Abstract
The flower of Hypericum calycinum, which appears uniformly yellow to humans, bears a UV pattern, presumably visible to insects. Two categories of pigments, flavonoids and dearomatized isoprenylated phloroglucinols (DIPs), are responsible for the UV demarcations of this flower. Flavonoids had been shown previously to function as floral UV pigments, but DIPs had not been demonstrated to serve in that capacity. We found the DIPs to be present in high concentration in the anthers and ovarian wall of the flower, suggesting that the compounds also serve in defense. Indeed, feeding tests done with one of the DIPs (hypercalin A) showed the compound to be deterrent and toxic to a caterpillar (Utetheisa ornatrix). The possibility that floral UV pigments fulfill both a visual and a defensive function had not previously been contemplated. DIPs may also serve for protection of female reproductive structures in other plants, for example in hops (Humulus lupulus). The DIPs of hops are put to human use as bitter flavoring agents and preservatives in beer.
Keywords: nectar guides‖pollination‖plant defense‖dearomatized phloroglucinols‖flavonoids
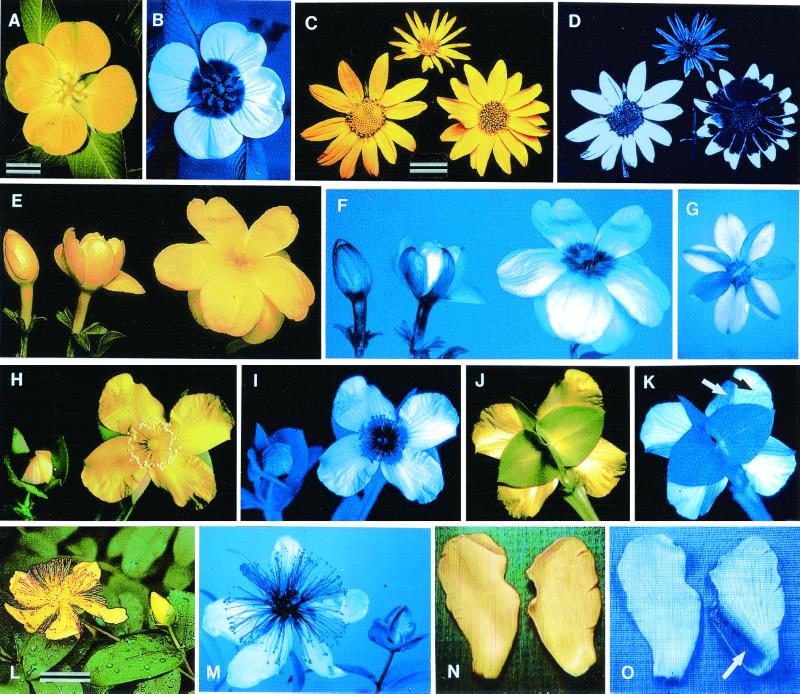
Many flowers have UV patterns, invisible to humans but visible to insects such as honeybees, whose visual sensitivity extends into the near UV region of the solar spectrum (1–8). These patterns are part of the visual gestalt of the flower and as such are meaningful to the pollinator (1, 5, 6). Not surprisingly, the patterns are displayed predominately on the exposed “facial” surface of the flower, where the pollinator makes its landing. The UV patterns sometimes differ in plants growing intermingled in potential competition (1, 5, 9) and may be part of the décor that makes each flower recognizably distinct and, as a consequence, potentially memorable to the pollinator (Fig. 1 C and D).
Figure 1.
Floral images in visible and UV light (the UV images are transduced into blue). (A and B) Ludwigia peruviana. (C and D) Chrysopsis villosa (Left), Helianthella quinqueneris (Top), and Viguiera multiflora (Right). (E and F) J. primulinum; bud opening into blossom. (G) Same, pressed flower in abaxial view. (H–K) H. edisonianum in frontal view beside bud and in abaxial view (arrows in K; white, portion of petal exposed in bud; black, portion of petal exposed in blossom). (L and M) H. calycinum flower, beside mature bud. (N and O) H. calycinum petals in frontal and abaxial view (the arrow in O points to the UV-absorbent zone on abaxial surface of petal). [Bars: A and C (1 cm); L (2 cm); E and H are slightly larger than natural size; N is at natural size.]
