Abstract
Purpose:
The purpose of this study was to determine whether pulmonary oligometastases from colorectal cancer have greater radioresistance than that of pulmonary oligometastases from other cancers and whether good local control can be achieved by dose escalation in stereotactic body radiotherapy.
Materials and Methods:
This systematic review and meta-analysis were conducted according to the preferred reporting items for systematic reviews and meta-analyses statement and methods. Studies were obtained from a database search of PubMed, Web of Science, and Google Scholar for publications using search terms designed to identify studies on “oligometastases,” “lung,” “stereotactic radiotherapy,” and “colorectal cancer.” For meta-analysis 1, studies that showed the number of local failures after stereotactic body radiotherapy for pulmonary metastases from colorectal carcinoma and other cancers were included. For meta-analysis2, studies in which a comparison was made of local control rates of pulmonary metastases from colorectal carcinoma by stereotactic body radiotherapy with a higher dose and that with a lower dose were included. A meta-analysis was performed using Mantel-Haenszel statics with the fixed or random-effect model by Review Manager 5.3.
Results:
Eighteen retrospective studies with 1920 patients with pulmonary oligometastases were used in meta-analysis 1. The local control rate in patients with pulmonary oligometastases from colorectal cancer was significantly lower than that in patients with pulmonary oligometastases from other cancers (odds ratio = 3.10, P < .00001). Next, 8 retrospective studies with 478 patients were included in meta-analysis 2 for dose escalation. Better local control was achieved by a higher prescription dose than by a lower prescription dose (odds ratio = 0.16, P < .00001).
Conclusion:
Our meta-analysis indicated that local control of pulmonary oligometastases from colorectal cancer by stereotactic body radiotherapy was significantly worse than that of pulmonary metastases from other cancers; however, our results also indicated that good local control of pulmonary oligometastases from colorectal cancer can be achieved by dose escalation.
Keywords: oligometastases, colorectal cancer, stereotactic radiotherapy, lung metastases, meta-analysis
Pulmonary oligometastases from colorectal cancer should be resected as much as possible.1 Stereotactic body radiotherapy (SBRT) for pulmonary oligometastases has been used commonly as an alternative method to metastomy in patients who cannot receive surgery; however, some studies have shown that pulmonary oligometastases from colorectal cancer are more difficult to control by SBRT than are pulmonary oligometastases from other cancers.2–4 On the other hand, some researchers have reported that there was no significant difference in local control.5 All of the studies were relatively small studies, and to the best of our knowledge, there has been no prospective study in which this issue was evaluated. Whether SBRT can be a practical alternative treatment to metastomy has remained controversial. We therefore evaluated local control by SBRT for pulmonary oligometastases from colorectal cancer compared to local control by SBRT for pulmonary oligometastases from other cancers using pooled analysis. There have been some studies showing that dose escalation could achieve better local control in patients who received SBRT for pulmonary oligometastases from colorectal cancer.4,6 Unfortunately, there has also been no prospective study on this issue. Since pulmonary oligometastases from colorectal cancer might have greater radioresistance, we also evaluated the efficacy of dose escalation in SBRT for pulmonary oligometastases from colorectal cancer using meta-analyses.
Purpose
The purpose of this study was to determine whether pulmonary oligometastases from colorectal cancer have greater radioresistance than that of pulmonary oligometastases from other cancers and whether good local control can be achieved by dose escalation in SBRT.
