Abstract
Introduction
Chronic kidney disease (CKD) and congestive heart failure (CHF) patients have higher serum B-type natriuretic peptide (BNP), which alters the test interpretation. We aim to define BNP cutoff levels to diagnose acute decompensated heart failure (ADHF) in CKD according to CHF subtype: heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF).
Material and methods
We reviewed 1,437 charts of consecutive patients who were admitted for dyspnea. We excluded patients with normal kidney function, without measured BNP, echocardiography, or history of CHF. BNP cutoff values to diagnose ADHF for CKD stages according to CHF subtype were obtained for the highest pair of sensitivity (Sn) and specificity (Sp). We calculated positive and negative likelihood ratios (LR+ and LR–, respectively), and diagnostic odds ratios (DOR), as well as the area under the receiver operating characteristic curves (AUC) for BNP.
Results
We evaluated a cohort of 348 consecutive patients: 152 had ADHF, and 196 had stable CHF. In those with HFpEF with CKD stages 3–4, BNP < 155 pg/ml rules out ADHF (Sn90%, LR– = 0.26 and DOR = 5.75), and BNP > 670 pg/ml rules in ADHF (Sp90%, LR+ = 4 and DOR = 6), with an AUC = 0.79 (95% CI: 0.71–0.87). In contrast, in those with HFrEF with CKD stages 3–4, BNP < 412.5 pg/ml rules out ADHF (Sn90%, LR– = 0.19 and DOR = 9.37), and BNP > 1166.5 pg/ml rules in ADHF (Sp87%, LR+ = 3.9 and DOR = 6.97) with an AUC = 0.78 (95% CI: 0.69–0.86). All LRs and DOR were statistically significant.
Conclusions
BNP cutoff values for the diagnosis of ADHF in HFrEF were higher than those in HFpEF across CKD stages 3–4, with moderate discriminatory diagnostic ability.
Keywords: acute decompensated heart failure, B-type natriuretic peptide, chronic kidney disease, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction
Introduction
Cardiovascular diseases are responsible for more than 50% of all deaths in patients with chronic kidney disease (CKD). Additionally, more than one-third of patients with congestive heart failure (CHF) have CKD [1, 2]. B-type natriuretic peptide (BNP) levels have been reported to be strongly associated with CHF independent of other predictors including renal function [3, 4]. Certain factors were found to be associated with elevated serum BNP levels, such as advancing age, female gender, high systolic blood pressure, and worsening renal and diastolic functions [5–7]. In patients with CKD, higher cutoffs of natriuretic peptides have been suggested; however, many of these initial studies excluded patients with an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 [8–11]. The other issue surrounding the cutoffs for natriuretic peptides has been the upper limit of BNP measurement in some of these studies. For example, in a subgroup from the Breathing Not Properly study [12], the upper limit of the BNP assay used in the study was 1300 pg/ml; however, about 9.2% of the total patients had a BNP value of 1300 pg/ml, thereby resulting in a weak (although statistically significant) correlation coefficient between BNP and estimated glomerular filtration rate (eGFR) [8]. Also, it is not clear how many patients who arrived at the emergency department (ED) had dyspnea exclusively secondary to heart failure with preserved ejection fraction (HFpEF); some studies have reported this number to be at least one-third [13]. Although patients with HFpEF usually have elevations in the median range of 413–445 [9, 13, 14], a study reported that up to one-third of patients can have a BNP level of more than 1,000 pg/ml [15]. The aim of our study was to examine different BNP cutoff levels for diagnosis of ADHF, according to CKD stage and CHF subtype for better utilization and interpretation of BNP in CKD patients.