In radial flowers, the UV-absorbing pigments responsible for the UV demarcation are often concentrated in the center of the flower, with the result that these flowers have the appearance of a bull's-eye in the UV (Fig. 1 A and B) (1, 5). Such an image has been shown to increase the attractiveness of a flower from a distance (10) and to help pollinators orient on the flower after landing (the dark UV-absorbing center of the flower can serve as a “nectar guide”) (5). In cases where the UV pigments of flowers have been characterized, they have been found to be flavonoids (11, 12), but there was no reason why other UV-absorbing compounds could not also serve in such a signaling capacity.
We report here on the UV pigments of Hypericum calycinum, a member of the Guttiferae (Fig. 1L). We found the pigments to include, beside two flavonoids, a mixture of dearomatized isoprenylated phloroglucinols (DIPs), compounds previously characterized from this plant (13, 14) but not shown to play an adaptive role. We were intrigued by the H. calycinum flower because, in addition to displaying a conventional UV bull's-eye pattern on the facial surface (Fig. 1M), it exhibited a rare trait that we had described earlier for other flowers, namely the possession of UV markings on the abaxial surface (15).
We had reported such adornment of the floral “rear” for two species of plant and speculated on its implications. In Jasminium primulinum, for example, the phenomenon is a consequence of differential UV demarcation of those petal parts exposed to the outside in the unopened bud. Whereas these exposed parts are UV-absorbent, the petal portions hidden in the bud that come into view only at maturity in the open blossom are UV-reflectant (Fig. 1 E and F). Buds, as a result, are kept different in appearance from the mature flowers, to the potential benefit of plant and pollinator alike, because the open flowers are thereby unambiguously advertised. The asymmetrical UV pattern that appears on the reverse of the flower (Fig. 1G) is the consequence of the irregular pattern of overlap of the petals in the bud. The phenomenon is identical in Hypericum edisonianum [misidentified as Hypericum peltatum in our earlier paper (ref. 15)], except that in this species (Fig. 1 H–K), the petals overlap in accord with a fixed pattern (imbricate aestivation). As a result of such overlap, the petals, on their reverse surface, are precisely divided into a UV-absorbent zone (Fig. 1K, white arrow), exposed in the bud, and a reflectant zone (Fig. 1K, black arrow), exposed in the blossom.
We found H. calycinum to show the same UV characteristics as H. edisonianum. In our efforts to characterize H. calycinum's floral UV pigments, we therefore extracted separately the UV-absorbing and -reflectant portions of its petals (Fig. 1 N and O). We also examined the reproductive parts of the flower, because these parts made up the UV-absorbing center of the flower and could therefore be expected to contain UV pigments. We examined anthers and filaments separately, as well as the ovaries. We found the UV pigments to be present in all UV-absorbing parts of the flower but unexpectedly found the DIPs to be deposited at extraordinarily high concentration in the ovarian wall. This observation raised the possibility that these compounds also served as antifeedants, possibly for protection of the developing seeds against insect herbivores. We found the DIPs also to be concentrated in the anthers, indicating that they might protect the pollen as well. Tests that we undertook with a caterpillar and one of the DIPs (hypercalin A) showed this compound to be not only deterrent but also toxic to the insect, thereby underscoring the possibility that DIPs serve in defense.
Materials and Methods
H. calycinum.
This plant is a common ornamental, native to Southeastern Europe. Flowers for our studies were obtained from cultivated plants in Ithaca, NY. The plant blooms throughout much of the summer, and the flowers last only about a day. The flowers are hypogynous, with five petals, numerous anthers, and compound pistil. The aestivation is imbricate, as it is apparently in Hypericum generally.
