Abstract
Total extracts of Drechslera rostrata and Eurotium tonophilum in addition of two isolated compounds from their cultures [di-2-ethylhexyl phthalate (H1) and 1,8-Dihydroxy-3-methoxy-6-methyl-anthraquinone (H2)] were tested for their antitumor activity using four human carcinoma cell lines. Antitumor activity was assessed by performing MTT assay to check the % cell viability. The % viability of HCT-116 (colon carcinoma), HeLa (cervical carcinoma), HEp-2 (larynx carcinoma) and HepG-2 (hepatocellular carcinoma) cells decreased after treatment with Drechslera rostrata and Eurotium tonophilum extracts, these effects were ranged from 059.0 ± 0.1 to 217.0 ± 0.3 µg/ml on all types of cancer cells. The best activity was recorded for Eurotium tonpholium extract (054.5 ± 0.3, 059.0 ± 0.5 and 059.0 ± 0.1 for HEp-2, Hela, and HepG-2 respectively). The isolated compounds (H1&H2) were found to be responsible about the activities because they recorded the lowest IC50 on tested cell lines with range of 9.5–20.3 μg/ml. Vinblastine sulphate was used as a reference standard and showed in vitro anticancer activity. This study demonstrated that all extracts and isolated compounds have antitumor activity against HCT-116, HeLa, HEp-2 and HepG-2 cells.
Keywords: Drechslera rostrata; Eurotium tonophilum; Di-2-ethylhexyl phthalate; 1,8-Dihydroxy-3-methoxy-6-methyl-anthraquinone; MTT assay; Human cancer cell lines
1. Introduction
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body and has become the most important health problem due effect on morbidity and mortality (Martin et al., 2013). Normal cancer chemotherapy has multidrug resistance (MDR) caused by overexpression of integral membrane transporters, which can decrease drug buildup inside the cell. MDR cells are resistant to cytotoxic effects of various chemotherapeutic agents (Ruefli et al., 2002, Shi et al., 2007). Developing new anticancer agents that are efficient to MDR cells is a reasonable approach to overcome MDR (Zhang et al., 2010).
Anticancer drugs can destroy tumors and arrest cancer progress but cancer treatment may damage healthy cells and tissues also (Liu et al., 2015). Thus, new anticancer agents from natural products are expected to play an important role in the development of more effective and safer drugs to inhibit the onset of cancer (Greenwell and Rahman, 2015).
Fungi contain some of the most unbelievable chemical factories known today. Accordingly, numerous bioactive agents such as mycotoxins, anticancer and antifungal agents have been reported in the literature (Frisvad, 2015, Frisvad et al., 2004). Despite there are many new compounds revealing meny biological activities but still being discovered, including well-known metabolites such as griseofulvin (Panda et al., 2005, Rebacz et al., 2007, Ho et al., 2001, Ronnest et al., 2009). The Large combinatorial libraries of fungal active compounds have not provided the estimated number of new chemical entities in addition it couldn’t explains why the field of natural products is currently assuming new prominence (Barnes et al., 2016). It has been estimated that approximately 1.5 million or likely as many as 3 million fungal species exist on Earth, of which only around 100,000 species have been described so far (Sharma et al., 2016, Hawksworth, 2012). A multitude of new species are likely to be discovered from miscellaneous habitats, such as soils and tropical forest plants, associated to insects and in the aquatic environment (Bladt et al., 2013).
Therefore, our aim was to study the antitumor activity of the extracts and isolated compounds from Drechslera rostrata and Eurotium tonophilum in human cancer cells.
2. Materials and methods
Fungi under investigation [Drechslera rostrata (DSM 62596) and Eurotium tonophilum (ATCC 16440)] were pushed from DSMZ (German Collection of Microorganisms and Cell Cultures). For culturing the fungi malt extract agar (MEA) medium (Zain et al., 2012) was used for cultivation of the fungal.
2.1. Fungal extraction and isolation of compounds
The mycelia mat (800 g) of each fungus (D. rostrata and E. tonophilum) was separately harvested, washed with distilled water, and extracted by refluxing in boiled ethanol (2 liter) for 3 h and filtered off, this process was repeated for three times. The combined filtrates were concentrated under reduced pressure at temperature not exceeding 35 °C. The obtained residues of D. rostrata (88 g) and E. tonophilum (90 g) were symbolized as D1 & E1.
