Abstract
In this study, we developed a validated HPTLC method for concurrent analysis of two natural antioxidant triterpenes, oleanolic acid (OA) and β-amyrin (BA) in the biologically active fractions (petroleum ether, toluene, chloroform, ethyl acetate and n-butanol) of aerial parts of three Hibiscus species (H. calyphyllus, H. deflersii and H. micranthus). The chromatography was conducted on normal HPTLC (ready to use glass-plate coated with silica gel 60 F254) plate with chloroform and methanol (97:3, V/V) used as mobile phase. The derivatization of the developed plate was done with p-anisaldehyde and scanned at λmax = 575 nm. Well resolved and intense peaks of OA and BA were obtained at Rf = 0.36 and 0.57, respectively. The linear regression equation/correlation coefficient (r2) for OA and BA were Y = 6.65x + 553.35/0.994 and Y = 9.177x + 637.23/0.998, respectively in the linearity range of 100–1200 ng/spot indicated good linear relationship. The low values of %RSD for intra-day/inter-day precision of OA (1.45–1.61/1.38–1.59) and BA (1.52–1.57/1.50–1.53) suggested that the method was precise. The recovery/RSD (%) values for OA and BA were found to be 99.21–99.62/1.39–1.95 and 98.75–99.70/1.56–1.80, respectively assures the reasonably good accuracy of the proposed method. Fifteen samples were analyzed to check the content of OA and BA by using the developed HPTLC methods. The content of OA in different samples were found to be 3.87 (HmP) > 1.212 (HcP) > 0.673 (HdC) > 0.493 (HdP) > 0.168 (HdE) > 0.059 (HcC) > 0.015 (HcE) > 0.008 (HmT) µg/mg of the dried weight of extract. However the content of BA was found as: 2.293 (HmP) > 1.852 (HdT) > 0.345 (HdC) > 0.172 (HmT) > 0.041 (HdE) > 0.008 (HcC) µg/mg of the dried weight of extract. Some Hibiscus species fractions exhibited good antioxidant potential like: HcE (IC50 = 17.6 ± 1.8) > HdB (IC50 = 32.16 ± 0.9) > HmP (IC50 = 80.4 ± 4.5) > HmT (IC50 = 99.7 ± 8.2) when compared with ascorbic acid (IC50 = 14.2 ± 0.5), while other fractions exhibited only mild antioxidant capability. The developed HPTLC method can be further exploited for analysis of these markers in the quality assessment of raw material as well as herbal formulations available in the market.
Keywords: Antioxidants, β-Amyrin, Hibiscus spp., HPTLC, Oleanolic acid, Triterpenes
1. Introduction
The genus Hibiscus (Malvaceae) represents around 275 species distributed in tropical and sub-tropical regions and many of which possess medicinal properties. Most Hibiscus species posses a distinct color pattern with the base of corolla (Lowry, 1976) and they are fairly widespread at medium altitudes in the western part of Saudi Arabia. Hibiscus calyphyllus (Hc) is a leafy shrub of 1 M height with wide simple serrate leaves and yellow flowers with dark red centre and found mainly in south-west part of Saudi Arabia, particularly NE of Jizan (Collenette, 1999).
H. deflersii (Hd) (Malvaceae) is an annual or perennial erect leafy straggly shrublet of 1 m height with narrow bright green dentate leaves having around 3 cm wide bright crimson-red flowers. It is grown as an ornamental plant and native to Ethiopia. The flowers are used as emollient and its infusion as a demulcent. Bronchial catarrh in India can be treated by the decoction of flowers of H. deflersii. Literature work revealed that H. deflersii possesses antidiarrhetic, antiphologistic and anticomplimentary activities. The leaves of H. deflersii were found very effective in heart disorders as well as in diabetes (Fryxell, 1980, Nadkarni, 1954, Lakshman et al., 2014).
Hibiscus micranthus (Hm) is a bushy leafy shrub of around 45 cm height having white flowers on short pedicels with very distinctive pea-size fruit capsules and distributed widely in Saudi Arabia, Ceylon, India and tropical Africa. In Saudi Arabia, H. micranthus is prevalently found from south to western part of Saudi Arabia (Kirtikar and Basu, 1984). The fruits and flowers of H. micranthus are used as antidiabetic (Kakrani et al., 2005) and anti-dandruff agent when applied topically and possesses laxative activity when taken orally (Tamilselvi et al., 2016). The plant has also been approved for its hematological, antipyretic, anti-inflammatory (Al-Yahya et al., 1987), antimicrobial, antiviral, antitumor (Jain et al., 1997), female antifertility, viralizing (Telefo et al., 1998) and anabolizing (Moundipa et al., 1999) effects. The antifungal and anti-tumor activity has been observed from roots of H. micranthus and also reported to possess good antiviral activity (Rekha, 2017). Literature reveals that H. micranthus possesses a wide range of phytochemicals such as phenolic acids, flavonoids, β-sitosterol, alkanes, fatty alcohols and acids (Jain et al., 1997). Chemical profiling of ethanol extract of H. micranthus roots by GC–MS revealed the presence of seventy nine compounds (Kumar et al., 2010). Available literature revealed that a fingerprint profile has been developed by HPTLC and rutin was analyzed by HPLC in H. micranthus (stem) hydro alcoholic extracts but there is no evidence yet available on the quantitative analysis of biomarkers using validated HPTLC method in H. micranthus extracts.