Materials and Methods
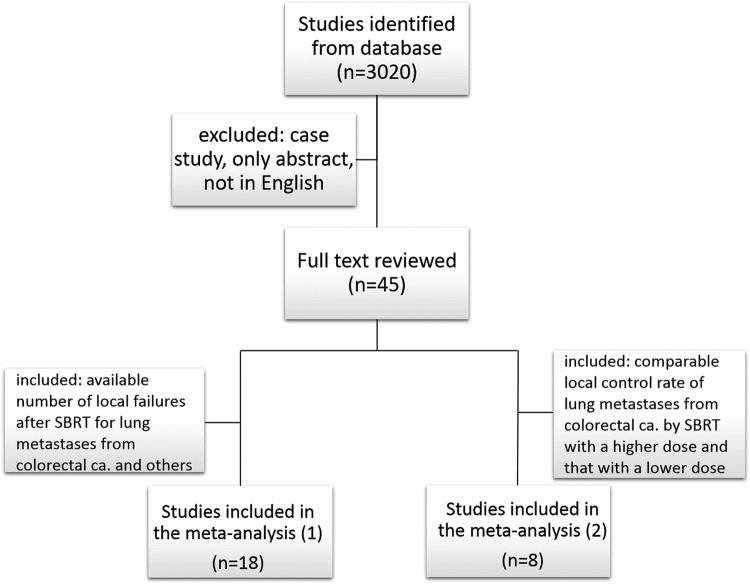
This systematic review and meta-analysis were conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and methods. Studies were obtained from a database search of PubMed, Web of Science, and Google Scholar for publications up until December 2017 using search terms designed to identify studies on “oligometastases,” “lung,” “stereotactic radiotherapy,” and “colorectal cancer.” The exclusion criteria were as follows: (1) case report, editorial, and specialist experience; (2) only abstract; and (3) articles written in languages other than English (Figure 1). Two investigators (K.J. and H.M.) selected trials independently for 2 meta-analyses to determine whether pulmonary oligometastases from colorectal cancer have greater radioresistance than pulmonary oligometastases from other cancers (meta-analysis 1) and whether good local control can be achieved by dose escalation in SBRT (meta-analysis 2). For meta-analysis 1, studies that showed the number of local failures after SBRT for lung metastases from colorectal carcinoma and other cancers were included. For meta-analysis 2, studies in which a comparison was made of local control rates for lung metastases from colorectal carcinoma by SBRT with a higher dose and that with a lower dose were included.
Figure 1.
Flow diagram of the search process.
The corresponding authors of the candidate studies were contacted via e-mail in the case of missing data or the requirement for additional information regarding their studies. A meta-analysis was performed using Mantel-Haenszel statics with the fixed or random-effect model by Review Manager 5.3 (Cochrane Collaboration, London, United Kingdom). Dichotomous data were calculated by the odds ratio (OR) with 95% confidence intervals (CIs).
The Q test was used to calculate the inconsistency index I 2 value. Due to the low sensitivity of the Cochrane Q test, the significance level α = 0.1 was used for conservation, with P > .1 indicating no statistical heterogeneity between studies and P < .1 indicating heterogeneity. Inconsistency index I2 was used to quantitatively evaluate heterogeneity. When I 2 was <25%, the fixed effect model was used for meta-analysis. When I 2 was more than 25% and less than 50%, the random effect model was used. When I 2was more than 50%, the source of the heterogeneity was analyzed first, and if there was no obvious clinical heterogeneity and the source of heterogeneity could not be found, the random effect model was used. A P < .05 was considered significant for all analyses.
Results
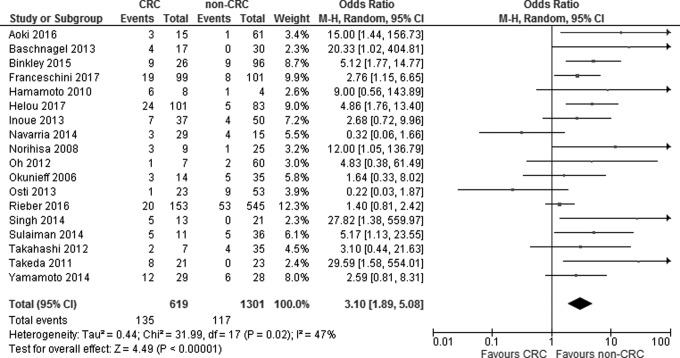
Figure 1 shows the results of the search strategy and all of the studies that were included and excluded. Data from 18 retrospective studies with 1920 patients were used in the meta-analysis. The patients included 619 patients with pulmonary oligometastases from colorectal cancer treated by SBRT and 1301 patients with pulmonary oligometastases from other cancers treated by SBRT (meta-analysis 1;2–5,7–20 Table 1). The local control rate in patients with pulmonary oligometastases from colorectal cancer was significantly lower than that in patients with pulmonary oligometastases from other cancers (OR = 3.17, 95% CI: 1.98-5.08, P < .00001) with substantial heterogeneity (P = .02, I2 = 47%; Figure 2). Funnel plots showed that there was no significant publication bias (Figure 3).
Table 1.