Material and methods
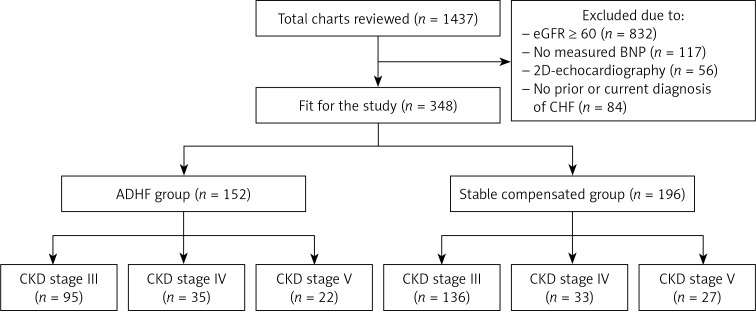
This was a retrospective study approved by our local institutional review board. We reviewed the charts of 1,437 patients admitted through the ED of St. Vincent Charity Medical Center/Case Western Reserve University, Cleveland, Ohio, with a chief complaint of “shortness of breath” or “dyspnea” from January 2013 to December 2015. ED notes, history and physical examination, daily progress notes, consult notes, discharge summaries and 2D echocardiography reports were all reviewed. We excluded 832 patients with an eGFR greater than or equal to 60 ml/min/1.73 m2, 117 patients without measured BNP, 56 patients without documented 2D echocardiography in the previous 3 months, and 84 patients with no prior or current final diagnosis of CHF (Figure 1). Patients were divided into two groups: the ADHF group, and the stable CHF group. The latter is a group of patients who are known to have CHF, but were found to be compensated, and the actual cause of the dyspnea that brought them to the ED was not related to ADHF, but to other factors (such as any acute lung disease).
Figure 1.
Structure of the study
ADHF – acute decompensated heart failure, BNP – B-type natriuretic peptide, CHF – congestive heart failure, CKD – chronic kidney disease, eGFR – estimated glomerular filtration rate.
The primary team (general internal medicine team) established the diagnosis relying on their clinical assessment, BNP level, and chest X-ray findings. We collected patients’ age, gender, race, body mass index, and eGFR with subsequent CKD stage, 2D echocardiography reports as well as the medical history of hypertension, diabetes, and atrial fibrillation. The plasma BNP concentration was measured using the ADVIA Centaur BNP immunoassay on the Siemens ADVIA Centaur XP system (Siemens Healthcare Diagnostics, Tarrytown, NY, USA), with a BNP reference range of < 2.0–5000 pg/ml. The eGFR was calculated by the modification of diet in renal disease formula. The stages of CKD were classified according to eGFR as follows: stage 3 with eGFR of 30–59 ml/min/1.73 m2, stage 4 with eGFR of 15–29 ml/min/1.73 m2, and stage 5 with eGFR of < 15 ml/min/1.73 m2 [16]. An ejection fraction of less than 50% was used to classify heart failure with reduced ejection fraction.
Statistical analysis
Differences between stable CHF and ADHF patient groups were tested with the χ2 test for categorical variables and with the t-test or Wilcoxon rank sum test for continuous variables. Within each CHF subtype (HFpEF and HFrEF), we tested the differences in BNP medians between the ADHF group and the stable compensated CHF group using the Wilcoxon rank sum test. This comparison was made separately for the individual CKD stages 3, 4, and 5, as well as for all stages combined. Statistical significance was accepted at a p-value of less than 0.05.
The area under the receiver operating characteristic (ROC) curve was utilized to assess the discriminative ability of BNP for detecting ADHF for CKD stages 3 and 4 in each CHF subtype (HFpEF and HFrEF). An area under ROC between 0.7 and 0.8 was described as moderate discriminative ability, and between 0.8 and 0.9 as optimal discriminative ability. The optimal BNP cutoff points were taken on the ROC curve for pre-specified values of sensitivity (Sn) and specificity (Sp) (both set at 90%) and for the highest pair of Sn and Sp. We then calculated positive and negative likelihood ratios (LR+ and LR–, respectively), and diagnostic odds ratios (DOR) for those BNP cutoff values. A 95% confidence interval (CI) for LR and DOR that excluded 1 was considered statistically significant.
