Abstract
Introduction
There are currently no reports available from a Polish clinical practice on heterozygous familial hypercholesterolemia (HeFH) management. The aim of this study was to test the efficacy of HeFH hypolipidemic treatment in a Polish outpatient metabolic clinic according to treatment targets outlined in the European Atherosclerosis Society (EAS) and European Society of Cardiology (ESC) guidelines.
Material and methods
This retrospective, observational study was performed on HeFH patients who attended their routine follow-up visits in the metabolic outpatient clinic in the period between April and September 2016. According to EAS/ESC guidelines, the goal and intensity of therapy were assigned individually for every patient based on cardiovascular (CV) risk (high or very high). The treatment target was achievement of low-density lipoprotein cholesterol (LDL-C) levels < 1.8 mmol/l for very high CV risk patients and < 2.6 mmol/l for high CV risk patients. A ≥ 50% decrease in LDL-C over the observation period was an additional outcome measure.
Results
In the overall group of 222 HeFH patients (mean age: 55.2 ±16.2 years, 72% women), LDL-C levels decreased on average by 52.6% (p < 0.001). More than half of the patients were treated with the maximum tolerated dose of statins. A total of 25.2% of patients attained target levels of LDL-C and 55.9% attained a ≥ 50% reduction in its concentration. Despite therapy, significantly elevated post-follow-up levels of LDL-C (> 4.1 mmol/l) remained in 14% of all patients.
Conclusions
Hypolipidemic therapy according to EAS/ESC guidelines was suboptimal for a significant number of HeFH patients. Additional clinical management should be considered.
Keywords: familial hypercholesterolemia, outpatient clinic, lipid-lowering therapy, efficacy,
Introduction
Heterozygous familial hypercholesterolemia (HeFH) is the most frequent genetic disorder. According to a meta-analysis by Pajak et al., HeFH affects 1 out of every 250 people in Poland [1]. This autosomal dominant genetic condition is characterized by congenitally elevated plasma levels of low-density lipoprotein cholesterol (LDL-C) [2, 3]. Familial hypercholesterolemia (FH) is caused by mutations in genes encoding the LDL receptor (LDLR) or, less frequently, apolipoprotein B (ApoB) and proprotein convertase subtilisin/kexin type 9 (PCSK9) [4, 5]. The HeFH is strongly associated with the development of premature atherosclerotic cardiovascular diseases [6]. Untreated patients with HeFH, in comparison to the general population, are known to be 4 times more likely to die from coronary artery disease [7].
In line with the European Atherosclerosis Society (EAS) and European Society of Cardiology (ESC) guidelines [8], as well as Polish guidelines [9] and statements [10, 11], HeFH is diagnosed according to Dutch Lipid Clinic Network (DLCN) criteria evaluating LDL-C plasma levels in patients and their first degree relatives, tendon xanthomas, as well as personal and family history of premature coronary heart disease and/or presence of causative genetic mutations which, although supportive of the diagnosis, are not obligatory to recognize FH. The diagnosis can be established as definite, probable, possible, or unlikely on the basis of points assigned to clinical features listed in the DLCN Scoring System.
Detection of HeFH is sufficient to assign the patient to a high cardiovascular (CV) risk group. When the HeFH diagnosis is accompanied by the presence of cardiovascular disease (CVD), the patient is assigned to the very high CV risk group. Patients from both groups need to be intensively treated with high doses of statins, often accompanied by ezetimibe [8]. According to many experts, HeFH is currently underdiagnosed and undertreated [12, 13]. Using currently available treatments, the recommended targets for LDL-C concentration are difficult to achieve in the majority of patients. This calls for the consideration of a new therapeutic approach.
Although we presently know the frequency of HeFH in the Polish adult population, there is still a lack of data on the clinical practice of FH management [1, 5].
In an effort to address this gap in knowledge, we present a retrospective observational study on patients with FH treated in a specialized outpatient metabolic clinic, including data on therapy regimen and treatment goal attainment according to available guidelines.
The aim of this study was to determine the proportion of patients who attained the therapeutic goals for hypercholesterolemia defined in the 2016 ESC/EAS guidelines [8]:
LDL-C levels of < 1.8 mmol/l (70 mg/dl) and < 2.6 mmol/l (100 mg/dl) for very high and high CV risk subpopulations, respectively.
At least 50% reduction in baseline LDL-C concentration.
