Abstract
Purpose
We explored whether advanced magnetic resonance (MR) imaging techniques could grade oligodendrogliomas.
Methods
Forty patients (age 9–61 years) with oligodendroglial tumors were selected. There were 23 patients with World Health Organization grade II (group 1) and 17 patients with grade III (group 2) tumors. Apparent diffusion coefficient (ADC) maps were calculated by b values of 0 and 1000 s/mm2. Dynamic susceptibility contrast (DSC) images were obtained during the first pass of a bolus of gadolinium-based contrast. These data were post-processed and cerebral blood volume (CBV) maps and permeability (PS) were calculated. MR spectroscopy was acquired after drawing a region of interest on the tumor using two-dimensional chemical shift imaging. Statistical analysis was performed using SPSS software.
Results
When the rPSmax was combined with the rCBVmax, there was a significant difference between the two groups (p ≤ 0.03) with area under the curve of 0.742 (95% CI: 0.412–0.904). rCBV, rADC, choline/creatine, and choline/NAA alone were able to differentiate between the two groups; however, they did not show any statistical difference with p values of ≤ 0.121, ≤ 0.722, and ≤ 0.582, respectively. A CBV PS product threshold of 0.53 provided a sensitivity of 80% and a specificity of 83.3% in detection of grade III tumors.
Conclusion
Combined rCBVmax and rPSmax can be utilized to grade oligodendrogliomas. ADC values, relative cerebral blood volume (rCBV), and MR spectroscopy alone can be utilized to differentiate between the two groups of oligodendrogliomas but without statistical significance.
Keywords: Apparent diffusion coefficient, cerebral blood volume, time to echo
Introduction
Gliomas can be classified as astroglial, oligodendroglial or oligoastrocytoma based on the cell population. Oligodendrogliomas together with mixed oligoastrocytomas constitute 5%–20% of all gliomas.1,2 However, in recently published consensus guidelines for nervous system tumor classification and grading, the International Society of Neuropathology proposes that both histopathologically diagnosed oligodendrogliomas and oligoastrocytomas with the 1 p/19q codeletion be classified as oligodendrogliomas.3 Tumors without the 1 p/19q codeletion would be classified as “diffuse glioma of the oligodendroglial phenotype” and as “diffuse astrocytoma” in cases of histopathological appearance of oligodendroglioma and oligoastrocytoma, respectively. With the incorporation of molecular information, the diagnosis of oligoastrocytomas would thus cease to exist; however, for the simplification of our current study, we still used the term oligoastrocytoma. The detectability of oligodendroglial and astroglial tumors with an oligodendroglial component is rising thanks to improved histopathologic techniques. However, in some situations such as tumor location in eloquent areas or deeper areas of the brain, complete removal of the tumor may not be possible, which may lead to misdiagnosis (mostly undergrading) on histopathology and may affect management and prognosis.4 The imaging characteristics on anatomical imaging have been described that are useful to differentiate oligodendroglial tumors from astrocytic tumors but have a limited role in grading these tumors.5 Advanced magnetic resonance imaging (MRI) and functional imaging techniques are being increasingly used in the clinical routine.6 Various authors have studied the role of computed tomography perfusion and permeability surface (PS),7 diffusion,8 MR spectroscopy,9 MR perfusion,10 and positron-emission tomography imaging,11 though there has been variable success in differentiating grades of oligodendrogliomas. The purpose of our study was to investigate and compare the diagnostic utility of combining various advanced imaging parameters in the differential diagnosis of the different grades of oligodendrogliomas. Various advanced imaging parameters that we studied include a quantitative apparent diffusion coefficient (ADC) value, relative cerebral blood volume (rCBV), and permeability surface area product (PS) values obtained from dynamic susceptibility contrast (DSC) perfusion imaging as well as choline-to-creatine (Chol/Cr) ratio and choline-to-N-acetylaspartate (Chol/NAA) ratio in MR spectroscopy.
Material and methods
Patient selection
From our database, we searched for patients with brain tumors with an oligodendroglial component. A total of 40 patients (age 9–61 years) matched our criteria and were found to have undergone advanced MRI. There were 23 patients with World Health Organization (WHO) grade II tumor or group 1 (16 patients with oligodendroglioma, OII and seven patients with oligoastrocytoma, OAII) and 17 patients with anaplastic/grade III tumors or group 2 (four patients with OIII and 13 patients with OAIII).
