Abstract
In this study, chitosan-assembled zinc oxide nanoparticle (CZNP) was successfully prepared for evaluated for its anticancer efficacy against cervical cancer cells. The CZNP particles were nanosized and spherical in shape. The zinc oxide nanoparticle (ZNP) and CZNP showed significant cytotoxicity in cervical cancer cells in a concentration-dependent manner. Results showed that the enhanced cytotoxicity was mainly attributed to the reactive oxygen species (ROS) generation in the cancer cells. The apoptosis assay further revealed that apoptosis was the main reason behind the cell killing effect of the zinc oxide nanoparticles. The apoptosis was further confirmed by the nuclear chromatin assay. Live dead assay showed increased red fluorescent cell for CZNP treated cancer cells. Overall, metal oxide present in nanoparticulate dimensions will be advantageous in imparting the cytotoxicity to cervical cancer cell.
Abbreviations: HPV, Human papillomavirus; CZNP, Chitosan-assembled zinc oxide nanoparticle; ROS, Reactive oxygen species; ZNO, Zinc oxide; ZNP, Zinc oxide nanoparticles
Keywords: Cervical cancer, Zinc oxide, Chitosan, Anticancer effect, Apoptosis
1. Introduction
The infection caused due to human papillomavirus (HPV) is the main reason behind the occurrence of cervical cancers (Lorusso et al., 2014). More than 90% of cervical cancer cases are due to the result of HPV infection and considered to be second common cancer in women. To be specific, HPV16 results in 50% of cervical cancers (Adefuye et al., 2014, Xu et al., 2015). The present treatment options includes chemotherapy, surgery and radiotherapy, however none of the treatment strategies were effective in controlling the cancer proliferation and reoccurrence (Zeng et al., 2013, Lv et al., 2015). Therefore, there is a need to develop alternative cancer killing agent while sparing the normal healthy cells in the body.
In this regard, inorganic chemicals draw the interest of researchers worldwide for their promising therapeutic efficiency in cancer cells (Medina et al., 2007). To be specific, zinc oxide is considered to be fatal to cancer cells, however, physical form and appearance of the metal or inorganic compounds has a great impact on the therapeutic efficacy (Hong et al., 2011). The nanotechnology field allows the fabrication of compounds at nano/microscale which decides the fate of the compounds in the biological system (Nag et al., 2011). Studies have shown that cancer cells are highly susceptible to zinc oxide (ZNO) toxicity in a time and concentration dependent manner (Ostrovsky et al., 2009). The fact that ZNO in the nanoparticulate form is more advantageous compared to that in the free metal forms. The cytotoxic effect of Zn2+ was mainly attributed to the delivery of the metal ion in the intracellular environment and which is possible only by the effective internalization of the metal ions in the cellular compartment (Premanathan et al., 2011, Gupta et al., 2011). Therefore, nanoparticulate zinc (ZNP) could be more beneficial to the cancer cell death. Hence it is conceivable idea that a strategy that can increase the intracellular uptake of zinc ion could be more beneficial (Limbach et al., 2007). The ZNP as such possess negative surface charge and therefore could have limited internalization.
In this perspective, surface modification of ZNP with a naturally derived chitosan is expected to improve the cellular internalization of the metal ions in the cancer cells (Ramasamy et al., 2014a, Ramasamy et al., 2014b, Tran et al., 2014). Chitosan is reported to possess high biodegradability, high biocompatibility, improved stability and low toxicity and low immunogenicity to the cells. The amine group of chitosan will improve the solubility and provide the hemocompatibility in the intracellular environment (Alkhader et al., 2016). In addition, positive surface of nanoparticles will its interaction with many cellular components which is highly anionic in nature. The mucoadhesive feature of chitosan will prolong the longer interaction of encapsulated component with the cells. The presence of chitosan on the particle surface will also help to open the tight intracellular junctions that might further increase the cellular uptake and thereby the therapeutic efficacy (Ramasamy et al., 2014a, Ramasamy et al., 2014b).