Material and methods
Patients and study design
The retrospective, observational study on the efficacy of HeFH treatment was performed in a public outpatient metabolic clinic in Warsaw and included patients who attended their routine follow-up visits in the period between April and September 2016 and had HeFH diagnosed based on DLCN score assessment. Only patients with either probable (DLCN score of 6–8 points) or definite (DLCN score > 8 points) HeFH were included in the study. The medical examination and outcome measurements analyzed in this study were performed at the time of inclusion (patient registration in the clinic) and on the last follow-up visit that took place in the previously mentioned period between April and September 2016. Because of the non-interventional nature of the study, the decision to use a particular type of hypolipidemic therapy regimen was made by the physicians. Efficacy of therapy was compared to the requirements from the 2016 ESC/EAS guidelines [8]. Patient medical and personal information was collected and used according to local regulations.
Statistical analysis
Descriptive statistics for continuous variables are presented as the median with first and third quartile or as the mean and standard deviation, depending on normality of the distribution. Categorical variables are presented as frequency and percentages. Significance of changes of LDL levels over the entire observation period was assessed using the paired Wilcoxon test. Significance of differences in categorical data was tested using the χ2 or Fisher’s exact test. All computations were done using R 3.3.1 statistical software.
For the subpopulation analyses, data were divided into the following sets:
Patients assigned to the very high CV risk subpopulation (patients with diagnosis of HeFH accompanied with CVD and/or diabetes). The goal target concentration of LDL-C for this subpopulation was < 1.8 mmol/l.
Patients assigned to the high CV risk subpopulation (patients with only the diagnosis of HeFH). The goal target concentration of LDL-C for this subpopulation was < 2.6 mmol/l.
Results
General characteristics
A total of 222 adult patients (mean age: 55.2 ±16.2 years, 72% women) treated in different years from 1993 to September 2016 were included in the study. A total of 73 patients included in the study were diagnosed with probable HeFH and 149 patients were diagnosed with definite HeFH. Known FH genetic mutations were found in 41.9% of the overall study population (Table I).
Table I.
Patient demographic and clinical characteristics in the overall population and gender subgroups
| Parameter | Overall | Women | Men |
|---|---|---|---|
| N (%) | 222 | 159 | 63 |
| Familial hypercholesterolemia diagnosis: | |||
| Points on Dutch Lipid Clinic scale: | |||
| 6–8 | 73 (32.9) | 51 (32.1) | 22 (34.9) |
| > 8 | 149 (67.1) | 108 (67.9) | 41 (65.1) |
| Genetic diagnosis | 93 (41.9) | 69 (43.4) | 24 (38.1) |
| Age at diagnosis: | |||
| Age at diagnosis [years] (mean (SD)) | 47.5 (15.8) | 50.5 (15.9) | 39.8 (12.7) |
| Age groups at diagnosis: | |||
| ≤ 30 | 40 (18.0) | 24 (15.1) | 16 (25.4) |
| 31–40 | 31 (14.0) | 16 (10.1) | 15 (23.8) |
| 41–50 | 34 (15.3) | 19 (11.9) | 15 (23.8) |
| 51–60 | 67 (30.2) | 52 (32.7) | 15 (23.8) |
| > 60 | 50 (22.5) | 48 (30.2) | 2 (3.2) |
| Age at last follow-up visit: | |||
| Age at last follow-up visit [years] (mean (SD)) | 55.2 (16.2) | 57.9 (16.4) | 48.2 (13.2) |
| Age groups at last follow-up visit: | |||
| ≤ 30 | 23 (10.4) | 17 (10.7) | 6 (9.5) |
| 31–40 | 26 (11.7) | 13 (8.2) | 13 (20.6) |
| 41–50 | 27 (12.2) | 12 (7.5) | 15 (23.8) |
| 51–60 | 46 (20.7) | 29 (18.2) | 17 (27.0) |
| > 60 | 100 (45.0) | 88 (55.3) | 12 (19.0) |
| Other risk factors and comorbidities: | |||
| BMI* [kg/m2]: | |||
| ≤ 18.5 | 1 (0.5) | 1 (0.6) | 0 (0.0) |
| 18.6–24.9 | 98 (44.3) | 81 (50.9) | 17 (27.4) |
| 25.0–29.9 | 82 (37.1) | 49 (30.8) | 33 (53.2) |
| ≥ 30.0 | 40 (18.1) | 28 (17.6) | 12 (19.4) |
| Smoking | 23 (10.4) | 13 (8.2) | 10 (15.9) |
| Diabetes | 22 (9.9) | 13 (8.2) | 9 (14.3) |
| Hypertension | 94 (42.3) | 71 (44.7) | 23 (36.5) |
| At least 1 CVD [MI/stroke/CABG/PTCA] | 32 (14.4) | 21 (32.2) | 11 (17.5) |
| MI | 13 (5.9) | 7 (4.4) | 6 (9.5) |
| Stroke | 7 (3.2) | 4 (2.5) | 3 (4.8) |
| CABG | 7 (3.2) | 5 (3.1) | 2 (3.2) |
| PCI | 22 (9.9) | 14 (8.8) | 8 (12.7) |
| CV risk group: | |||
| Very high | 45 (20.3) | 28 (17.6) | 17 (27.0) |
| High | 177 (79.7) | 131 (82.4) | 46 (73.0) |
| Observation period: | |||
| Duration of observation [years] (mean (SD)) | 7.70 (5.48) | 7.41 (5.32) | 8.43 (5.82) |
BMI – body mass index, CABG – coronary artery bypass graft, CV – cardiovascular, CVD – cardiovascular disease, MI – myocardial infarction, PCI – percutaneous coronary intervention, SD – standard deviation.