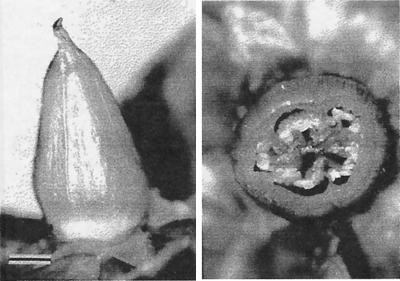
The buds mature slowly, turning from green to yellow over a period of days and remaining in the yellow state for 2–3 days before floral opening. The opening itself takes place over a period of 1–2 h, soon after dawn. The flowers remain bright yellow for much of the day, but by evening the petals are already partly shriveled and the anthers and filaments have begun to turn light brown. At any one time in midsummer, the plants typically bear a mixture of buds and flowers at all stages of development. As the flower senesces and the petals and stamens wilt away, the ovary gradually enlarges into a cone-shaped structure (Fig. 2). Within the five cells of the ovary, the placentation is axial.
Figure 2.
Ovary in profile view and in cross section. Structure is shown as it appears on about the fifth day after wilting of the flower. (Bar = 2 mm.)
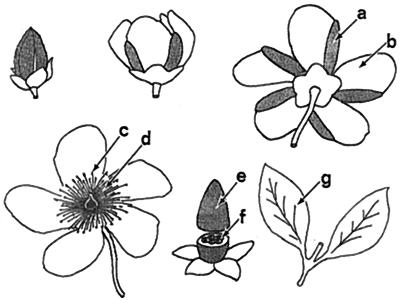
For purposes of chemical analysis, we extracted the following floral components (Fig. 3): (a) UV-absorbent part of the petals; (b) UV-reflectant part of the petals; (c) anthers; (d) filaments; (e) ovarian wall; and (f) ovarian contents (partitions, and developing seeds). We also extracted the leaves (g). For determination of concentrations, means were calculated based on analyses of two to five extracts per component.
Figure 3.
Development from bud to blossom and beyond in H. calycinum. UV-absorbent parts are shaded or shown in black. Note that the UV-absorbent zones (a) on the abaxial side of the petals correspond to petal areas exposed to the outside in the bud. (b) UV-reflectant zone of petal, (c) anthers, (d) filaments, (e) ovarian wall, (f) ovarian contents, and (g) leaves. The ovary is here depicted in the stage shown in Fig. 2, corresponding to about the fifth day after blooming.
Samples e and f were obtained from ovaries of flowers that had bloomed several days beforehand (Fig. 3). The two samples were prepared by cutting the ovaries transversely into slices and then separating each slice into wall and contents by severing the partitions along their lines of attachment to the wall.
UV Viewing.
UV photography was carried out with a 35-mm camera and UV-transmitting lens and filter (maximum transmission 366 nm) by using UV-sensitive film (Kodak EPY). UV video-viewing made use of the same lens and filter and was effected by the technique previously described (15, 16).
Chemical Analyses.
The samples to be extracted were first dried over calcium sulfate in a vacuum dessicator at room temperature for 48 h. The dried material was crushed and stirred in methanol for 24 h, at which point the resulting slurry was filtered and concentrated to give the crude extract. Standard solutions of the extracts were prepared in methanol for HPLC analysis.
HPLC analyses were carried out by using a Hewlett–Packard 1090 II liquid chromatograph (4.6 × 250 mm Supelco 5 μ ODS (octadecylsilane) analytical column, eluted at a flow rate of 1.0 ml/min; 10 × 250 mm Supelco 5 μ ODS preparative column eluted at a flow rate of 4.0 ml/min) equipped with a diode array detector set to monitor 350, 300, and 250 nm. Isocratic elution with acetonitrile containing 0.5% formic acid was used for compounds 3-7. For compounds 1 and 2, gradient elution started with a solvent composition of 20% acetonitrile and 80% water (both containing 0.5% formic acid) for 3 minutes and progressed to a final composition of 100% acetonitrile (containing 0.5% formic acid) after 10 minutes. NMR spectra (1H, 13C, double quantum-filtered correlation spectroscopy, distortionless enhancement by polarization transfer, gradient-enhanced heteronuclear multiple quantum coherence, gradient-enhanced heteronuclear shift correlations via multiple bond connectivities, and nuclear Overhauser effect spectroscopy) were recorded in benzene-d6, DMSO-d6, methanol-d4, or chloroform-d at 298 K by using a Varian UNITY+ (500 MHz proton, 126 MHz carbon) and a Varian XL (400 MHz proton, 101 MHz carbon) spectrometer. Mass spectra were obtained by using a Micromass (Manchester, U.K.) Quattro I tandem mass spectrometer operated in both positive and negative ion electrospray modes. UV spectra were taken in methanol by using a Spectronic (Westbury, NY) Genesys 2 spectrophotometer.