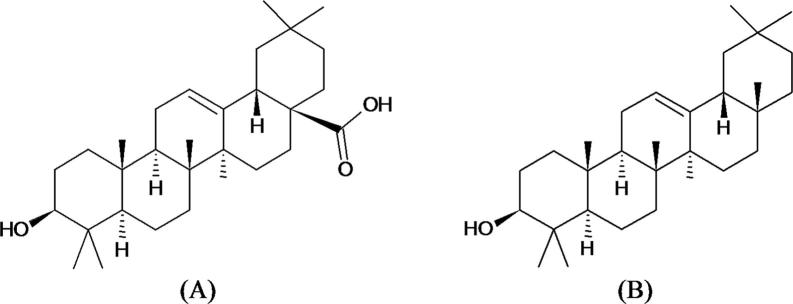
As evident from earlier reports the aerial parts of selected Hibiscus species (H. calyphyllus, H. deflersii and H. micranthus) mainly contain β-ionone, flavonoid and phenolic acids, oleic acid and other organic acids along with their esters (Kumar et al., 2011). As part of our research based on the reported phytochemicals, we selected three species of genus Hibiscus (H. calyphyllus, H. deflersii and H. micranthus) for quantitative analysis of biomarkers oleanolic acid (Fig. 1A) and β-amyrin (Fig. 1B) by using validated HPTLC method as well as evaluation of their antioxidant potential.
Fig. 1.
Chemical structure of biomarkers oleanolic acid (A) and β-amyrin (B).
2. Experimental
2.1. Plant material
Aerial parts of three different species of genus Hibiscus; H. calyphyllus (Voucher specimen number: HA-234), H. deflersii (Voucher specimen number: HA-567) and H. micranthus L. (Voucher specimen number: HA-16,240) were collected from Jabal As-Sahla', As-Sarawat mountains (18°42′0″N 42°13′50″E), Asir region of Saudi Arabia in March 2009 and authenticated by Dr. Mohamed Yousef, taxonomist at Pharmacognosy Department, College of Pharmacy, King Saud University. Voucher specimens were deposited in the herbarium, Department of Pharmacognosy. Aerial parts of plant sample were thoroughly washed broken into small pieces and evenly distributed in aluminum trays. The samples were dried in shade at normal temperature, powdered and stored in airtight containers for further use.
2.2. Extraction of plant material
The dried powder of selected plant species were weighed (400 g) and soaked in 95% ethanol (1 L) for 48 h at room temperature with occasional stirring. In each experiment, process of extraction was repeated three times under similar conditions. The ethanolic extracts were then filtered using Whatman filter paper and the filtrates were combined. The obtained filtrates were concentrated under reduced pressure using rotavapour to get dark green solid mass. The obtained yields for H. calyphyllus, H. deflersii and H. micranthus were 12.23 g (3.06%, w/w), 10.21 g (2.6%, w/w) and 12.01 g (3.0%, w/w), respectively. The obtained extracts of H. calyphyllus, H. deflersii and H. micranthus were dissolved in a mixture of methanol and water (7:3) and successively partitioned three times with petroleum ether, toluene, chloroform, ethyl acetate and n-butanol to obtain the respective fractions. The yield of each fraction was calculated as H. calyphyllus [petroleum ether fraction (HcP; 2.25 g); toluene fraction (HcT; 3.21 g); chloroform fraction (HcC; 4.30 g); ethyl acetate fraction (HcE; 2.8 g) and n-butanol fraction (HcB; 2.21 g)]; H. deflersii [petroleum ether fraction (HdP; 2.19 g); toluene fraction (HdT; 3.10 g); chloroform fraction (HdC; 2.56 g); ethyl acetate fraction (HdE; 3.01 g) and n-butanol fraction (HdB; 1.98 g)] and H. micranthus [petroleum ether fraction (HmP; 2.21 g); toluene fraction (HmT; 2.96 g); chloroform fraction (HmC; 2.95 g); ethyl acetate fraction (HmE; 3.86 g) and n-butanol fraction (HmB; 2.01 g)] and stored in refrigerator at 4 °C until the time of use.
2.3. Apparatus and reagents
The two biomarkers, oleanolic acid (OA) and β-amyrin (BA) were purchased from Sigma Aldrich (USA) and the solvents of analytical grade from BDH (UK). The glass-backed silica gel 60F254 plates for the HPTLC analysis were purchased from Merck (Germany). Furthermore, biomarkers OA and BA along with extract sample were applied band wise to the chromatographic plates using method described by Siddiqui et al. (2016), with slight modification.