Conventional MRI
All MRI examinations were performed on a 1.5-T whole-body MR unit (Signa; GE Medical Systems, Milwaukee, WI). The conventional MRI examinations included unenhanced T1-weighted imaging (T1-WI), fluid-attenuated inversion-recovery (FLAIR) imaging. Post-contrast T1-WIs in three planes were obtained following acquisition of DSC imaging.
Diffusion imaging
Diffusion-weighted (DW) images (repetition time (TR)/echo time (TE)/number of excitations (NEX), 10,000/125/1; B = 1000) were acquired before injection of intravenous contrast. A 5-mm section thickness with 1-mm spacing, a field of view of 24 cm, and a matrix size of 128 × 128 were obtained. ADC maps were calculated from DW images using b values of 0 and 1000 s/mm2. Areas showing minimum ADC values were determined for each section in which the tumor was visible. At least four regions of interest (ROIs) measuring 0.09–0.44 cm2 were drawn within the lesions by a single author blinded to the histopathologic results. Gross areas of necrosis, calcification, and artifacts were avoided. A single ROI was drawn in the normal-appearing white matter (NAWM). The normalized ADC was calculated by dividing the minimum ADC measurement in the lesion by the ADC measurement in NAWM (rADCmin). Areas of abnormal WM were avoided, as were areas of immature myelination in infants.12
DSC
DSC images were obtained by using a gradient-recalled T2*-weighted echo-planar imaging sequence. Parameters used were TR/TE 1500/50 ms, flip angle 80 degrees, NEX 1, matrix size 128 × 96, and section thickness 6 mm. The relative cerebral blood volume (rCBV) maps were calculated using an algorithm for deconvolution. Raw perfusion-weighted MR data was processed offline on the Lund perfusion program. Implementation of a correction algorithm for T1 effects from blood-brain barrier leakage, as described in Haselhorst et al.,13 was applied in all cases. Before starting the contrast agent injection, the first 10 acquisitions of a total of 60 image volumes were selected to establish a pre-contrast baseline. After acquisition of the first 10 image volumes, a total of 0.15 mmol/kg of body weight gadopentetate dimeglumine was injected at a rate of 5 ml/s followed by a 20-ml bolus of saline injection at the same rate of 5 ml/s through an 18 - or 20-G intravenous catheter. A total of 12 contiguous axial sections were chosen for the analysis on the basis of lesion extent determined by the pre-contrast T2-FLAIR images.
rCBV measurement
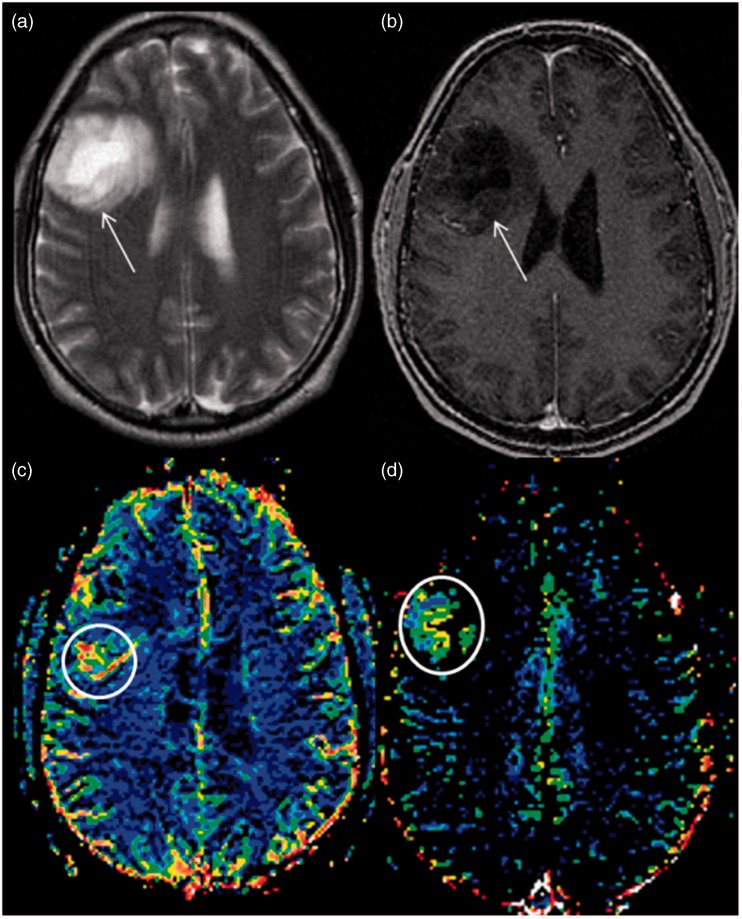
Post-processing of images was conducted on a different workstation by one author (RM), who was blinded to the histological findings at the time of analysis. Reference was made with conventional imaging during calculation of rCBV. ROIs were drawn around the most enhancing part of the tumor on contrast-enhanced T1-WI axial images and images were transferred onto co-registered DSC perfusion maps (Figure 1(c)). Multiple ROIs of 30–50 mm2 were drawn over four or five hot perfused spots, and the highest value, i.e. rCBVmax, was picked. Raw data of perfusion images as well as T1-WI and T2-weighted images were used to ensure that ROIs did not include apparent blood vessels or any hemorrhage. Another ROI with an approximate size of 30–50 mm2 was drawn in the contralateral NAWM as a standard internal reference. The rCBV ratio was obtained by dividing the rCBV values of the lesion by the contralateral NAWM.14
Figure 1.