Characteristics of Studies Included in the Meta-Analysis 1.
| Author | No. of Patients | No. of Failures | Median Follow-up Period | Dose/Fraction | Median BED10 | Local Control Rate | |
|---|---|---|---|---|---|---|---|
| Aoki7 | CRC* | 15 | 3 | 31.7 months | 50 Gy/5 fractions | 100 GyBED | 3 years: 47.6% |
| non-CRC | 61 | 1 | 3 years: 97.5% | ||||
| Baschnagel8 | CRC | 17 | 4 | 27.6 months | 60 Gy/4 fractions | 132 GyBED | 2 years: 80% |
| non-CRC | 30 | 0 | 2 years: 100% | ||||
| Binkley9 | CRC | 26 | 9 | 22 months | 25 Gy/1 faraction or 50 Gy/4 fractions | 85 GyBED | 2 years: 57.6% |
| non-CRC | 96 | 9 | 2 years: 90.1% | ||||
| Franceschini10 | CRC | 99 | 19 | 24.2 months | 48 Gy/4 fractions | 105.6 GyBED | 3 years: 75.7% |
| non-CRC | 101 | 8 | 3 years: 88.2% | ||||
| Hamamoto12 | CRC | 8 | 6 | 19 months | 48 Gy/4 fractions | 105.6 GyBED | 25% |
| non-CRC | 4 | 1 | 75% | ||||
| Helou4 | CRC | 101 | 24 | 22 months | 52 Gy/4 fractions | 119.6 GyBED | 2 years: 76.4% |
| non-CRC | 83 | 5 | 2 years: 91.7% | ||||
| Inoue13 | CRC | 37 | 7 | NA | 48 Gy/4 fractions | 105.6 GyBED | 81% |
| non-CRC | 50 | 4 | 92% | ||||
| Navarria14 | CRC | 29 | 3 | 18 months | 48 Gy/4 fractions | 105.6 GyBED | 89.7% |
| non-CRC | 15 | 4 | 73.3% | ||||
| Norihisa21 | CRC | 9 | 3 | 27 months | 48 Gy/4 fractions | 105.6 GyBED | 66.7% |
| non-CRC | 25 | 1 | 96% | ||||
| Oh15 | CRC | 7 | 1 | 21 months | 60 Gy/5 fractions | 132 GyBED | 85.7% |
| non-CRC | 60 | 2 | 96.7% | ||||
| Okunieff16 | CRC | 14 | 3 | 14.9 months | 50 Gy/ 10 fractions | 75 GyBED | 78.6% |
| non-CRC | 35 | 5 | 85.7% | ||||
| Osti5 | CRC | 23 | 1 | 15 months | 30 Gy/1 fraction | 120 GyBED | 95.7% |
| non-CRC | 53 | 9 | 83.0% | ||||
| Rieber11 | CRC | 153 | 20 | 14.3 months | NA | 84.4 GyBED | 86.9% |
| non-CRC | 545 | 53 | 90.3% | ||||
| Singh17 | CRC | 13 | 5 | 16.7 months | 50 Gy/5 fractions | 100 GyBED | 61.50% |
| non-CRC | 21 | 0 | 100% | ||||
| Sulaiman18 | CRC | 11 | 5 | 17 months | NA | 110 GyBED | 54.50% |
| non-CRC | 36 | 5 | 86.10% | ||||
| Takahashi19 | CRC | 7 | 2 | 20 months | 48 Gy/4 fractions | 105.6 GyBED | 2 years: 67% |
| non-CRC | 35 | 4 | 2 years: 89% | ||||
| Takeda3 | CRC | 21 | 8 | 29 months | 50 Gy/5 fractions | 100 GyBED | 2 years: 73% |
| non-CRC | 23 | 0 | 15 months | 2 years: 94% | |||
| Yamamoto2 | CRC | 29 | 12 | 35 months | 48 Gy/4 fractions | 105.6 GyBED | 2 years: 25.5% |
| non-CRC | 28 | 6 | 2 years: 70.0% |
Abbreviations: BED, biological effective dose; CRC, colorectal cancer; NA, not available.
Figure 2.
Forest plot showing the association between local control rate and subgroup (colorectal cancer vs others).
Figure 3.
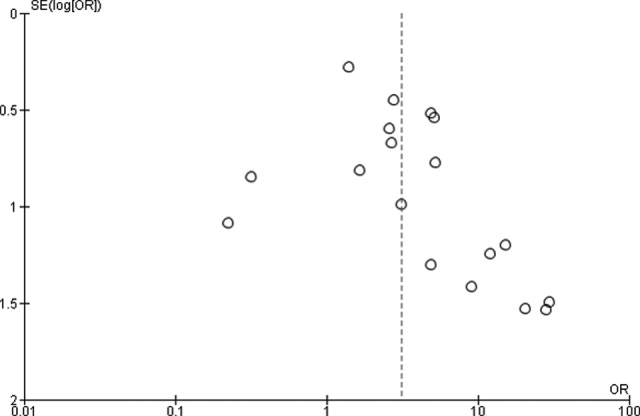
Funnel plots for publication bias for local control in patients with pulmonary metastases from colorectal cancer compared with that in patients with pulmonary metastases from other cancers.