2.4. HPTLC instrumentation and conditions
The HPTLC analyses of OA and BA in samples (15 fractions) were carried out on pre-coated 20 × 10 cm HPTLC plates and almost similar instrumentation and conditions were used as described by Siddiqui et al. (2015) with slight modifications.
2.5. Preparation of standard stock solutions
The stock solutions of OA and BA (1 mg/mL) were prepared in chloroform, following further dilution with chloroform to provide seven different concentrations ranging from 10 to 120 μg/mL. 10 μL of each dilution of both biomarkers were applied on the HPTLC plate through the microliter syringe to provide a linearity range of 100–1200 ng/band.
2.6. Method validation
The latest ICH guidelines (2005) were being followed for the validation of developed HPTLC method. The parameters observed for validation were the determination of limit of detection (LOD), limit of quantification (LOQ), linearity range, precision, recovery as accuracy and robustness.
2.7. Antioxidant assay
The antioxidant activity of extract was determined at the Regional Center for Mycology and Biotechnology (RCMB) at Al-Azhar University by the DPPH free radical scavenging assay in triplicate and average values were considered.
2.7.1. DPPH free radical scavenging assay
The evaluation of all the fifteen fractions of H. calyphyllus, H. deflersii and H. micranthus was done for antioxidant activities in terms of quantity by free-radical scavenging ability against DPPH according to the method described by Lee et al. (2013), with minor modification to suite 96-well microtitre plate format. In brief, 40 μL of DPPH (0.2 mM in methanol) was mixed with 100 μL of different concentrations (31.25, 62.5, 125, 250 and 500 μg/mL) of each fraction in wells of a 96-well microtitre plate. Pure solvent along with same amount of DPPH was used as control to rule out the effect of solvent. After 30 min incubation in dark at 25 °C, the decrease in absorbance (Abs) was measured immediately with a UV–visible spectrophotometer (Milton Roy, Spectronic 1201) at λ = 515 nm using microtitre plate reader. The absorbance of the DPPH radical without antioxidant (control) and the reference compound (ascorbic acid) were also measured. All the observations were made in triplicate and average was considered for each observation. The percentage radical scavenging activity was calculated according to the formula:
The IC50 value of each fraction was calculated and reported in Table 6.
Table 6.
The estimated IC50 (μg/mL) values of different fractions of H. calyphyllus, H. deflersii, and H. micranthus for antioxidant potential.
| IC50 (μg/mL) values of different fractions of H. calyphyllus |
IC50 (μg/mL) values of different fractions of H. deflersii |
IC50 (μg/mL) values of different fractions of H. micranthus |
|||
|---|---|---|---|---|---|
| Fractions | IC50 (µg/ml) ± SD | Fractions | IC50 (µg/ml) ± SD | Fractions | IC50 (µg/ml) ± SD |
| HcT | 207.5 ± 1.3 | HdT | 125 ± 2.5 | HmT | 99.7 ± 8.2 |
| HcP | 122 ± 0.8 | HdP | 196.5 ± 0.8 | HmP | 80.4 ± 4.5 |
| HcC | 150.9 ± 1.2 | HdC | 301.5 ± 0.6 | HmC | 164 ± 1.2 |
| HcE | 17.6 ± 1.8 | HdE | 120 ± 9.2 | HmE | 139.6 ± 1.8 |
| HcB | 292.9 ± 3.6 | HdB | 32.16 ± 0.9 | HmB | 106.6 ± 4.5 |
| Ascorbic acid | 14.2 ± 0.5 | Ascorbic acid | 14.2 ± 0.5 | Ascorbic acid | 14.2 ± 0.5 |
2.8. Statistical analysis
One-way analysis of variance (ANOVA) followed by Dunnet’s test for the estimation of total variation was used for statistical analysis. Results were expressed as mean ± SD. P < .05 was considered as significant.