For statistical analyses, we used SPSS software (IBM Corp., IBM SPSS Statistics for Windows, Version 22.0, released 2013).
Results
A total of 348 patients were enrolled in the study. Of those 348, 152 patients had a final diagnosis of ADHF (ADHF group), and 196 patients were found to have compensated CHF (stable CHF group). Of the 152 patients of the ADHF group, 95 patients had CKD stage 3; 35 patients had CKD stage 4, and 22 patients had CKD stage 5 (with or without end-stage renal disease). Among the 196 patients of stable compensated group, 136 patients had CKD stage 3; 33 patients had CKD stage 4, and 27 patients had CKD stage 5 (Table I). For most variables, there were no significant differences between the stable and ADHF groups. In both groups, females were slightly more numerous than males; the patients were elderly and mildly obese. While the majority of the patients had hypertension (more than 90% in both groups), only half of them had diabetes mellitus. A significantly higher proportion of African American, smokers, and HFrEF patients were found in the ADHF group. Finally, higher median BNP levels were found in the ADHF group in comparison to the stable group (p < 0.001).
Table I.
Demographics of included patients
| Parameter | Stable compensated group (n = 196) | ADHF group (n = 152) | P-value |
|---|---|---|---|
| CKD stage, n (%): | 0.31 | ||
| 3 | 136 (69) | 95 (63) | |
| 4 | 33 (17) | 35 (23) | |
| 5 | 27 (14) | 22 (14) | |
| Sex, n (%): | 0.26 | ||
| Male | 76 (39) | 68 (45) | |
| Female | 120 (61) | 84 (55) | |
| Age, mean ± SD [years] | 70.62 ±14.1 | 70.66 ±12.2 | 0.98 |
| BMI, mean ± SD [kg/m2] | 31.58 ±8.9 | 31.13 ±8.5 | 0.63 |
| Hypertension, n (%) | 182 (93) | 140 (92) | 0.79 |
| Diabetes mellitus, n (%) | 105 (54) | 82 (54) | 0.94 |
| African American, n (%) | 131 (67) | 117 (77) | 0.04 |
| Atrial fibrillation, n (%) | 48 (24) | 31 (20) | 0.37 |
| Smoking history, n (%) | 99 (51) | 94 (62) | 0.04 |
| CHF subtype, n (%): | < 0.001 | ||
| HFrEF | 53 (27) | 88 (58) | |
| HFpEF | 143 (73) | 64 (42) | |
| BNP, median (IQR) [pg/ml] | 230 (106–557.8) | 1052 (500.3–2064.5) | < 0.001 |
ADHF – acute decompensated heart failure, BMI – body mass index, BNP – B-type natriuretic peptide, CHF – congestive heart failure, CKD – chronic kidney disease, HFpEF – heart failure with preserved ejection fraction, HFrEF – heart failure with reduced ejection fraction.
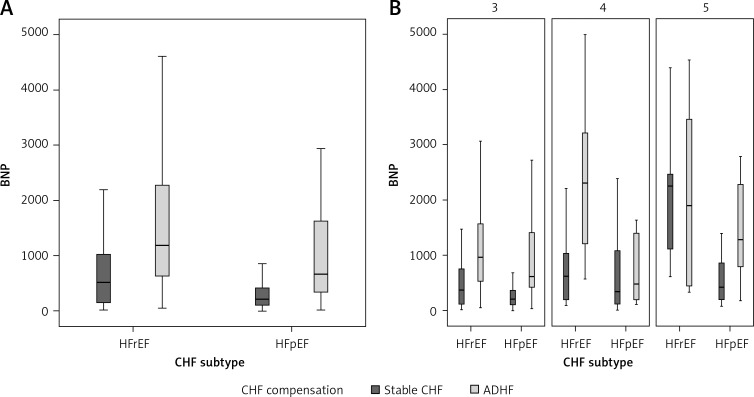
Median BNP levels between the stable compensated group and the ADHF group according to CHF subtype were statistically significant for all combined CKD stages (Table II). In HFpEF, the median BNP level was 212 (interquartile range (IQR): 103–419 pg/ml) in the stable group and 671 (IQR: 32–1626 pg/ml) in the ADHF group. In HFrEF, the median BNP level was 513 (IQR: 148–1065 pg/ml) in the stable group and 1185 (IQR: 615–2285.8 pg/ml) in the ADHF group. The same comparison was also statistically significant in each CKD stage, with the exception of comparisons between patients with CKD stage 4 with HFpEF and patients with CKD stage 5 with HFrEF (Table II, Figure 2).