Among men, there is 1 missing BMI value.
The mean age at diagnosis (at registration in the clinic) of the overall patient population was 47.5 ±15.8 years. Patients aged > 50 years at diagnosis were overrepresented in the study population. In the subpopulation of women, the oldest patients aged > 60 years at diagnosis were more abundant in comparison to the subpopulation of men (Table I).
More than half of the patients were obese or overweight. The prevalence of obesity was similar among genders, but in the group of men, being overweight was significantly more prevalent. A total of 23 patients (10.4%) were active smokers (Table I).
On the basis of concomitant CVD and/or diabetes, 45 (20.3%) patients were assigned to the very high CV risk subgroup. Based on a diagnosis of HeFH without concomitant CVD and/or diabetes, 177 (79.7%) patients were assigned to the high CV risk subpopulation (Table I).
The observation period was equal to the duration of treatment for each individual patient. Due to the limited number of patients and the nature of clinical practice, we were unable to include only patients with equal observation periods in this study. Therefore, the average observation period was 7.7 ±5.48 years (Table I). Patients treated for a short period, defined as > 1 year and 1–2 years, constituted 4.1% and 9.5% of the overall group, respectively. Patients with longer treatment periods of 2–5 years, 5–10 years, and 10–23 years, were more abundant and constituted 30.2%, 20.2%, and 33.8% of the overall group, respectively (data not shown).
Pharmacotherapy
A total of 204 (91.9%) patients were treated with statins. The subpopulations of patients treated with statin monotherapy and combination therapy were of similar size (Table II). There were 12 patients treated with ezetimibe or fenofibrate alone and 6 patients who were not treated with any hypolipidemic drugs.
Table II.
Patients treated with high, moderate or low doses of statins either used in monotherapy or in combination with ezetimibe or fenofibrate
| Parameter | Statin therapy overall | Statin monotherapy | Statin with ezetimibe | Statin with fenofibrate | P-value | |
|---|---|---|---|---|---|---|
| Number of patients | N (%) | 204 | 91 (44.6) | 99 (48.5) | 14 (6.9) | |
| Number of patients on different doses of statin N (%) | High | 116 (56.9) | 39 (42.9) | 71 (71.7) | 6 (42.9) | < 0.001 |
| Moderate | 81 (39.7) | 48 (52.7) | 27 (27.3) | 6 (42.9) | ||
| Low | 7 (3.4) | 4 (4.4) | 1 (1.0) | 2 (14.3) |
Doses of statins were applied and assigned as high, moderate, or low based on the guidelines by the Blood Cholesterol Expert Panel from the American College of Cardiology (ACC) and the American Heart Association (AHA) [14].
High, moderate, and low doses of statins, as defined by the Blood Cholesterol Expert Panel from the American College of Cardiology (ACC) and the American Heart Association (AHA), were used for the treatment of 116 (56.9%) patients, 81 (39.7%) patients, and 7 (3.4%) patients of 204 patients receiving statin therapy (either in monotherapy or in combination), respectively [14]. Intensive high-dose therapy was used for the treatment of 39 out of 91 patients in the statin monotherapy subpopulation and 71 out of 99 patients treated with statins and ezetimibe. Patients treated with the combination of statins and ezetimibe received high doses of statins more frequently than those on statin monotherapy or those treated with combination therapy of statin and fenofibrate. Only 6 patients were treated with a high dose of statins in combination with fenofibrate (Table II).
A total of 33 patients were treated with high doses of atorvastatin while 30 patients were treated with moderate doses; 34 of those patients received atorvastatin in combination with ezetimibe (28 patients) or fenofibrate (6 patients). Rosuvastatin was taken by 124 patients, of whom 80 and 44 used high and moderate doses, respectively; 74 of those patients took rosuvastatin in combination with ezetimibe (68 patients) or fenofibrate (6 patients). Significantly more patients on high doses of statins were treated with rosuvastatin than with other statins (Table III).