The total extracts (22 g each) were separately dissolved in ethanol and applied on the top of column (5 × 150 cm) packed with 100 g silica gel. Elution was carried out using chloroform -methanol (95:5 v/v), 150 hundred fractions (100 ml each) were collected, the similar fractions were collected together (according to color and number of spots) and concentrated using reduced pressure as previously described, they reduced into two sub fraction for each column. Both symbolized as DF and ET for D. rostrata and E. tonophilum. respectively.
DF fractions (12 g) were collected together were individually introduced to the top of a glass column (3 × 160 cm) packed with 360 g silica gel G. Elution were carried out using system Ethyl acetate: Methanol: Water (30: 5: 4 v/v/v), 110 fractions (100 ml each) were collected; each was concentrated under reduced pressure to a small volume. Similar fractions were collected together and re-applied on to other columns for final purification. H1 was isolated (Awaad et al., 2014).
The sub-fractions ET (9) were dissolved in ethanol and applied on the top of column (5 × 150 cm) packed with 300 g silica gel G, Benzene: ethyl acetate (86:14 v/v) was used as eluent. Sixty fractions (100 ml each) were collected, similar fraction were collected together (according to color and number of spot) and concentrated as previously described, to produced two sub-groups with semi purified compounds for final purifications each fraction was reapplied on other columns packed with silica gel G and eluted with benzene: chloroform (90:10 v/v) from which compound H2 was isolated (Awaad et al., 2014).
2.2. Cell culture
The tested human carcinoma cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The cells were grown on RPMI-1640 medium supplemented with 10% heat inactivated fetal calf serum, 1% L-glutamine, and 50 µg/ml gentamycin. The cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 incubator (Shel lab 2406, USA) and were sub-cultured two to three times a week.
2.3. Antitumor activity assay
For antitumor assays, the tumor cell lines were suspended in buffer and added to the medium at concentration 5 × 104 cell/well in Corning® 96-well tissue culture plates, then incubated for 24 h. The tested compounds were then added into 96-well plates (six replicates) to achieve seven concentrations for each compound. Six vehicle controls with media or 0.5% DMSO were run for each 96 well plate as a control. After incubating for 24 h, the numbers of viable cells were determined by the MTT test. Briefly, the media was removed from the 96 well plate and replaced with 100 µl of fresh culture RPMI 1640 medium without phenol red then 10 µl of the 12 mM MTT (Sigma) stock solution (5 mg of MTT in 1 mL of PBS) to each well including the untreated controls. The 96 well plates were then incubated at 37 °C and 5% CO2 for 4 h. An 85 µl aliquot of the media was removed from the wells, and 50 µl of DMSO was added to each well and mixed thoroughly with the pipette and incubated at 37 °C for 10 min. Then, the optical density was measured at 590 nm with the microplate reader (SunRise, TECAN, Inc, USA) to determine the number of viable cells.
The percentage of viability was calculated as:
Where ODt is the mean optical density of wells treated with the tested sample and ODc is the mean optical density of untreated cells.
The relation between surviving cells and drug concentration is plotted to get the survival curve of each tumor cell line after treatment with the specified compound. The 50% inhibitory concentration (IC50), the concentration required to cause toxic effects in 50% of intact cells, was estimated from graphic plots of the dose response curve for each conc. using Graphpad Prism software (San Diego, CA, USA) (Mosmann, 1983, Elaasser et al., 2011).
2.4. Statistical analysis
Data were expressed as mean ± S. D. Statistical analysis was done by using GraphPad Prism 5 (San Diego, CA, USA).
3. Results and discussion
3.1. Isolated compounds
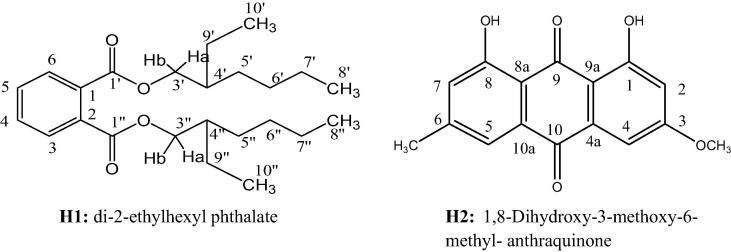
Two compounds were isolated from mycelial mat of D. rostrate (H1) and E. tonophilum (H2) using different spectroscopic analysis including 1HNMR, 13CNMR, HMBC, HMQC and EI-MS (Fig. 1).