Axial T2-weighted (a) and T1 post-gadolinium (b) magnetic resonance images at the level of the corona radiata showing a large, non-enhancing T2-hyperintense tumor in the right frontal lobe (white arrows). The relative cerebral blood volume (c) and relative permeability surface area maps (d) show increased perfusion and permeability, respectively (white circles).
PS
The same DSC software generates PS mapping along with other perfusion parameters. PS was calculated by the first-pass model described by Weisskoff et al.15 This method determines PS, which characterizes the diffusion of some of the contrast agent from the tumor vessels into the interstitium due to a deficient blood-brain barrier. PS values were selected separately and independently from the ROIs of the rCBV values from PS maps (Figure 1(d)). A total of four ROIs with maximum PS values were placed on the leakage maps. The maximum value of the four ROIs was normalized to contralateral WM, and maximum relative permeability surface product (rPSmax) was obtained.
MR spectroscopy
Two-dimensional chemical shift imaging MR spectroscopy was acquired by having water saturation with point-resolved saturation (PRESS) with a TE of 144 and placing a voxel on the enhancing area on the T1-post-gadolinium images or on the solid portion of the lesion on T2-FLAIR images. Spectroscopic data were analyzed using the automated analysis software provided by the vendor. Voxels with the highest Chol/Cr and Cho/NAA ratio were selected to represent the tumor.
Statistical analysis
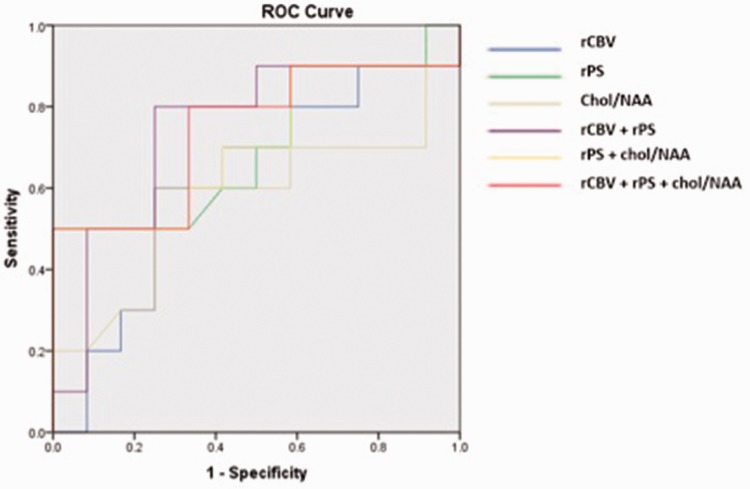
Statistical analysis was performed on IBM SPSS statistics, version 24.0 (IBM, Chicago, IL) software, and the Mann–Whitney test was used to test the statistical mean values and the standard deviation (SD). Receiver operating characteristic (ROC) curve was analyzed for various parameters with statistically significant differences in regard to their ability to differentiate different grades of oligodendrogliomas (Figure 2). The area under the curve (AUC) of various MR parameters was computed to determine which continuous variables were the most predictive for differentiating grades (Table 1). The p values of all MR variables were calculated by utilizing a one-way analysis of variance (ANOVA) test. A p value of < 0.05 was considered statistically significant. Optimum thresholds were chosen from ROC curves to define the maximum sensitivity and specificity.