3. Results and discussion
3.1. HPTLC method development and validation
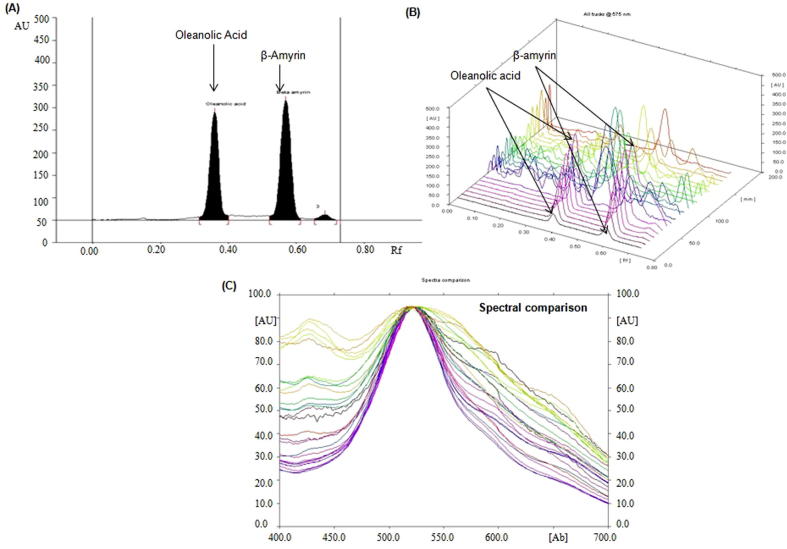
The mobile phase was selected by the rigorous exercise of permutation-combination of many solvents in different proportions. The combination of chloroform and methanol in the proportion of 97:3 (v/v) was found to be the optimized mobile phase for the development and quantitative analysis of OA and BA. An intense, sharp and compact peak of OA and BA were found at Rf = 0.36 ± 0.001 and 0.57 ± 0.002, respectively (Fig. 2A). The proposed method separated the biomarkers OA and BA as well as different constituents of selected Hibiscus species fractions (Fig. 2B). The saturation time for the saturation of developing chamber and volume of mobile phase was found to be 20 min and 20 mL, respectively. The recognition of the bands of the extracts were established by overlaying their spectra along with the spectra of standard OA and BA (Fig. 2C). The regression equation/correlation coefficient (r2) for biomarkers OA and BA were found as Y = 6.651x + 553.35/0.994 and Y = 9.177x + 637.23/0.998, respectively in the linearity range 100–1200 ng/spot while the limit of detection (LOD)/limit of quantification (LOQ) for OA and BA were found as 11.47/34.78 ng and 15.29/46.35 ng, respectively (Table 1). The recoveries as accuracy study for the proposed method was recorded (Table 2). The recovery/RSD (%) for biomarkers OA and BA were found as 99.21–99.62/1.39–1.95 and 98.75–99.70/1.56–1.80, respectively. The intra-day and inter-day precision for the proposed method was recorded in Table 3 and the % RSD for intra-day/inter-day precisions (n = 6) of biomarkers OA and BA were recorded as 1.45–1.61/1.38–1.59 and 1.52–1.57/1.50–1.53, respectively, which exhibits the good precision of the proposed method. Some small intentional changes were made in for mobile phase volume, composition, saturation time etc. to check the robustness of the proposed method. The data reported in Table 4 show low values of SD and % RSD which indicate that the proposed method was robust and not significantly affected by slight changes in the experimental environment.
Fig. 2.
Quantification of oleanolic acid and β-amyrin in different fractions of H. calyphyllus, H. deflersii and H. micranthus by HPTLC. (A) Chromatogram of standards oleanolic acid (Rf = 0.36; 800 ng/spot) and β-amyrin (Rf = 0.57; 800 ng/spot) at 575 nm. (B) 3-D display of all tracks at 575 nm. (C) Spectral comparison of all tracks at 575 nm.
Table 1.
Rf, Linear regression data for the calibration curve of oleanolic acid and β-amyrin (n = 6).
| Parameters | Oleanolic acid (OA) | β-Amyrin (BA) |
|---|---|---|
| Linearity range (ng/spot) | 100–1200 | 100–1200 |
| Regression equation | Y = 6.651X + 553.35 | Y = 9.177X + 637.23 |
| Correlation (r2) coefficient | 0.994 | 0.998 |
| Slope ± SD | 6.651 ± 0.023 | 9.177 ± 0.042 |
| Intercept ± SD | 553.35 ± 11.93 | 637.23 ± 12.85 |
| Standard error of slope | 0.009 | 0.017 |
| Standard error of intercept | 4.87 | 5.24 |
| Rf | 0.36 ± 0.001 | 0.57 ± 0.001 |
| LOD (ng) | 11.47 | 15.29 |
| LOQ (ng) | 34.78 | 46.35 |
Table 2.
Recovery as accuracy studies of the proposed HPTLC Method (n = 6).
| Percent (%) of oleanolic acid and β-amyrin added to analyte | Theoretical concentration of oleanolic acid and β-amyrin (ng/μL) | Concentration found (ng/μL) ± SD |
%RSD |
% Recovery |
|||
|---|---|---|---|---|---|---|---|
| Oleanolic acid | β-amyrin | Oleanolic acid | β-amyrin | Oleanolic acid | β-amyrin | ||
| 0 | 200 | 199.25 ± 2.77 | 198.84 ± 3.11 | 1.39 | 1.56 | 99.62 | 99.41 |
| 50 | 300 | 297.64 ± 4.39 | 296.25 ± 4.69 | 1.47 | 1.58 | 99.21 | 98.75 |
| 100 | 400 | 398.16 ± 6.37 | 398.84 ± 6.71 | 1.59 | 1.68 | 99.54 | 99.70 |
| 150 | 500 | 497.45 ± 9.74 | 497.47 ± 8.97 | 1.95 | 1.80 | 99.49 | 99.49 |
Table 3.