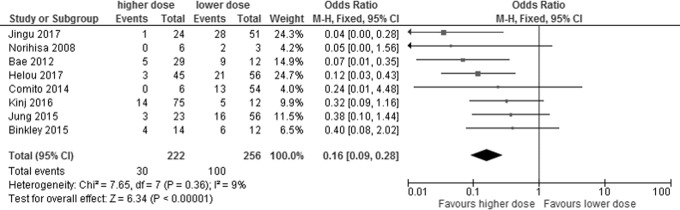
Among the studies on SBRT for pulmonary metastases from colorectal cancer, 8 retrospective studies with 478 patients were included in the meta-analysis for dose escalation: 222 patients who were treated with a higher dose and 256 patients who were treated with a lower dose (meta-analysis [2])4,6,9,20–24 (Table 2). Better local control was achieved by a higher prescription dose than by a lower prescription dose (OR = 0.16, 95% CI: 0.09-0.28, P < .00001) with no statistical heterogeneity (P = .36, I2 = 9%; Figure 4). Funnel plots showed that there was no significant publication bias (Figure 5).
Table 2.
Characteristics of Studies Included in the Meta-Analysis 2.
| Author | Median Follow-up Period | Higher Dose Group | Lower Dose Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median BED10 | No. of Patients | No. of Failures | Local Control Rate | Median BED10 | No. of Patients | No. of Failures | Local Control Rate | ||
| Jingu6 | 28 months | 132 GyBED | 24 | 1 | 3 years: 95.5% | 105.6 GyBED | 51 | 28 | 3 years: 59.6% |
| Norihisa20 | 27 months | 132 GyBED | 6 | 0 | 3 years: 100% | 105.6 GyBED | 3 | 2 | NA |
| Bae21 | 28 months | 180 GyBED | 29 | 5 | 3 years: 69% | 124.8 GyBED | 12 | 9 | 3 years: 49% |
| Helou4 | 22 months | 150 GyBED | 45 | 3 | 2 years: 90% | 119.6 GyBED | 56 | 21 | 2 years: 70% |
| Kinj22 | 33 months | 180 GyBED | 75 | 14 | 2 years: 82.1% | 87.5 GyBED | 12 | 5 | 2 years: 57.1% |
| Comito23 | 24 months | 180 GyBED | 6 | 0 | 3 years: 100% | 105.6 GyBED | 54 | 13 | 3 years: 70% |
| Jung24 | 42.8 months | 150 GyBED | 23 | 3 | 3 years: 84% | 105.6 GyBED | 56 | 16 | 3 years: 64.6% |
| Binkley9 | 22 months | 112.5 GyBED | 14 | 4 | 2 years: 62.5% | 87.5 GyBED | 12 | 6 | 2 years: 16.7% |
Abbreviations: BED, biological effective dose; NA, not available.
Figure 4.
Forest plot showing the association between local control rate and subgroup (higher dose vs lower dose) in patients with pulmonary oligometastases from colorectal cancer.
Figure 5.
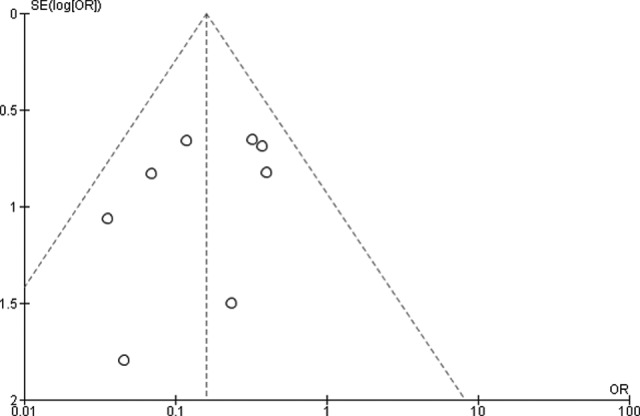
Funnel plots for publication bias for local control with higher dose in patients with pulmonary metastases from colorectal cancer compared to that of lower dose in patients with pulmonary metastases from other cancers.