Figure 2.
Receiver operating characteristic curve (ROC) for all magnetic resonance parameters.
rCBV: relative cerebral blood volume; rPS: relative permeability surface area; Chol/NAA: choline/N-acetylaspartate.
Table 1.
Areas under the receiver operating characteristic analysis curve for the parameters of interest for distinguishing grade II and grade III gliomas.
| MR parameters | AUC | SE | 95% CI |
|---|---|---|---|
| rCBV | 0.617 | 0.125 | 0.371–0.863 |
| rPS | 0.704 | 0.118 | 0.472–0.936 |
| rADC | 0.496 | 0.136 | 0.229–0.762 |
| Chol/NAA | 0.571 | 0.133 | 0.310–0.832 |
| Chol/Cr | 0.550 | 0.128 | 0.298–0.802 |
| rCBV + rPS | 0.742 | 0.122 | 0.412–0.904 |
| rPS + Chol/NAA | 0.708 | 0.120 | 0.456–0.922 |
| rCBV + rPS + Chol/NAA | 0.742 | 0.122 | 0.416–0.910 |
MR: magnetic resonance; AUC: area under the curve; SE: standard error; rCBV: relative cerebral blood volume; rADC: relative apparent diffusion coefficient; Chol/Cr: choline/creatine; Chol/NAA: choline/N-acetylaspartate; rPS: relative permeability surface area; CI: confidence interval.
Results
The mean values and the SDs of rADCmin, Cho/Cr, Cho/NAA, rPSmax, and rCBVmax and results of the t test for grade II and grade III are detailed in Table 2. For the purpose of simplification we will use minimum rADCmin as ADC, rPSmax as rPS, and rCBVmax as rCBV interchangeably. The mean values of ADC were lower in grade III as compared to grade II but did not show a statistically significant difference (p = 0.121). The rPS, rCBV, Cho/Cr, and Cho/NAA ratios were higher in grade III (Table 2). The mean values of rPS demonstrated a significant statistical difference (p = 0.007) between grade II and grade III gliomas (Table 2). The rCBV, Cho/Cr, and Cho/NAA did not show a significant difference (Table 2). The ROC analysis for rCBV, rPS, Chol/NAA, rCBV + rPS, and rPS + Chol/NAA between grade II and grade III gliomas is shown in Figure 2.
Table 2.
Comparison of the grade II and grade III glioma groups with regards to the MR parameters of interest.
| MR parameters | Grades | N | Mean | SD | p value (ANOVA) |
|---|---|---|---|---|---|
| rCBV | II | 22 | 2.1747 | 1.66948 | 0.236 |
| III | 16 | 2.8400 | 1.69428 | ||
| rADC | II | 22 | 1.4080 | 0.32212 | 0.121 |
| III | 13 | 1.2061 | 0.42525 | ||
| Chol/Cr | II | 12 | 2.9840 | 2.78563 | 0.847 |
| III | 10 | 3.2440 | 3.47464 | ||
| Chol/NAA | II | 12 | 3.0665 | 1.67774 | 0.203 |
| III | 10 | 5.0615 | 4.93579 | ||
| rPS | II | 22 | 0.7767 | 1.04450 | 0.007 |
| III | 15 | 3.7054 | 4.65106 |
MR: magnetic resonance; rCBV: relative cerebral blood volume; rADC: relative apparent diffusion coefficient; Chol/Cr: choline/creatine; Chol/NAA: choline/N-acetylaspartate; rPS: relative permeability surface area; SD: standard deviation.
