Short abstract
Objective
Previous studies of neuropathic pain have suggested that the P2X4 purinoceptor (P2X4R) in spinal microglia is essential for maintaining allodynia following nerve injury. However, little is known about its role in inflammatory soup-induced trigeminal allodynia, which closely mimics chronic migraine status. Here, we determined the contributions of P2X4R and related signaling pathways in an inflammatory soup-induced trigeminal allodynia model.
Methods
P2X4R gene and protein levels in the trigeminal nucleus caudalis were analyzed following repeated dural inflammatory soup infusions. p38, brain-derived neurotrophic factor, excitatory amino acid transporter 3, c-Fos, and calcitonin gene-related peptide protein levels in the trigeminal nucleus caudalis, as well as trigeminal sensitivity, were assessed among the different groups. Immunofluorescence staining was used to detect protein localization and expression in the trigeminal nucleus caudalis.
Results
Repeated inflammatory dural stimulation induced trigeminal hyperalgesia and the upregulation of P2X4R. Immunofluorescence revealed that P2X4R was expressed in trigeminal nucleus caudalis microglial cells. Blockage of P2X4R produced an anti-nociceptive effect, which was associated with an inhibition of inflammatory soup-induced increases in p38, brain-derived neurotrophic factor, excitatory amino acid transporter 3, c-Fos, and calcitonin gene-related peptide protein levels. The tyrosine receptor kinase B antagonist ANA-12 reversed trigeminal allodynia and the upregulation of excitatory amino acid transporter 3, c-Fos, and calcitonin gene-related peptide, whereas the agonist 7,8-dihydroxyflavone exacerbated these effects. Double immunostaining indicated that p38 and brain-derived neurotrophic factor were mainly expressed in microglial cells, whereas excitatory amino acid transporter 3 was primarily expressed in trigeminal nucleus caudalis neurons.
Conclusions
These data indicate that microglial P2X4R is involved in the regulation of excitatory amino acid transporter 3 via brain-derived neurotrophic factor-tyrosine receptor kinase B signaling following repeated inflammatory dural stimulation. Microglial P2X4R activation and microglia–neuron interactions in the trigeminal nucleus caudalis may play a role in the pathogenesis of migraine chronicity, and the modulation of P2X4R activation might be a potential therapeutic strategy.
Keywords: P2X4-receptor, excitatory amino acid transporter 3, brain-derived neurotrophic factor-tyrosine receptor kinase B signaling, chronic migraine, trigeminal nucleus caudalis
Introduction
P2X4 purinoceptor (P2X4R) upregulation in spinal microglia is thought to be involved in peripheral nerve injury-induced allodynia.1 Recent work by Fried et al.,2 studying blood–brain barrier (BBB) permeability, observed microglial activation coupled with increased BBB permeability following repeated dural inflammatory stimulation. However, the role of microglia, as well as P2X4R, in the pathogenesis of inflammatory soup (IS)-induced trigeminal allodynia, which closely mimics chronic migraine status, remains unclear.
Following peripheral nerve injury (PNI), P2X4R activation promotes the synthesis and release of brain-derived neurotrophic factor (BDNF) from microglia through the activation of p38-mitogen-activated protein kinase (p38-MAPK).3,4 The interaction between microglial P2X4R and neurons was further confirmed to be an important link in neuropathic pain via BDNF-tyrosine receptor kinase B (TrkB) signaling.3–6 Here, we investigated whether signaling between microglial P2X4R and neurons is also an essential link in chronic migraine status and whether BDNF also plays a crucial role in this process.
The role of microglial P2X4R in regulating glutamate receptor activation and GABAergic inputs has been thoroughly investigated in neuropathic pain.4,5,7,8 However, little evidence has been reported for its role in modulating excitatory amino acid transporters (EAATs) in the pathogenesis of neuropathic pain, as well as in migraine chronicity. Sodium-dependent EAATs are divided into five subtypes, namely, EAAT1-5, of which EAAT3 (EAAC1) is expressed in brain stem nuclei and is primarily localized in neurons, with high levels observed at postsynaptic sites.9,10 Therefore, EAAT3 may reasonably play a larger role in regulating regional glutamate (Glu) concentrations rather than global concentrations, which are primarily regulated by glial EAAT1 (GLAST) and EAAT2 (GLT-1) through recycling Glu into glutamine.10,11 Consistent with this hypothesis, recent studies demonstrated that a selective EAAT3 inhibitor produced anti-nociceptive effects in rats following periphery nerve injury, whereas EAAT1-2 inhibitors exacerbated pain behaviors by increasing extracellular glutamate.12 Accordingly, the effect of P2X4R on the regulation of EAAT3 expression and their potential relation to the development of trigeminal allodynia were explored in this study.
Previous studies have reported that expression of spinal EAATs is regulated by neurotrophic factors through the activation of Trk receptors and intracellular MAPK in neuropathic pain,13 though the exact mechanism remains unclear. Recent work in a depression model demonstrated that the regulation of glial GLT-1 via BDNF-TrkB signaling was involved in the antidepressant effects of ketamine.14 Therefore, the role of BDNF-TrkB signaling in the modulation of EAAT3 expression, as well as their possible effects on the development of trigeminal allodynia, were studied here.
To this end, we established an IS rat model of trigeminal allodynia, which closely mimics chronic migraine status. In this rat model, repeated dural stimulation with IS was administered to mimic repetitive dural nociceptor activation and the clinical features of migraineurs.15,16 In the present study, we first examined the localization and expression changes of P2X4R, p38, BDNF, and EAAT3. We then investigated whether EAAT3 is modulated by P2X4R through the administration of a P2X4R nonselective inhibitor, TNP-ATP. Finally, we examined the role of BDNF in the regulatory effect of P2X4R on EAAT3 using ANA-12 and 7,8-dihydroxyflavone (DHF), a selective TrkB receptor antagonist and agonist, respectively. The protein levels of calcitonin gene-related peptide (CGRP), a key neuropeptide implicated in the activation of trigeminovascular system,17–19 and c-Fos, a commonly used marker of neuronal activation after pain stimulation,20–23 were also analyzed.
Materials and methods
Animals
Adult male Sprague-Dawley rats (250–300 g, n = 87) were used for the experiments. Rats were housed in a temperature- (23 ± 2°C) and humidity-controlled (50% ± 10%) room with ad libitum access to water and food, under a 12-h light/dark cycle. All procedures performed on the animals were approved by the Ethics Committee of the Department of Medical Research at the First Affiliated Hospital of Chongqing Medical University.
Experimental design
Rats were randomly assigned to seven different experimental groups, as shown in Table 1. Two experiments were then conducted in rat models of trigeminal allodynia induced by repeated dural IS infusions.
Table 1.
Schematic representation of experimental groups, administration time (day, D), and sample size (number, n) of rats per group.
| Experimental group | D1–7 | D8 | RT-PCR (n) | WB (n) | IF (n) |
|---|---|---|---|---|---|
| Control (CON) | PBS | — | 6 | 6 | 3 |
| IS | IS | — | 6 | 6 | 3 |
| IS+PBS | IS | PBS | 6 | ||
| IS+TNP | IS | TNP-ATP (30 nmol) | 6 | 3 | |
| IS | TNP-ATP (60 nmol) | 6 | |||
| IS+DMSO | IS | DMSO | 6 | ||
| IS+ANA | IS | ANA-12 (100 nmol) | 6 | 3 | |
| IS | ANA-12 (200 nmol) | 6 | |||
| IS+DHF | IS | 7,8-DHF (20 nmol) | 6 | 3 | |
| IS | 7,8-DHF (40 nmol) | 6 |
RT-PCR: real-time polymerase chain reaction; WB: western blot; IF: immunofluorescence; IS: inflammatory soup; PBS: phosphate-buffered saline; DHF: 7,8-dihydroxyflavone; DMSO: dimethylsulfoxide.
Experiment 1
Rats in groups 1 to 4 were used to test the role of P2X4R and related pathways in IS-induced allodynia and the regulation of EAAT3. The P2X4R inhibitor 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate tetrasodium salt (TNP-ATP) was dissolved in phosphate-buffered saline (PBS) and slowly administered (5 μl/rat, intracerebroventricular injection (i.c.v.)) across two doses using a microinfusion pump (WPI, Sarasota, FL, USA) the next day after seven days of IS infusions. The doses for TNP-ATP used in the experiment were based on previous studies, which demonstrated clear inhibition of P2X4R.1,24 As a control, rats in group 3 received the same volume of PBS (pH 7.4).