Precision of the proposed HPTLC Method (n = 6).
| Concentration of standards added (ng/spot) | Oleanolic acid |
β-Amyrin |
||||||
|---|---|---|---|---|---|---|---|---|
| Intra-day precision |
Inter-day precision |
Intra-day precision |
Inter-day precision |
|||||
| Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | |
| 200 | 199.25 ± 2.89 | 1.45 | 196.69 ± 2.73 | 1.38 | 197.78 ± 3.02 | 1.52 | 195.60 ± 2.94 | 1.50 |
| 400 | 398.16 ± 6.27 | 1.57 | 398.62 ± 6.12 | 1.53 | 398.96 ± 6.23 | 1.56 | 393.51 ± 6.01 | 1.52 |
| 600 | 597.21 ± 9.67 | 1.61 | 597.21 ± 9.54 | 1.59 | 597.35 ± 9.43 | 1.57 | 596.26 ± 9.13 | 1.53 |
Table 4.
Robustness of the proposed HPTLC Method (n = 6).
| Optimization condition | Oleanolic acid (300 ng/band) |
β-Amyrin (300 ng/band) |
||
|---|---|---|---|---|
| SD | %RSD | SD | %RSD | |
| Mobile phase composition (Chloroform: methanol) | ||||
| (97:3) | 4.59 | 1.54 | 4.81 | 1.65 |
| (96.5:3.5) | 4.62 | 1.56 | 4.89 | 1.66 |
| (97.5:2.5) | 4.55 | 1.53 | 4.92 | 1.68 |
| Mobile phase volume (for saturation) | ||||
| (18 mL) | 4.72 | 1.59 | 4.71 | 1.61 |
| (20 mL) | 4.79 | 1.61 | 4.79 | 1.63 |
| (22 mL) | 4.82 | 1.62 | 4.85 | 1.66 |
| Duration of saturation | ||||
| (10 min) | 4.51 | 1.51 | 4.83 | 1.65 |
| (20 min) | 4.58 | 1.54 | 4.89 | 1.66 |
| (30 min) | 4.68 | 1.57 | 4.94 | 1.68 |
3.2. HPTLC analysis of biomarkers OA and BA in different fractions of selected Hibiscus species
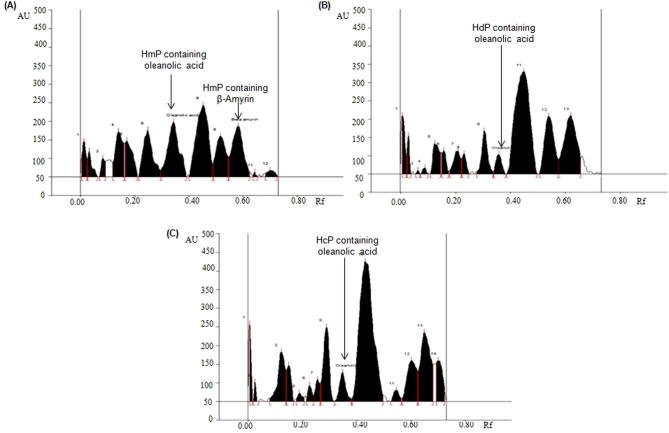
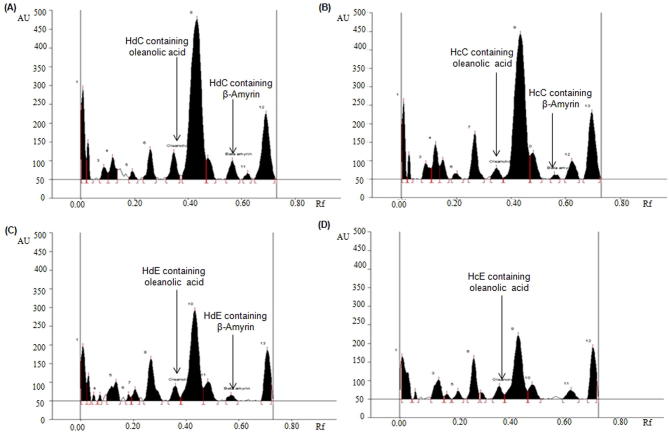
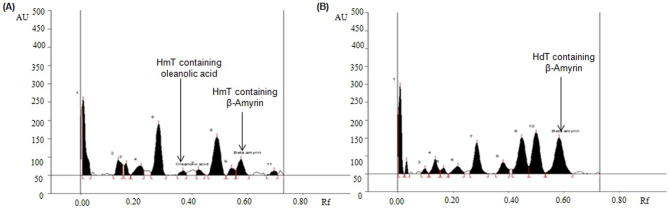
The developed HPTLC method was used for the concurrent analysis of biomarkers OA and BA in the different fractions of selected Hibiscus species (Table 5; Fig. 3, Fig. 4, Fig. 5). By applying the above developed method the quantity of biomarker OA and BA in HmP was found as 3.87 and 2.29 μg/mg, respectively of the dried weight of extracts (Fig. 3A). The quantity of OA in HdP and HcP and was found as 0.49 μg/mg and 1.21 μg/mg, respectively (Fig. 3B and C) with respect to the dried weight of the extract, but BA was altogether absent in HdP as well as HcP. As evident from the Fig. 4 HdC, HcC, HdE and HcE fractions showing very little or insignificant amount of both OA as well as BA. HmT also exhibited low amount of OA and BA (Fig. 5A) while HdT showed good amount of BA but no OA (Fig. 5B). The previous literature indicates that the authors reporting the quantification of oleanolic acid and β-amyrin by HPTLC method for the first time in the petroleum ether, toluene, chloroform, ethyl acetate and n-butanol fractions of H. calyphyllus, H. deflersii and H. micranthus.
