Abstract
Introduction
Infantile colic is a common pediatric problem. The cause of infantile colic remains unclear. Treatment options are limited. Evidence suggests that probiotics might offer some benefit. The aim of the study was to systematically assess the effectiveness of probiotics supplementation in the management of infantile colic.
Material and methods
MEDLINE and the Cochrane Library were searched up to April 2016 for randomized controlled trials (RCTs) evaluating the efficacy of probiotics (any well-defined strain) compared with placebo for the management of infantile colic. The outcome measures of interest were treatment success and the duration of crying at the end of the intervention.
Results
Seven RCTs (471 participants) were included. Compared with placebo the administration of Lactobacillus reuteri DSM 17938 at a daily dose of 108 CFU was associated with the treatment success (relative risk = 1.67, 95% CI: 1.10–2.81, number needed to treat 5, 95% CI: 4–8) and reduced crying times at the end of the intervention (mean difference: –49 min, 95% CI: –66 to –33); however, the effect was mainly seen in exclusively breastfed infants. Other probiotics (single or in combinations) were studied in single trials only.
Conclusions
Some probiotics, primarily L. reuteri DSM 17938, may be considered for the management of infantile colic. Data on other probiotics are limited.
Keywords: children, microbiota, infants
Introduction
According to the Rome III criteria for functional gastrointestinal disorders, infantile colic is diagnosed in infants younger than 4 months of age if all of the following symptoms occur: paroxysms of irritability, fussing or crying that starts and stops without obvious cause; episodes lasting 3 or more hours per day and occurring at least 3 days per week for at least 1 week; and no failure to thrive [1]. Infantile colic is a common problem which affects around 20% of infants [2] and often leads to parental anxiety and frustration. The cause of infantile colic remains unclear. However, a number of possible factors have been suggested such as increased painful intestinal contractions, lactose intolerance, food hypersensitivity, gas, parental misinterpretation of the normal crying pattern, and altered gut microbiota (dysbiosis) [3, 4]. In the management of infantile colic, dietary, pharmacological, behavioral interventions, and complementary and/or alternative therapies are used. However, data on their effectiveness are limited [5, 6]. Considering that dysbiosis may play a role in the pathogenesis of infantile colic and that the gut microbiota of infants with colic differs from that in unaffected infants [7], there is an interest in gut microbiota modification, including the use of probiotics, for the management of infantile colic. Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [8].
The aim of this review was to systematically evaluate current evidence on the efficacy of probiotics for the treatment of infantile colic.
Material and methods
We performed a systematic review of randomized controlled trials (RCTs) that compared probiotics (any well-characterized strain, in any dose) with placebo, in healthy full-term infants with infantile colic who were less than 6 months of age. The Cochrane Library and MEDLINE databases were searched up to April 2016. The main search text word terms and MESH headings used were as follows: excessive crying, infantile colic, colic, treatment, use, management, probiotic*, Lactobacillus, Lactobacillus reuteri, L. reuteri, bab*, infant*, newborn*, kid*, ped*. Only studies published in English were included. The primary outcomes were treatment success (defined as the percentage of children who achieved a reduction in the daily average crying time > 50%) and the duration of crying time (both at the end of intervention). The Cochrane Collaboration’s tool for assessing risk of bias in included trials, which assesses randomization and allocation of participants, blinding of participants, personnel and outcome assessors, and incomplete or selective reporting, was used.
Statistical analysis
The data were analyzed using the Review Manager (RevMan) computer program (Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The binary measure for individual studies and pooled statistics is reported as the relative risk (RR) between the experimental and control groups with the 95% confidence interval (CI). The continuous measure for individual studies is reported as the mean difference (MD) with 95% CI. Statistical heterogeneity was quantified by I2. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity. For pooling, random effects models were used, if appropriate. As it is known that not all probiotics are equal, and pooling data on different probiotics has been repeatedly questioned, data on each probiotic strain (or their combinations) are reported separately. The number needed to treat was calculated using the StatsDirect Statistical Software (Version 3.0.150, 01.05.2015).