Periorbital pressure thresholds were tested before IS or PBS infusions and 1 h after drug treatment, after which the animals were sacrificed. Following completion of the final threshold tests, other post-treatment assessments included real-time polymerase chain reaction (RT-PCR), western blotting, and immunofluorescence (IF).
Experiment 2
Rats in groups 1, 2, and 5 to 7 were used to investigate the underlying mechanisms by which P2X4R regulates the expression of EAAT3. The TrkB receptor specific antagonist N-[2-[[(Hexahydro-2-oxo-1H-azepin-3-yl)amino]carbonyl]phenyl]-benzo[b]thiophene-2-carboxamide (ANA-12; 5 μl/rat, i.c.v.) or the agonist 7,8-DHF (5 μl/rat, i.c.v.) were dissolved in dimethylsulfoxide and slowly administered across two doses the next day after seven days of IS infusions. The doses for ANA-12 and 7,8-DHF were based on previous findings that reported reliable effects.25–27 Rats in group 5 received the same volume of dimethylsulfoxide as a control.
Periorbital pressure thresholds were tested before IS or PBS infusions and 1 h after drug treatment, after which the animals were sacrificed. Following completion of the final threshold tests, other post-treatment assessments included western blotting and IF.
Craniotomy and cannula fixation
Surgical procedures were performed as described previously.15,16 Rats were fasted of food and water for 12 h before surgery to prevent abdominal dilation. Under anesthesia with 10% chloral hydrate (4 ml/kg, intraperitoneally), rats were placed in a stereotactic frame (ST-51603; Stoelting Co., Chicago, IL, USA). Following local infiltration anesthesia with lidocaine (0.1 g/5 ml), an incision was made to expose the skull completely. A 1-mm diameter craniotomy (+1.5 mm from bregma, +1.5 mm lateral) was carefully performed using a burr drill, avoiding any damage to the dura mater.15 Next, a stainless-steel cannula with a plastic cap (Guide Cannula for Rat, Item No.: 900–0062-001, O.D.: 0.64 mm; RWD Life Science, Shenzhen, China) was placed just above the skull and fixed with dental acrylic. Rats were allowed to recover for 1 week and then randomly assigned for the subsequent experiments.
Infusion of IS or saline
Rats were placed in a transparent glass chamber (22 × 22 × 30 cm) that allowed for free movement during infusion. The IS contained 1 mM each of histamine, serotonin, bradykinin, and 0.1 mM prostaglandin E2 in PBS, pH 7.4 (Sigma-Aldrich, St. Louis, MO, USA) (adapted from Strassman et al.28). We steadily delivered 2 μl of IS or PBS over 5 min through a microinfusion pump that was attached to the top of the cannula via a polyethylene tube (PE50 Tubing, Item No.: 900–0062-301, 0.97 × 0.58 mm, RWD Life Science, Shenzhen, China). The same procedure was repeated once daily for seven days (from Day 1 to Day 7, Table 1).
Tactile sensory testing
After a habituation period of 10 min in the chamber, rats were tested for basal periorbital pressure thresholds prior to infusion and periorbital pressure thresholds 1 h after drug treatment. Pressure thresholds were determined by applying an electronic von Frey monofilament (Electrovonfrey, model no.: 2391, IITC Inc., Woodland Hills, CA, USA) to the periorbital region of the face over the rostral portion of the eye, as reported by Oshinsky and Gomonchareonsiri.29 The assigned force values of the von Frey device ranged from 0 to 800 g. The von Frey stimuli were gradually enhanced to determine the response threshold. A positive response was suggested when the rat stroked its face with the ipsilateral forepaw, quickly retracted its head from the stimulus, or vocalized. The pressure thresholds were recorded automatically and were determined three times at each site with an interval of at least 1 min.
Quantitative RT-PCR
Rats were sacrificed under chloral hydrate anesthesia. The trigeminal nucleus caudalis (TNC), 1 to 5 mm from the obex, according to the atlas by Paxinos and Watson,30 was rapidly separated and used for further analysis.31 Total RNA was extracted from TNC segments using the RNAiso Plus reagent (Takara) following the manufacturer’s instructions. Next, cDNA was synthesized using the PrimeScript™ RT reagent kit (Takara, Tokyo, Japan). RT-PCR was performed on a CFX96 Touch thermocycler (Bio-Rad) using the SYBR® Premix Ex Taq™ II (Takara). The following specific primers (Sangon Biotech, Shanghai, China) were used as follows: P2X4R_forward: 5′-TCG TGT GGG AAA AGG GCT AC-3′, P2X4R_reverse: 5′-GTC TGG TTC ACG GTG ACG AT-3′; GAPDH_forward: ATG ACT CTA CCC ACG GCA AGC T-3′, GAPDH_reverse: 5′-GGA TGC AGG GAT GAT GTT CT-3′. Relative gene expression was normalized to the internal reference GAPDH using the 2–ΔΔCTmethod.
Western blotting
Fresh tissue samples from TNC segments were homogenized in radioimmunoprecipitation assay buffer containing a mixture of proteinase inhibitors (Beyotime, China) and phosphatase inhibitors (Boster, China) at 4°C for 1.5 h. Protein concentrations were determined using the BCA protein assay kit (Beyotime, China). Protein samples were separated via 10% sodium dodecyl sulfate-polyacrylamide electrophoresis, and western blotting was performed using the following antibodies: P2X4R (Abcam; 1:800), pp38 (Santa Cruz; 1:500), p38 (Santa Cruz; 1:1000), BDNF (Santa Cruz; 1:300), EAAT3 (Bioss; 1:500), CGRP (Abcam; 1:2000), c-Fos (Santa Cruz; 1:500), and β-actin (Proteintech; 1:5000) as a loading control.
IF staining
Rats were anesthetized with chloral hydrate and subjected to cardiac perfusion with 200 ml of 0.9% saline, followed by 250 ml of 4% paraformaldehyde in 0.1 M PBS. The areas from the medulla oblongata to the first cervical cord were removed,31,32 fixed overnight in 4% paraformaldehyde, transferred to 30% sucrose in 0.1 M PBS until they sank to the bottom, frozen, and then serially and transversely sectioned (10 μm thick) through the segment of the TNC (approximately 1–5 mm from the obex, Figure 1) on a cryostat (Leica). For immunostaining, sections were rinsed three times in 0.1 M PBS before being incubated with 0.3% Triton X-100 for 10 min and 10% normal goat serum for 30 min at 37°C. Sections were incubated overnight at 4°C with primary antibodies against P2X4R (rabbit polyclonal antibody, 1:800, Abcam), pp38 (mouse monoclonal antibody; 1:100), BDNF (mouse monoclonal antibody; 1:100), EAAT3 (rabbit polyclonal antibody, 1:500, Bioss), p-TrkB (mouse monoclonal antibody; 1:100), CGRP (mouse monoclonal antibody, 1:500), and c-Fos (mouse monoclonal antibody, 1:4000). Markers for microglia (Iba-1; goat polyclonal antibody, 1:500, Abcam), astrocytes (glial fibrillary acidic protein, mouse monopoly antibody, 1:100, Abcam), and neurons (neuronal nuclei; mouse monoclonal antibody, 1:500, Abcam) were used to identify the types of positive cells. After being rinsed three times in PBS, sections were incubated overnight at 4°C with the corresponding secondary antibodies: cy3-conjugated anti-goat IgG (1:60, Proteintech), fluorescein isothiocyanate-conjugated anti-mouse IgG (1:60, Proteintech), fluorescein isothiocyanate-conjugated anti-rabbit IgG (1:60, Proteintech), or cy3-conjugated anti-rabbit IgG (1:60, Proteintech). The sections were then incubated with 6′-diamidino-2-phenylindole staining solution (Beyotime, China) at 37°C for 8 min after being rinsed three times with PBS. Negative-control sections were incubated with PBS instead of the corresponding primary antibodies and showed no positive signals. Sections were mounted and observed on a fluorescence microscope (Leica). The number of P2X4R-, c-Fos-, and EAAT3-immunoreactive cells in the TNC was measured using Image J software (version 1.8.0_112). Three images randomly selected under high-power magnification (200×) were obtained for TNC per sample.