Table 5.
HPTLC analysis of Oleanolic acid and β-amyrin in different fractions of H. calyphyllus, H. deflersii and H. micranthus.
| S. No. | Samples | Oleanolic acid content (µg/mg of dried weight of extract) | β-amyrin content (µg/mg of dried weight of extract) |
|---|---|---|---|
| 1 | H. micranthus petroleum ether fraction (HmP) | 3.87 | 2.29 |
| 2 | H. deflersii petroleum ether fraction (HdP) | 0.49 | Not detected |
| 3 | H. calyphyllus petroleum ether fraction (HcP) | 1.21 | Not detected |
| 4 | H. micranthus toluene fraction (HmT) | 0.008 | 0.17 |
| 5 | H. deflersii toluene fraction (HdT) | Not detected | 1.85 |
| 6 | H. calyphyllus toluene fraction (HcT) | Not detected | Not detected |
| 7 | H. micranthus chloroform fraction (HmC) | Not detected | Not detected |
| 8 | H. deflersii chloroform fraction (HdC) | 0.673 | 0.345 |
| 9 | H. calyphyllus chloroform fraction (HcC) | 0.059 | 0.008 |
| 10 | H. micranthus ethyl acetate fraction (HmE) | Not detected | Not detected |
| 11 | H. deflersii ethyl acetate fraction (HdE) | 0.168 | 0.041 |
| 12 | H. calyphyllus ethyl acetate fraction (HcE) | 0.015 | Not detected |
| 13 | H. micranthus n-butanol fraction (HmB) | Not detected | Not detected |
| 14 | H. deflersii n-butanol fraction (HdB) | Not detected | Not detected |
| 15 | H. calyphyllus n-butanol fraction (HcB) | Not detected | Not detected |
Fig. 3.
Chromatogram of oleanolic acid and β-amyrin estimation in petroleum ether fractions of H. calyphyllus, H. deflersii and H. micranthus at 575 nm [mobile phase: chloroform: methanol (97:3)]. (A) H. micranthus Petroleum ether fraction [HmP (oleanolic acid, spot 7, Rf = 0.36; β-amyrin, spot 10, Rf = 0.57)]; (B) H. deflersii Petroleum ether fraction [HdP (oleanolic acid, spot 10, Rf = 0.36)]; (C) H. calyphyllus Petroleum ether fraction [HcP (oleanolic acid, spot 9, Rf = 0.36)].
Fig. 4.
Chromatogram of oleanolic acid and β-amyrin estimation in the dichloromethane and ethyl acetate fractions of H. calyphyllus and H. deflersii at 575 nm [mobile phase: chloroform: methanol (97:3)]. (A) H. deflersii dichloromethane fraction [HdC (oleanolic acid, spot 7, Rf = 0.36; β-amyrin, spot 10, Rf = 0.57)]; (B) H. calyphyllus dichloromethane fraction [HcC (oleanolic acid, spot 8, Rf = 0.36; β-amyrin, spot 11, Rf = 0.57)]; (C) H. deflersii ethylacetate fraction [HdE (oleanolic acid, spot 9, Rf = 0.36; β-amyrin, spot 12, Rf = 0.57)]; (D) H. calyphyllus ethylacetate fraction [HcE (oleanolic acid, spot 8, Rf = 0.36)].
Fig. 5.
Chromatogram of oleanolic acid and β-amyrin estimation in the toluene extract of H. micranthus and H. deflersii at 575 nm [mobile phase: chloroform: methanol (97:3)]. (A) H. micranthus toluene extract [HmT (oleanolic acid, spot 6, Rf = 0.36; β-amyrin, spot 10, Rf = 0.57)]; (B) H. deflersii toluene extract [HdT (β-amyrin, spot 10, Rf = 0.57)].
High-performance thin-layer chromatography (HPTLC) is a more precise, calibrated and automated TLC which has many advantages in comparison to other techniques like high performance liquid chromatography (HPLC) and other chromatographic methods in the analysis of different markers. HPTLC can be used for simultaneous identification and quantification for multiple markers whether UV active or not. A wide range of stationary phases has broadened the application of HPTLC for a variety of samples contrary with the separation on bare silica gel.