Thus far, main aim of present study was to study an alternative therapeutic agent that can improve the therapeutic efficacy in cervical cancer cells. Towards this purpose, we have prepared chitosan-supported zinc oxide nanoparticles (CZNP) and studied its physicochemical and biological effect in HeLa cervical cancer cells. The particle size was measured using dynamic light scattering technique while surface morphology was evaluated using transmission electron microscopy (TEM). The anticancer effect of formulation was studied by means of MTT assay and Annexin-V/PI assay.
2. Materials and methods
2.1. Materials
Zinc acetate dihydrate (Zn[CH3COO]2·2H2O, 99–100%, was purchased from Merck Chemicals, Germany. Tocopherol acetate, Polyvinyl alcohol (PVA) and chitosan (85% deacetylated, low molecular weight) were purchased from Sigma-Aldrich, China. All other chemicals are used any further modifications and present as reagent grade.
2.2. Preparation of chitosan-modified zinc oxide nanoparticles
In the first step, zinc oxide nanoparticle was prepared by adding 3 g of zinc acetate and 1.5 g of tocopherol together and heated at 70 °C until it forms a homogenous solution. The solution was then heated at 120 °C to remove all the organic solvents and forms gel. The gel was then pyrolyzed at 400 °C for 5 h and the final ZNO nanoparticle is ready and zinc acetate salt is calcinated during the pyrolysis process. The chitosan-coated ZNP nanoparticle was prepared by mixing 1 ml of ZNO nanoparticle dispersion into 1 ml of chitosan solution at a weight ratio of 5:1 and incubated for 12 h. The chitosan-coated ZNO nanoparticle was then separated by centrifugation and particles were resuspended (Akhtar et al., 2012).
2.3. Zeta potential and particle size analysis
The particle sizes and zeta potential of the particles was determined using Malvern Zetasizer (Zeta ZS NANO, Malvern Instruments, UK). The formulations were suitably diluted and evaluated by dynamic light scattering (DLS) method. The study was performed in 25 °C and performed in triplicate.
2.4. Particle morphology
The particle morphology was determined using transmission electron microscopy (TEM). The particle dispersion was suitably diluted and counter stained with 2% phosphotungistic acid and placed in copper grid which is coated with carbon. The particles were dried under the infra-red light and morphology was studied using H-7500, Hitachi, Japan.
2.5. Cell culture and cytotoxicity analysis
The human cervical cell carcinoma (HeLa) was cultured in RPMI-1640 medium. The growth medium was supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic mixture. The cells were grown ambient conditions.
The cytotoxic potential of individual formulation was estimated by MTT assay. The cells were seeded in 96-well plate and incubated for 24 h. The cells were then treated with respective formulations (ZNP and CZNP) and incubated for 24 h. The cells were washed two times with PBS and 10 µl of MTT solution was added. The MTT solution was prepared with a final concentration of 5 mg/ml. The cells were then incubated for 3 h and then supernatant was removed. The cells were added with 100 µl of DMSO and incubated for 30 min. The formazan crystal was extracted by this way. The absorbance of the individual well plate was studied at 570 nm by means of microplate reader (ELISASCAN, Transasia Bio-Medicals Ltd, India).
2.6. Apoptosis assay
The HeLa cells were plated in 12-well plate and incubated for 24 h. Next day, cells were exposed with ZNP and SZNP and incubated for 24 h. The cells were extracted next day using trypsinization process. The trypsin is added to each and incubated for 3 min. The trypsin was neutralized by the addition of serum media and centrifuged to collect the cell pellets. The cells were washed two times with PBS. Subsequently, cells were incubated with 2 µl of Annexin-V and 2 µl of PI and kept aside for 15 min. The volume was made up to 1 ml and analyzed using FACS Calibur flow cytometer.
