmacol. 2017;133:86–96. doi: 10.1016/j.bcp.2016.11.019. [DOI] [PubMed] [Google Scholar]
- Chandra J., Kuhn D.M., Mukherjee P.K., Hoyer L.L., McCormick T., Ghannoum M.A. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier M., Medioni E., Prêcheur I. Inhibition of Candida albicans yeast-hyphal transition and biofilm formation by Solidago virgaurea water extracts. J. Med. Microbiol. 2012;61:1016–1022. doi: 10.1099/jmm.0.041699-0. [DOI] [PubMed] [Google Scholar]
- Davis R.B., Pizzini L.C., Bara E.J. The condensation of aromatic nitro compounds with arylacetonitriles. 111. Some ortho- and meta-substituted nitrobenzenes. J. Org. Chem. 1961;26:4270–4274. [Google Scholar]
- De Bernardis F., Molinari A., Boccanera M., Stringaro A., Robert R., Senet J.M., Arancia G., Cassone A. Modulation of cell surface-associated mannoprotein antigen expression in experimental candidal vaginitis. Infect. Immun. 1994;62:509–519. doi: 10.1128/iai.62.2.509-519.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny W.A., Rewcastle G.W., Baguley B.C. Potential antitumor agents. 59. Structure-activity relationships for 2-phenylbenzimidazole-4-carboxamides, a new class of minimal; DNA-intercalating agents which may not act via topoisomerase II. J. Med. Chem. 1990;33:814–819. doi: 10.1021/jm00164a054. [DOI] [PubMed] [Google Scholar]
- Domańska K., Zieliński R., Kubiński K., Sajnaga E., Masłyk M., Bretner M., Szyszka R. Different properties of four molecular forms of protein kinase CK2 from Saccharomyces cerevisiae. Acta Biochim. Pol. 2005;52:947–951. [PubMed] [Google Scholar]
- Dorgan E., Denning D.W., McMullan R. Burden of fungal disease in Ireland. J. Med. Microbiol. 2015;64:423–426. doi: 10.1099/jmm.0.000020. [DOI] [PubMed] [Google Scholar]
- Fekkar A., Meyer I., Brossas J.Y., Dannaoui E., Palous M., Uzunov M., Nguyen S., Leblond V., Mazier D., Datry A. Rapid emergence of echinocandin resistance during Candida kefyr fungemia treatment with caspofungin. Antimicrob. Agents. Chemother. 2013;57:2380–2382. doi: 10.1128/AAC.02037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B., Muraglia R., Dietz J.P., Sobel J.D., Wagner J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: results from an internet panel survey. J. Low Genit. Tract Dis. 2013;17:340–345. doi: 10.1097/LGT.0b013e318273e8cf. [DOI] [PubMed] [Google Scholar]
- Goldin N., Heyfets A., Reischer D., Flescher E. Mitochondria-mediated ATP depletion by anti-cancer agents of the Jasmonate family. J. Bioenergetics Biomembr. 2007;39:51–57. doi: 10.1007/s10863-006-9061-y. [DOI] [PubMed] [Google Scholar]
- Gow N.A., Brown A.J., Odds F.C. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 2002;5:366–371. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- Heintz-Buschart A., Eickhoff H., Hohn E., Bilitewski U. Identification of inhibitors of yeast-to-hyphae transition in Candida albicans by a reporter screening assay. J. Biotechnol. 2013;164:137–142. doi: 10.1016/j.jbiotec.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Hong Z., Li J.-J., Chen G., Jiang H.-J., Yang X.-F., Pan H., Su W.-K. Solvent-free mechanochemical synthesis of arylcyanomethylenequinone oximes from phenylacetonitriles and 4-unsubstituted nitroaromatic compounds using KF/nano-gamma-Al2O3 as catalyst. RSC Adv. 2016;6:13581. [Google Scholar]
- Huang Z.X., Zhao P., Zeng G.S., Wang Y.M., Sudbery I., Wang Y. Phosphoregulation of Nap1 plays a role in septin ring dynamics and morphogenesis in Candida albicans. MBio. 2014;5:e00915–e1013. doi: 10.1128/mBio.00915-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen I.D., Wilson D., Wächtler B., Brunke S., Naglik J.R., Hube B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti. Infect. Ther. 2012;10:85–93. doi: 10.1586/eri.11.152. [DOI] [PubMed] [Google Scholar]
- Kabir M.A., Hussain M.A., Ahmad Z. Candida albicans: a model organism for studying fungal pathogens. ISRN Microbiol. 2012;2012:538694. doi: 10.5402/2012/538694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplancikli Z.A., Turan-Zitouni G., Revial G., Guven K. Synthesis and study of antibacterial and antifungal activities of novel 2-[[(benzoxazole/benzimidazole-2-yl)sulfanyl] acetylamino]thiazoles. Arch. Pharm. Res. 2004;27:1081–1085. doi: 10.1007/BF02975108. [DOI] [PubMed] [Google Scholar]
- Klopffleisch K., Issinger O.G., Niefind K. Low-density crystal packing of human protein kinase CK2 catalytic subunit in complex with resorufin or other ligands: a tool to study the unique hinge-region plasticity of the enzyme without packing bias. Acta Crystallogr. D. Biol. Crystallogr. 2012;68:883–892. doi: 10.1107/S0907444912016587. [DOI] [PubMed] [Google Scholar]
- Konstantinidou N., Morrissey J.P. Co-occurence of filamentation defects and impaired biofilms in Candida albicans protein kinase mutants. FEMS Yeast Res. 2015;15 doi: 10.1093/femsyr/fov092. [DOI] [PubMed] [Google Scholar]
