Abstract
Melanotic neuroectodermal tumour of infancy is an uncommon pigmented neoplasm of neural crest origin. It was first described in 1918 by Krompecher, known as congenital melanocarcinoma at that time. Although it is generally agreed upon that it is a benign entity, it is locally aggressive and has a significant recurrent risk, reported to be between 10–15%. There have also been prior reports of malignant behaviour in these tumours, although extremely rare. The majority of cases of this tumour (about 70%) arise from the maxilla and its occurrence in the cranial vault represents approximately 15.6% of cases. We describe a rare case of melanotic neuroectodermal tumour of infancy, with simultaneous involvement of the cranial vault and petrous temporal bone, in a four-month-old child, complicated by post-surgical pseudo-meningocele. This case illustrates the diagnostic dilemma in differentiating reactive osseous sclerosis from direct tumour infiltration, both of which can occur in the context of melanotic neuroectodermal tumour of infancy. The discussion places emphasis on differential diagnoses and useful radiological features to assist in clinching the diagnosis of melanotic neuroectodermal tumour of infancy.
Keywords: Melanotic neuroectodermal tumour of infancy, cranial vault mass, pigmented neoplasm, pseudo-meningocele
Introduction
Melanotic neuroectodermal tumour of infancy (MNTI) is an uncommon pigmented neoplasm of neural crest origin. The term ‘melanotic neuroectodermal tumour of infancy’ was introduced for the first time in 1966.1 Although it is generally agreed upon that it is a benign entity, it is locally aggressive and has a significant recurrent risk, reported to be between 10-15%.2 There have also been prior reports of malignant behaviour in these tumours, although extremely rare. The majority of cases (about 70%) arise from the maxilla and only approximately 15.6% arise from the cranial vault.1,2 Patients often present with a rapidly growing soft tissue mass that displaces rather than infiltrates adjacent structures. The main imaging differential diagnoses for a cranial vault mass in infants, aside from MNTI, includes metastatic neuroblastoma, Langerhans’ cell histiocytosis (LCH), Ewing's sarcoma, intradiploic cavernous hemangioma and intracranial infantile hemangiopericytoma (HPC).3,4 Herein, we describe a rare case of MNTI, with simultaneous involvement of the cranial vault and petrous temporal bone, in a four-month-old child, complicated by post-surgical encephalocele. This case illustrates the diagnostic dilemma in differentiating reactive osseous sclerosis in surrounding bone (in this case, the left petrous temporal bone) from direct tumour infiltration, both of which can occur in the context of MNTI. Follow-up scan one year after surgery showed interval reduction in the extent of osseous sclerosis within the left petrous temporal bone, in keeping with reactive sclerosis and less likely to represent residual tumour. The discussion emphasises on differential diagnoses and useful radiological features to assist in clinching the diagnosis of MNTI.
Case description
A four-month-old child presented with a rapidly growing lump behind her left ear. The child was otherwise well. Physical examination revealed a bony lump with no evidence of overlying inflammatory skin changes. Neurological examination was also unremarkable. No dysmorphic features were noted.