2.7. Hoechst 33342 nuclear assay
The HeLa cells were seeded in 12-well plate at a seeding level of 3 × 105 cells/well. Next day, cells were exposed with ZNP and SZNP and incubated for 24 h. Next day, cells were washed with PBS and fixed with 4% paraformaldehyde (PFA). The cells were then stained with Hoechst 33342 (10 µG/ML) for 15 min. The cells were again washed three times to remove all the adhering dye. The cells were cell with nuclear condensation was then observed under fluorescence microscope.
2.8. Live/dead assay
The HeLa cells were seeded in 12-well plate at a seeding level of 3 × 105 cells/well. Next day, cells were exposed with ZNP and SZNP and incubated for 24 h. After 24 h incubations, cells were washed and treated with 1 µg of Calcein AM and Ethidium homodimer-1 as a respective live and dead cell staining agent for 20 min. The cells were washed and mounted on a glass slide and the borders are sealed. The cells are then observed under fluorescence microscope.
2.9. Statistical analysis
All statistical analyses were performed using One-way ANOVA followed by Dunnet’s or Bonferronni’s post hoc tests for statistical significance. The results are expressed as the mean ± standard deviation and a statistical difference of p < .05 was considered significant.
3. Results
3.1. Particle size and zeta potential characterization
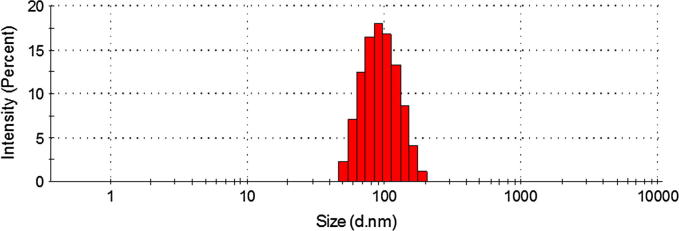
The particle size characterization of ZNP and CZNP is important from systemic administration perspective. It is generally considered that nanosized particles are more efficacious compared to that of microsized particles. In this regard, average particle size of ZNP was ∼30 nm with narrow dispersity index. After chitosan coating the particle size increased to ∼100 nm indicating the presence of polymer structure on the ZNP particles (Fig. 2). The increase in particle size also accompanied with zeta potential change. The zeta potential of ZNP was −12.5 ± 1.2 mV while it converted to +21.5 ± 1.35 mV after chitosan coating. Overall, nanosized particles of ∼100 nm size will be beneficial for cellular internalization and anticancer efficacy as reported in numerous literature.
Fig. 2.
Particle size distribution of CZNP using dynamic light scattering (DLS) technique.
3.2. Particle morphology
The particle morphology was evaluated using TEM. The particles were perfectly spherical in nature and uniformly spread on the copper grid. It can be seen that a core-shell type of structure was not visible however a definitive coating of polymers on the ZNP particle could be seen (Fig. 3). It must be mentioned that the particle size observed from TEM was smaller compared to that of DLS. The obvious difference in particle size was attributed to the fact that DLS measures the hydrodynamic state while TEM measures the dried state.
Fig. 3.
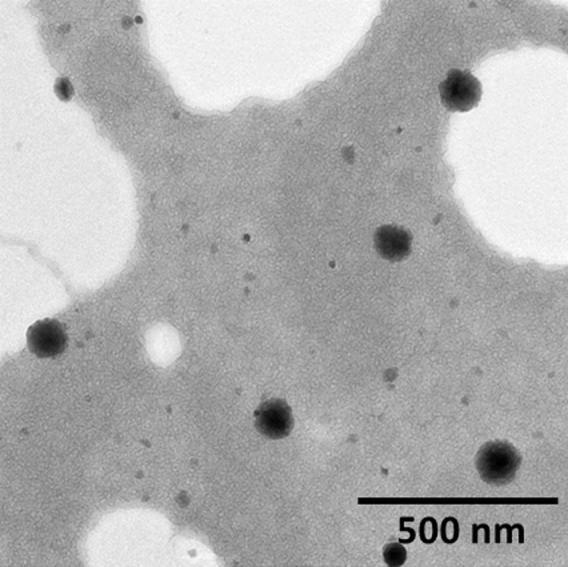
Transmission electron microscope (TEM) image of CZNP.