The rCBVmax of grade II (23 patients) and grade III tumors (17 patients) was found to be 2.17 ± 1.66 SD and 2.84 ± 1.69 SD, respectively. The one-way ANOVA test (Table 2) showed no significant difference between the two groups with a p value of 0.236. However, the rPSmax was found to be low in the low-grade oligodendroglial lesions that showed high rCBV. When the rPSmax obtained from the DSC maps was combined with the rCBVmax, there was a significant difference between the two groups (p ≤ 0.03) with AUC of 0.742 (95% confidence interval (CI): 0.412–0.904) (Table 1). The ADC of grade II (22 patients) and grade III tumors (13 patients) was found to be 1.40 ± 0.322 SD and 1.206 ± 0.425 SD, respectively. There was no statistical difference between these two groups with a p value ≤ 0.121. The Chol/Cr ratio of grade II (12 patients) and grade III tumor (10 patients) was found to be 2.98 ± 2.7 SD and 3.24 ± 3.47 SD, respectively. Again, there was no statistical difference between the two groups with a p value ≤ 0.847. The Chol/NAA ratio of grade II (12 patients) and grade III tumor (10 patients) was found to be 3.06 ± 1.67 SD and 5.06 ± 4.93 SD, respectively. Also, there was no statistical difference between the two groups with a p value ≤ 0.203. The lactate peak was found in 25% of the low-grade group and 50% of the high-grade group. A CBV threshold of 1.69 provided a sensitivity of 70% and a specificity of 58.3%. rPSmax of 0.20 provided a sensitivity of 60% and a specificity of 58.3%. The CBV permeability product threshold of 0.53 provided a sensitivity of 80% and a specificity of 83.3% in detection of grade III tumors.
Discussion
There has been increased detection of oligodendroglial tumors thanks to improved histopathology techniques; however, in some situations, such as tumors in eloquent or deeper areas of brain, complete removal is not possible, which may lead to undergrading. Differentiation of oligodendroglioma grading is important from a prognostic and management perspective.4 So, there has been increased emphasis on imaging for differentiation tumors grading. Various studies have reported variable success in differentiating low-grade oligodendrogliomas from high-grade oligodendrogliomas. We tried to compare the role of various advanced imaging modalities in grading of these tumors. In this study we have explored the role of MR perfusion, MR permeability, DWI, and MR spectroscopy.
rCBV, which is linearly correlated with microvascular area, is the most widely used parameter for glioma grading. It has been reported that high-grade glioma can be differentiated from low-grade glioma with 95% sensitivity with an rCBV ratio cutoff value of 1.75.16 However, these findings are not applicable to reliably differentiate between high- and low-grade oligodendrogliomas, which may have markedly elevated rCBV even when low grade. As seen in previous studies, we found high rCBV in low-grade tumors.8 This finding of high rCBV is considered to be at least in part attributed to the focal presence of so-called “chicken wire”-like vessels, characteristically seen on histopathological examination, seen in these low-grade tumors.17 This is due to widely used methods of measuring maximum rCBV, which can give a false high rCBV value corresponding to focally increased vascularity. We believe this can be rectified if instead of taking the highest of four to five hot-spot values (CBVmax), a mean of four to five hot spots were to be taken into consideration for measurement (CBVmean), as described elsewhere.18 So, focally elevated rCBV does therefore not necessarily indicate a high-grade tumor in oligodendroglioma if rCBVmax is used as a parameter of perfusion. We found rCBV in the grade III tumor to be slightly higher than grade II but the difference was not significant. Our results corroborate the studies by Jain et al.19 and Lev et al.20
Our study showed a potential role of PS. This method characterizes the leakage of the contrast agent from the tumor vessels into the interstitium due to a deficient blood-brain barrier. It has been proposed that vascular permeability occurring with increasing glioma grade can be evaluated noninvasively by using in vivo perfusion imaging techniques.19 It has further been suggested that tumor vascular leakiness as depicted by PS maps could be a marker of leaky and immature vessels and probably represent sites of active angiogenesis. This also has been shown to correlate with increased vascular endothelial growth factor expression and microvascular endothelial proliferation. It is likely that this microvascular endothelial proliferation has more permeability than chicken wire-like vessels seen in low-grade oligodendroglioma.19 In our study, both rPS and rCBV values revealed strong association with high-grade anaplastic oligodendroglial tumors showing higher rPS and rCBV as compared with low-grade tumors. We found that rPSmax is more useful than rCBV. Also, when rPSmax is combined with rCBVmax, it provides better accuracy in predicting the grade of these tumors.
Diffusion restriction is typically absent in oligodendroglioma.21 Average ADC values are reported to be lower in high-grade than in low-grade glioma.21 However, DWI could not reliably distinguish grade II oligodendroglioma from grade III anaplastic oligodendroglioma because of overlap of average ADC values.22 In a high-grade tumor, this is at least in part due to the coexistence of vasogenic edema and necrosis resulting in high ADC, and vital tumor with high cellularity resulting in lower ADC. Although few studies have been successful in differentiating low-grade tumors from high-grade,8 research has been quite variable from study to study. Better results to reliably distinguish high-grade from low-grade glioma can be obtained using a multiparametric approach such as minimum ADC (ADCmin) value combined with other advanced and conventional MRI data.23 Alternatively, the mean of four areas of ADC values (ADCmean) rather than the minimum of four ADC values (ADCmin) may improve differentiation. Further studies are needed to validate the significance of CBVmean and ADCmean in differentiating grades of gliomas.