Results
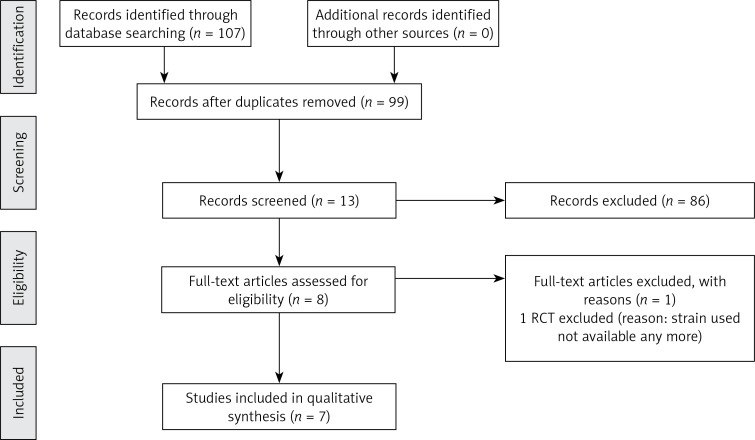
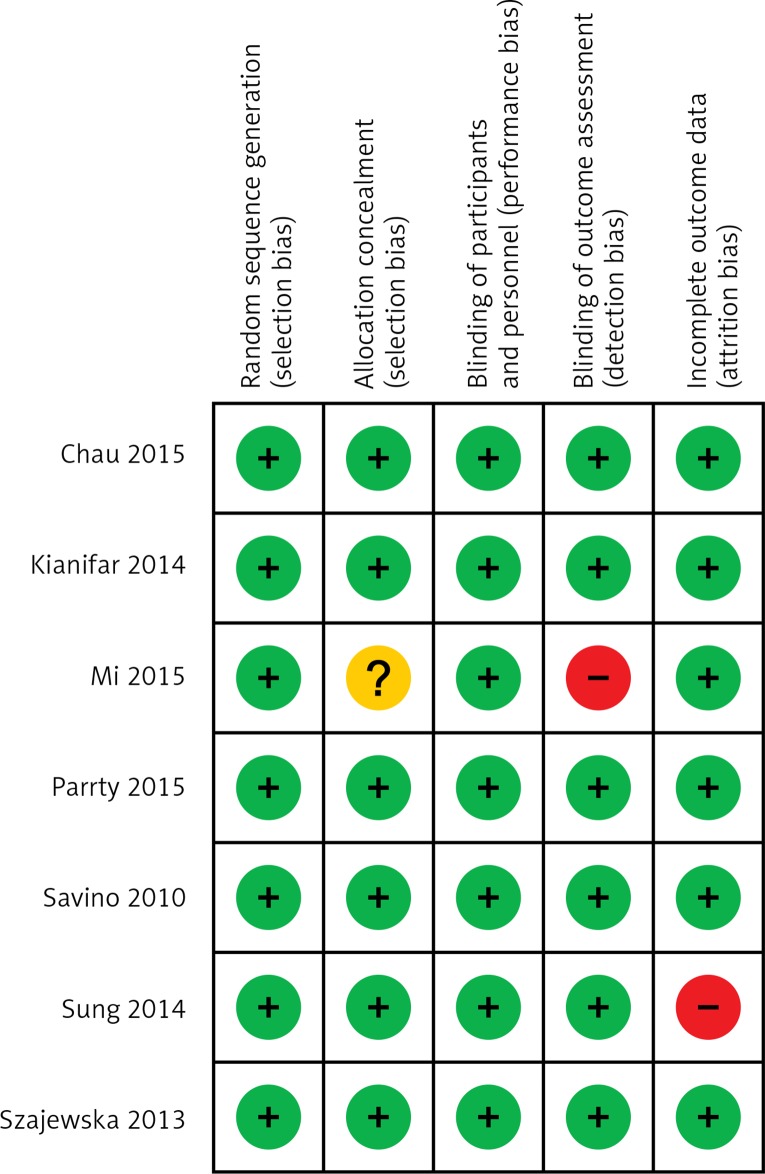
For a flow diagram documenting the identification process for the eligible trials (Figure 1). The characteristics of the seven included RCTs [9–15] that recruited a total of 471 infants are summarized in Table I. With one exception [13], all trials were double-blind and placebo controlled. All RCTs were published in English. Figure 2 presents the assessment of the risk of bias in included trials. All trials were generally considered as having ‘low risk of bias’.