3.3. Cytotoxic effect of nanoparticles
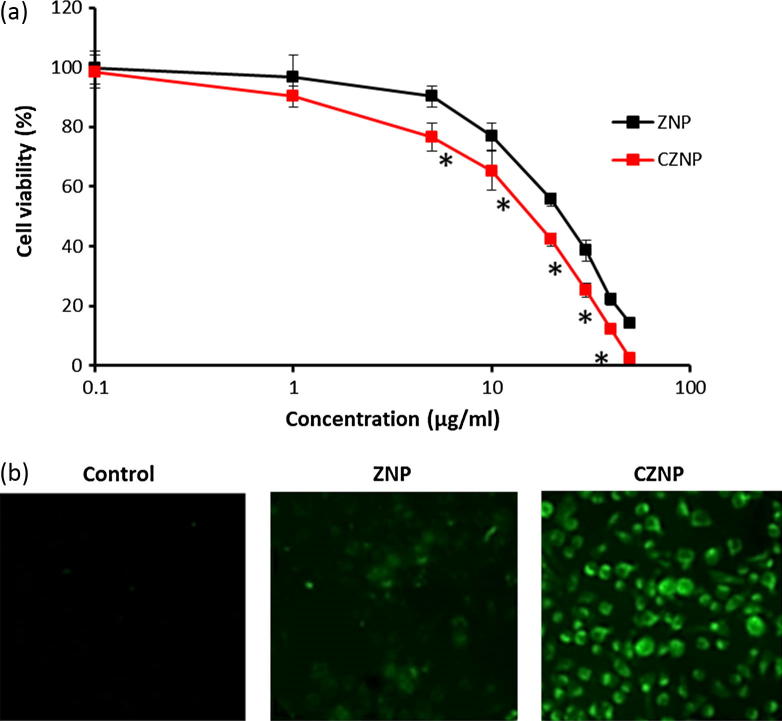
The cytotoxic effect of ZNP and CZNP was evaluated by means of MTT assay. The results clearly showed the typical concentration-dependent cytotoxic effect of ZNP and CZNP particles on the cervical cancer cells (Fig. 4a). It should be noted that CZNP showed significantly higher toxicity compared to that of ZNP in cervical cancer cells. The result is consistent with the DCF fluorescence intensity of the present study (Fig. 4b). The CZNP result in maximum green fluorescence compared to any other group indicating the high ROS generation capacity of CZNP.
Fig. 4.
(a) Cytotoxicity potential of ZNP and CZNP in HeLa cancer cells using MTT assay (b) reactive oxygen species (ROS) generation capacity of individual nanoparticles by fluorescence method.
3.4. Apoptosis assay
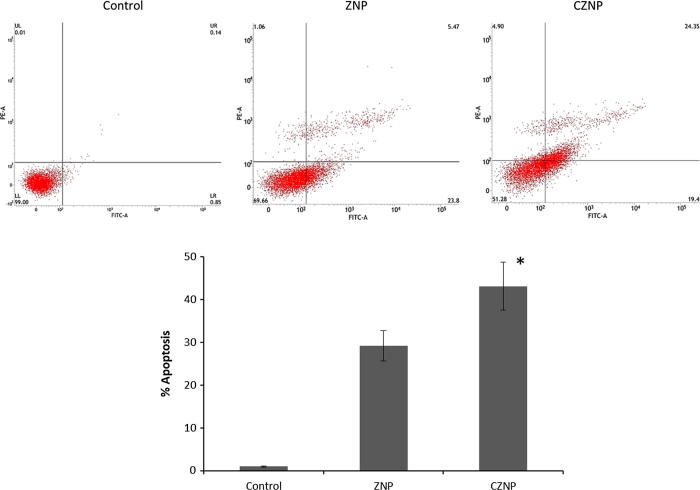
It has been reported that nanoparticles could efficiently internalize the cancer cells and induce cell death via apoptosis and necrosis mechanisms (Rana, 2008). Induction of apoptosis is considered to be main mechanism behind cell death (AshaRani et al., 2009). In order to verify whether or not cell death caused by ZNO was due to apoptosis, we have performed annexin-V/PI based apoptosis assay (Fig. 5). Results revealed that ZNP and CZNP induced a remarkable apoptosis of cancer cells. To be specific, CZNP induced a significantly greater apoptosis of cancer cells compared to that of ZNP in cervical cancer cells indicating the superior anticancer potential of chitosan-coated ZNP.