The role of MR spectroscopy in oligodendroglioma tumor grading is also variable from study to study. It has been proposed that MR spectroscopic imaging can discriminate high-grade from low-grade neoplasms by measuring cellular turnover and necrosis.24 The typical spectrum of oligodendroglioma shows moderately elevated Cho and decreased NAA without a lactate peak.21 The absence of a lipid/lactate peak favors oligodendroglioma over anaplastic oligodendroglioma.21 However, in our study, lactate peak was found in 25% in the low-grade group and 50% in the high-grade group. Moreover in one study, high-grade oligodendrogliomas were distinguished from low-grade with 100% sensitivity and 83% specificity based on a Cho/Cr ratio threshold of 2.33,9 although our study found a high Cho/Cr ratio in grade III tumors but the difference was not statistically significant from grade II due to overlap. Our results are similar to other previous studies that could not find a statistically significant difference.25 Diagnostic accuracy may be further enhanced if perfusion imaging can be used to guide the placement of ROIs for MR spectroscopy measurement.26
Limitations
We did not assess conventional MRI features in our series of oligodendroglial tumors, because this information has been previously reported.27 This study had a relatively small sample size, and we have not analyzed pure and mixed oligodendroglial tumors separately. We did not use a pre-infusion loading dose of contrast material although we did perform leakage correction. Information about isocitrate dehydrogenase mutation and 1p/19q-codeletion has not been included in our study as it was available in very few cases, so correlation of different MRI parameters with oligodendroglioma genotype was not conducted in the present study, as has been described elsewhere.28–31
Conclusion
rPSmax could be useful in grading oligodendroglial tumors. The accuracy of detection can be increased by the rCBVmax and rPSmax product, which may act as a noninvasive predictor of tumor grade. ADCmin and other metabolic maps provided information on the cellularity and metabolic composition of oligodendroglial tumors but, although they could differentiate between the two groups, none of the parameters alone showed a statistically significant difference.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas. Cancer 2006; 106: 1358–1363. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy BJ, Propp JM, Davis FG, et al. Time trends in oligodendroglial and astrocytic tumor incidence. Neuroepidemiology 2008; 30: 34–44. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Burger P, et al. International Society of Neuropathology–Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol 2014; 24: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebrun C, Fontaine D, Ramaioli A, et al. Long-term outcome of oligodendrogliomas. Neurology 2004; 62: 1783–1787. [DOI] [PubMed] [Google Scholar]
- 5.Koeller KK, Rushing EJ. Oligodendroglioma and its variants: Radiologic-pathologic correlation. Radiographics 2005; 25: 1669–1688. [DOI] [PubMed] [Google Scholar]
- 6.van den Bent MJ. Anaplastic oligodendroglioma and oligoastrocytoma. Neurol Clin 2007; 25: 1089–1109. [DOI] [PubMed] [Google Scholar]
- 7.Ding B, Ling HW, Chen KM, et al. Comparison of cerebral blood volume and permeability in preoperative grading of intracranial glioma using CT perfusion imaging. Neuroradiology 2006; 48: 773–781. [DOI] [PubMed] [Google Scholar]
- 8.Khalid L, Carone M, Dumrongpisutikul N, et al. Imaging characteristics of oligodendrogliomas that predict grade. AJNR Am J Neuroradiol 2012; 33: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spampinato MV, Smith JK, Kwock L, et al. Cerebral blood volume measurements and proton MR spectroscopy in grading of oligodendroglial tumors. AJR Am J Roentgenol 2007; 188: 204–212. [DOI] [PubMed] [Google Scholar]
- 10.Whitmore RG, Krejza J, Kapoor GS, et al. Prediction of oligodendroglial tumor subtype and grade using perfusion weighted magnetic resonance imaging. J Neurosurg 2007; 107: 600–609. [DOI] [PubMed] [Google Scholar]
- 11.Jansen NL, Schwartz C, Graute V, et al. Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [18F] FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro Oncol 2012; 14: 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginat DT, Mangla R, Yeaney G, et al. Diffusion-weighted imaging for differentiating benign from malignant skull lesions and correlation with cell density. AJR Am J Roentgenol 2012; 198: W597–W601. [DOI] [PubMed] [Google Scholar]
- 13.Haselhorst R, Kappos L, Bilecen D, et al. Dynamic susceptibility contrast MR imaging of plaque development in multiple sclerosis: Application of an extended blood-brain barrier leakage correction. J Magn Reson Imaging 2000; 11: 495–505. [DOI] [PubMed] [Google Scholar]
- 14.Mangla R, Singh G, Ziegelitz D, et al. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology 2010; 256: 575–584. [DOI] [PubMed] [Google Scholar]
- 15.Weisskoff RM, Boxerman JL, Sorensen AG, et al. Simultaneous blood volume and permeability mapping using a single Gd-based contrast injection. In: Proceedings of the second annual meeting of the Society of Magnetic Resonance, San Francisco, CA, USA, 6 August 1994, pp.6-12.