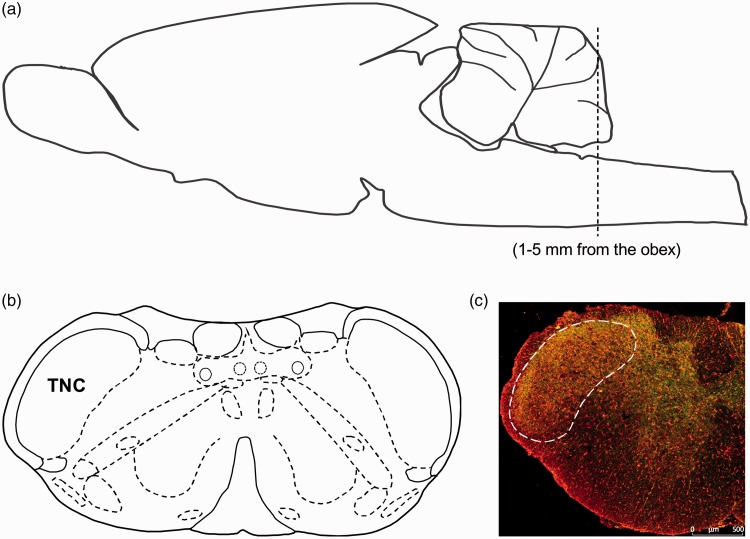
Figure 1.
Schematic representation of the brain region for TNC. (a) Side view of the rat brain to show the region of the TNC for analysis. The brain region for TNC was analyzed along the brainstem at approximately 1 to 5 mm from the obex. (b) The coronal view shows the location of TNC in the rat brain. (c) Immunofluorescence staining of coronal section representing the brain region for TNC (Scale bar = 500 μm). (a) and (b) are adapted from George Paxinos and Charles Watson.30
Statistical analysis
Data represent the mean ± standard error of the mean (SEM). SPSS 20.0 was used for statistical analysis. Differences between two groups were analyzed using the independent samples T test. Differences in mechanical thresholds were analyzed using two-way analysis of variance (ANOVA), and differences among other variables were analyzed using one-way ANOVA, both followed by a Tukey’s multiple comparison tests. Differences were considered statistically significant at P < 0.05.
Results
Decreased baseline mechanical thresholds following repeated dural stimulation
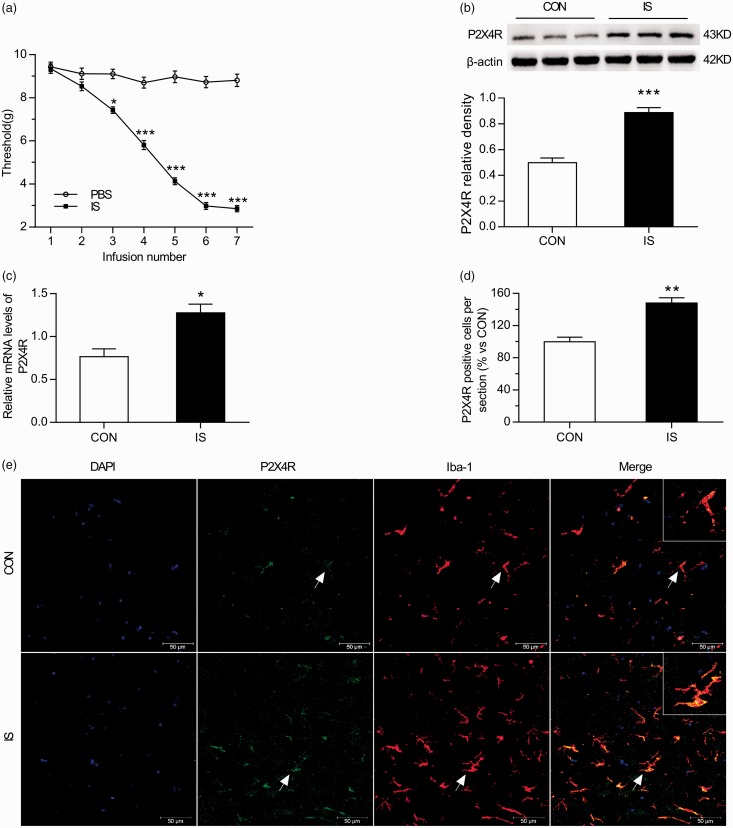
To investigate changes in the mechanical threshold following repeated dural IS infusions, we examined the baseline periorbital withdrawal thresholds using a von Frey monofilament prior to daily infusion with IS or PBS. Periorbital thresholds before infusions were not significantly different between the two groups (two-way ANOVA, P > 0.05; Figure 2(a)). However, the periorbital threshold decreased significantly in the IS group compared with the control group after the second infusion (two-way ANOVA, P < 0.05; Figure 2(a)). In addition, the periorbital thresholds reached a low level (∼3.0 g) after the fifth IS infusion onto the dura, suggesting the establishment of mechanical allodynia.
Figure 2.
Trigeminal sensitivity and P2X4R expression in the TNC increase with repeated dural stimulation. (a) Decreased baseline mechanical thresholds following repeated dural stimulation with IS. The basal mechanical thresholds of periorbital decreased in the IS group and were significantly different from the PBS group after the second infusion. (b) WB for P2X4R expression in the TNC (upper panel) revealed that P2X4R protein level was upregulated in the IS group compared with the CON group. Quantification (lower panel) of WB experiments normalized to actin control indicated an approximate twofold increase in IS group. (c) RT-PCR for P2X4R in TNC revealed that P2X4R mRNA level was significantly increased in the IS group compared with the CON group. All data were normalized to GAPDH controls. (e) Co-localization images of P2X4R (green) with Iba-1 (red) in the TNC reveal increased microglial activation and P2X4R expression in the IS group compared with the CON group. Blue indicates DAPI immunoreactivity, green indicates P2X4R immunoreactivity, red indicates Iba-1 immunoreactivity, and yellow indicates the merged signal. (d) Histogram showed the statistical result of P2X4R expression in TNC (e). Data represent the means ± SEM. Statistical analyses in (a) were performed by two-way ANOVA, followed by a Tukey test; *P < 0.05, ***P < 0.001 (n = 12 per group). Statistical analyses in ((b), (c) and (d)) were performed by independent samples T test; *P < 0.05, **P < 0.01, ***P < 0.001 (n = 6 per group in (b) and (c); n = 3 per group in (d)). Arrows indicate cells shown in the top right corner of images at approximately 4X magnification. Scale bar = 50 μm.
RT-PCR: real-time polymerase chain reaction; WB: western blot; IS: inflammatory soup; PBS: phosphate-buffered saline; CON: control.