Fig. 1.
The isolated compounds (H1 & H2) from Drechslera rostrata and Eurotium tonophilum.
H1: Colorless oil, (950 mg), Rf = 0.40 in system (n-hexane- benzene 30:70 v/v), b.p. = 385 °C; The ESI-MS at m/z 413 [M + Na]+ (100%), 390 g/mol, [M + H]+ ion 391 (9.1), [M]+ 390, [M2 + Na]+ 803 (5.4%).1H-NMR in CDCl3: δ; 0.94 (3H, t, J = 7.2 Hz,H8′, H8″), 0.93 (3H, t, J = 7.2 Hz, H10′,H10″), 1.25–1.40 (6H, m, (H-5′,5″), (H- 6′, 6″ and (H-7′,7″), 1.50–1.42 (4H, m, H-9′,9″), 1.69 (2H, septet, J = 6 HZ, H-4′, 4″), 4.10 (2H, dd,J H3′b, H4′ = 6.1 HZ and JH3′b, H3′a = 10.9 Hz,H-3′b,3″b), 4.26 (2H,dd, J H3′a, H4′ = 5.7 HZ and JH3′a, H3′b = 10.9 HZ, H-3′a,3″a), 7.6 (2H, m, H4,H5) and 7.74 (2H, m, H3,H6).13C-NMR and DEPT in CDCl3: δ; 10.97 (C-10′,10″) and 14.07 (C-8′,8″)] δ; 22.97 (C-7′,7″), 23.24 (C-9′,9″), 28.90 (C-6′,6″) and 30.35 C-5′,5″), δ; 38.72 (C-4′,4″)], [δ 128.83 (C-3,6) and 130.86 (C-4,5), δ; 132.38 (C-1, C-2)] δ; 168.10 (C-1′, 1″). Spectroscopic data analysis (1H-NMR.13C-NMR and DEPT COSY, HSQC and HMBC) was compared with published (Rao et al., 2000, Amade et al., 1994), this compound is identified as; di-2-ethylhexyl phthalate.
H2: Orange to yellow needle crystals (550 mg), Rf = 0.67 in system (Benzene- chloroform 80:20 v/v), m.p. = 204–205 °C. 1H-NMR in CDCl3, showed at δ 2.45(S, CH3) at δ (3.94, S, OCH3), at δ 6.73 (1H, d, J = 2.55 Hz, H-2), δ 7.1 (1H, S, H-7), δ 7.41 (1 H, d, J = 2.55 Hz, H-4), δ 7.55 (1H, S,H-5) δ12.12, (S, 8-OH), δ 12.32 (S, 1-OH).13C- NMR and DEPT; the13C-NMR in CDCl3, δ 22.20 (CH3-6) and δ 56.12 (OCH3-3) δ 106.76, 108.25, 121.32 and 124.53] δ 110.09, 113.93, 133.22, 135.23, 148.47, 162.49, 165.18, 166.53, 181.05, 190.78.By comparing the obtained spectroscopic data analysis with published (Jo et al., 2011), this compound is identified as 1,8-Dihydroxy-3-methoxy-6-methyl- anthraquinone.