- 16.Law M, Yang S, Wang H, et al. Glioma grading: Sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 2003; 24: 1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 17.Burger P. Tumors of the central nervous system, Washington, DC: American Registry of Pathology, 2007. [Google Scholar]
- 18.Goyal P, Kumar Y, Gupta N, et al. Usefulness of enhancement-perfusion mismatch in differentiation of CNS lymphomas from other enhancing malignant tumors of the brain. Quant Imaging Med Surg 2017; 7: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain R, Griffith B, Alotaibi F, et al. Glioma angiogenesis and perfusion imaging: Understanding the relationship between tumor blood volume and leakiness with increasing glioma grade. AJNR Am J Neuroradiol 2015; 36: 2030–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: Confounding effect of elevated rCBV of oligodendroglimoas. AJNR Am J Neuroradiol 2004; 25: 214–221. [PMC free article] [PubMed] [Google Scholar]
- 21.Osborn A. Osborn’s brain imaging pathology anatomy, Salt Lake City, UT: Amirsys Inc, 2012. [Google Scholar]
- 22.Al-Okaili RN, Krejza J, Wang S, et al. Advanced MR imaging techniques in the diagnosis of intraaxial brain tumors in adults. Radiographics 2006; 26(Suppl 1): S173–S189. [DOI] [PubMed] [Google Scholar]
- 23.Young GS. Advanced MRI of adult brain tumors. Neurol Clin 2007; 25: 947–973. [DOI] [PubMed] [Google Scholar]
- 24.Xu M, See SJ, Ng WH, et al. Comparison of magnetic resonance spectroscopy and perfusion-weighted imaging in presurgical grading of oligodendroglial tumors. Neurosurgery 2005; 56: 919–926. [PubMed] [Google Scholar]
- 25.Fellah S, Caudal D, De Paula AM, et al. Multimodal MR imaging (diffusion, perfusion, and spectroscopy): Is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? AJNR Am J Neuroradiol 2013; 34: 1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chawla S, Wang S, Wolf RL, et al. Arterial spin-labeling and MR spectroscopy in the differentiation of gliomas. AJNR Am J Neuroradiol 2007; 28: 1683–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White ML, Zhang Y, Kirby P, et al. Can tumor contrast enhancement be used as a criterion for differentiating tumor grades of oligodendrogliomas? AJNR Am J Neuroradiol 2005; 26: 784–790. [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkinson MD, du Plessis DG, Smith TS, et al. Histological growth patterns and genotype in oligodendroglial tumours: Correlation with MRI features. Brain Engl 2006; 129(Pt 7): 1884–1891. [DOI] [PubMed] [Google Scholar]
- 29.Megyesi JF, Kachur E, Lee DH, et al. Imaging correlates of molecular signatures in oligodendrogliomas. Clin Cancer Res 2004; 10: 4303–4306. [DOI] [PubMed] [Google Scholar]
- 30.Chawla S, Krejza J, Vossough A, et al. Differentiation between oligodendroglioma genotypes using dynamic susceptibility contrast perfusion-weighted imaging and proton MR spectroscopy. AJNR Am J Neuroradiol 2013; 34: 1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapoor GS, Gocke TA, Chawla S, et al. Magnetic resonance perfusion-weighted imaging defines angiogenic subtypes of oligodendroglioma according to 1p19q and EGFR status. J Neurooncol 2009; 92(3): 373–386. [DOI] [PubMed] [Google Scholar]