Table III.
Patients on high, moderate, or low doses of different statins either used as monotherapy or in combination with ezetimibe or fenofibrate
| Parameter | Atorvastatin | Fluvastatin | Rosuvastatin | Simvastatin | P-value | |
|---|---|---|---|---|---|---|
| Doses of statins | High | 33 (52.4) | 0 (0.0) | 80 (64.5) | 3 (18.8) | < 0.001 |
| Moderate | 30 (47.6) | 0 (0.0) | 44 (35.5) | 7 (43.8) | ||
| Low | 0 (0.0) | 1 (100.0) | 0 (0.0) | 6 (37.5) | ||
| Statin monotherapy | 29 (46.0) | 1 (100.0) | 50 (40.3) | 11 (68.8) | 0.118 | |
| Combination therapy | Statin + ezetimibe | 28 (44.4) | 0 (0.0) | 68 (54.8) | 3 (18.8) | |
| Statin + fenofibrate | 6 (9.5) | 0 (0.0) | 6 (4.8) | 2 (12.5) |
Changes in lipid levels
Throughout the observation period, the average concentrations of LDL-C, total cholesterol (TC), and triglyceride (TG) in the overall study population decreased significantly by 52.6%, 40.5%, and 17.95%, respectively (p < 0.001) (Table IV). Conversely, hypolipidemic therapy had no effect on the serum concentration of high-density lipoprotein cholesterol (HDL-C). Except for a significantly greater reduction in TC and TG in the subpopulation of men vs. women (–44.8% vs. –39.2% (TC) and –28.6% vs. –14.3% (TG), respectively; p < 0.05; Table IV), no gender-specific differences in the hypolipidemic profile were found.
Table IV.
Serum concentration of lipids – LDL-C, TC, TG, and HDL-C – at the inclusion visit and last follow-up visit in the overall population and gender subgroups, as well as the percentage reduction in serum levels throughout the observation period
| Parameter | Overall study population (N = 222) | Women (n = 159) | Men (n = 63) |
|---|---|---|---|
| TC [mmol/l] Median (interquartile range; Q1–Q3) | |||
| Inclusion visit | 8.63 (7.78–9.46) | 8.50 (7.78–9.38) | 8.81 (7.75–9.65) |
| Last follow-up visit | 5.13 (4.55–5.85) | 5.19 (4.74–5.87) | 4.81 (4.22–5.56) |
| % | –40.50 (–49.38 – –31.05)* | –39.20 (–47.25 – –29.40)^ | –44.80 (–52.35 – –36.15)^ |
| LDL-C [mmol/l] Median (interquartile range; Q1–Q3) | |||
| Inclusion visit | 6.20 (5.43–7.24) | 6.05 (5.44–6.99) | 6.54 (5.39–7.53) |
| Last follow-up visit | 2.92 (2.42–3.59) | 2.92 (2.47–3.51) | 2.92 (2.36–3.86) |
| % | –52.60 (–63.68 – –40.15)* | –51.20 (–63.50 – –39.85) | –58.50 (–65.30 – –42.30) |
| HDL-C [mmol/l] Median (interquartile range; Q1–Q3) | |||
| Inclusion visit | 1.47 (1.24–18.1) | 1.60 (1.37–1.91) | 1.27 (1.09–1.42) |
| Last follow-up visit | 1.49 (1.29–1.76) | 1.60 (1.33–1.89) | 1.32 (1.07–1.43) |
| % | –1.00 (–12.28 – 12.38) | –1.60 (–12.25 – 11.70) | 2.20 (–11.35 – 13.25) |
| TG [mmol/l] Median (interquartile range; Q1–Q3) | |||
| Inclusion visit | 1.38 (1.01–2.10) | 1.28 (0.98–2.00) | 1.62 (1.13–2.32) |
| Last follow-up visit | 1.21 (0.86–1.64) | 1.21 (0.85–1.62) | 1.15 (0.92–1.64) |
| % | –17.95 (–38.18 – 9.97)* | –14.30 (–31.45 – 14.85)^ | –28.60 (–45.30 – 4.75)^ |
LDL-C – low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, TC – total cholesterol, TG – triglyceride.
Percentage reduction of concentration through the entire observation period; Statistical significance set at p < 0.001.
Difference in percentage reduction through the entire observation period between gender subpopulations. Statistical significance set at p < 0.05.