3.2. Antitumor activity assay
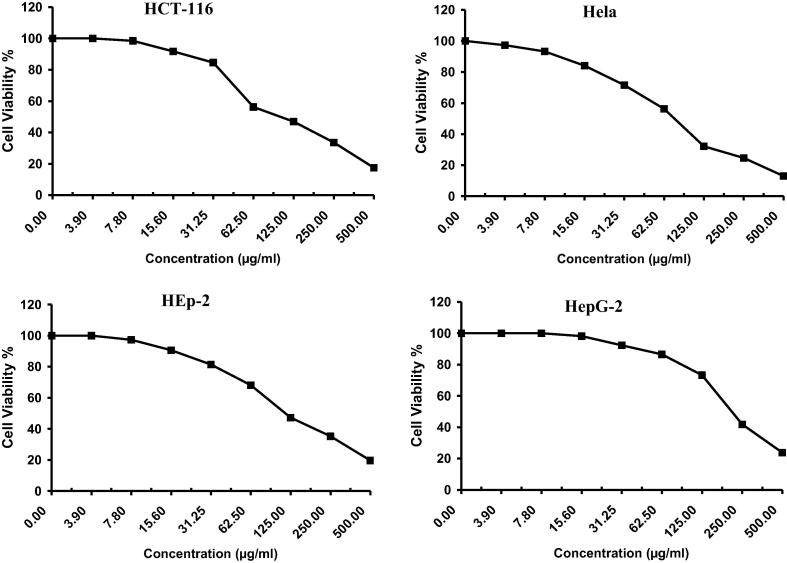
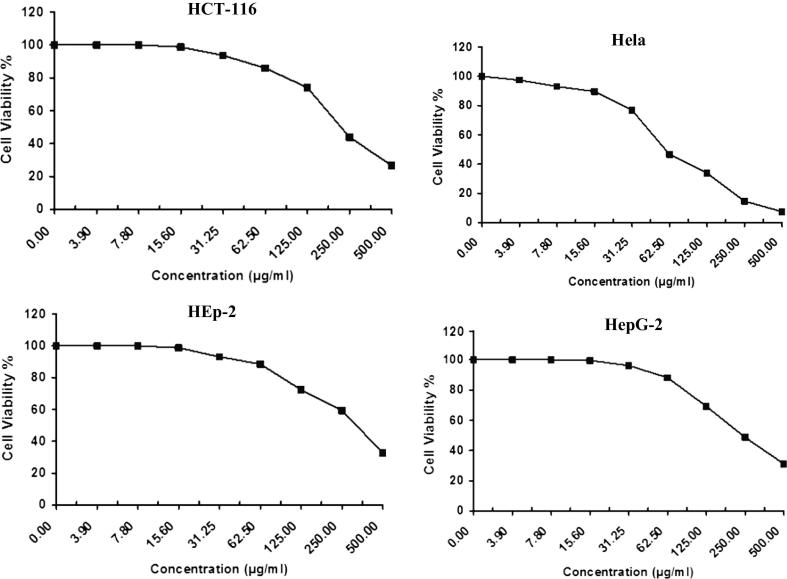
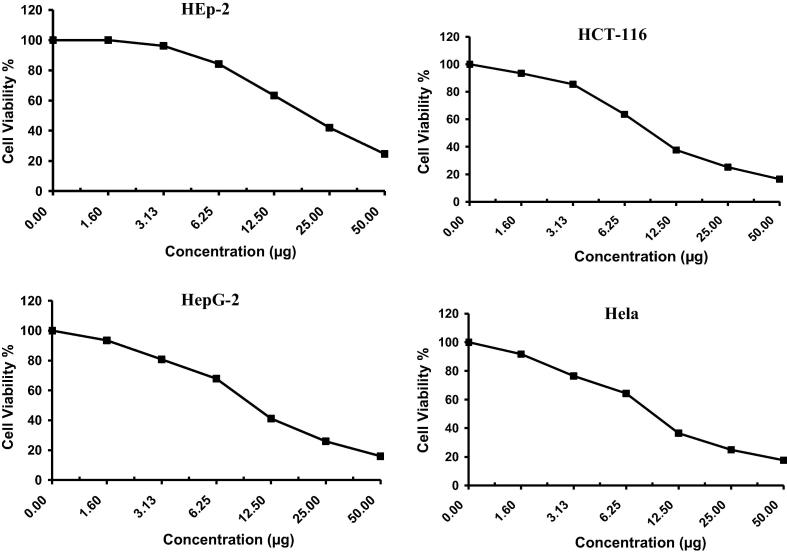
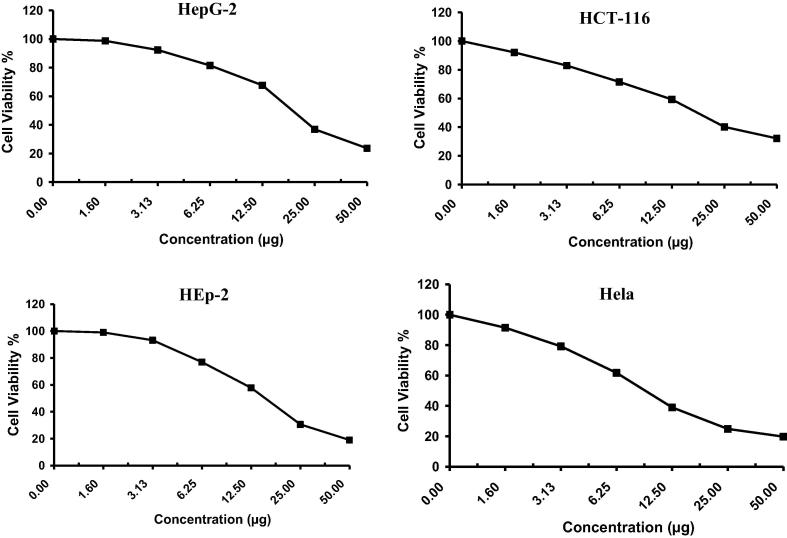
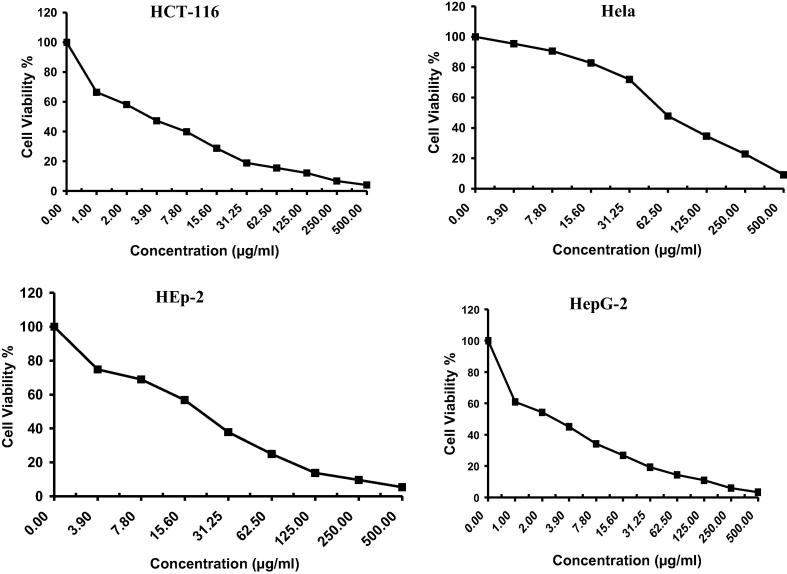
The anticancer activity of Drechslera rostrata, Eurotium tonophilum extracts in addition to the isolated compounds H1 & H2 were evaluated on the basis of its protective effects on cell viability is shown in Fig. 2, Fig. 3, Fig. 4, Fig. 5 & Table 1. The anticancer activity of Vinblastine sulphate as a reference standard was also studied and shown in Fig. 6 & Table 1.
Fig. 2.
The antitumor activity of Drechslera rostrata extract on four cell lines.
Fig. 3.
The antitumor activity of Eurotium tonpholium extract on four cell lines.
Fig. 4.
The antitumor activity of compound H1 on four cell lines.