P2X4R expression and microglial activation increases with repeated dural stimulation
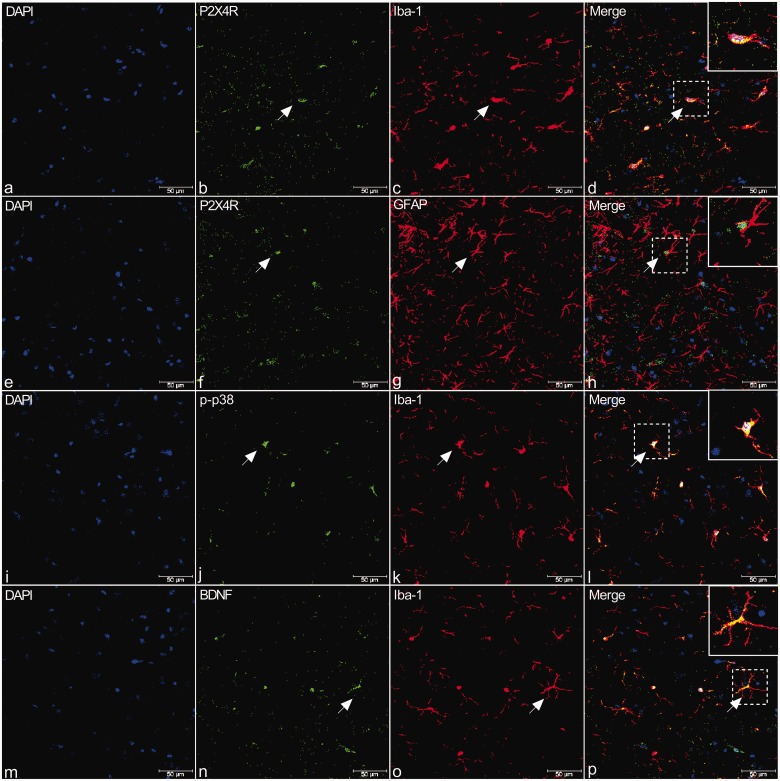
P2X4R mRNA and protein levels in the TNC of rats were examined after seven days of dural IS or PBS infusions. As shown in Figure 2(b) to (d), P2X4R expression was significantly increased in the IS group compared with the control (CON) group as found by RT-PCR (Independent samples T test, P < 0.05; Figure 2(c)), western blot (Independent samples T test, P < 0.001; Figure 2(b)), and IF (Independent samples T test, P < 0.01; Figure 2(d) to (e)). IF analysis revealed increased Iba-1 positive cells in TNC in the IS group compared with the CON group (ANOVA, P < 0.01; Supplemental Figure 1). Moreover, P2X4R expression was present in Iba-1-but not in glial fibrillary acidic protein-immunoreactive cells (Figure 4(a) to (h)). These results suggested that microglial activation and P2X4R expression were definitely increased in TNC microglia in rats stimulated by repeated dural infusion of IS.
Figure 4.
Double immunostaining of P2X4R (b, f, green), p-p38 (j, green), and BDNF (n, green) with Iba-1 (c, k, o, red), a microglial marker, or GFAP (g, red), an astrocyte marker, in the TNC of rats. (a) to (h) Immunofluorescence (IF) in TNC from rats revealed that P2X4R is present in Iba-1-but not in GFAP-immunoreactive cells (shown by arrows in a–h). (i) to (p) Similar to P2X4R, IF in TNC revealed that p-p38 and BDNF staining were observed primarily in Iba-1-positive cells (shown by arrows in i–p). Arrows indicate cells shown in the top right corner of images at approximately 4X magnification. Scale bar = 50 μm.
BDNF: brain-derived neurotrophic factor; DAPI: 6′-diamidino-2-phenylindole; P2X4R: P2X4 purinoceptor; GFAP: glial fibrillary acidic protein.
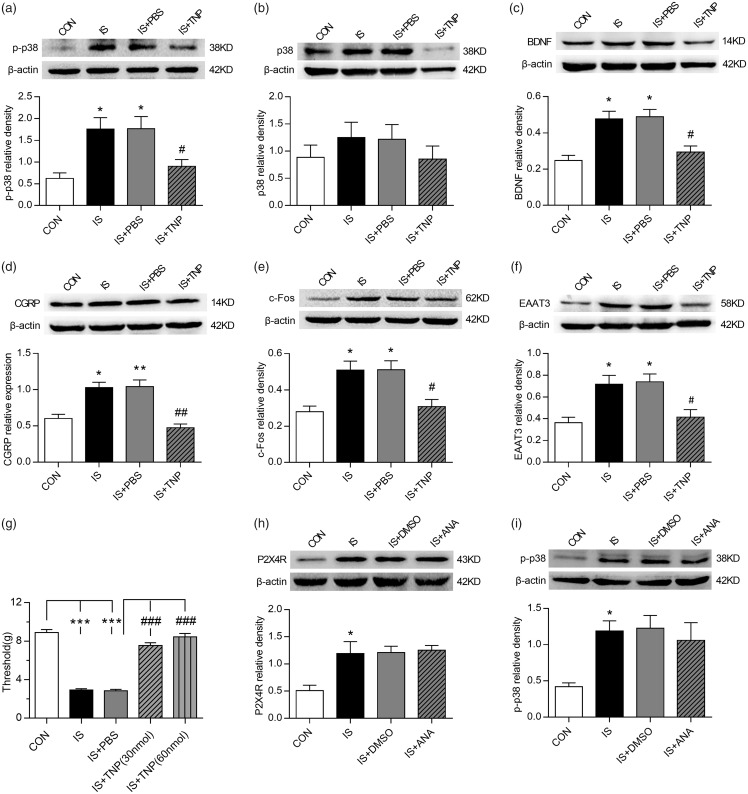
TNP-ATP treatment reverses trigeminal allodynia and microglial activation following repeated dural stimulation
To study whether P2X4R is relevant to the mechanical allodynia induced by repeated dural stimulation, the P2X4R antagonist TNP-ATP was administered the next day after the final IS infusion, and the mechanical threshold was detected using electronic von Frey monofilaments 1 h later. TNP-ATP administration significantly increased the periorbital pressure threshold (ANOVA, P < 0.001; Figure 3(g)). The IS+TNP-ATP (30 nmol) and IS+TNP-ATP (60 nmol) groups did not differ significantly (ANOVA, P > 0.05; Figure 3(g)). These results suggest that the P2X4R antagonist TNP-ATP alleviated mechanical allodynia in a concentration-independent manner, and the low-dose group was therefore selected for the subsequent experiments. IF analysis revealed that TNP-ATP (30 nmol) treatment reversed IS-induced Iba-1 positive cells increase in TNC compared with the IS group (ANOVA, P < 0.05; Supplemental Figure 1).
Figure 3.
P2X4R and related signaling pathways were involved in EAAT3 regulation and trigeminal allodynia following repeated dural stimulation. (a) WB for p-p38 expression in the TNC (upper panel) revealed that p-p38 protein level was increased in the IS group compared with the CON group. TNP-ATP (30 nmol) treatment repressed its expression as compared to the group of IS+PBS. Quantification (lower panel) of WB experiments was normalized to actin control. (b) WB for p38 expression in the TNC (upper panel) revealed no evident difference among the four groups. Quantification (lower panel) of WB experiments was normalized to actin control. (c) to (f): WB for BDNF (c), CGRP (d), c-Fos (e), EAAT3 (f) expression in the TNC (upper panel) revealed that BDNF, CGRP, c-Fos, and EAAT3 were all upregulated following repeated dural stimulation as compared to control. TNP-ATP (30 nmol) treatment decreased their protein levels compared with the group of IS+PBS. There were no obvious difference between the group of IS and IS +PBS. Quantification (lower panel) of all WB experiments was normalized to actin control. (g) TNP-ATP treatment significantly increased the basal periorbital pressure thresholds compared with IS+PBS group. The IS+TNP-ATP (30 nmol) and IS+TNP-ATP (60 nmol) groups showed no obvious difference in anti-nociceptive effect. (h) and (i): WB for P2X4R (h), p-p38 (i) expression in the TNC (upper panel) revealed that ANA-12 (100 nmol) treatment did not reverse IS-induced P2X4R and p-p38 upregulation. Quantification (lower panel) of WB experiments was normalized to actin control. Data represent the mean ± SEM. Statistical analyses were performed by one-way ANOVA, followed by a Tukey test; *P < 0.05, **P < 0.01, ***P < 0.001 vs. CON, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. IS+PBS (n = 6 per group).
WB: western blot; IS: inflammatory soup; CON: control; PBS: phosphate-buffered saline; BDNF: brain-derived neurotrophic factor; CGRP: calcitonin gene-related peptide; EAAT3: excitatory amino acid transporter 3.
P2X4R and related signaling pathways were involved in EAAT3 regulation and trigeminal allodynia following repeated dural stimulation
The protein levels of p-p38, p38, and BDNF were detected in the TNC of rats after seven days of dural IS stimulation and administration of the P2X4R inhibitor TNP-ATP. The expression of p-p38 and BDNF were strikingly upregulated following repeated dural stimulation, compared with the CON group (ANOVA, P < 0.05; Figure 3(a) to (c)). Moreover, TNP-ATP significantly suppressed p-p38 and BDNF expression in the TNC of rats (ANOVA, P < 0.05; Figure 3(a) to (c)). Because EAAT3 is involved in the glutamate-induced neuroplasticity underlying persistent pain,12 we further examined the regulatory role of microglial P2X4R in EAAT3, CGRP, and c-Fos expression. EAAT3, CGRP, and c-Fos expression in the TNC segments of rats was significantly increased in the IS group compared with the CON group (ANOVA, P < 0.05; Figure 3(d) to (f)). Furthermore, TNP-ATP administration reduced EAAT3, CGRP, and c-Fos expression (ANOVA, P < 0.05; Figure 3(d) to (f)). Immunostaining revealed that p38 and BDNF were primarily expressed in microglial cells (Figure 4(i) to (p)), while EAAT3 was expressed in TNC neurons and activated microglial cells (Figure 5(a) to (l)).