Fig. 5.
Annexin-V/PI based apoptosis assay on HeLa cervical cancer cells. The apoptosis assay was carried out by flow cytometer.
3.5. Hoechst 22242-based nuclear assay
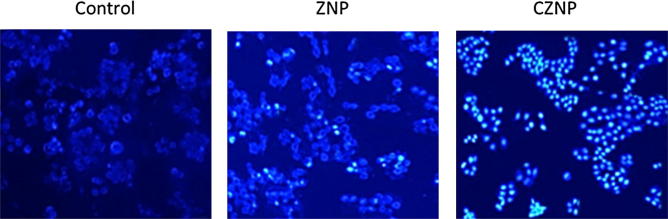
The apoptosis was further confirmed by Hoechst 33342 nuclear staining assay. The apoptosis is generally associated with biochemical and physical changes in the cell nucleus and cell cytoplasm (Fig. 6). Results showed that CZNP and ZNP treated cells were round and showed remarkable fluorescence which is indicative of the cell apoptosis.
Fig. 6.
Hoechst 33342 based nuclear staining assay. The cell morphology was studied by means of fluorescence microscope.
3.6. Live dead assay
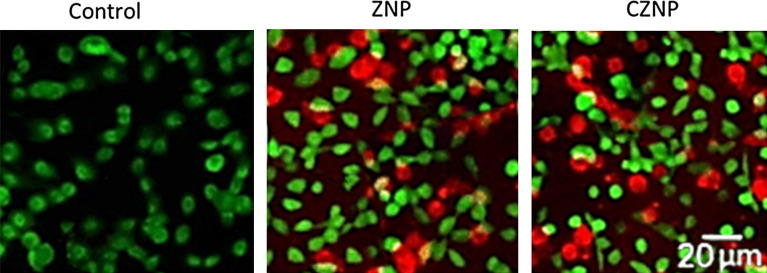
The cell killing of the ZNP and CZNP was further confirmed by live/dead assay. The cells were treated with respective formulations and studied the live and dead cells after the staining with live/dead staining agents (calcein AM and ethidium bromide) (Fig. 7). As seen, control cells were largely whereas ZNP and CZNP treated cells showed remarkable red fluorescence indicating the presence of dead cells. CZNP treated cells showed large number of dead cells indicating its high anticancer potential.
Fig. 7.
Live dead assay of HeLa cells after treatment with ZNP and CZNP. The assay was performed by staining with live dead staining agents. Scale bar: 20 µm.
4. Discussion
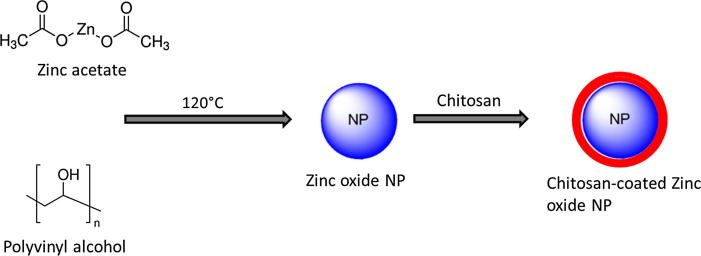
Inorganic chemicals draw the interest of researchers worldwide for their promising therapeutic efficiency in cancer cells. Studies have shown that cancer cells are highly susceptible to zinc oxide (ZNO) toxicity in a time and concentration dependent manner. The fact that ZNO in the nanoparticulate form is more advantageous compared to that in the free metal forms. Several reports suggest the anticancer effect of ZNO NP in different types of cancer cells including breast, colon, bone and so on. Thus far, main aim of present study was to study an alternative therapeutic agent that can improve the therapeutic efficacy in cervical cancer cells. Towards this purpose, we have prepared chitosan-supported zinc oxide nanoparticles (CZNP) and studied its physicochemical and biological effect in HeLa cervical cancer cells (Fig. 1).