Discussion
First, our results showed that it was more difficult to control pulmonary oligometastases from colorectal cancer by SBRT than pulmonary oligometastases from other cancers.
Some investigators have reported that metastases from colorectal cancer have radioresistance. Laarhoven et al showed that metastases of colorectal cancer contain large amounts of hypoxic cells compared to those in metastases of other cancers and are therefore radioresistant25; however, this must be only one of the reasons for local control by SBRT for metastases from colorectal cancer being poor. In our previous study, we showed that pulmonary oligometastases from colon cancer was more difficult to control by SBRT than those from rectal cancer.6 This may be due to molecular differences (eg, KRAS and BRAF status and microsatellite instability); however, the exact reasons are also unknown.
Next, the present meta-analysis indicated that dose escalation was important for local control of pulmonary oligometastases from colorectal cancer as well as hepatic oligometastases26; however, the appropriate total dose and appropriate dose per fraction in SBRT for pulmonary oligometastases from colorectal cancer have still not been determined. In past studies, there were notable differences in prescription methods (eg, for the isocenter and for the periphery of the planning target volume [PTV]) as well as in total dose and dose per fraction. In some studies, 2- to 3-year local control rates by 100 to 105.6 GyBED10, calculated using the linear-quadratic (LQ) model with α/β = 10 Gy, with prescription for the isocenter were 24% to 75.7%,2,4,7,10,19,24,27 while in other studies, 2- to 3-year local control rates by 95.8 to 150 GyBED10 with prescription for the periphery of the PTV were 52.7% to 100%,3,8–10,15,21,22,24,27-31,32 although there were many variations in prescription methods for the periphery of the PTV. Klement reported that the α–β ratio of pulmonary metastases from noncolorectal cancer was 21.6 and that the α–β ratio of pulmonary metastases from colorectal cancer was 43.1.33 If the α–β ratio of pulmonary metastases from colorectal cancer is very high as Klement reported, both the total dose and dose per fraction are important. He recommended more than 3 × 17 Gy to be given over a course of 5 days to the isocenter in order to control 90% of metastases from colorectal cancer after 1 year. It is difficult to determine the appropriate prescription dose because there are many differences among studies; however, the present meta-analysis suggested that better local control would be achieved by a higher dose. We recommend a prescription dose of >100 Gy of BED10 to the periphery of the PTV in SBRT for pulmonary oligometastases from colorectal cancer. In some past studies, oligometastases including those in the liver, lung, and lymph nodes were analyzed collectively. However, Ahmed et al showed that control of liver metastases was more difficult than that of lung metastases.34 Furthermore, Ahmed et al and Fode et al revealed that pulmonary metastases could be controlled more easily than metastases in other sites.28,35 Thus, investigation that includes oligometastases in several organs is not appropriate. In the present study, we therefore used data only for patients with pulmonary oligometastases.
A retrospective study by the Japanese Radiation Oncology Study Group showed, by multivariate analysis, that adjuvant chemotherapy after SBRT was a favorable prognostic factor for local control in patients with pulmonary oligometastases from colorectal cancer.6 Thibault et al also showed by multivariate analysis that previous chemotherapy improved local control of lung metastases treated by SBRT.36 Systemic therapy with SBRT might improve not only overall survival but also local control; however, the safety and efficacy of systemic therapy with SBRT have still not been established. Prospective studies on SBRT concurrent with systemic therapy including molecular targeted drug therapy for oligometastases are needed.
There was a major limitation in the present study. Most of the data used for analyses were from retrospective studies except for a few phase II studies, which were relatively small-scale studies, because there were no randomized trials to evaluate our queries. However, to the best of our knowledge, this is the first pooled analysis in patients treated by SBRT for pulmonary oligometastases from colorectal cancer. Prospective large randomized trials are needed.
Conclusion
Our meta-analysis indicated that local control of pulmonary oligometastases from colorectal cancer by SBRT was significantly worse than that of pulmonary metastases from other cancers; however, the results of the present study also indicated that good local control of pulmonary oligometastases from colorectal cancer can be achieved by dose escalation.
Acknowledgments
We are very grateful to Dr Helou, Dr Rieber, Prof Guckenberger, Prof Okunieff, Prof Milano, Dr Thibault, Prof Cheung, Dr Matsuo, and Dr Norihisa for providing unpublished data.