Figure 1.
Flow diagram of studies included in the systematic review
Table I.
Characteristics of included trials
| Study | Population | Inclusion criteria | Intervention (dose/duration of intervention) | Comparison | Primary outcomes |
|---|---|---|---|---|---|
| Probiotics: | |||||
| Chau et al., 2015 [12] | N = 52 BF |
|
L. reuteri DSM 17938 (108 CFU for 21 days) | Placebo | Reduction in the duration of average crying and fussing times, from baseline to end of treatment to < 3 h per day |
| Mi et al. 2015 [13] | N = 42 exclusively or predominantly (> 50%) BF |
|
L. reuteri DSM 17938 (108 CFU for 21 days) | Placebo | Reduction in crying time |
| Savino et al. 2010 [9] | N = 50 exclusively BF |
|
L. reuteri DSM 17938 (108 CFU for 21 days) | Placebo | Reduction of average crying time to < 3 h a day on day 21 |
| Sung et al. 2014 [11] | N = 167 BF or FF |
|
L. reuteri DSM 17938 (108 CFU for 28 days) | Placebo | Daily duration of cry or fuss at 1 mo (min/day) |
| Szajewska et al. 2013 [10] | N = 80 exclusively or predominantly (> 50%) BF |
|
L. reuteri DSM 17938 (108 CFU for 21 days) | Placebo | Duration of crying and treatment success defined as percentage of children achieving a reduction in the daily average crying time ≥ 50% during the study |
| Pärtty et al. 2015 [14] | N = 30 BF & FF |
|
L. rhamnosus GG 4.5 × 109 CFU/day for 28 days | Placebo | Daily crying time |
| Synbiotics: | |||||
| Kianifar et al. 2014 [15] | N = 50 BF |
|
Synbiotic sachet containing 1 billion CFU (L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. infantis, L. bulgaricus, and fructooligosaccharides) for 30 days | Placebo | At least 50% reduction in average daily crying time |
BF – breastfed infants, FF – formula-fed infants.
Figure 2.
Risk of bias in included trials
Five included RCTs involving 349 infants assessed the effect of administration of L. reuteri DSM 17938 at a daily dose of 108 colony forming units (CFU) given for 21 days [9, 10, 12, 13] or 28 days [11]. Four RCTs involved only infants with infantile colic who were breastfed exclusively or predominantly [9, 10, 12 13], and one RCT involved both breastfed and formula-fed infants [11].
One RCT involving 30 breastfed and formula-fed infants evaluated the effectiveness of Lactobacillus GG at a daily dose of 4.5 × 109 CFU administered for one month in addition to the cow’s milk elimination diet either in infants or in breastfeeding mothers compared with placebo (microcrystalline cellulose) [14].
One RCT evaluated the use of a synbiotic containing L. casei, L. rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, L. acidophilus, B. infantis and L. bulgaricus at a daily dose of 1 billion CFU, and fructooligosaccharides compared with placebo administered for 30 days in 50 breastfed infants with infantile colic [15].