Table II.
Median BNP level in stable and ADHF groups in both HFpEF and HFrEF
| Variable | HFpEF | HFrEF | ||||
|---|---|---|---|---|---|---|
| Stable | ADHF | P-value* | Stable | ADHF | P-value* | |
| Combined CKD | 212 (103–419) n = 143 |
671 (32–1626) n = 64 |
< 0.001 | 513 (148–1065) n = 53 |
1185 (615–2285.8) n = 88 |
< 0.001 |
| CKD 3 | 197 (94.3–348.8) n = 100 |
612 (420–1546) n = 35 |
< 0.001 | 369 (104.5–760.8) n = 36 |
957 (522.3–1593) n = 60 |
< 0.001 |
| CKD 4 | 340 (91.8–1080.3) n = 22 |
474 (179–1510.5) n = 13 |
0.207 | 624 (155–1160) n = 11 |
2297.5 (1182.5–3253.8) n = 22 |
0.002 |
| CKD 5 | 419 (180–940) n = 21 |
1270 (740.8–2367.8) n = 16 |
0.005 | 2248.5 (985–2942.3) n = 6 |
1884 (401.5–3727.5) n = 6 |
0.749 |
Data are median (interquartile range)
P-value was calculated using Wilcoxon rank sum test. ADHF – acute decompensated heart failure, CKD – chronic kidney disease, HFpEF – heart failure with preserved ejection fraction, HFrEF – heart failure with reduced ejection fraction.
Figure 2.
Boxplots for median BNP in HFrEF and HFpEF in combined CKD stages (A) and each CKD stage (B)
CKD – chronic kidney disease, HFpEF – heart failure with preserved ejection fraction, HFrEF – heart failure with reduced ejection fraction.
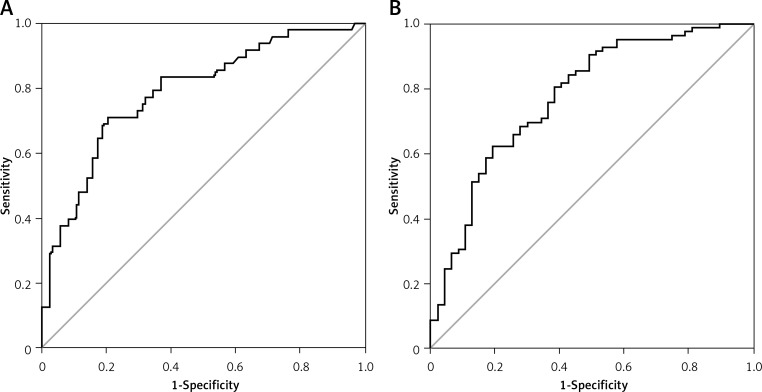
The optimal cutoff levels for CKD stages 3 and 4 according to CHF subtype are shown in Table III. In HFpEF, a BNP cutoff at 155 pg/ml defining a Sn of 90% had statistically significant LRs and DOR. Similarly, a BNP cutoff at 670 pg/ml defining a Sp of 90% had statistically significant LRs and DOR. A combination of the highest Sn and Sp (71% and 80%, respectively) had a cutoff of 415.5 pg/ml and also had significant LRs and DOR. The AUC for BNP in HFpEF was 0.79 (95% CI: 0.71–0.865) (Figure 3). In HFrEF, BNP cutoffs were at 412.5 pg/ml for Sn of 90% and 1166.5 pg/ml for Sp of 87%; both had significant LRs and DOR. A combination of the highest Sn and Sp (62% and 81%, respectively) had a cutoff of 937.5 pg/ml and significant LRs and DOR. The AUC for BNP in HFrEF was 0.78 (95% CI: 0.69–0.86) (Figure 3).