Fig. 5.
The antitumor activity of compound H2 on four cell lines.
Table 1.
The IC50 values of Drechslera rostrata and Eurotium tonophilum extracts and isolated compounds on four cell lines.
| Tested extract | Cell line | |||
|---|---|---|---|---|
| IC50 (µg/ml) | ||||
| HCT-116 (Colon carcinoma) | Hela (Cervical carcinoma) | HEp-2 (Larynx carcinoma) | HepG-2 (Hepatocellular carcinoma) | |
| Drechslera rostrata | 104.0 ± 0.4 | 078.7 ± 0.5 | 117.0 ± 0.2 | 217.0 ± 0.3 |
| Eurotium tonophilum | 125.0 ± 0.2 | 059.0 ± 0.5 | 054.5 ± 0.3 | 059.0 ± 0.1 |
| H1 | 9.5 ± 0.4 | 17.5 ± 0.4 | 20.3 ± 0.4 | 10.4 ± 0.4 |
| H2 | 18.6 ± 0.4 | 9.5 ± 0.4 | 16.1 ± 0.4 | 19.7 ± 0.4 |
| Vinblastine Sulphate | 3.5 ± 0.2 | 59.7 ± 2.1 | 21.2 ± 0.9 | 2.93 ± 0.3 |
Values are expressed as mean ± SEM of 6 determinants. Symbols ND represents not determined.