3.3. Antioxidant potential of different fractions of H. calyphyllus, H. Deflersii and H. Micranthus
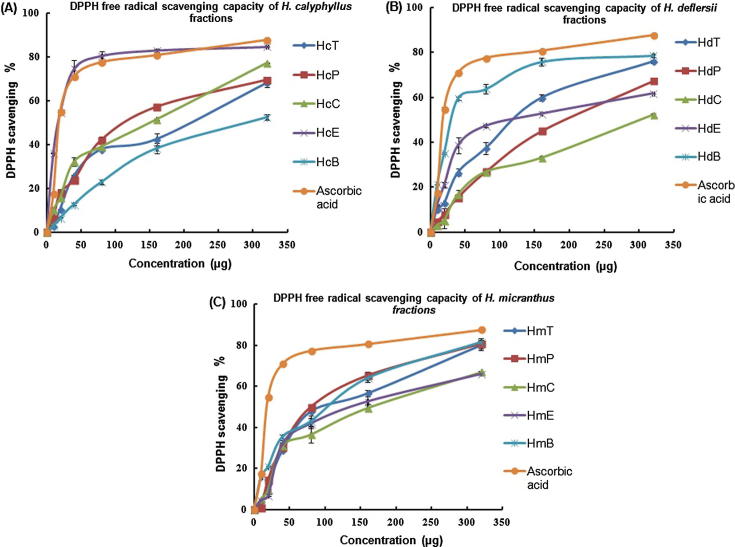
As far as the antioxidant potential is concerned ethyl acetate fraction of H. calyphyllus was emerged as most significant [HcE (IC50 = 17.6 ± 1.8); Fig. 6A] among all the fractions of H. calyphyllus, H. deflersii and H. micranthus compared to the standard ascorbic acid (IC50 = 14.2 ± 0.5). These findings also supports the previous literature about antioxidant potential of oleanolic acid with mechanism of significant inhibition in the production of nonenzymatic glycative products, pentosidine and carboxymethyllysine (CML) and it is reported that oleanolic acid exhibited greater antioxidant activity than alpha-tocopherol at different pH. Oleanolic acid also reported to possesses a dose-dependent effect on superoxide anion scavenging activity, chelating effect, xanthine oxidase inhibition activity, and reducing power (Yin and Chan, 2007). The experimental findings also indicate that OA and BA are not the only factors regulating anti-oxidant capabilities otherwise H. micranthus would have been the most potent antioxidant being highest contents of OA and BA present in it. H. deflersii also exhibited moderate antioxidant capabilities [HdB (IC50 = 32.16 ± 0.9); Fig. 6B] but H. micranthus [HmP (IC50 = 80.4 ± 4.5), HmT (IC50 = 99.7 ± 8.2); Fig. 6C] showed least usefulness as anti-oxidant. Some previous findings also depicted the antioxidant capabilities of β-amyrin acetate (Fabiyi et al., 2012). The data obtained from Table 6 exhibited that the butanol fractions free from OA and BA (HcB and HmB) also possess antioxidant potential, that too HdB showed significant IC50 value as antioxidant. On the other side HmP which possess highest amount of OA and BA also showed moderate antioxidant capabilities with IC50 value 80.4. These findings suggest that OA and BA are active antioxidant biochemicals but the emergence of HcE as most potent antioxidant among the selected Hibiscus species indicates the presence of many other phytochemicals as antioxidants.
Fig. 6.
DPPH free radical scavenging activity of different concentrations (10–320 µg/mL) of different fractions of H. calyphyllus, H. deflersii and H. micranthus. Values are means of three experiments. (A) DPPH free radical scavenging capacity of H. calyphyllus fractions. (B) DPPH free radical scavenging capacity of H. deflersii fractions. (C) DPPH free radical scavenging capacity of H. micranthus fractions.
4. Conclusion
The authors developed HPTLC method for simultaneous analysis of oleanolic acid and β-amyrin in H. calyphyllus, H. deflersii and H. micranthus for the first time. The proposed method may be further applied for quality analysis of the raw material as well as herbal formulation claiming the presence of oleanolic acid and β-amyrin. Stability studies and degradation kinetics of herbal formulation having oleanolic acid and β-amyrin can also be performed by the method developed by the authors. Exploration of new genera or species possessing oleanolic acid and can be possible by the proposed method. The Hibiscus species can also be considered for antioxidant activity because the experimental findings suggest that H. calyphyllus possesses significant antioxidant potential.
Acknowledgments
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research and Research Centre, College of Pharmacy, King Saud University, Riyadh, Kingdom of Saudi Arabia for providing the facilities to carry out these studies.
Acknowledgments
Conflict of interest
The authors declare that they do not have any conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-yahya M.A., Tariq M., Parmar N.S., Ageel A.M. Pharmacological investigations of Hibiscus micranthus Linn., a febrifuge used in Saudi Arabian folk medicine. Phytother. Res. 1987;1(2):73–75. [Google Scholar]
- Collenette, S., 1999. Wild Flowers of Saudi Arabia, National Commission for Wildlife Conservation and Development (NCWCD), 1st ed. Kingdom of Saudi Arabia, pp. 554–555.