Treatment success at end of intervention
Lactobacillus reuteri DSM 17938
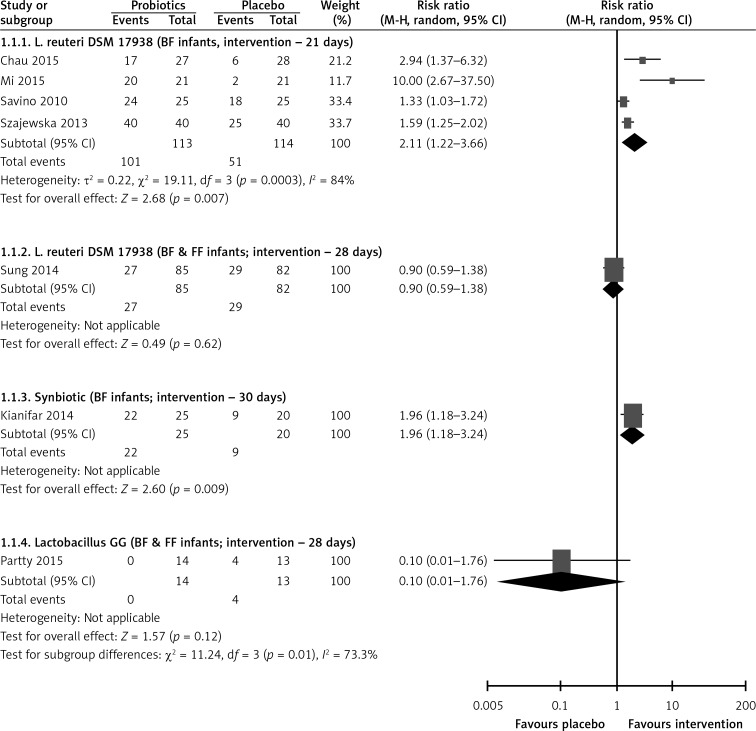
Pooled data from five RCTs [9–13] or 28 days showed that compared with placebo the administration of L. reuteri DSM 17938 increased the treatment success (RR = 1.67, 95% CI: 1.10 to 2.51, NNT = 5, 95% CI: 4–8). The effect of L. reuteri DSM 17938 was seen in breastfed infants (4 RCTs, RR = 2.11, 95% CI: 1.22–3.66, NNT = 2, 95% CI: 2 to 25), but not in breastfed and formula-fed infants (1 RCTs, RR = 0.9, 95% CI: 0.59–1.38) (Figure 3).
Figure 3.
Effect of probiotics on treatment success at end of intervention
BF – breastfed infants, FF – formula-fed infants.
Lactobacillus GG
One RCT [14] showed that compared with placebo the administration of Lactobacillus GG had no significant effect on the treatment success at the end of the intervention (RR = 0.10, 95% CI: 0.01–1.76) (Figure 3).
Synbiotic
One RCT [15] found that compared with placebo the administration of a synbiotic containing L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. infantis, L bulgaricus and fructooligosaccharides increased the treatment success at the end of the intervention (RR = 1.96, 95% CI: 1.18 to 3.24, NNT = 3, 95% CI: 2–16) (Figure 3).