Fig. 6.
The antitumor activity of Vinblastine sulphate as Reference Standard on four cell lines.
Drechslera rostrata extract showed an IC50 value of 104.0 μg/ml, 78.7 μg/ml, 117.0 μg/ml and 217.0 μg/ml against colon carcinoma cells, cervical carcinoma cells, larynx carcinoma cells and hepatocellular carcinoma cells, respectively.
Eurotium tonpholium extract showed an IC50 value of 125.0 μg/ml, 59.0 μg/ml, 54.5 μg/ml and 59.0 μg/ml against colon carcinoma cells, cervical carcinoma cells, larynx carcinoma cells and hepatocellular carcinoma cells, respectively.
H1 showed an IC50 value of 9.5 μg/ml, 17.5 μg/ml, 20.3 μg/ml and 10.4 μg/ml against colon carcinoma cells, cervical carcinoma cells, larynx carcinoma cells and hepatocellular carcinoma cells, respectively.
H2 showed an IC50 value of 18.6 μg/ml, 9.5 μg/ml, 16.1 μg/ml and 19.7 μg/ml against colon carcinoma cells, cervical carcinoma cells, larynx carcinoma cells and hepatocellular carcinoma cells, respectively.
Reference standard, Vinblastine sulphate showed an IC50 value of 3.5 μg/ml, 59.7 μg/ml, 21.2 μg/ml and 2.93 μg/ml against colon carcinoma cells, cervical carcinoma cells, larynx carcinoma cells and hepatocellular carcinoma cells, respectively.
Drechslera rostrate extract, Eurotium tonophilum extract, H1 and H2 showed a dose-dependent inhibitory effect on the growth of colon carcinoma cells, cervical carcinoma cells, larynx carcinoma cells and hepatocellular carcinoma cells.
4. Discussion and conclusion
Natural products have provided the most important successes in the chemotherapy of cancer. Most of the major anticancer compounds are obtained from natural products which includes plants and microorganisms (Olano et al., 2009). Earlier reports showed that the secondary metabolites from fungi provided an important group of new biological agents and having low toxicity on normal cells. Further, the secondary metabolites are low molecular weight compounds, exhibiting a potential anticancer activity. It is wondered that biological activity of the fungi extracts is associated with the endogenous environment of the plant (Engel and Evens, 2006). It is observed that the existing anticancer drugs have a limited selectivity and high toxicity.
Researchers in the molecular and cellular biology are regularly identifying novel potential targets, which are specific or selective for cancer cells (Kohn et al., 1996). The effect of the extracts of endophytic fungi isolated from mangrove plants on the cancer cells as well as their effects on topoisomerase was reported (Cai et al., 2010). The anticancer activity of compounds isolated from marine endophytic fungus Aspergillus terreus was reported (Suja et al., 2014). A new Topo I isomerase inhibitor, (+)−3, 3, 7, 7, 8. 8- hexahydroxy-5, 5-dimethylbianthraquinone was recently isolated from mangrove endophytic fungi (Tan et al., 2008).
The di-esters of 1, 2-benzenedicarboxylic acid (phthalic acid), commonly known as phthalates, are a group of man-made chemicals (Hauser and Calafat, 2005).
There are many researches reporting isolation of phthalates from bacterial strains, streptomycetes, fungi, algae and plants. These compounds seem to play an important role in many areas (Prabukumar et al., 2015).
Although these compounds are not novel isolates, it is the first time that their antitumor activity is described in colon carcinoma cells, cervical carcinoma cells, larynx carcinoma cells and hepatocellular carcinoma cells.
In our study, effects on cell viability/proliferation were assessed by an MTT assay and all extracts and compounds tested significantly decreased cell viability and proliferation in one or more of the cell lines tested. The anti-proliferative effect of the compounds could be due to induction of cell death and/or cell cycle arrest.
Hela (cervical carcinoma) cell line was the most sensitive line to Drechslera rostrata extract and H2, HEp-2 (larynx carcinoma) cell line was the most sensitive line to Eurotium tonophilum extract and HCT-116 (colon carcinoma) cell line was the most sensitive line to H1 and Vinblastine sulphate. In fact, almost all the extracts and compounds, proved to have an antitumor activity in a dose-dependent manner in these cell lines. The IC50 value of H1 and H2 are found to be most promising compare to the extracts in all the four cell lines. The IC50 value of H1 was found to be 9.5 μg/ml against colon carcinoma cells. The IC50 value of H2 was found to be 9.5 μg/ml against cervical carcinoma cells.