Fig. 1.
Schematic presentation of preparation chitosan-coated zinc oxide nanoparticles.
The enhanced cytotoxic effect of CZNP was mainly attributed to the enhanced cellular internalization of CZNP in the cancer cells. The chitosan present on the surface of the nanoparticle might increase the interaction of particles to the negatively charged cancer cells and thereby increased the concentration of particles in the intracellular environment. The increased concentration of Zn2+ might contributed to the oxidative stress and thereby increased the reactive oxygen species (ROS) level and induced the cytotoxic effect. Several other reports also suggest that ZNO is involved in the ROS generation. Moreover, several researchers observed increased lipid peroxides in the cancer cells which give rise to more free radicals and damage the biomolecules such as DNA and other biostructures in the cell. The result is consistent with the DCF fluorescence intensity of the present study. The intracellular ROS generation is considered to be main mechanism behind the cell killing effect. It is believed that ROS generation when comes beyond capacity of the cell results in oxidative damage and cell killing effects. The increased ROS results in the up-regulation of p-53 mediated signaling pathways to DNA damage and regulate the cell proliferation. Overall, CZNP exhibited a superior toxicity in cervical cancer cells (Guo et al., 2008). To be specific, CZNP induced a significantly greater apoptosis of cancer cells compared to that of ZNP in cervical cancer cells indicating the superior anticancer potential of chitosan-coated ZNP.
Hoechst staining showed that CZNP and ZNP treated cells were round and showed remarkable fluorescence which is indicative of the cell apoptosis. In the early stage of apoptosis, cells round up and lose contacts with the neighbouring cells and started shrinking. Followed by, endoplasmic reticulum dilates and forms the vesicles. Similarly in the nucleus, chromatin condenses to form a compact masses and nuclear condensation will happen (Lanone et al., 2009). In the present study, morphological difference in the cell morphology is the indicative of the significant toxic effect of the formulations.
Overall, chitosan-assembled zinc oxide nanoparticle was successfully prepared for evaluated for its anticancer efficacy against cervical cancer cells. Results showed that the enhanced cytotoxicity was mainly attributed to the reactive oxygen species (ROS) generation in the cancer cells. The apoptosis assay further revealed that apoptosis was the main reason behind the cell killing effect of the zinc oxide nanoparticles. Live dead assay showed increased red fluorescent cell for CZNP treated cancer cells. Overall, metal oxide present in nanoparticulate dimensions will be advantageous in imparting the cytotoxicity to cervical cancer cell. Based on the success proof-of-concept, detailed study on animal models will be the subject of our futuristic studies.
Competing interest
The authors report no declarations of interest.