Figure 5.
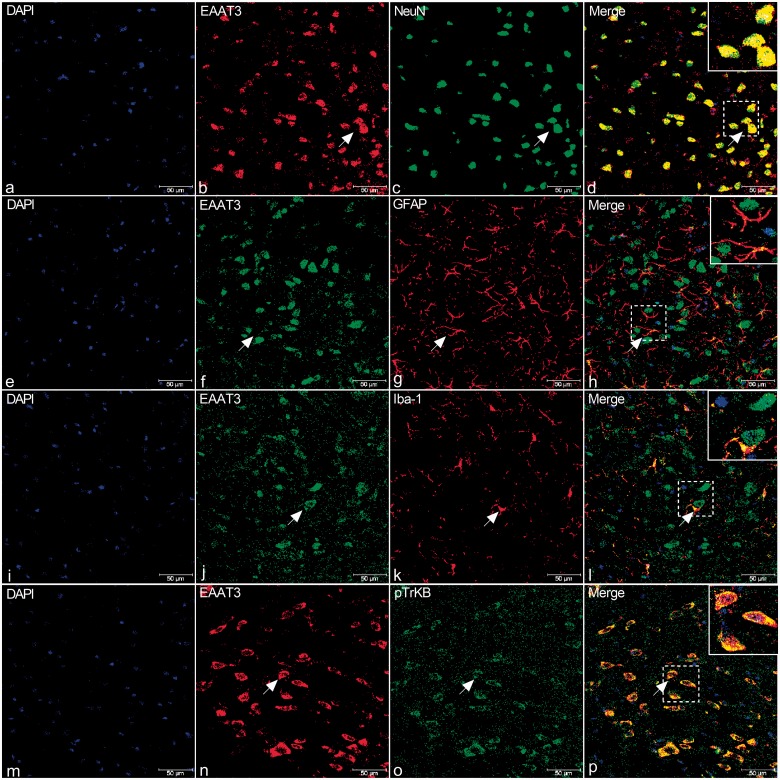
Double immunostaining of EAAT3 (b, n, red; f, j, green) with NeuN (c, green), GFAP (g, red), Iba-1 (k, red), and p-TrkB (o, green) in the TNC of rats. (a) to (l) Immunofluorescence (IF) in TNC of rats revealed that EAAT3 is present primarily in NeuN-positive cells (shown by arrows in a-d). There was relatively less expression in Iba-1-positive cells (i) to (l), and expression in GFAP-positive neurons was not detected (e) to (h). (m) to (p) IF in TNC of rats revealed that EAAT3 (n) and TrkB (o) co-expressed (yellow, p). Arrows indicate cells shown in the top right corner of images (d, h, l, p) at approximately 4X magnification. Scale bar = 50 μm.
DAPI: 6′-diamidino-2-phenylindole; EAAT3: excitatory amino acid transporter 3; GFAP: glial fibrillary acidic protein; TrkB: tyrosine receptor kinase B.
To further verify the establishment of the signaling cascade from P2X4R activation to BDNF release, we examined the effect of ANA-12 on P2X4R and p-p38 expression in TNC. ANA-12 treatment did not cause obvious changes in protein expression of P2X4R as well as p-p38 (ANOVA, P > 0.05; Figure 3(h) and (i)). The effect of TNP-ATP on trigeminal allodynia and p-p38, BDNF, CGRP, c-Fos, and EAAT3 expression in CON rats were also examined. Results showed that TNP-ATP (30 nmol) did not cause significant changes in the periorbital threshold or p-p38, BDNF, CGRP, c-Fos, and EAAT3 expression as compared to the group of CON+PBS (ANOVA, P > 0.05; Supplemental Figure 2(a) to (f)).
BDNF-TrkB pathways were involved in trigeminal allodynia and EAAT3 regulation following repeated dural stimulation
To further study whether BDNF is closely related to the mechanical allodynia induced by repeated IS stimulation, the TrkB antagonist ANA-12 and agonist 7,8-DHF were administered the next day after seven times of IS infusions. Electronic von Frey monofilaments were applied to test the mechanical threshold 1 h after drug intervention. Periorbital thresholds were markedly increased after ANA-12 administration and significantly decreased following 7,8-DHF administration (ANOVA, P < 0.05; Figure 6(a)). The low-dose and high-dose groups did not differ significantly, suggesting that ANA-12 treatment reverses mechanical allodynia by blocking the action of BDNF, whereas 7,8-DHF exacerbates this effect, in a concentration-independent manner. Therefore, the low-dose groups were selected for the subsequent experiments.
Figure 6.
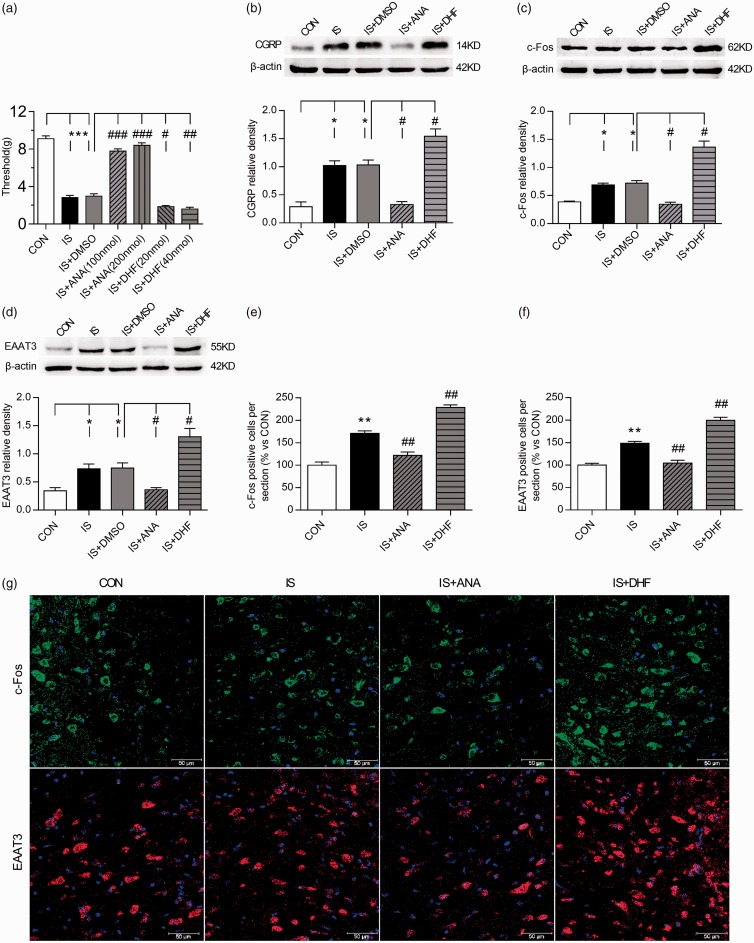
BDNF-TrkB pathways were involved in trigeminal allodynia and EAAT3 regulation following repeated dural stimulation. (a) ANA-12 treatment significantly increased the basal periorbital thresholds, while 7,8-DHF further reduce the thresholds as compared to the group of IS+DMSO. The low-dose and high-dose groups of ANA-12 as well as 7,8-DNF did not differ significantly. (b) to (d): WB for CGRP (b), c-Fos (c), and EAAT3 (d) expression in the TNC (upper panel) revealed that CGRP, c-Fos, and EAAT3 protein levels were all increased following repeated dural stimulation as compared to control. ANA-12 (100 nmol) treatment decreased their expression, while 7,8-DHF (20 nmol) caused a further increase compared with the group of IS+DMSO. There were no obvious difference between the group of IS and IS +DMSO. Quantification (lower panel) of all WB experiments was normalized to actin control. (g) Representative immunofluorescence samples of c-Fos and EAAT3 in the TNC in the CON, IS, IS+ANA (100 nmol), and IS+DHF (20 nmol) groups. Scale bar = 50 μm. (e) and (f) Histograms showed the statistical results of c-Fos and EAAT3 expression in TNC (g). Data represent the mean ± SEM. Statistical analyses were performed by one-way ANOVA, followed by a Tukey test; *P < 0.05, **P < 0.01, ***P < 0.001 vs. CON, #P < 0.05, ##P < 0.01, ###P < 0.001 vs. IS or IS+DMSO (n = 6 per group in a ∼ d; n = 3 per group in e and f).