This study demonstrates that the compounds H1 and H2 obtained from the fungus Drechslera rostrata (DSM 62596) and Eurotium tonophilum (ATCC 16440) have significant antitumor properties, as evaluated by performing MTT assay to check the % cell viability against HCT-116 (colon carcinoma), HeLa (cervical carcinoma), HEp-2 (larynx carcinoma) and HepG-2 (hepatocellular carcinoma) cells.
Acknowledgements
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amade P., Mallea M., Bouaicha N. Isolation, structural identification and biological activity of two metabolites produced by Penicillium Olsonii Bainier and Sartory. J. Antibiotics. 1994;47(2):201–207. doi: 10.7164/antibiotics.47.201. [DOI] [PubMed] [Google Scholar]
- Awaad A.S., Al-Zaylaee H.M., Alqasoumi S.I., Zain M.E., Aloyan E.M., Alafeefy A.M., Awad E.S., El-Meligy R.M. Anti-leishmanial activities of extracts and isolated compounds from Drechslera rostrata and Eurotium tonpholium. Phytother. Res. 2014;28(5):774–780. doi: 10.1002/ptr.5096. [DOI] [PubMed] [Google Scholar]
- Barnes E.C1., Kumar R., Davis R.A. The use of isolated natural products as scaffolds for the generation of chemically diverse screening libraries for drug discovery. Nat. Prod. Rep. 2016;33(3):372–381. doi: 10.1039/c5np00121h. [DOI] [PubMed] [Google Scholar]
- Bladt T.T., Frisvad J.C., Knudsen P.B., Larsen T.O. Anticancer and antifungal compounds from Aspergillus Penicillium and other filamentous fungi. Molecules. 2013;18(9):11338–11376. doi: 10.3390/molecules180911338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Bingya, Ezeogu Lewis, Zellmer Lucas, Baofa Yu., Ningzhi Xu., Liao Dezhong Joshua. Cancer Med. 2015;4(9):1394–1403. doi: 10.1002/cam4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Xiaoli L., Shining Z., Junping G., Shuiping W., Xiaoming L., Zhigang S., Yongcheng L. Cytotoxic and topoisomerase I inhibitory activities from extracts of endophytic fungi isolated from mangrove plants in Zhuhai China. J. Ecol. Nat. Environ. 2010;2(2):017–024. [Google Scholar]
- Sharma Deeksha, Pramanik Avijit, Agrawal Pavan Kumar. Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D.Don. 3. Biotech. 2016;6(2):210. doi: 10.1007/s13205-016-0518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaasser M.M., Abdel-Aziz M.M., El-Kassas R.A. Antioxidant, antimicrobial, antiviral and antitumor activities of pyranone derivative obtained from Aspergillus candidus. J. Microbiol. Biotech. Res. 2011;1(4):5–17. [Google Scholar]
- Engel R.H., Evens A.M. Oxidative stress and apoptosis: a new treatment paradigm in cancer. Frontiers Biosci. 2006;11:300–312. doi: 10.2741/1798. [DOI] [PubMed] [Google Scholar]
- Frisvad J.C. Taxonomy, chemo diversity, and chemoconsistency of Aspergillus, Penicillium, and Talaromyces species. Front Microbiol. 2015;12(5):773. doi: 10.3389/fmicb.2014.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004;49:201–241. [Google Scholar]
- Greenwell M., and Rahman P., 2015. Medicinal Plants: Their Use in Anticancer Treatment. Int J Pharm Sci Res. 1; 6(10): 4103–4112. [DOI] [PMC free article] [PubMed]
- Hauser R., Calafat A.M. Phthalates and human health. Occup Environ. 2005;62(11):806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth D.L. Global species numbers of fungi: are tropical studies and molecular approaches contributing to a more robust estimate? Biodivers. Conserv. 2012;21:2425–2433. [Google Scholar]
- Ho Y.S., Duh J.S., Jeng J.H., Wang Y.J., Liang Y.C., Lin C.H., Tseng C.J., Yu C.F., Chen R.J., Lin J.K. Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int. J. Cancer. 2001;91:393–401. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1070>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Jo G., Shin S.Y., Lee Y., Hyun J., Dong K.S., Park J.C., Kim H.S., Lee Y.H., Lim Y. A compound isolated from Rumex japonicas induces early growth response gene-1 expression. J. Korean Soc. Appl. Biol. Chem. 2011;54(4):637–643. [Google Scholar]