Abbreviations
- CI
confidence interval
- OR
odds ratio
- PRISMA
preferred reporting items for systematic reviews and meta-analyses
- PTV
planning target volume
- SBRT
Stereotactic body radiotherapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a Grant-in-Aid for Scientific Research (B); 16672039 from the Ministry of Education, Science, Sports, and Culture of Japan
ORCID iD: Keiichi Jingu, MD, PhD  http://orcid.org/0000-0002-7032-1577
http://orcid.org/0000-0002-7032-1577
Haruo Matsushita, MD, PhD  http://orcid.org/0000-0002-8615-2165
http://orcid.org/0000-0002-8615-2165
References
- 1. National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology for colon cancer. v.2. 2017. https://www.nccn.org/professionals/physician_gls/default.aspx#site.
- 2. Yamamoto T, Jingu K, Shirata Y, et al. Outcomes after stereotactic body radiotherapy for lung tumors, with emphasis on comparison of primary lung cancer and metastatic lung tumors. BMC Cancer. 2014;14:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takeda A, Kunieda E, Ohashi T, Aoki Y, Koike N, Takeda T. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol. 2011;101(2):255–259. [DOI] [PubMed] [Google Scholar]
- 4. Helou J, Thibault I, Poon I, et al. Stereotactic ablative radiation therapy for pulmonary metastases: histology, Dose, and Indication Matter. Int J Radiat Oncol Biol Phys. 2017;98(2):419–427. [DOI] [PubMed] [Google Scholar]
- 5. Osti MF, Carnevale A, Valeriani M, et al. Clinical outcomes of single dose stereotactic radiotherapy for lung metastases. Clin Lung Cancer. 2013;14(6):699–703. [DOI] [PubMed] [Google Scholar]
- 6. Jingu K, Matsuo Y, Onishi H, et al. Dose escalation improves outcome in stereotactic body radiotherapy for pulmonary oligometastases from colorectal cancer. Anticancer Res. 2017;37(5):2709–2713. [DOI] [PubMed] [Google Scholar]
- 7. Aoki M, Hatayama Y, Kawaguchi H, et al. Stereotactic body radiotherapy for lung metastases as oligo-recurrence: a single institutional study. J Radiat Res. 2016;57(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baschnagel AM, Mangona VS, Robertson JM, Welsh RJ, Kestin LL, Grills IS. Lung metastases treated with image-guided stereotactic body radiation therapy. Clin Oncol (R Coll Radiol). 2013;25(4):236–241. [DOI] [PubMed] [Google Scholar]
- 9. Binkley MS, Trakul N, Jacobs LR, et al. Colorectal histology is associated with an increased risk of local failure in lung metastases treated with stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):1044–1052. [DOI] [PubMed] [Google Scholar]
- 10. Franceschini D, Cozzi L, De Rose F, et al. Role of stereotactic body radiation therapy for lung metastases from radio-resistant primary tumours. J Cancer Res Clin Oncol. 2017;143(7):1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rieber J, Streblow J, Uhlmann L, et al. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases—A pooled analysis of the German working group “stereotactic radiotherapy.” Lung Cancer. 2016;97:51–58. [DOI] [PubMed] [Google Scholar]
- 12. Hamamoto Y, Kataoka M, Yamashita M, et al. Local control of metastatic lung tumors treated with SBRT of 48 Gy in four fractions: in comparison with primary lung cancer. Jpn J Clin Oncol. 2010;40(2):125–129. [DOI] [PubMed] [Google Scholar]
- 13. Inoue T, Oh RJ, Shiomi H, Masai N, Miura H. Stereotactic body radiotherapy for pulmonary metastases. Prognostic factors and adverse respiratory events. Strahlenther Onkol. 2013;189(4):285–292. [DOI] [PubMed] [Google Scholar]
- 14. Navarria P, Ascolese AM, Tomatis S, et al. Stereotactic body radiotherapy (SBRT) in lung oligometastatic patients: role of local treatments. Radiat Oncol. 2014;9(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oh D, Ahn YC, Seo JM, et al. Potentially curative stereotactic body radiation therapy (SBRT) for single or oligometastasis to the lung. Acta Oncol. 2012;51(5):596–602. [DOI] [PubMed] [Google Scholar]
- 16. Okunieff P, Petersen AL, Philip A, et al. Stereotactic body radiation therapy (SBRT) for lung metastases. Acta Oncol. 2006;45(7):808–817. [DOI] [PubMed] [Google Scholar]