Duration of crying at end of intervention
Lactobacillus reuteri DSM 17938
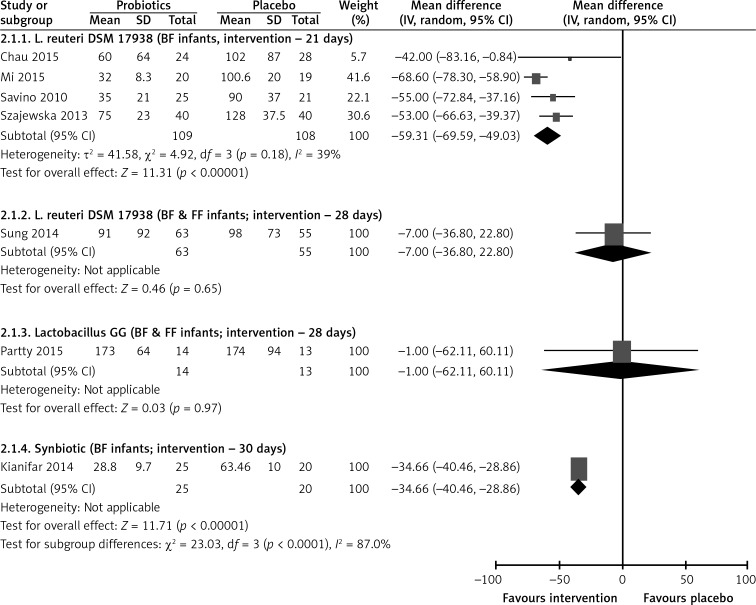
The pooled results of five RCTs [9–13] showed that administration of L. reuteri DSM 17938 compared with placebo reduced the duration of crying at the end of the intervention by almost 50 min (MD = –50, 95% CI: –66 to –33) (Figure 4).
Figure 4.
Effect of probiotics on duration of crying (min) at end of intervention
BF – breastfed infants, FF – formula-fed infants.
Lactobacillus GG
At the end of the intervention, based on diaries there was no difference in the daily crying time between the probiotic and the placebo group (MD = 1 min, 95% CI: –62 to 60) (Figure 4).
Synbiotic
One RCT [15] showed that administration of a synbiotic containing L. casei, L. rhamnosus, S. ther-mophilus, B. breve, L. acidophilus, B. infantis, L. bulgaricus and fructooligosaccharides compared with placebo reduced the duration of crying at the end of the intervention by almost 35 min (MD = –35, 95% CI: –40 to –29) (Figure 4).
Discussion
The aim of this review was to summarize current evidence on the effectiveness of probiotics supplementation in the management of infantile colic. We focused on two primary outcomes only, i.e. treatment success (defined as the percentage of children who achieved a reduction in the daily average crying time > 50%) and the duration of crying time, as being clinically important. Both outcomes were assessed at the end of the intervention period. Administration of L. reuteri DSM 17938 at a daily dose of 108 CFU was associated with treatment success and reduced crying times; however, the effect was mainly seen in breastfed infants. Data are inconclusive with regard to the efficacy of Lactobacillus GG. Findings from a single trial suggest the efficacy of a synbiotic consisting of L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. infantis, L. bulgaricus and fructooligosaccharides.
The extensive and systematic literature search is one of the strengths of this systematic review. We focused exclusively on specific strains, and abstained from pooling data on various probiotic strains. The methodological quality of included trials was assessed. However, only a limited number of trials were available, and a limited number of probiotics were studied. Some of the included studies were limited with respect to the study population. For example, L. reuteri DSM 17938 was mainly studied in breast-fed infants; data on formula-fed infants are limited and do not support the use of L. reuteri DSM 17938 in this group of infants. On the other hand, prevalence of infantile colic is similar in breastfed and formula-fed infants. Thus, the generalizability of the findings should not be limited. One important limitation of the included studies was the lack of an objective way to assess the duration of crying in infants. With one exception [11] the investigators fully relied on parents’ reports on the duration of crying recorded in the diaries. The precision and validity of such reporting may be questioned.
Previously one of us co-authored a systematic review which concluded that the administration of L. reuteri DSM 17938 is likely to reduce crying time in infants with infantile colic in breast-fed infants, but not in formula-fed infants. Thus, our current findings are in line with the results of the previously published review [16]. However, compared with a 2014 systematic review focusing on L. reuteri only, in this review we included more trials (3 vs. 7 respectively) involving also other probiotics and more patients. Thus, our analysis more precisely defines the role of probiotics for treating infantile colic. A more recent systematic review by Harb et al. [17] focused on interventions in breast-fed infants only and concluded that probiotics, in particular L. reuteri, appear effective for reducing colic, although there are limitations to these findings.