Table III.
BNP cutoff levels for each CKD stage according to CHF subtype
| Variable | BNP cut-off | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR– (95% CI) | DOR (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|---|---|
| HFpEF in CKD 3 and 4 | 155 | 90% (0.82–0.95) |
39% (0.29–0.49) |
1.48 (1.25–1.75) |
0.26 (0.14–0.48) |
5.75 (2.67–12.39) |
0.79 (0.71–0.87) |
| 415.5 | 71% (0.61–0.80) |
80% (0.71–0.87) |
3.55 (2.35–5.36) |
0.36 (0.26–0.50) |
9.79 (5.10–18.82) |
||
| 670 | 40% (0.30–0.50) |
90% (0.82–0.95) |
4 (2.12–7.55) |
0.67 (0.56–0.79) |
6 (2.79–12.91) |
||
| HFrEF in CKD 3 and 4 | 412.5 | 90% (0.82–0.95) |
51% (0.41–0.61) |
1.84 (1.49–2.27) |
0.19 (0.11–0.36) |
9.37 (4.37–20.07) |
0.78 (0.69–0.86) |
| 937.5 | 62% (0.52–0.72) |
81% (0.72–0.88) |
3.26 (2.12–5.03) |
0.47 (0.36–0.61) |
6.67 (3.51–12.66) |
||
| 1166.5 | 51% (0.41–0.61) |
87% (0.79–0.93) |
3.9 (2.28–6.75) |
0.56 (0.45–0.70) |
6.97 (3.45–14.06) |
AUC – area under the curve, BNP – B-type natriuretic peptide, CI – confidence interval, CKD – chronic kidney disease, DOR – diagnostic odds ratios, HFpEF – heart failure with preserved ejection fraction, HFrEF – heart failure with reduced ejection fraction, LR– – negative likelihood ratio, LR+ – positive likelihood ratio.
Figure 3.
ROC curve for patients with CKD stages 3 and 4. A – Patients with HFpEF, AUC = 0.79 (95% CI: 0.71–0.87). B – Patients with HFrEF, AUC = 0.78 (95% CI: 0.69–0.86)
CKD – chronic kidney disease, HFpEF – heart failure with preserved ejection fraction, HFrEF – heart failure with reduced ejection fraction, ROC – receiver operating characteristic.
Discussion
In our study, we found that the BNP cutoff values for diagnosing ADHF were upshifted more by the heart failure subtype (HFpEF vs. HFrEF) than by the CKD stage itself, driven by BNP production rather than clearance unless it reaches the end stage.
BNP cutoff values for the diagnosis of ADHF in HFrEF were higher than those in HFpEF across CKD stages 3–4. Specific BNP cutoff points were associated with statistically significant LRs and DOR, suggesting their utility in the diagnosis decision-making, and the discriminatory ability of BNP for diagnosing ADHF was moderate to optimal for both HF types.