Antifeedant Assay.
The larva of the moth Utetheisa ornatrix (henceforth referred to as Utetheisa) has an avidity for pyrrolizidine alkaloids, bitter chemicals that it ordinarily obtains from its foodplants, stores systemically, and uses in defense (17). Larvae that have been raised on pyrrolizidine alkaloid-free diet will consume virtually any chewable material provided it is laced with pyrrolizidine alkaloid. Such larvae can be put to the test in the assessment of potency of suspected feeding deterrents. All one needs to do is determine whether addition of the test substance to an alkaloid-treated food item reduces the acceptability of the item.
The assay we designed involved presenting individual Utetheisa larvae with two discs of filter paper [7-mm diameter; 3.43 ± 0.11 (SD) mg per disk, n = 25]. One disk (control) was treated by addition of 50 μg of monocrotaline (a pyrrolizidine alkaloid used in its free base form). The other disk (experimental) was treated by addition of 50 μg of monocrotaline plus 25 μg of hypercalin A (5). The chemicals were added to the discs in solution (each in 2 μl of methanol); to equalize the amount of solvent applied to the two discs, the controls received a supplement of 2 μl of methanol. The larvae (n = 18) were midsize (16–18 days old) and had been reared in accord with a standard protocol (17) on pyrrolizidine alkaloid-free diet (so-called pinto bean diet). Each larva was confined to a Petri dish (8.8-cm diameter), having been transferred directly from its rearing chamber. To keep the two discs in place in the dish, they were presented impaled on upside-down thumb tacks. The tests lasted 48 h, at the end of which the discs were weighed, and the condition of the larvae checked. A disk was considered to have been sampled by a larva if it weighed less than 3.32 mg (that is, mean disk weight minus 1 SD of the mean).
Other Species of Hypericum.
Using UV photography and/or video-viewing, we examined the inflorescences of a number of other species of Hypericum, including H. perforatum, H. fasciculatum, H. cumulicola, H. reductum, H. kalmianum, and H. frondosum.
Results
UV Pattern.
The Hypericum species we examined, including H. calycinum, all showed the same floral UV characteristics. In all species, the petal surfaces exposed in the buds were UV-absorbent, with the consequence that the buds were UV-absorbent in their entirety and distinct from the open flowers. It was predicted that the species should all have the abaxial surfaces of the petals subdivided into UV-absorbent and -reflectant parts, and this turned out to be the case. The flowers in all species were also similar in facial view. The pistil and stamens were consistently UV-absorbent, whereas the unfurled petals were consistently UV-reflectant. H. calycinum is in no way exceptional (Fig. 1 L–O). Therefore, we expected the UV pigments in the H. calycinum flower to be concentrated in parts a, c, d, and e (Fig. 3; Scheme S1).
Scheme 1.
Identification and Quantification of UV Pigments.
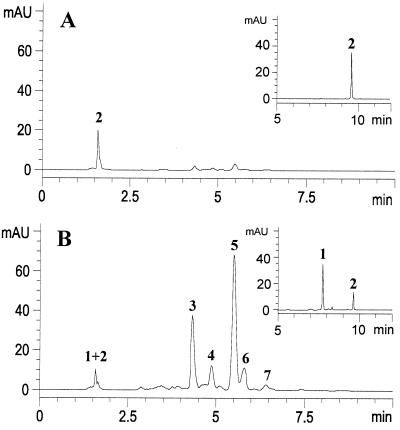
The HPLC system set to monitor at a wavelength of 350 nm provided a convenient means for detecting and isolating the pigments responsible for the observed UV patterns. This analysis revealed the relative contribution made by each pigment to the absorption at this wavelength (to which insects are known to be visually sensitive) (4). The UV-absorbent and -reflectant petal parts gave virtually equal amounts of crude extract per dry weight of material. Comparison of the chromatographs obtained from the standardized methanolic extracts of the two petal samples revealed two major and three minor components that were prominent in the UV-absorbent extract but virtually missing from that of the UV-reflectant regions (Fig. 4). A small amount of UV-absorbing material was also apparent in the solvent front, which on further HPLC analysis revealed two additional minor UV-active pigments. Sufficient material for further chemical analysis of all seven compounds was obtained by preparative HPLC.