- Fabiyi O.A., Atolani O., Adeyemi O.S., Olatunji G.A. Antioxidant and Cytotoxicity of β-Amyrin acetate fraction from Bridelia ferruginea Leaves. Asian Pac. J. Trop. Biomed. 2012:S981–S984. [Google Scholar]
- Fryxell P.A. A revision of the American species of Hibiscus sect. Bombicella. Tech. Bull. USDA. 1980;1624:1–52. [Google Scholar]
- International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human use, Harmonised Triplicate Guideline on Validation of Analytical Procedures: Text and Methodology Q2 (R1), Complementary Guideline on Methodology incorporated in November 2005 by the ICH Steering Committee, IFPMA, Geneva.
- Jain R., Arora R., Jain S.C. Chemical constituents and bioactivity studies of Hibiscus micranthus Linn. Indian J. Pharm Sci. 1997:91–93. [Google Scholar]
- Kakrani H.N., Kakrani B.H., Saluja A.K. Traditional treatment of diabetes through herbs in Kutch district, Gujrat state. Planta Indica. 2005;1(1):16–21. [Google Scholar]
- Kirtikar K.R., Basu B.D. 2nd ed. Periodical Expert Book Agency; Delhi: 1984. Indian Medicinal Plants; p. 293. vol.1. [Google Scholar]
- Kumar A.K., Setty R.S., Narsu L. Pharmacognostic and phytochemical investigations of stems of Hibiscus micranthus Linn. Pharmacognosy J. 2010;2(15):21–30. [Google Scholar]
- Kumar A.K., Setty R.S., Narsu L. GC-MS Analysis of n-Hexane Extracts of Hibiscus micranthus Linn. Asian J. Chem. 2011;23(2):561–565. [Google Scholar]
- Lakshman S., Jyothi A.B., Mounica V., Ravi Kumar A., Subbu Rathinam K.M., Rajesh K. Antidiabetic activity of methonolic extract of in streptozotocin induced diabetic rats Hibiscus deflersii. Int. Res. J. Pharm. App. Sci. 2014;4(1):1–3. [Google Scholar]
- Lee Y.J., Kim D.B., Lee J., Cho J.H., Kim B., Choi H.S. Antioxidant activity and anti-adipogenic effects of wild herbs mainly cultivated in Korea. Molecules. 2013;18:12937–12950. doi: 10.3390/molecules181012937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry J.B. Floral anthocyanins of some Malesian Hibiscus species. Phytochemistry. 1976;15:1395–1396. [Google Scholar]
- Moundipa F.P., Kamtchouing P., Koueta N., Tantchou J., Foyang N.P., Mbiapo F.T. Effects of aqueous extracts of Hibiscus macranthus and Basella alba in mature rat testis function. J. Ethnopharmacol. 1999;65:133–139. doi: 10.1016/s0378-8741(98)00207-4. [DOI] [PubMed] [Google Scholar]
- Nadkarni, A.K., 1954. Indian Materia Medica. Bombay, p. 631.
- Rekha T. Comparative pharmacological study of Aerial parts and roots of ethanolic extract of Hibiscus micranthus Linn. J. Med. Pharm. All. Sci. 2017;1:581–587. [Google Scholar]
- Siddiqui N.A., Alam P., Al-Rehaily A.J., Al-Oqail M.M., Parvez M.K. Simultaneous quantification of biomarkers Bergenin and menisdaurin in the methanol extract of aerial parts of flueggea virosa by validated HPTLC densitometric method. J. Chromatogr. Sci. 2015;53:824–829. doi: 10.1093/chromsci/bmu231. [DOI] [PubMed] [Google Scholar]
- Siddiqui N.A., Mothana R.A., Alam P. Quantitative determination of alliin in dried garlic cloves and products by high-performance thin-layer chromatography. Trop. J. Pharm. Res. 2016;15(8):1759–1765. [Google Scholar]
- Tamilselvi S.S., Venkatachalapathi A., Paulsamy S. Ethnomedicinal plants used by irula tribes of maruthamalai hills of coimbatore district, western ghats, India. Int. J. Pharm. Bio Sci. 2016;7(3):533–553. [Google Scholar]
- Telefo P.B., Moundipa P.F., Tehana A.N., Tchouanguep C.D., Mbiapo F.T. Effects of an aqueous extract of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus, and Justicia insularis on some biochemical and physiological parameters of reproduction in immature female rat. J. Ethnopharmacol. 1998;63:193–200. doi: 10.1016/s0378-8741(98)00062-2. [DOI] [PubMed] [Google Scholar]
- Yin M.C., Chan K.C. Nonenzymatic antioxidative and antiglycative effects of oleanolic acid and ursolic acid. J. Agric. Food Chem. 22. 2007;55(17):7177–7181. doi: 10.1021/jf071242m. [DOI] [PubMed] [Google Scholar]