The presence of CKD increases the overall prevalence of CHF [8] and also appears to impact the optimum cutoffs for BNP required for its diagnosis. There are few reports of BNP cutoffs for diagnosis of CHF in patients with CKD. McCullough et al. reported a weak correlation between renal function and BNP, with cutoffs of BNP at 225 pg/ml in patients with eGFR of 15–29 ml/min, and 201.2 pg/ml in patients with eGFR of 30–59 ml/min for diagnosing CHF [8]. Another study of 182 patients with all stages of CKD (including patients on dialysis) admitted with shortness of breath suggested BNP levels ≥ 858.8 pg/ml as diagnostic of CHF (AUC 0.82) [11]. Jafri et al. suggested cutoffs of BNP at 300 pg/ml and NT-proBNP at 4502 pg/ml, respectively, for diagnosing systolic heart failure (left ventricular ejection fraction < 40%) in an overall sample of 190 patients [10].
Several studies have suggested cardiac causes for the increase in plasma BNP levels in patients with end stage renal disease on dialysis [8, 16–18], as well as those with CKD [19, 20]. BNP has also been shown to correlate with eGFR, especially in patients without CHF (r = –0.31) as compared to patients with CHF (r = –0.17; p < 0.0001), most likely secondary to increased blood volume and left ventricular wall tension [8, 17, 18]. Interestingly, in patients without CHF the onset and presence of left ventricular overload has been shown to influence plasma BNP regardless of the severity of renal dysfunction, whereas even marked elevation of serum creatinine did not result in incremental elevation of BNP in patients without left ventricular overload [19]. Yet another study using direct sampling of BNP from the aortic root and coronary sinus has shown that decreased clearance of BNP is a more important reason for BNP elevation in CKD patients with CHF; however, this study did not have many patients with eGFR < 30 [20, 21]. In spite of these data, eGFR has not been shown to confound with interpretation of BNP levels, especially in patients with BNP > 500 pg/ml, in whom nearly 90% had CHF as the primary diagnosis after evaluation of shortness of breath in the ED [8]. Most BNP has also not been shown to be renally cleared and therefore most likely represents a counter-regulatory response to increased wall tension from volume overload in patients with CKD [22].
BNP levels are also usually found to be higher in patients with reduced ejection fraction than those with preserved ejection fraction [9, 14]. However, there is a marked overlap limiting the value of BNP in reliably differentiating the two heart failure subtypes (AUC = 0.66, 95% CI: 0.61–0.72) [13]. In our study the discriminative ability of BNP in differentiating ADHF and stable CHF had a significantly larger AUC than that study, and for both preserved and reduced EF types. In a retrospective study of 421 patients admitted with a diagnosis of HFpEF, 28% had BNP > 1000 pg/ml and these patients were usually older with impaired renal function and greater use of antihypertensive medications such as thiazides and spironolactone. However, one-fifth of patients with BNP > 1000 pg/ml had normal renal function and were older, with a lower ejection fraction [15]. We found that the BNP cutoff value in CKD patients is comparable to non-CKD patients in HFpEF; thus, the clinical utility of BNP in CKD is comparable to the non-CKD population as reported previously from a sub-analysis of the Breathing-Not-Properly study [7].
In our study, the comparison of median BNP levels between compensated and ADHF groups was statistically significant for all corresponding and combined CKD stages except CKD stage 4 in the HFpEF group and CKD stage 5 in the HFrEF group. We attribute the latter to the comparatively smaller patient sample in the respective CKD stage 4 and 5 groups. All BNP cutoff points in our study were associated with significant LRs and DOR for diagnosing ADHF in both preserved and reduced CHF subtypes. These BNP cutoffs may help the treating physician in his or her decision making as a value below or above such a cutoff will help decrease or increase, respectively, the probability that a given patient has ADHF. Needless to say, the BNP level should not be used as a diagnostic tool alone; rather it gives extra information when the clinical diagnosis is uncertain.
It is important to mention that the majority of the patients were African Americans (71%), who have been found to have lower BNP levels compared to other ethnicities [23], and this makes the study population distinct from the total population. Furthermore, none of the patients was taking neprilysin inhibitors (such as sacubitril), which would decrease BNP clearance [24].