Figure 4.
Comparison of extract of (A) UV-reflectant petal parts and (B) UV-absorbent petal parts (H. calycinum) by HPLC, with detection at 350 nm, showing the order of elution of pigments 1–7 (MAU, milliabsorbance units). Both extracts were injected as standard solutions consisting of 4.0 mg of crude extract per milliliter of methanol. The elution was carried out by using acetonitrile containing 0.5% formic acid. Pigments 1 and 2, which coeluted under these conditions (the peak at 1.6 min), were subsequently resolved in a separate HPLC step (Insets).
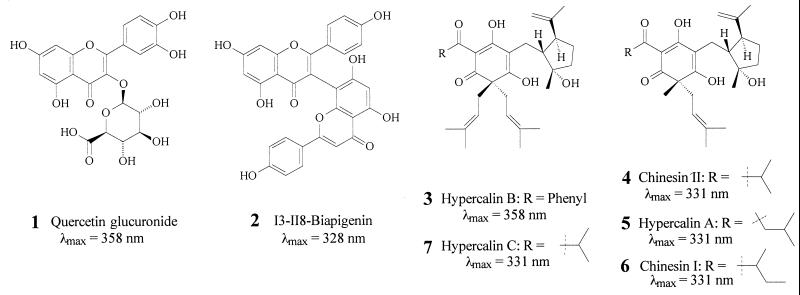
Two-dimensional NMR spectroscopic analysis of the two major HPLC fractions, coupled with molecular mass information obtained by electrospray ionization mass spectrometry, revealed these fractions to contain the DIPs hypercalin B (3) and hypercalin A (5), respectively, compounds previously isolated from H. calycinum (14). Minor components 4, 6, and 7 were obtained in amounts and at levels of purity inadequate for definitive NMR structure elucidation. Nevertheless, their mass spectral and UV spectral data suggest that 4 is chinesin II, and 7 is hypercalin C, also previously isolated from H. calycinum (14). Compound 6 was identified as chinesin I, which had been isolated from another Hypericum (13). The one-dimensional 1H NMR spectra of these fractions are consistent with the published data for these compounds.
The earliest eluting pigment was suspected to be a uronic acid derivative of quercetin on the basis of its collisionally induced fragmentation pattern in both positive and negative ion electrospray mass spectrometry as well as its 1H NMR spectra (18). Key evidence for the identity of the uronic acid moiety included the presence of strong nuclear Overhauser effect correlations between H-1′′, H-3′′, and H-5′′, as well as the observed coupling constants of 7.5 Hz for H-1′′ and H-2′′, and 9.9 Hz for H-4′′ and H-5′′. These results are consistent with a β-glucuronic acid residue in the chair conformation with all five ring protons axial. The presence of an HMBC (heteronuclear shift correlations via multiple bond connectivities) correlation between H-1′′ and C-3 allowed the pigment's complete assignment as quercetin 3-O-β-d-glucoronide (1). The second pigment to elute showed mass spectra and 1H NMR spectra in accord with those reported for the dimeric flavonoid, I3-II8-biapigenin (2) (19). Both of these flavonoids have been reported to occur in other species of Hypericum (19, 20).