- Kumamoto C.A., Vinces M.D. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell. Microbiol. 2005;7:1546–1554. doi: 10.1111/j.1462-5822.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- Lo H.J., Köhler J.R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G.R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- Low C.F., Chong P.P., Yong P.V., Lim C.S., Ahmad Z., Othman F. Inhibition of hyphae formation and SIR2 expression in Candida albicans treated with fresh Allium sativum (garlic) extract. J. Appl. Microbiol. 2008;105:2169–2177. doi: 10.1111/j.1365-2672.2008.03912.x. [DOI] [PubMed] [Google Scholar]
- Magaldi S., Mata S., Hartung C., Verde G., Deibis L., Roldán Y., Marcano C. In vitro susceptibility of 137 Candida sp. isolates from HIV positive patients to several antifungal drugs. Mycopathologia. 2001;149:63–68. doi: 10.1023/a:1007237711099. [DOI] [PubMed] [Google Scholar]
- McCarty T.P., Pappas P.G. Invasive Candidiasis. Infect. Dis. Clin. North Am. 2016;30:103–124. doi: 10.1016/j.idc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Messier C., Epifano F., Genovese S., Grenier D. Inhibition of Candida albicans biofilm formation and yeast-hyphal transition by 4-hydroxycordoin. Phytomedicine. 2011;18:380–383. doi: 10.1016/j.phymed.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Nyirjesy P., Sobel J.D. Vulvovaginal candidiasis. Obstet. Gynecol. Clin. North Am. 2003;30:671–684. doi: 10.1016/s0889-8545(03)00083-4. [DOI] [PubMed] [Google Scholar]
- Peters B.M., Palmer G.E., Nash A.K., Lilly E.A., Fidel P.L., Noverr M.C. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect. Immun. 2014;82:532–543. doi: 10.1128/IAI.01417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M.A. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012;125:S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Pierre F., Chua P.C., O'Brien S.E., Siddiqui-Jain A., Bourbon P., Haddach M., Michaux J., Nagasawa J., Schwaebe M.K., Stefan E., Vialettes A., Whitten J.P., Chen T.K., Darjania L., Stansfield R., Anderes K., Bliesath J., Drygin D., Ho C., Omori M., Proffitt C., Streiner N., Trent K., Rice W.G., Ryckman D.M. Discovery and SAR of 5-(3-chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer. J. Med. Chem. 2011;54:635–654. doi: 10.1021/jm101251q. [DOI] [PubMed] [Google Scholar]
- Roca I., Akova M., Baquero F., Carlet J., Cavaleri M., Coenen S., Cohen J., Findlay D., Gyssens I., Heure O.E., Kahlmeter G., Kruse H., Laxminarayan R., Liébana E., López-Cerero L., MacGowan A., Martins M., Rodríguez-Baño J., Rolain J.M., Segovia C., Sigauque B., Taconelli E., Wellington E., Vila J. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Blas G., Richard A C. Caister Academic Press; 2004. Pathogenic fungi: Host Interactions and Emerging Strategies for Control. [Google Scholar]
- Sandini S., La Valle R., De Bernardis F., Macrì C., Cassone A. The 65 kDa mannoprotein gene of Candida albicans encodes a putative beta-glucanase adhesin required for hyphal morphogenesis and experimental pathogenicity. Cell. Microbiol. 2007;9:1223–1238. doi: 10.1111/j.1462-5822.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- Saville S.P., Lazzell A.L., Monteagudo C., Lopez-Ribot J.L. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell. 2003;2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J.D. Pathogenesis of recurrent vulvovaginal candidiasis. Curr. Infect. Dis. Rep. 2002;4:514–519. doi: 10.1007/s11908-002-0038-7. [DOI] [PubMed] [Google Scholar]
- Sobel J.D. Management of recurrent vulvovaginal candidiasis: unresolved issues. Curr. Infect. Dis. Rep. 2006;8:481–486. doi: 10.1007/s11908-006-0023-7. [DOI] [PubMed] [Google Scholar]
- Sobel J.D., Wiesenfeld H.C., Martens M., Danna P., Hooton T.M., Rompalo A., Sperling M., Livengood C., Horowitz B., Von Thron J., Edwards L., Panzer H., Chu T.C. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004;351:876–883. doi: 10.1056/NEJMoa033114. [DOI] [PubMed] [Google Scholar]
- Sudbery P., Gow N., Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–324. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Sudbery P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011;9:737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- Tong Y., Tang J. Candida albicans infection and intestinal immunity. Microbiol. Res. 2017;198:27–35. doi: 10.1016/j.micres.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Vediyappan G., Dumontet V., Pelissier F., d'Enfert C. Gymnemic acids inhibit hyphal growth and virulence in Candida albicans. PLoS One. 2013;8:e74189. doi: 10.1371/journal.pone.0074189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Raniga P.P., Lane S., Lu Y., Liu H. Hyphal chain formation in Candida albicans: Cdc28-Hgc1 phosphorylation of Efg1 represses cell separation genes. Mol. Cell. Biol. 2009;29:4406–4416. doi: 10.1128/MCB.01502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2014. Antimicrobial resistance global report on surveillance 2014: City.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.