Our study has several limitations. First, the study has a retrospective design. Second, patients with no prior or current final diagnosis of CHF were excluded from the study population; hence, we cannot comment on the degree of overlap in BNP between CKD patients with HFpEF and those without CHF. Third, there was no independent adjudication or confirmation of the clinical diagnosis of CHF; BNP was part of the diagnostic evaluation. Fourth, patients with troponin elevation from acute myocardial infarction/ischemia were not excluded from the study. Fifth, it should be noted that the relationship between CKD stage and BNP differs from that with NT-proBNP, as the latter seemed to predict ADHF more accurately [10]; hence our findings should not be extrapolated to NT-proBNP. Finally, over the last 3 decades, prevalence of LV systolic dysfunction has been trending down, while the mean LVEF as well as the diagnosis of HFpEF have been trending up. That would make most studied CHF patients belong to the HFpEF group [25]. Furthermore, 71% of our study sample were African Americans, who are known to have more frequent HFpEF (around 73% of CHF patients). Also, HFpEF has a better prognosis than HFrEF [26]. That made it hard to recruit a higher number of patients with HFrEF; thus, our analysis is limited by the small sample size when stratified by heart failure subtype and CKD stage. Therefore, the proposed cut-off should be further tested in prospective studies with larger samples.
In conclusion, BNP cutoff values for the diagnosis of ADHF in HFrEF were higher than those in HFpEF across CKD stages 3–4. The discriminatory ability of BNP for diagnosing ADHF was moderate for both CHF types. Overall, specific BNP cutoff points had a good test performance.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Vanderheyden M, Bartunek J, Filippatos G, Goethals M, Van Vlem B, Maisel A. Cardiovascular disease in patients with chronic renal impairment: role of natriuretic peptides. Congest Heart Fail. 2008;14:38–42. doi: 10.1111/j.1751-7133.2008.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Schaub JA, Coca SG, Moledina DG, Gentry M, Testani JM, Parikh CR. Amino-terminal pro-B-type natriuretic peptide for diagnosis and prognosis in patients with renal dysfunction: a systematic review and meta-analysis. JACC Hear Fail. 2015;3:977–89. doi: 10.1016/j.jchf.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis GS, Felker GM, Tang WHW. A test in context: critical evaluation of natriuretic peptide testing in heart failure. J Am Coll Cardiol. 2016;67:330–7. doi: 10.1016/j.jacc.2015.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu WH, Chen LY, Dai HL, Chen JH, Chen Y, Fang LZ. Correlation between B type natriuretic peptide and metabolic risk factors. Arch Med Sci. 2016;12:334–40. doi: 10.5114/aoms.2015.57001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feola M, Lombardo E, Testa M, Avogadri E, Piccolo S, Vado A. Prognostic factors of mid-term clinical outcome in congestive heart failure patients discharged after acute decompensation. Arch Med Sci. 2012;8:462–70. doi: 10.5114/aoms.2012.29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karasek D, Sinkiewicz W, Błażejewski J. Relationship between B-type natriuretic peptide serum level, echocardiographic TEI index and the degree of diastolic dysfunction in patients with heart failure with preserved systolic function. Arch Med Sci. 2011;7:449–56. doi: 10.5114/aoms.2011.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41:571–9. doi: 10.1053/ajkd.2003.50118. [DOI] [PubMed] [Google Scholar]
- 9.Niizuma S, Iwanaga Y, Yahata T, et al. Impact of left ventricular end-diastolic wall stress on plasma B-type natriuretic peptide in heart failure with chronic kidney disease and end-stage renal disease. Clin Chem. 2009;55:1347–53. doi: 10.1373/clinchem.2008.121236. [DOI] [PubMed] [Google Scholar]