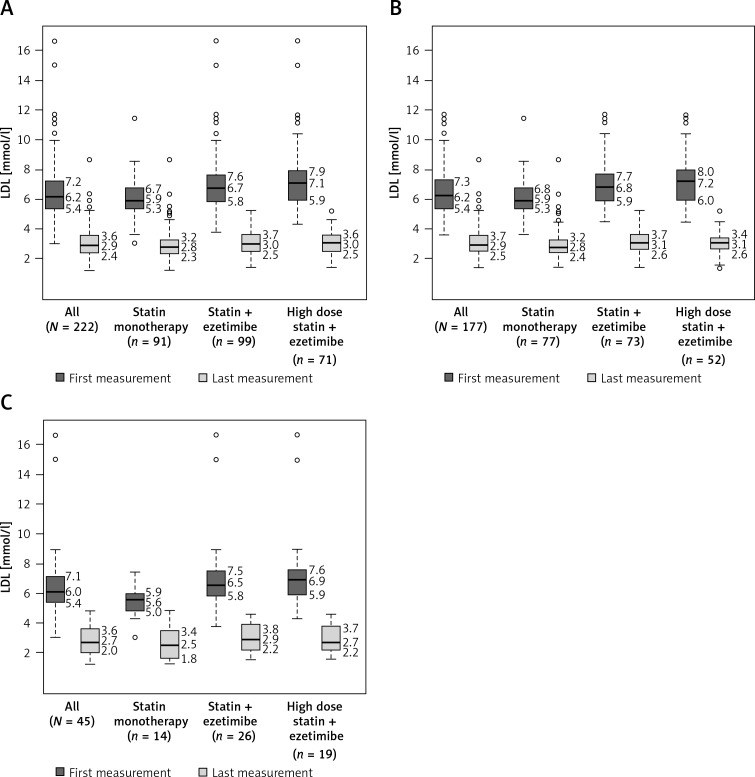
The distribution of LDL-C levels in the overall population and different CV risk patients treated with either statin monotherapy or combination therapy with ezetimibe at the registration visit and at the last follow-up visit is shown in Figure 1.
Figure 1.
Distribution of initial and post-follow-up serum LDL-C molar concentration levels in patients treated with different statin regimens. A – Overall population. B – High cardiovascular risk subpopulation. C – Very high cardiovascular risk subpopulation. Median values (50th percentile) are shown as bands inside each box. The box top–bottom values are defined by the 25th (Q1) and 75th (Q3) percentile. The ends of the whiskers represent the values less than or greater than the median by the value of 3 times the difference between the median and corresponding quartile (Q1 or Q3). Outliers are defined as numbers less than or greater than the median by more than 3 times the difference between the median and corresponding quartile (Q1 or Q3). They are shown as transparent circles
Attainment of therapeutic treatment goals
In the overall study population, irrespective of therapy regimen, the target LDL-C concentration was achieved in 56 (25.2%) patients, including 6 (13.3%) patients in the very high CV risk group and 50 (28.2%) patients in the high CV risk group (data not shown). Attainment of this therapeutic target among subpopulations of patients treated with either statin monotherapy or with statin and ezetimibe was less frequent in patients with very high CV risk as compared to patients with high CV risk (Table V).
Table V.
Attainment of LDL-C therapeutic goals depending on the CV risk and ≥ 50% reduction in LDL-C concentration among subpopulations treated with statin monotherapy or in combination with ezetimibe according to statin dose
| Type of therapy regimen | Dose of statins | Attainment of LDL-C < 1.8 mmol/l N (%)* | Attainment of LDL-C < 2.6 mmol/l N (%)* | Attainment of therapeutic goal: ≥ 50% reduction in LDL-C N (%)* | |
|---|---|---|---|---|---|
| Very high CV risk | High CV risk | Very high CV risk | High CV risk | ||
| Statin monotherapy | 4 (28.6) | 29 (37.7) | 8 (57.1) | 44 (57.1) | |
| Statin monotherapy | High | 3 (27.3) | 11 (39.3) | 6 (54.5) | 18 (64.3) |
| Moderate | 1 (33.3) | 17 (37.8) | 2 (66.7) | 25 (55.6) | |
| Low | 0 (0) | 1 (25.0) | 0 (0) | 1 (25.0) | |
| Statin in combination with ezetimibe | 1 (3.8) | 17 (23.3) | 18 (69.2) | 43 (58.9) | |
| Statin in combination with ezetimibe | High | 1 (5.3) | 10 (19.2) | 14 (73.7) | 33 (63.5) |
| Moderate | 0 (0) | 6 (30.0) | 4 (57.1) | 9 (45.0) | |
| Low | 0 (0) | 1 (100.0) | 0 (0) | 1 (100.0) | |
The percentages in parentheses represent the prevalence of patients with target levels of LDL-C in CV risk subpopulations on particular therapy regimens.