IS: inflammatory soup; CON: control; DMSO: dimethylsulfoxide; DHF: 7,8-dihydroxyflavone; CGRP: calcitonin gene-related peptide; EAAT3: excitatory amino acid transporter 3.
Although previous studies have indicated that EAAT3 expression is regulated by neurotrophic factors, the underlying mechanisms remain unclear. We further investigated the regulatory effects of BDNF on EAAT3, CGRP, and c-Fos expression. The TrkB antagonist ANA-12 significantly reduced EAAT3, CGRP, and c-Fos expression in the TNC compared with the IS group (ANOVA, P < 0.05; Figure 6(b) to (g)). Whereas administration of the TrkB agonist 7,8-DHF further elevated EAAT3, CGRP, and c-Fos expression (ANOVA, P < 0.05; Figure 6(b) to (g), the typical IF samples for c-Fos and EAAT3 were showed in Figure 6(g)).
The effect of ANA-12 and 7,8-DHF on trigeminal allodynia and CGRP, c-Fos, and EAAT3 expression in CON rats were also examined. Results showed that ANA-12 (100 nmol) did not cause significant changes in the periorbital threshold or CGRP, c-Fos, and EAAT3 expression as compared to the group of CON+PBS (ANOVA, P > 0.05; Supplemental Figure 2(a, d–f)). 7,8-DHF (20 nmol) treatment evidently decreased the thresholds while increased the expression of CGRP, c-Fos, and EAAT3 as compared to the group of CON+PBS (ANOVA, P < 0.05; Supplemental Figure 2(a, d–f)).
Discussion
Previous studies of neuropathic pain have reported that P2X4R in spinal microglia is crucial for maintaining PNI-induced allodynia.1,4,6 The present study reveals a potential role for microglial P2X4R in the regulation of EAAT3 in the IS rat model of trigeminal allodynia. Specifically, the activation of microglial P2X4R may cause p38 activation and eventually promote EAAT3 expression via BDNF-TrkB signaling following repeated dural inflammatory stimulation. Therefore, microglial activation may play a role in the pathogenesis of migraine chronicity.
Decreased baseline mechanical thresholds following repeated dural stimulation
Repeated infusion of IS onto the dura is a commonly used rat model of migraine.15 Chemical stimulation of the dura is believed to activate the trigeminovascular system and lead to trigeminal allodynia that is similar to the common symptoms observed in migraineurs.28 In the present study, periorbital thresholds decreased significantly following the second IS infusion and reached to a low level (∼3.0 g) after the fifth infusion, suggesting the progression to trigeminal allodynia. Recent reports have focused on two widely used migraine model induced by repeated dural stimulation with IS.15,29 One model involves seven successive days of dural IS infusions.15,16 as described in the present study. This model mimics several clinical features displayed by migraineurs, such as decreased basal thresholds, decreased routine physical activity, increased resting behavior, and the pharmacological effectiveness of migraine treatment.15,16 In the other model, rats are infused with IS onto the dura, and the same procedure is repeated three times per week for up to 4 weeks.2,29 Repeated infusions of IS in the two animal models both induce a lasting decrease (low threshold state) in periorbital basal pressure thresholds. Therefore, repeated dural stimulation with IS may produce a chronic state of trigeminal activation and may therefore be suitable for elucidating the potential mechanisms underlying chronic migraine status.
P2X4R expression and microglial activation increase with repeated dural stimulation
Previous evidence of microglial activation in migraine model was provided by a study of BBB permeability, in which microglial activation was detected along with increased BBB permeability following repeated dural inflammatory stimulation.2 In line with this study, our experiments demonstrated that repetitive IS application increased the number of Iba-1 positive cells in TNC which could be reversed by TNP-ATP treatment. Microglial P2X4R is known to play an essential role in the chronic pain conditions gating PNI and morphine-induced hyperalgesia.1,8 Our findings revealed that repeated dural stimulation induced a strong upregulation of P2X4R in the TNC, compared with only low expression in control animals. We also observed that the induction of P2X4R expression in the TNC following repeated dural stimulation was confined to microglia. Thus, increased P2X4R expression in activated TNC microglia might be involved in chronic migraine.
P2X4R and related pathways were involved in trigeminal allodynia and EAAT3 regulation following repeated dural stimulation
The interaction between microglial P2X4R and neurons has been confirmed to be a vital link in neuropathic pain, and p38-BDNF is a crucial signaling pathway involved in this process.3–6 In the present study, our results demonstrated that pp38 and BDNF expression in the TNC was markedly increased following repeated dural stimulation, while TNP-ATP treatment prevented p38-BDNF signaling, as well as trigeminal allodynia. Our results also indicated that p38 and BDNF were expressed in TNC microglia. Although the sources of p38 and BDNF elucidated here are not in complete agreement with the findings reported for neuropathic pain.3,5 p38-BDNF signaling regulated by microglial P2X4R is nevertheless likely implicated in chronic migraine.
BDNF from P2X4R-positive microglia plays an important role in PNI and morphine-induced hyperalgesia by regulating the activity of the NMDA receptor4,7 and downregulating the potassium-chloride cotransporter KCC2 in dorsal horn neurons.5,8,33 Yet the role of microglial P2X4R in regulating EAATs in the pathogenesis of migraine chronicity, as well as in neuropathic pain, remains to be completely clarified. Five subtypes of EAATs have been determined to date, of which EAAT1-2 are primarily observed in glial cells and play an essential role in extracellular glutamate homeostasis in the central nervous system.34 EAAT3 is primarily expressed in neurons, with high expression at postsynaptic sites9,10 that is believed to primarily modulate regional Glu concentrations.10,11 Since the role of EAAT4 and EAAT5 are still unclear, we investigated only neuronal EAAT3 in the present study. EAAT3 was primarily expressed in TNC neurons, consistent with previous findings.9 Moreover, our results demonstrated that EAAT3 was partially expressed in TNC microglia. Although most studies demonstrate that EAAT3 is neuronal, several groups have reported EAAT3 expression in glial cells.34,35 These studies found that EAAT3 gene and protein expression increased with microglial activation.35 Our results suggested the involvement of microglial P2X4R in the regulation of EAAT3 in TNC, whereby blocking P2X4R prevented the upregulation of EAAT3 induced by repeated dural stimulation.
Similar to our findings, Kerui GongM demonstrated that sustained administration of morphine for seven days resulted in hyperalgesia and upregulated EAAT3 in dorsal root ganglia neurons.36 Previous studies of neuropathic pain also reported that spinal EAAT3 exhibits a biphasic change following chronic constriction nerve injury (CCI), with an initial upregulation followed by downregulation.13,37 An explanation for EAAT3 downregulation may be that CCI results in degenerative changes in primary afferents.13 The time course of the late phase downregulation was thought to resemble changes in spinal CGRP and substance P expression following CCI.38 No loss of trigeminal nerve primary afferents has been reported in studies of chronic migraine. The upregulation of EAAT3, which may be different from the changes of glial EAAT1-2, can thus be reasonably concluded to participate in the pathogenesis of migraine chronicity.
BDNF-TrkB pathways were involved in trigeminal allodynia and EAAT3 regulation following repeated dural stimulation
A previous study using intrathecal injections of K252a, a nonselective inhibitor for Trk receptors, reported that spinal EAATs were regulated by neurotrophic factors via Trk receptor activation following PNI.13 Trk receptors include subtypes TrkA, TrkB, and TrkC, which are activated by nerve growth factor, BDNF, and neurotrophin-3, respectively.13 Serum levels of neurotrophic factors, specifically BDNF, are increased during migraine attacks compared with headache-free intervals.39,40 BDNF is thought to exert a crucial role in pain modulation and central sensitization after acting on its receptor.5,7,24,44,45 Activation of Trk receptors then initiates downstream cascades, including MAPK activation, to promote the expression of several proteins.41,42 Recently, GLT-1 expression in astrocytes was found to be regulated by BDNF-TrkB signaling in a rat model of depression.14 Our findings demonstrated that BDNF-TrkB signaling is involved in the development of trigeminal allodynia and the modulation of EAAT3, given that TrkB inhibitor treatment remarkably prevented the mechanical allodynia and the expression of EAAT3. Similar to our results, the work by Burgos-Vega et al.43 also showed a role for BDNF using ANA-12 and TrkB-Fc in behavioral responses following dural stimulation.