In conclusion, this systematic review confirms that the administration of L. reuteri DSM 17938 is likely to show a benefit in infants with infantile colic, especially in breast-fed infants. More studies, especially in formula-fed infants, are needed. Studies evaluating the effectiveness of other probiotics and/or prebiotics are needed, as preliminary results with some of them are promising.
Conflict of interest
Radoslaw Dryl declares no conflict of interest. Hanna Szajewska has participated as a clinical investigator and/or speaker for companies manufacturing probiotics.
References
- 1.Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. Childhood functional gastrointestinal disorders: neonate-toddler. Gastroenterology. 2006;130:1519–26. doi: 10.1053/j.gastro.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 2.Vandenplas Y, Abkari A, Bellaiche M, et al. Prevalence and health outcomes of functional gastrointestinal symptoms in infants from birth to 12 months of age. J Pediatr Gastroenterol Nutr. 2015;61:531–7. doi: 10.1097/MPG.0000000000000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung V, Collett S, de Gooyer T, Hiscock H, Tang M, Wake M. Probiotics to prevent or treat excessive infant crying: systematic review and meta-analysis. JAMA Pediatr. 2013;167:1150–7. doi: 10.1001/jamapediatrics.2013.2572. [DOI] [PubMed] [Google Scholar]
- 4.Dobson D, Lucassen PLBJ, Sampler S. Manipulative therapy for infantile colic (Protocol) Cochrane Database Syst Rev. 2003;4:CD004796. doi: 10.1002/14651858.CD004796.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savino F, Ceratto S, De Marco A, Cordero di Montezemolo L. Looking for new treatments of infantile colic. Ital J Pediatr. 2014;40:53–9. doi: 10.1186/1824-7288-40-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olafsdottir E, Aksnes L, Fluge G, Berstad A. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr. 2002;91:45–50. doi: 10.1080/080352502753457932. [DOI] [PubMed] [Google Scholar]
- 7.de Weerth C, Fuentes S, Puylaert P, de Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics. 2013;131:550–8. doi: 10.1542/peds.2012-1449. [DOI] [PubMed] [Google Scholar]
- 8.Hill C, Guarner F, Reid G, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 9.Savino F, Cordisco L, Tarasco V, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:526–33. doi: 10.1542/peds.2010-0433. [DOI] [PubMed] [Google Scholar]
- 10.Szajewska H, Gyrczuk E, Horvath A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2013;162:257–62. doi: 10.1016/j.jpeds.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:2107. doi: 10.1136/bmj.g2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chau K, Lau E, Greenberg S, et al. Probiotics for infantile colic: a randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. J Pediatr. 2015;166:74–8. doi: 10.1016/j.jpeds.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Mi GL, Zhao L, Qiao DD, Kang WQ, Tang MQ, Xu JK. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: a prospective single blind randomized trial. Antonie Van Leeuwenhoek. 2015;107:1547–53. doi: 10.1007/s10482-015-0448-9. [DOI] [PubMed] [Google Scholar]
- 14.Pärtty A, Lehtonen L, Kalliomäki M, Salminen S, Isolauri E. Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: a randomized, controlled trial. Pediatr Res. 2015;78:470–5. doi: 10.1038/pr.2015.127. [DOI] [PubMed] [Google Scholar]
- 15.Kianifar H, Ahanchian H, Grover Z, et al. Synbiotic in the management of infantile colic: a randomised controlled trial. J Paediatr Child Health. 2014;50:801–5. doi: 10.1111/jpc.12640. [DOI] [PubMed] [Google Scholar]
- 16.Urbańska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327–37. doi: 10.1007/s00431-014-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harb T, Matsuyama M, David M, Hill RJ. Infant colic-what works: a systematic review of interventions for breast-fed infants. J Pediatr Gastroenterol Nutr. 2016;62:668–86. doi: 10.1097/MPG.0000000000001075. [DOI] [PubMed] [Google Scholar]