In the overall study population, the target of a ≥ 50% reduction in LDL-C levels was achieved by 124 (55.9%) patients, including 30 (66.7%) patients in the very high CV risk group and 94 (53.1%) patients in the high CV risk group.
Notably, none of the 22 patients diagnosed with diabetes attained any of the therapeutic targets.
Apart from the result showing that higher doses of statins were significantly more efficacious at lowering LDL-C levels (Table VI), we did not find any statistically significant differences in the reduction of LDL-C concentration comparing subpopulations of high and very high CV risk patients or patients on different therapy regimens. Attainment of therapeutic targets was not found to be related to CV risk, gender, other patient characteristics, or type of therapy.
Table VI.
Absolute and percentage reduction in serum LDL-C concentration (mmol/l) in relation to statin dose (high, moderate or low); median (interquartile range; Q1–Q3)
| Parameter | High | Moderate | Low | P-value |
|---|---|---|---|---|
| N | 116 | 81 | 7 | |
| Absolute change [mmol/l] | –3.64 (–4.66, –2.58) | –2.95 (–3.80, –2.27) | –2.66 (–2.94, –2.12) | 0.001 |
| Percentage change | –57.95 (–66.00, –45.05) | –52.45 (–59.98, –41.10) | –46.05 (–52.53, –38.35) | 0.019 |
Notably, despite intensive therapy, significantly elevated levels of LDL-C (> 4.1 mmol/l) remained in 14% of the overall patient population after treatment. Of those patients, 38.7% were unsuccessfully treated with high doses of statins (either in monotherapy (5 patients) or in combination with ezetimibe (7 patients)). Dangerously high levels of post-treatment LDL-C (> 5.2 mmol/l) remained in 9 patients belonging to the high CV risk subpopulation (4.1% of the overall study population). None of the patients in the subpopulation of very high CV risk had post-follow-up LDL-C levels > 5.2 mmol/l, suggesting that the most intensive statin therapy was more efficacious (Table VII).
Table VII.
Prevalence of patients who were treated with high doses of statins and remained with high post-therapy levels of LDL-C in subpopulations defined by CV risk and statin monotherapy or statin-ezetimibe combination therapy. The thresholds for the LDL-C concentrations were adopted from the current Polish statement [20] and current European guidelines [21]
| Type of treatment used | LDL-C concentration at last follow-up visit [mmol/l] | All patients N (%) | High cardiovascular risk patients N (%) | Very high cardiovascular risk patients N (%) |
|---|---|---|---|---|
| Overall population | > 4.1* | 31 (14.0) | 24 (13.6) | 7 (15.6) |
| > 5.2** | 9 (4.1) | 9 (5.1) | 0 (0) | |
| High doses of statin in monotherapy | > 4.1* | 5 (12.8) | 3 (10.7) | 2 (18.2) |
| > 5.2** | 2 (5.1) | 2 (7.1) | 0 (0) | |
| High doses of statins in combination with ezetimibe | > 4.1* | 7 (9.9) | 4 (7.7) | 3 (15.8) |
| > 5.2** | 1 (1.4) | 1 (1.9) | 0 (0) |
The tolerance of statins was addressed by surveying the patients about known adverse effects (AEs). Most of the patients (167, 75.2%) reported no drug-related complications. A total of 6 (2.7%) patients refused statin therapy. A total of 49 (22.1%) patients reported AEs, mainly pain and muscular weakness, which are the most frequent AEs of statin treatment [15]. Nevertheless, in the subgroup of patients reporting AEs, creatine kinase (CK) did not increase more than 4 times the upper limit of normal (ULN) and therefore therapy was not discontinued. Features of statin-induced hepatotoxicity were not observed in our patients. We did not observe any new, unknown AEs of any type of statin used.