- 17. Singh D, Chen Y, Hare MZ, et al. Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung. J Thorac Dis. 2014;6(4):369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sulaiman NS, Fujii O, Demizu Y, et al. Particle beam radiation therapy using carbon ions and protons for oligometastatic lung tumors. Radiat Oncol. 2014;9:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takahashi W, Yamashita H, Niibe Y, Shiraishi K, Hayakawa K, Nakagawa K. Stereotactic body radiotherapy for metastatic lung cancer as oligo-recurrence: an analysis of 42 cases. Pulm Med. 2012;2012:454107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72(2):398–403. [DOI] [PubMed] [Google Scholar]
- 21. Bae SH, Kim MS, Cho CK, et al. High dose stereotactic body radiotherapy using three fractions for colorectal oligometastases. J Surg Oncol. 2012;106(2):138–143. [DOI] [PubMed] [Google Scholar]
- 22. Kinj R, Bondiau PY, François E, et al. Radiosensitivity of colon and rectal lung oligometastasis treated with stereotactic ablative radiotherapy. Clin Colorectal Cancer. 2017;16(3):e211–e220. [DOI] [PubMed] [Google Scholar]
- 23. Comito T, Cozzi L, Clerici E, et al. Stereotactic ablative radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: a safe and effective approach. BMC Cancer. 2014;14:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung J, Song SY, Kim JH, et al. Clinical efficacy of stereotactic ablative radiotherapy for lung metastases arising from colorectal cancer. Radiat Oncol. 2015;10:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Laarhoven HW, Kaanders JH, Lok J, et al. Hypoxia in relation to vasculature and proliferation in liver metastases in patients with colorectal cancer. Int J Radiat Oncol Biol Phys. 2006;64(2):473–482. [DOI] [PubMed] [Google Scholar]
- 26. Kim MS, Yoo SY, Cho CK, et al. Stereotactic body radiation therapy using three fractions for isolated lung recurrence from colorectal cancer. Oncology. 2009;76:212–219. [DOI] [PubMed] [Google Scholar]
- 27. Carvajal C, Navarro-Martin A, Cacicedo J, Ramos R, Guedea F. Stereotactic body radiotherapy for colorectal lung oligometastases: preliminary single-institution results. J BUON. 2015;20(1):158–165. [PubMed] [Google Scholar]
- 28. Ahmed KA, Fulp WJ, Berglund AE, et al. Differences between colon cancer primaries and metastases using a molecular assay for tumor radiation sensitivity suggest implications for potential oligometastatic SBRT patient selection. Int J Radiat Oncol Biol Phys. 2015;92(4):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lausch A, Sinclair K, Lock M, et al. Determination and comparison of radiotherapy dose responses for hepatocellular carcinoma and metastatic colorectal liver tumours. Br J Radiol. 2013;86(1027):20130147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Filippi AR, Badellino S, Ceccarelli M, et al. Stereotactic ablative radiation therapy as first local therapy for lung oligometastases from colorectal cancer: a single-institution cohort study. Int J Radiat Oncol Biol Phys. 2015;91(3):524–529. [DOI] [PubMed] [Google Scholar]
- 31. Ricco A, Davis J, Rate W, et al. Lung metastases treated with stereotactic body radiotherapy: the RSSearch® patient registry’s experience. Radiat Oncol. 2017;12(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takeda A, Sanuki N, Tsurugai Y, Oku Y, Aoki Y. Stereotactic body radiotherapy for patients with oligometastases from colorectal cancer: risk-adapted dose prescription with a maximum dose of 83-100 Gy in five fractions. J Radiat Res. 2016;57(4):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klement RJ. Radiobiological parameters of liver and lung metastases derived from tumor control data of 3719 metastases. Radiother Oncol. 2017;123(2):218–226. [DOI] [PubMed] [Google Scholar]
- 34. Ahmed KA, Caudell JJ, El-Haddad G, et al. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95(5):1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fode MM, Høyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol. 2015;114(2):155–160. [DOI] [PubMed] [Google Scholar]
- 36. Thibault I, Poon I, Yeung L, et al. Predictive factors for local control in primary and metastatic lung tumours after four to nine fraction stereotactic ablative body radiotherapy: a single institution’s comprehensive experience. Clin Oncol (R Coll Radiol). 2014;26(11):713–719. [DOI] [PubMed] [Google Scholar]