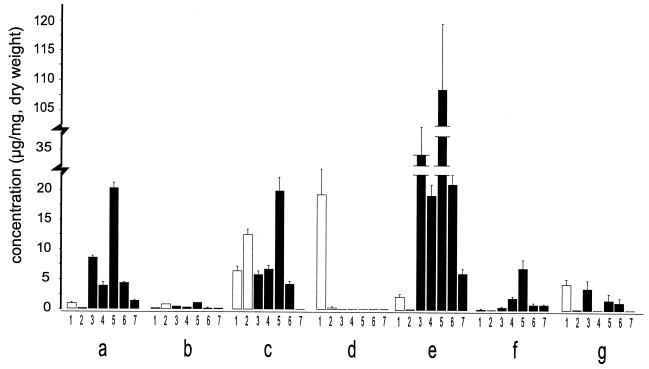
Compounds 1, 2, 3, and 5 were isolated in sufficient quantity and high enough purity to be used as quantification standards. However, noting that compounds 4, 6, and 7 possess the same chromophore as 5 and hence expectedly similar molar UV absorption, it was deemed appropriate to use compound 5 as a standard for the HPLC quantification of compounds 4-7. Calibration curves for compounds 1, 2, 3, and 5, based on UV absorption at 350 nm and corrected for purity (determined by quantitative proton NMR analysis), were constructed and found to be linear throughout the region of interest. On the basis of their spectroscopic data, a determination was made of the absolute amounts of UV pigments 1-7 present in the floral components and leaves (Fig. 3 a–g) of H. calycinum. These values, expressed as concentrations, are shown in Fig. 5. The data confirm what we predicted from the UV imagery of the flower and provide the added information that the DIPs are present at high concentration in the anthers and at very high concentration in the wall (but not the contents) of the ovary. Moreover, they indicate that the UV absorption of the filaments, evidenced by their darkness in the UV image (Fig. 1M), is attributable to the presence of one of the flavonoids only, rather than to the combined presence of flavonoids and DIPs.
Figure 5.
Concentration of flavonoids (open bars) and hypercalins (solid bars) in floral parts and leaves of H. calycinum. Letters designate part in accord to labeling in Fig. 3. Numbers give compounds in accord to designation in text. Values are given as mean + SD. Sample sizes (i.e., number of extracts analyzed) were as follows: a and b (n = 2); d and g (n = 4); c, e, and f (n = 5).
Antifeedant Assay.
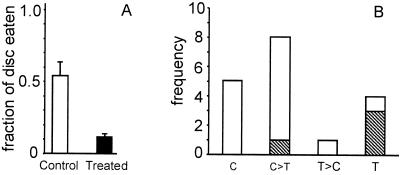
The larvae ate a significantly greater fraction of the control disk than of the experimental disk (Wilcoxon signed rank test, P = 0.0043). On average, the control discs lost about half their mass, whereas the experimental discs lost little more that one-tenth of theirs (Fig. 6A).
Figure 6.
(A) Portion of control and of treated discs consumed by Utetheisa larvae in feeding test with hypercalin A. n = 18 per category. Values are given as mean + SEM. (B) Frequency distribution of choices made by Utetheisa larvae in feeding tests with hypercalin A. C, fed exclusively on control disk; C>T, fed predominantly on control disk; T>C, fed predominantly on treated disk; T, fed exclusively on treated disk. Striped units indicate larvae that died.
A plot of the relative fate of the two discs per trial is presented in Fig. 6B. Although in most tests (n = 13) the larvae fed either exclusively or preferentially on the control disk, they showed the reverse preference in the remaining tests (n = 5). Three of the five larvae in the latter category died before the end of the experiment, presumably as a consequence of hypercalin ingestion. The dose of hypercalin taken in by these larvae, calculated from the amount of disk consumed, was in the range of 1.8–5.4 μg. There was also a fatality among the group of larvae that showed preference for the control disk. The amount of hypercalin ingested by that larva was 1.8 μg.
Discussion
There seems little doubt that the DIPs (3–7) are centrally involved in the demarcation of the floral UV pattern of H. calycinum. In fulfilling that decorative role, they appear to operate in conjunction with the two flavonoids, 1 and 2. The DIPs and the flavonoids both have the capacity to appeal visually to pollinators, given that they both show light absorption in the 340- to 380-nm region of the UV that is discernable to insects (4). The relative ratio of the two types of compounds in the various floral parts is variable. Although the DIPs dominate in the ovarian wall and are present in substantial concentration in the UV-absorbing petal parts and in the anthers, they are virtually absent from the filaments. The filaments make up the only UV-absorbing part in which the absorption is due primarily to one flavonoid.