Discussion
Our study is the first to describe the effects of routine clinical management of HeFH in Poland. Retrospectively analyzing patient history, we found that only 25.2% of patients attained the target levels of LDL-C, including 6 (13.3%) patients in the very high CV risk group and 50 (28.2%) patients in the high CV risk group. In the overall study population, the target of a ≥ 50% reduction in LDL-C levels was achieved by 124 (55.9%) patients, including 30 (66.7%) patients in the very high CV risk group and 94 (53.1%) patients in the high CV risk group. The detailed prospective cohort studies of Perez de Isla et al. [16], who analyzed Spanish FH patient populations, and Brunham et al. [17], who analyzed FH patients in British Columbia, showed that therapeutic target attainment was rare and was achieved only in 10% of patients – significantly less than in our study. On the other hand, the results of a large, cross-sectional study performed on a well-described Dutch population by Pijlman et al. [18] were more similar to ours and showed that the treatment goal for LDL-C < 2.5 mmol/l was achieved in 21% of patients. Another French, retrospective study performed on 1,669 patients showed that only 10.4% of patients reached the target level of LDL-C < 2.6 mmol/l [19]. As different values of therapeutic target attainment are present in the literature, one may conclude that the results are largely influenced by study design, length of observation period, and different target level definitions (i.e. European vs. American). Nevertheless, the conclusions of these studies are similar to ours and point to the striking inadequacy of the routinely available treatment.
According to the Polish statement on when to use PCSK9 inhibitors, one of the indications is to start this type of therapy in patients with an LDL-C level > 4.1 mmol/l (160 mg/dl), despite intensive hypolipidemic therapy with high doses of statins [20]. In our study, 5 (12.8%) patients treated with high-dose statin monotherapy and 7 (9.9%) patients treated with high doses of statins in combination with ezetimibe fulfilled these criteria.
As stated in the first EAS/ESC consensus statement on PCSK9 inhibitors, for patients with HeFH but without CVD or diabetes (high CV risk patients) treated with maximally tolerated efficacious statin therapy in combination with ezetimibe and still characterized by LDL-C > 5.2 mmol/l (200 mg/dl), PCSK9 inhibitors may also be considered [21]. In our study, only one high CV risk patient was applicable for this treatment.
We found that hypolipidemic therapy in men was more efficacious than similar therapy in women. The subpopulation of men attained a significantly stronger reduction in TC as well as a lower concentration of TC and TG at the last follow-up visit. As the recent huge meta-analysis focused on the differences in therapeutic outcomes of statin therapy among sexes proved that statins are of similar effectiveness in both men and women, one may hypothesize that the differences we have observed are caused by a non-interventional study design and related to statistical bias [22]. Moreover, in our patient population, the starting levels of TG were much lower in the subpopulation of women compared to the subpopulation of men, which would clearly explain the observed differences in the percentage reduction in TG levels.
The inadequacy of the currently used treatment is further supported by the fact that none of the 22 diabetic patients in the study group attained therapeutic goals. Diabetes is known to be associated with a significantly increased risk of CVD. Therefore, patients with accompanying HeFH need to be carefully monitored for hypolipidemic therapy progress and attainment of a strict < 1.8 mmol/l (70 mg/dl) therapeutic target [8].
There are some limitations to our study, including its retrospective study design [23]. In addition, the duration of the therapy period and the mode of therapy used varied within our study cohort.
In conclusion, this was the first observational study in a Polish patient population treated for HeFH by routine clinical management. The results revealed that therapy based on EAS/ESC guidelines was suboptimal for a significant number of patients. The intensification of therapy is based on the physician’s decisions and patient agreement. Patients are often afraid of adverse events related to higher doses of statins, particularly after reading leaflets [24]. The combination therapy of statins and ezetimibe could be applied to a large number of patients to better address their needs. However, in some patients from our study, the cost of therapy was an obstacle.
Nonetheless, despite adherence to high-intensity therapy with statins in combination with ezetimibe, some HeFH patients do not achieve target levels of LDL-C; although fortunately, this may be improved by including PCSK9 inhibitors with their treatment. For patients at very high risk, LDL apheresis is another therapeutic option. However, there is potential to avoid this procedure with the use of PCSK9 inhibitors [25].
Acknowledgments
All authors were involved in interpretation of the data, critical revision of the manuscript, and final approval of the version to be published. Medical writing support was provided by Proper Medical Writing Sp. z o.o., Warsaw, Poland and sponsored by Sanofi.
Conflict of interest
LKL and BC received honoraria for advisory boards from Angen and Sanofi. BC received honoraria for advison board from MSD.