- 10.Jafri L, Kashif W, Tai J, et al. B-type natriuretic peptide versus amino terminal pro-B type natriuretic peptide: selecting the optimal heart failure marker in patients with impaired kidney function. BMC Nephrol. 2013;14:117. doi: 10.1186/1471-2369-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JW, Kim MS, Kim JS, et al. Relationship between serum brain natriuretic peptide and heart function in patients with chronic kidney disease. Korean J Intern Med. 2008;23:191–200. doi: 10.3904/kjim.2008.23.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 13.Maisel AS, McCord J, Nowak RM, et al. Bedside B-type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction: results from the breathing not properly multinational study. J Am Coll Cardiol. 2003;41:2010–7. doi: 10.1016/s0735-1097(03)00405-4. [DOI] [PubMed] [Google Scholar]
- 14.Jaubert MP, Armero S, Bonello L, et al. Predictors of B-type natriuretic peptide and left atrial volume index in patients with preserved left ventricular systolic function: an echocardiographic-catheterization study. Arch Cardiovasc Dis. 2010;103:3–9. doi: 10.1016/j.acvd.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Tate S, Griem A, Durbin-Johnson B, Watt C, Schaefer S. Marked elevation of B-type natriuretic peptide in patients with heart failure and preserved ejection fraction. J Biomed Res. 2014;28:255–61. doi: 10.7555/JBR.28.20140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int. 2009;76:S1–2. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 17.Buckley MG, Sethi D, Markandu ND, Sagnella GA, Singer DR, MacGregor GA. Plasma concentrations and comparisons of brain natriuretic peptide and atrial natriuretic peptide in normal subjects, cardiac transplant recipients and patients with dialysis-independent or dialysis-dependent chronic renal failure. Clin Sci. 1992;83:437–44. doi: 10.1042/cs0830437. [DOI] [PubMed] [Google Scholar]
- 18.Nomura H, Hayashi T, Esaki T, et al. Standardization of plasma brain natriuretic peptide concentrations in older Japanese – relationship to latent renal dysfunction and ischemic heart disease. J Am Geriatr Soc. 2002;50:1504–9. doi: 10.1046/j.1532-5415.2002.50405.x. [DOI] [PubMed] [Google Scholar]
- 19.Takami Y, Horio T, Iwashima Y, et al. Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am J Kidney Dis. 2004;44:420–8. [PubMed] [Google Scholar]
- 20.Sagnella GA. Measurement and significance of circulating natriuretic peptides in cardiovascular disease. Clin Sci. 1998;95:519–29. doi: 10.1042/cs0950519. [DOI] [PubMed] [Google Scholar]
- 21.Tsutamoto T, Wada A, Sakai H, et al. Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol. 2006;47:582–6. doi: 10.1016/j.jacc.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 22.Akiba T, Tachibana K, Togashi K, Hiroe M, Marumo F. Plasma human brain natriuretic peptide in chronic renal failure. Clin Nephrol. 1995;44:S61–4. [PubMed] [Google Scholar]
- 23.Gupta DK, Daniels LB, Cheng S, Criqui MH, Maisel AS, Lima JA. Differences in natriuretic peptide levels by race/ethnicity (from the multi-ethnic study of atherosclerosis) Am J Cardiol. 2017;120:1008–15. doi: 10.1016/j.amjcard.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yandrapalli S, Aronow WS, Mondal P, Chabbott DR. The evolution of natriuretic peptide augmentation in management of heart failure and the role of sacubitril/valsartan. Arch Med Sci. 2017;13:1207–16. doi: 10.5114/aoms.2017.68813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasan RS, Xanthakis V, Lyass A, et al. Epidemiology of left ventricular systolic dysfunction and heart failure in the Framingham study: an echocardiographic study over 3 decades. JACC: cardiovascular imaging. JACC Cardiovasc Imaging. 2018;11:1–11. doi: 10.1016/j.jcmg.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta DK, Shah AM, Castagno D, et al. Heart failure with preserved ejection fraction in African Americans: the ARIC (Atherosclerosis Risk In Communities) study. JACC Hear Fail. 2013;1:156–63. doi: 10.1016/j.jchf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]