The finding that DIPs are present at high concentrations in the anthers and the ovarian wall raised the possibility that these compounds served a second function. It made sense to presume that both pollen and developing seeds might be in need of protection, and the finding that hypercalin A was deterrent and toxic to Utetheisa larvae suggested that the second function was defense. The ovarian wall in particular appears to be most heavily protected. The concentration of DIPs in that tissue, amounting in the aggregate to ≈20% of dry weight, is remarkable.
The question arises whether the explanation originally offered (15) for the occurrence of UV patterns on the rear of flowers is correct and, in particular, whether that explanation applies to Hypericum flowers. In our view, the notion that a plant benefits from displaying its buds in a different color from its blossoms need not be discarded. Indeed, with its mature flowers clearly distinguishable, a plant may be of increased “interest” to a pollinator. Yet, it is possible also that by impregnating the outside of the bud (that is, the exposed surfaces of the petals) with DIPs, the plant is protecting the buds, just as it protects its reproductive structures by use of the chemicals. The decorative and protective functions, of course, could both be operating in the bud. In the mature flower, we would argue, the duality of function is almost certainly at play. It is then that the DIPs could be envisioned, on the one hand, to help draw the pollinator, and on the other, to discourage the herbivore.
That hypercalin A seemed to be lethal to a fraction of the larvae in our assay is of interest. It would obviously have been desirable to investigate the toxicity of these compounds in more detail. Had we had enough material to do so, we might have been able to explain why the compounds are present at such high concentration in the ovarian wall of H. calycinum.
The apparent use of DIPs for defense in H. calycinum may not be without parallel. These compounds have been shown to be present in extracts of the female inflorescences of hops (Humulus lupulus) and to be deterrent to both mites (21) and aphids (22). Interestingly, it is these very substances in hops that are known for their bitterness to humans, as well as for their antimicrobial properties and that are used because of these qualities as additives to beer (23).
One wonders whether the DIPs fulfill a defensive role in the leaves as well. If indeed they are able to provide protection at the low concentrations in which they occur in the leaves, one could argue that they evolved first as defensive agents and that they were only secondarily “co-opted” to fulfill their pigmentary role in flowers. In fact, the DIPs in the leaves could do more than protect against herbivores. Given their antibiotic potency (13), the compounds could serve as antimicrobial agents as well. The flavonoids could themselves also play defensive roles. The compounds occur in both flowers and leaves and have been shown in a number of instances to be deterrent to insects (24–27).
Hypercalins and chinesins are distinct chemically from any other floral UV pigments so far characterized. Compounds previously assigned such pigmentary function are all flavonoids and include flavonol and chalcone glycosides (11, 12).
The discovery that an insect antifeedant should be stored at high concentration in the ovary wall of a flower raises the question whether the phenomenon is of more general occurrence. It seems unlikely that such a “logical” way of protecting developing seeds should be of rare occurrence. If the phenomenon exemplified by H. calycinum, as well as possibly hops, should be commonplace, a comparative chemical study of the ovarian wall of plants could be of value. Although the search could, in some cases, lead to no more than the rediscovery of bioactive materials already known from the chemical study of fruits, this need not be the rule. In their early developmental stages, plant ovaries could well have defensive chemical profiles of their own.
Acknowledgments
Some of the Hypericum species were obtained from Cornell Plantations, Ithaca, NY. We are indebted to the staff of the Plantations, particularly Raylene Gardner, for help and numerous courtesies. Donald Rakow, Director of the Plantations, Melody Siegler of Emory University, and Koji Nakanishi of Columbia University provided helpful comments on the manuscript. This study was supported in part by Grants AI02908 and GM53830 from the National Institutes of Health.