References
- 1.Pajak A, Szafraniec K, Polak M, et al. Prevalence of familial hypercholesterolemia: a meta-analysis of six large, observational, population-based studies in Poland. Arch Med Sci. 2016;12:687–96. doi: 10.5114/aoms.2016.59700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raal FJ, Santos RD. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis. 2012;223:262–8. doi: 10.1016/j.atherosclerosis.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Santos RD, Gidding SS, Hegele RA, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4:850–61. doi: 10.1016/S2213-8587(16)30041-9. [DOI] [PubMed] [Google Scholar]
- 4.Di Taranto MD, D’Agostino MN, Fortunato G. Functional characterization of mutant genes associated with autosomal dominant familial hypercholesterolemia: integration and evolution of genetic diagnosis. Nutr Metab Cardiovasc Dis. 2015;25:979–87. doi: 10.1016/j.numecd.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Sharifi M, Walus-Miarka M, Idzior-Walus B, et al. The genetic spectrum of familial hypercholesterolemia in south-eastern Poland. Metabolism. 2016;65:48–53. doi: 10.1016/j.metabol.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turgeon RD, Barry AR, Pearson GJ. Familial hypercholesterolemia: review of diagnosis, screening, and treatment. Can Fam Physician. 2016;62:32–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Marks D, Thorogood M, Neil HAW, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168:1–14. doi: 10.1016/s0021-9150(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 8.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Atherosclerosis. 2016;253:281–344. doi: 10.1016/j.atherosclerosis.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Banach M, Jankowski P, Jozwiak J, et al. PoLA/CFPiP/PCS guidelines for the management of dyslipidaemias for family physicians 2016. Arch Med Sci. 2017;13:1–45. doi: 10.5114/aoms.2017.64712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rynkiewicz A, Cybulska B, Banach M, et al. Management of familial heterozygous hypercholesterolemia: position paper of the Polish Lipid Expert Forum. J Clin Lipidol. 2013;7:217–21. doi: 10.1016/j.jacl.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Mysliwiec M, Walczak M, Malecka-Tendera E, et al. Management of familial hypercholesterolemia in children and adolescents. Position paper of the Polish Lipid Expert Forum. J Clin Lipidol. 2014;8:173–80. doi: 10.1016/j.jacl.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Nordestgaard BG, Chapman MJ, Humphries SE, et al. European Atherosclerosis Society consensus panel. Familial hypercholesterolemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary artery disease. Eur Heart J. 2013;34:3478–90. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallejo-Vaz AJ, Kondapally Seshasai SR, Cole D, et al. Familial hypercholesterolaemia: a global call to arms. Atherosclerosis. 2015;243:257–9. doi: 10.1016/j.atherosclerosis.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA Cholesterol Guideline Panel. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med. 2014;160:339–43. doi: 10.7326/M14-0126. [DOI] [PubMed] [Google Scholar]
- 15.Banach M, Rizzo M, Toth PP, et al. Statin intolerance – an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11:1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez de Isla L, Alonso R, Watts GF, et al. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia. J Am Coll Cardiol. 2016;67:1278–85. doi: 10.1016/j.jacc.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Brunham LR, Cermakova L, Lee T, et al. Contemporary trends in the management and outcomes of patients with familial hypercholesterolemia in Canada: a prospective observational study. Can J Cardiol. 2017;33:385–92. doi: 10.1016/j.cjca.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Pijlman AH, Huijgen R, Verhagen SN, et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis. 2010;209:189–94. doi: 10.1016/j.atherosclerosis.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Béliard S, Carreau V, Carrié A, et al. Improvement in LDL-cholesterol levels of patients with familial hypercholesterolemia: Can we do better? Analysis of results obtained during the past two decades in 1669 French subjects. Atherosclerosis. 2014;234:136–41. doi: 10.1016/j.atherosclerosis.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Cybulska B, Gaciong Z, Hoffman P, et al. Severe hypercholesterolaemia – when to use the proprotein convertase subtilisin-kexin type 9 protease inhibitors (PCSK9 inhibitors)? Polish Society of Cardiology experts’ group statement. Kardiol Pol. 2016;74:394–8. doi: 10.5603/KP.2016.0051. [DOI] [PubMed] [Google Scholar]
- 21.Landmesser U, Chapman MJ, Farnier M, et al. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J. 2017;38:2245–55. doi: 10.1093/eurheartj/ehw480. [DOI] [PubMed] [Google Scholar]
- 22.Fulcher J, O’Connell R, Voysey M, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 23.Hulley SB, Cummings SR, Browner WS, et al. Designing clinical research. Lippincott Williams & Wilkins; 2013. p. 8. [Google Scholar]
- 24.Banach M, Stulc T, Dent R, Toth PP. Statin non-adherence and residual cardiovascular risk: there is need for substantial improvement. Int J Cardiol. 2016;225:184–96. doi: 10.1016/j.ijcard.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 25.Moriarty PM, Parhofer KG, Babirak SP, et al. Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur Heart J. 2016;37:3588–95. doi: 10.1093/eurheartj/ehw388. [DOI] [PMC free article] [PubMed] [Google Scholar]