- Kohn A.D., Summers S.A., Birnbaum M.J., Roth R.A. Expression of a constitutely active Akt Ser/Thr kinase in 3T3 –L1 adipocytes stimulates glucose uptake and glucose transport 4 translocation. J. Biol. Chem. 1996;271(49):31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Imm. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Olano C., Mendez C., Jose A. Salas antitumor compounds from marine actinomycetes. Mar. Drugs. 2009;7(2):210–248. doi: 10.3390/md7020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D., Rathinasamy K., Santra M.K., Wilson L. Kinetic suppression of microtubule dynamic instability by griseofulvin: implications for its possible use in the treatment of cancer. Proc Natl Acad Sci USA. 2005;102:9878–9883. doi: 10.1073/pnas.0501821102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabukumar S., Rajkuberan C., Ravindran K., Sivaramakrishnan S. Isolation and characterization of endophytic fungi from medicinal plant Crescentia cujete L. and their antibacterial, antioxidant and anticancer properties. J. Pharm. Pharm. Sci. 2015;7(11):316–321. [Google Scholar]
- Rao G., Kumar P., Dhandapani V., Krishna T., Hayashi T. Constituents of Cassia auriculata. Fitoter. 2000;71:82–83. doi: 10.1016/s0367-326x(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Rebacz B., Larsen T.O., Clausen M.H., Rønnest M.H., Loffler H., Ho A.D., Kramer A. Identification of griseofulvin as an inhibitor of centrosomal clustering in a phenotype-based screen. Cancer Res. 2007;67:6342–6350. doi: 10.1158/0008-5472.CAN-07-0663. [DOI] [PubMed] [Google Scholar]
- Ronnest M.H., Rebacz B., Markworth L., Terp A.H., Larsen T.O., Kramer A., Clausen M.H. Synthesis and structure-activity relationship of griseofulvin analogues as inhibitors of centrosomal clustering in cancer cells. J. Med. Chem. 2009;52:3342–3347. doi: 10.1021/jm801517j. [DOI] [PubMed] [Google Scholar]
- Ruefli A.A., Tainton K.M., Darcy P.K., Smyth M.J., Johnstone R.W. P-glycoprotein inhibits caspase-8 activation but not formation of the death inducing signal complex (disc) following Fas ligation. Cell Death Differ. 2002;9:1266–1272. doi: 10.1038/sj.cdd.4401081. [DOI] [PubMed] [Google Scholar]
- Shi Z., Peng X.X., Kim I.W., Shukla S., Si Q.S., Robey R.W., Bates S.E., Shen T., Ashby C.R., Jr, Fu L.W., Ambudkar S.V., Chen Z.S. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- Suja M., Vasuki S., Sajitha N. Anticancer activity of compounds isolated from marine endophytic fungus Aspergillus terreus. World J. Pharm. Pharm. Sci. 2014;3:661–672. [Google Scholar]
- Tan D.S.P., Marchio C., Filho J.S.R. Hereditary breast cancer: from molecular pathology to tailored therapies. J. Clin. Pathol. 2008;61:1073–1082. doi: 10.1136/jcp.2008.057950. [DOI] [PubMed] [Google Scholar]
- Martin Tracey A., Lin Ye., Sanders Andrew J., Jane Lane., Wen G. Jiang., 2013. Cancer Invasion and Metastasis: Molecular and Cellular Perspective
- Zhang J.Y., Tao L.Y., Liang Y.J., Chen L.M., Mi Y.J., Zheng L.S., Wang F., She Z.G., Lin Y.C., To K.K., Fu L.W. Anthracenedione derivatives as anticancer agents isolated from secondary metabolites of the mangrove endophytic fungi. Marine Drugs. 2010;8(4):1469–1481. doi: 10.3390/md8041469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain M.E., Awaad A.S., Al-Outhman1 MR., El-Meligy RM. Antimicrobial activities of Saudi Arabian desert plants. Phytopharmacology. 2012;2(1):106–113. [Google Scholar]