Abbreviation
- DIPs
dearomatized isoprenylated phloroglucinols
References
- 1.von Frisch K. The Dance Language and Orientation of Bees. Cambridge, MA: Harvard Univ. Press; 1967. [Google Scholar]
- 2.von Helversen O. J Comp Physiol. 1972;80:439–472. [Google Scholar]
- 3.Menzel R, Blakers M. J Comp Physiol. 1976;108:11–33. [Google Scholar]
- 4.Menzel R, Backhaus W. In: The Perception of Colour. Gouras P, editor. Boca Raton, FL: CRC Press; 1991. pp. 262–293. [Google Scholar]
- 5.Daumer K. Z vergl Physiol. 1958;41:49–110. [Google Scholar]
- 6.Barth F G. Insects and Flowers. Princeton, NJ: Princeton Univ. Press; 1985. [Google Scholar]
- 7.Kevan P G. In: The Pollination of Flowers by Insects. Richards A J, editor. London: Academic; 1978. pp. 51–78. [Google Scholar]
- 8.Kevan P G, Banker H G. Annu Rev Entomol. 1983;28:407–453. [Google Scholar]
- 9.Eisner T, Silberglied R E, Aneshansley D, Carrel J E, Howland H C. Science. 1969;166:1172–1174. doi: 10.1126/science.166.3909.1172. [DOI] [PubMed] [Google Scholar]
- 10.Free J B. Behaviour. 1970;37:269–285. [Google Scholar]
- 11.Thompson W R, Meinwald J, Aneshansley D, Eisner T. Science. 1972;177:528–530. doi: 10.1126/science.177.4048.528. [DOI] [PubMed] [Google Scholar]
- 12.Dement W A, Raven P H. Nature (London) 1974;252:705–706. [Google Scholar]
- 13.Nagai M, Tada M. Chem. Lett. 1987. 1337–1340. [Google Scholar]
- 14.Decosterd L A, Stoeckli-Evans H, Chapuis J C, Sordat B, Hostettmann K. Helv Chim Acta. 1989;72:1833–1845. [Google Scholar]
- 15.Eisner T, Eisner M, Aneshansley D. Proc Natl Acad Sci USA. 1973;70:1002–1004. doi: 10.1073/pnas.70.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisner T, Aneshansley D J, Eisner M. Bioscience. 1988;38:496–498. [Google Scholar]
- 17.Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1995;92:50–55. doi: 10.1073/pnas.92.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber B, Leinhard H, Rast D M. Phytochemistry. 1995;40:433–437. [Google Scholar]
- 19.Hansen S H, Jensen A G, Cornett C, Bjornsdottir I, Taylor S, Wright B, Wilson I D. Anal Chem. 1999;71:5235–5241. [Google Scholar]
- 20.Butterweck V, Juergenliemk G, Nahrstedt A, Winterhoff H. Planta Medica. 2000;66:3–6. doi: 10.1055/s-2000-11119. [DOI] [PubMed] [Google Scholar]
- 21.Jones G, Campbell C A M, Pye B J, Maniar S P, Mudd A. Pesticide Science. 1996;47:165–169. [Google Scholar]
- 22.Powell G, Hardie J, Pickett J A. Entomol Exp Appl. 1997;84:189–193. [Google Scholar]
- 23.Moir M. J Am Soc Brew Chem. 2000;58:131–146. [Google Scholar]
- 24.Echeverri F, Cardona G, Torres F, Pelaez C, Quiñones W, Renteria E. Phytochemistry. 1991;30:153–155. [Google Scholar]
- 25.Russell G B, Bowers W S, Keesing V, Niemeyer H M, Wevenet T, Vasanthaverni S, Wratten S D. J Chem Ecol. 2000;26:41–56. [Google Scholar]
- 26.Mullin C A, Alfatafta A A, Harman J L, Everett S L, Serino A A. J Agric Food Chem. 1991;39:2293–2299. [Google Scholar]
- 27.Simmonds M S J, Blaney W M, Delle-Monache F, Marini-Bettolo G B. J Chem Ecol. 1990;16:365–380. doi: 10.1007/BF01021771. [DOI] [PubMed] [Google Scholar]