Recent studies of neuropathic pain showed that sex differences exist in the pathological mechanism of pain between males and females.44,45 They found that the involvement of spinal microglial BDNF in the induction of mechanical allodynia after nerve injury was male specific.44 This is the limitation of the present study that female rats did not contain in the study yet migraine occurs predominantly in female humans. So, female animals should be included in future studies to evaluate the sex differences in the pathogenesis of migraine in order to extract conclusions relevant to female patients.
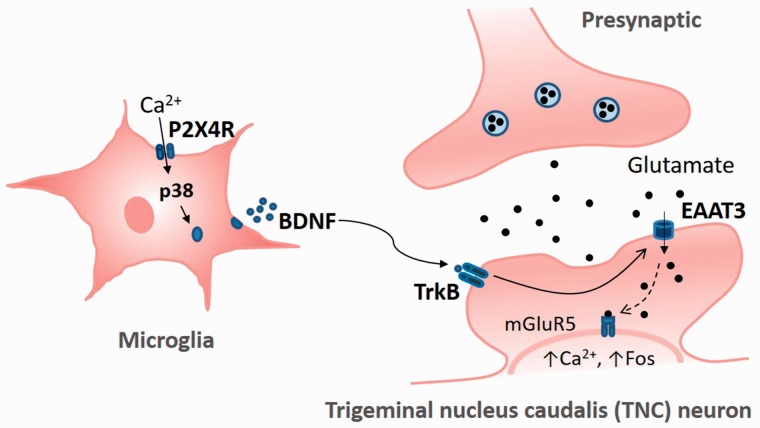
In summary, our results suggest a potential role for microglial P2X4R in an IS-induced trigeminal allodynia model that regulates EAAT3 expression via BDNF-TrkB signaling, as shown in Figure 7. Repeated dural inflammatory stimulation induces the activation of TNC microglial cells and a prominent increase in P2X4R expression. The activation of P2X4R in the brain regions which including TNC is essential for trigeminal allodynia following repeated dural IS infusions. Pharmacological blockade of P2X4R evidently prevents trigeminal allodynia, suggesting that IS-induced trigeminal allodynia depends on the activation of microglial P2X4R and related signaling pathways. Furthermore, the activation of microglial P2X4R and BDNF-TrkB signaling increased EAAT3 expression, which may further cause the activation of the intracellular metabotropic glutamate 5 receptor (mGluR5) by transporting glutamate into the cell, as shown in a recent study of neuropathic pain.12 Accumulating evidence suggests that mGluR5 is a crucial mediator of glutamate-induced neuroplasticity underlying persistent pain.46–50 Since blocking P2X4 or TrkB receptors prevents the upregulation of EAAT3 and trigeminal allodynia, pharmacological blockade of these receptors may represent a potential therapeutic approach for treating trigeminal allodynia, as seen in migraineurs. More studies are needed to further investigate the precise mechanisms underlying microglia-neuron interactions in the pathophysiology of chronic migraine.
Figure 7.
Schematic representation of the potential mechanisms by which the P2X4 receptor in activated microglia modulates EAAT3 expression in the TNC in an IS-induced trigeminal allodynia model. Following repeated dural inflammatory stimulation, P2X4R expression is elevated in TNC microglia. Upregulation of microglial P2X4R promotes the activation of p38-MAPK and the synthesis and release of BDNF. By acting on its high-affinity receptor, TrkB, BDNF upregulates the glutamate transporter EAAT3. Glutamate influx through EAAT3 may further activate intracellular metabotropic glutamate 5 receptor (mGluR5), which is essential for enhanced Ca2+-dependent Fos and plays a crucial role in the glutamate-induced neuroplasticity underlying persistent pain.12,46–50
EAAT3: excitatory amino acid transporter 3; TrkB: tyrosine receptor kinase B; P2X4R: P2X4 purinoceptor; BDNF: brain-derived neurotrophic factor.
Supplemental Material
Supplemental material, Supplemental Figures for P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia by Chaoyang Liu, Yixin Zhang, Qing Liu, Li Jiang, Maolin Li, Sha Wang, Ting Long, Wei He, Xueying Kong, Guangcheng Qin, Lixue Chen, Yuhong Zhang and Jiying Zhou in Molecular Pain
Author Contributions
Jiying Zhou, Yuhong Zhang, Lixue Chen, and Guangcheng Qin supervised the experiment design. Chaoyang Liu conceived the study. Chaoyang Liu and Sha Wang performed most of the experiments, analyzed the data, and wrote the manuscript. Qing Liu, Ting Long, and Wei He assisted with some WB experiments. Li Jiang and Maolin Li participated in some behavioral experiments. Yixin Zhang and Xueying Kong provided advice on histological experiments. Jiying Zhou and Yuhong Zhang revised the manuscript. All of the authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the research grants from the National Natural Science Foundation of China (No: 81671092).
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003; 424: 778–783. [DOI] [PubMed] [Google Scholar]
- 2.Fried NT, Maxwell CR, Elliott MB, Oshinsky ML. Region-specific disruption of the blood-brain barrier following repeated inflammatory dural stimulation in a rat model of chronic trigeminal allodynia. Cephalalgia 2017; 38: 674–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci 2009; 29: 3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 2008; 28: 11263–11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coull JAM, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005; 438: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 6.Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci 2012; 15: 1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, Cai J, Feng ZB, Jin ZR, Liu BH, Zhao HY, Jing HB, Wei TJ, Yang GN, Liu LY, Cui YJ, Xing GG. BDNF contributes to spinal long-term potentiation and mechanical hypersensitivity via Fyn-mediated phosphorylation of NMDA receptor GluN2B subunit at tyrosine 1472 in rats following spinal nerve ligation. Neurochem Res 2017; 42: 2712–2729. [DOI] [PubMed] [Google Scholar]
- 8.Ferrini F, Trang T, Mattioli T-AM, Laffray S, Del’Guidice T, Lorenzo L-E, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu J-M, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat Neurosci 2013; 16: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Janssen WGM, Rothstein JD, Morrison JH. Differential synaptic localization of the glutamate transporter EAAC1 and glutamate receptor subunit GluR2 in the rat hippocampus. J Comp Neurol 2000; 418: 255–269. [PubMed] [Google Scholar]
- 10.Bjorn-Yoshimoto WE, Underhill SM. The importance of the excitatory amino acid transporter 3 (EAAT3). Neurochem Int 2016; 98: 4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albrecht J, Sidoryk-Węgrzynowicz M, Zielińska M, Aschner M. Roles of glutamine in neurotransmission. Neuron Glia Biol 2010; 6: 263–276. [DOI] [PubMed] [Google Scholar]
- 12.Vincent K, Cornea VM, Jong Y-JI, Laferrière A, Kumar N, Mickeviciute A, Fung JST, Bandegi P, Ribeiro-da-Silva A, O’Malley KL, Coderre TJ. Intracellular mGluR5 plays a critical role in neuropathic pain. Nat Commun 2016; 7: 10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci 2003; 23: 2899–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W-X, Wang J, Xie Z-M, Xu N, Zhang G-F, Jia M, Zhou Z-Q, Hashimoto K, Yang J-J. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology 2016; 233: 405–415. [DOI] [PubMed] [Google Scholar]
- 15.Melo-Carrillo A, Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia 2013; 33: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 16.Dong X, Guan X, Chen K, Jin S, Wang C, Yan L, Shi Z, Zhang X, Chen L, Wan Q. Abnormal mitochondrial dynamics and impaired mitochondrial biogenesis in trigeminal ganglion neurons in a rat model of migraine. Neurosci Lett 2017; 636: 127–133. [DOI] [PubMed] [Google Scholar]
- 17.Edvinsson L. The trigeminovascular pathway: role of CGRP and CGRP receptors in migraine. Headache 2017; 57: 47–55. [DOI] [PubMed] [Google Scholar]
- 18.Miller S, Liu H, Warfvinge K, Shi L, Dovlatyan M, Xu C, Edvinsson L. Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 2016; 328: 165–183. [DOI] [PubMed] [Google Scholar]