Acknowledgement
This study was supported from the funding grant of Zhengzhou University People's Hospital & Henan Province People's Hospital, China.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akhtar M.J., Ahamed M., Kumar S., Majeed Khan M.A., Ahmad J., Alrokayan S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012;7:845–857. doi: 10.2147/IJN.S29129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AshaRani P.V., Low K.M.G., Hande M.P., Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- Adefuye A., Katz A.A., Sales K.J. The regulation of inflammatory pathways and infectious disease of the cervix by seminar fluid. Patholog. Res. Int. 2014;2014:748740. doi: 10.1155/2014/748740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhader E., Billa N., Roberts C.J. Mucoadhesive chitosan-pectinate nanoparticles for the delivery of curcumin to the colon. AAPS PharmSciTech. 2016;2016 doi: 10.1208/s12249-016-0623-y. [DOI] [PubMed] [Google Scholar]
- Hong H., Shi J., Yang Y., Zhang Y., Engle J.W., Nickles R.J., Wang X., Cai W. Cancer-targeted optical imaging with fluorescent zinc oxide nanowires. Nano Lett. 2011;11:3744–3750. doi: 10.1021/nl201782m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N.K., Sharma V., Dixit V.K. Development and characterization of chitosan coated poly-(ε-caprolacone) nanoparticulate system for effective immunization against influenza. Vaccine. 2011;29:9026–9037. doi: 10.1016/j.vaccine.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Guo D., Wu C., Jiang H., Li Q., Wang X., Chen B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. J. Photochem. Photobiol. B. 2008;93:119–126. doi: 10.1016/j.jphotobiol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Lanone S., Rogerieux F., Geys J., Dupont A., Maillot-Marechal E., Boczkowski J., Lacroix G., Hoet P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part Fibre Toxicol. 2009;6:14. doi: 10.1186/1743-8977-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbach L.K., Wick P., Manser P., Grass R.N., Bruinink A., Stark W.J. Exposure of engineered nanoparticles to human lung epithelial cells: influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 2007;41:4158–4163. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- Lorusso D., Petrelli F., Coinu A., Raspagliesi F., Barni S. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol. Oncol. 2014;133:117–123. doi: 10.1016/j.ygyno.2014.01.042. [DOI] [PubMed] [Google Scholar]
- Lv L., Guo Y., Shen Y. Intracellularly degradable, self-assembled amphiphilic block copolycurcumin nanoparticles for efficient in vivo cancer chemotherapy. Adv. Healthcare Mater. 2015;4:1496–1501. doi: 10.1002/adhm.201500075. [DOI] [PubMed] [Google Scholar]
- Medina C., Santos-Martinez M.J., Radomski A., Corrigan O.I., Radomski M.W. Nanoparticles: pharmacological and toxicological significance. Br. J. Pharmacol. 2007;150:552–558. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag N., Bhattacharya A., Gupta R.K., Manivannan K., Kahol P.K., Wanekaya A.K., Delong R.K., Ghosh K. Glucose stabilized ZnO nanoparticles for biomedical applications. J. Nanosci. Lett. 2011;1:172–178. [Google Scholar]
- Ostrovsky S., Kazimirsky G., Gedanken A., Brodie C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009;2:882–890. [Google Scholar]
- Premanathan M., Karthikeyan K., Jeyasubramanian K., Manivannan G. Selective toxicity of ZnO nanoparticles toward Gram positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotech. Biol. Med. 2011;7:184–192. doi: 10.1016/j.nano.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Ramasamy T., Tran T.H., Cho H.J. Chitosan-based polyelectrolyte complexes as potential nanoparticulate carriers: physicochemical and biological characterization. Pharm. Res. 2014;31:1302–1314. doi: 10.1007/s11095-013-1251-9. [DOI] [PubMed] [Google Scholar]
- Ramasamy T., Haidar Z.S., Tran T.H. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 2014;10:5116–5127. doi: 10.1016/j.actbio.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Rana S.V. Metals and apoptosis: recent developments. J. Trace Elem. Med. Biol. 2008;22:262–284. doi: 10.1016/j.jtemb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Tran T.H., Choi J.Y., Ramasamy T. Hyaluronic acid-coated solid lipid nanoparticles for targeted delivery of vorinostat to CD44 overexpressing cancer cells. Carbohyd. Polym. 2014;114:407–415. doi: 10.1016/j.carbpol.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Xu L., Liu J.H., Zhang J., Zhang N., Wang Z.H. Blockade of autophagy aggravates endoplasmic reticulum stress and improves paclitaxel cytotoxicity in human cervical cancer cells. Cancer Res. Treat. 2015;47:313–321. doi: 10.4143/crt.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Tao W., Mei L., Huang L., Tan C., Feng S.S. Cholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancer. Biomaterials. 2013;34:6058–6067. doi: 10.1016/j.biomaterials.2013.04.052. [DOI] [PubMed] [Google Scholar]