- 19.Karsan N, Goadsby PJ. Calcitonin gene-related peptide and migraine. Curr Opin Neurol 2015; 28: 250–254. [DOI] [PubMed] [Google Scholar]
- 20.Knyihár-Csillik E, Toldi J, Mihály A, Krisztin-Péva B, Chadaide Z, Németh H, Fenyő R, Vécsei L. Kynurenine in combination with probenecid mitigates the stimulation-induced increase of c-fos immunoreactivity of the rat caudal trigeminal nucleus in an experimental migraine model. J Neural Transm 2007; 114: 417–421. [DOI] [PubMed] [Google Scholar]
- 21.Lukács M, Warfvinge K, Tajti J, Fülöp F, Toldi J, Vécsei L, Edvinsson L. Topical dura mater application of CFA induces enhanced expression of c-fos and glutamate in rat trigeminal nucleus caudalis: attenuated by KYNA derivate (SZR72). J Headache Pain 2017; 18: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knyihár-Csillik E, Toldi J, Krisztin-Péva B, Chadaide Z, Németh H, Fenyő R, Vécsei L. Prevention of electrical stimulation-induced increase of c-fos immunoreaction in the caudal trigeminal nucleus by kynurenine combined with probenecid. Neurosci Lett 2007; 418: 122–126. [DOI] [PubMed] [Google Scholar]
- 23.Ter Horst GJ, Meijler WJ, Korf J, Kemper RHA. Trigeminal nociception-induced cerebral Fos expression in the conscious rat. Cephalalgia 2001; 21: 963–975. [DOI] [PubMed] [Google Scholar]
- 24.He Y-Q, Lang X-Q, Lin L, Ji L, Yuan X-Y, Chen Q, Ran Y-M, Chen H-S, Li L, Wang J-M, Wang Z-G, Gregersen H, Zou D-W, Liang H-P, Yang M. P2X3 receptor-mediated visceral hyperalgesia and neuronal sensitization following exposure to PTSD-like stress in the dorsal root ganglia of rats. Neurogastroenterol Motil 2017; 29: e12976. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Walwyn W, Ennes HS, Kim H, McRoberts JA, Marvizón JCG. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur J Neurosci 2014; 39: 1439–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahbaie P, Liang D-Y, Shi X-Y, Sun Y, Clark JD. Epigenetic regulation of spinal cord gene expression contributes to enhanced postoperative pain and analgesic tolerance subsequent to continuous opioid exposure. Mol Pain 2016; 12: 174480691664195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blugeot A, Rivat C, Bouvier E, Molet J, Mouchard A, Zeau B, Bernard C, Benoliel J-J, Becker C. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J Neurosci 2011; 31: 12889–12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996; 384: 560–564. [DOI] [PubMed] [Google Scholar]
- 29.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache 2007; 47: 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed Orlando: Academic Press, 1997. [Google Scholar]
- 31.Di W, Shi X, Lv H, Liu J, Zhang H, Li Z, Fang Y. Activation of the nuclear factor E2-related factor 2/antioxidant response element alleviates the nitroglycerin-induced hyperalgesia in rats. J Headache Pain 2016; 17: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu B, Wang S, Qin G, Xie J, Tan G, Zhou J, Chen L. Protein kinase C gamma contributes to central sensitization in a rat model of chronic migraine. J Mol Neurosci 2017; 63: 131–141. [DOI] [PubMed] [Google Scholar]
- 33.Hildebrand ME, Xu J, Dedek A, Li Y, Sengar AS, Beggs S, Lombroso PJ, Salter MW. Potentiation of synaptic GluN2B NMDAR currents by Fyn kinase is gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep 2016; 17: 2753–2765. [DOI] [PubMed] [Google Scholar]
- 34.Liang J, Takeuchi H, Doi Y, Kawanokuchi J, Sonobe Y, Jin S, Yawata I, Li H, Yasuoka S, Mizuno T, Suzumura A. Excitatory amino acid transporter expression by astrocytes is neuroprotective against microglial excitotoxicity. Brain Res 2008; 1210: 11–19. [DOI] [PubMed] [Google Scholar]
- 35.Liang J, Chao D, Sandhu HK, Yu Y, Zhang L, Balboni G, Kim D H, Xia Y. δ-Opioid receptors up-regulate excitatory amino acid transporters in mouse astrocytes. Br J Pharmacol 2014; 171: 5417–5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong K, Bhargava A, Jasmin L. GluN2B N-methyl-D-aspartate receptor and excitatory amino acid transporter 3 are upregulated in primary sensory neurons after 7 days of morphine administration in rats: implication for opiate-induced hyperalgesia. Pain 2016; 157: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Lim G, Yang L, Sung B, Mao J. Downregulation of spinal glutamate transporter EAAC1 following nerve injury is regulated by central glucocorticoid receptors in rats. Pain 2006; 120: 78–85. [DOI] [PubMed] [Google Scholar]
- 38.Kajander KC, Xu J. Quantitative evaluation of calcitonin gene-related peptide and substance P levels in rat spinal cord following peripheral nerve injury. Neurosci Lett 1995; 186: 184–188. [DOI] [PubMed] [Google Scholar]
- 39.Tanure MTA, Gomez RS, Hurtado RCL, Teixeira AL, Domingues RB. Increased serum levels of brain-derived neurotropic factor during migraine attacks: a pilot study. J Headache Pain 2010; 11: 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer M, Wille G, Klien S, Shanib H, Holle D, Gaul C, Broessner G. Brain-derived neurotrophic factor in primary headaches. J Headache Pain 2012; 13: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagemann C, Blank JL. The ups and downs of MEK kinase interactions. Cell Signal 2001; 13: 863–875. [DOI] [PubMed] [Google Scholar]
- 42.Ji R-R, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci 2002; 22: 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burgos-Vega CC, Quigley LD, Avona A, Price T, Dussor G. Dural stimulation in rats causes brain-derived neurotrophic factor-dependent priming to subthreshold stimuli including a migraine trigger. Pain 2016; 157: 2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin J-S, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji R-R, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci 2015; 18: 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mapplebeck JC, Beggs S, Salter MW. Sex differences in pain: a tale of two immune cells. Pain 2016; 157: S2–S6. [DOI] [PubMed] [Google Scholar]
- 46.Dogrul A, Ossipov MH, Lai J, Malan TP, Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci Lett 2000; 292: 115–118. [DOI] [PubMed] [Google Scholar]
- 47.Fisher K, Lefebvre C, Coderre TJ. Antinociceptive effects following intrathecal pretreatment with selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol, Biochem Behav 2002; 73: 411–418. [DOI] [PubMed] [Google Scholar]
- 48.Sotgiu ML, Bellomi P, Biella GE. The mGluR5 selective antagonist 6-methyl-2-(phenylethynyl)-pyridine reduces the spinal neuron pain-related activity in mononeuropathic rats. Neurosci Lett 2003; 342: 85–88. [DOI] [PubMed] [Google Scholar]
- 49.Vincent K, Wang SF, Laferrière A, Kumar N, Coderre TJ. Spinal intracellular metabotropic glutamate receptor 5 (mGluR5) contributes to pain and c-fos expression in a rat model of inflammatory pain. Pain 2017; 158: 705–716. [DOI] [PubMed] [Google Scholar]
- 50.Purgert CA, Izumi Y, Jong Y-JI, Kumar V, Zorumski CF, O’Malley KL. Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. J Neurosci 2014; 34: 4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Figures for P2X4-receptor participates in EAAT3 regulation via BDNF-TrkB signaling in a model of trigeminal allodynia by Chaoyang Liu, Yixin Zhang, Qing Liu, Li Jiang, Maolin Li, Sha Wang, Ting Long, Wei He, Xueying Kong, Guangcheng Qin, Lixue Chen, Yuhong Zhang and Jiying Zhou in Molecular Pain