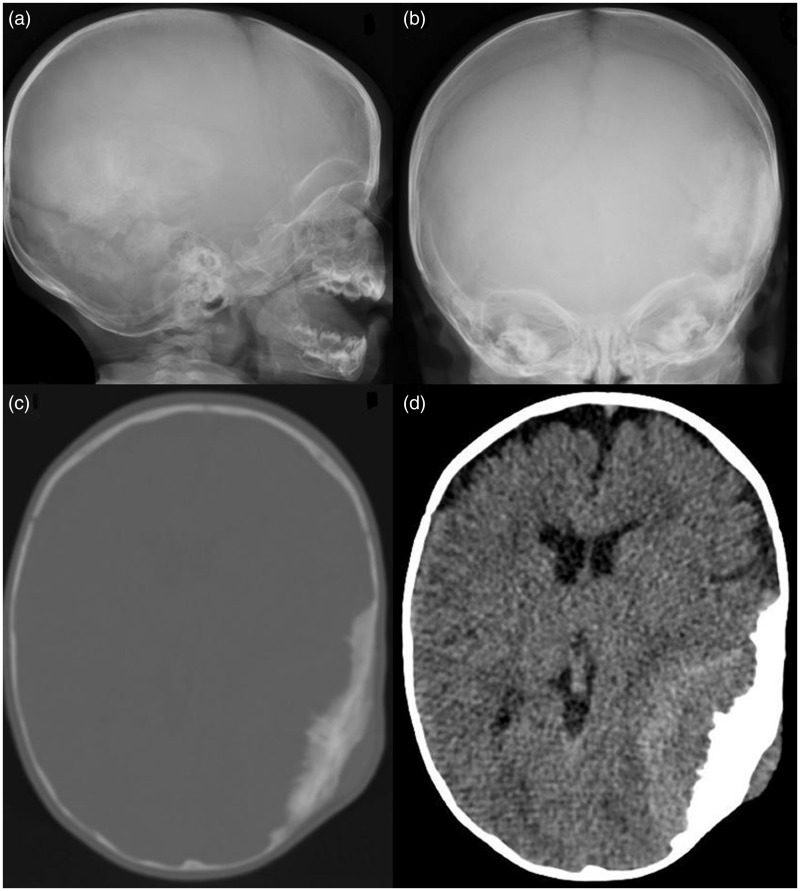
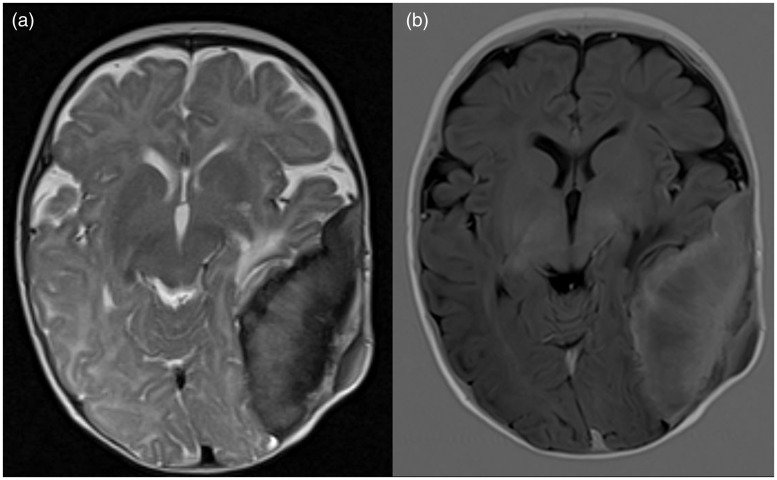
Skull radiographs were acquired and showed a sclerotic lesion in the left parieto-occipito-temporal cranial vault (Figure 1(a) and (b)). Computed tomography (CT) and magnetic resonance imaging (MRI) of the head were subsequently performed for further lesion characterization. CT demonstrated a solitary osseous-based lesion with marked spiculated periosteal reaction and mildly hyperdense soft tissue component abutting the inner and outer tables of the left cranial vault (Figure 1(c) and (d)). On MRI, the soft tissue component showed a hyperintense signal on T1-weighted images and a mildly hypointense signal on T2-weighted images. The mass effect upon the underlying left temporo-parietal lobes was observed with mild vasogenic oedema within the left temporal lobe (Figure 2).
Figure 1.
Lateral (a) and frontal (b) skull radiographs showed a sclerotic lesion in the left parieto-occipito-temporal cranial vault. Computed tomography (CT) demonstrated a solitary osseous-based lesion with marked spiculated periosteal reaction (c) and mildly hyperdense soft tissue component abutting the inner and outer tables of the left cranial vault (d).
Figure 2.
On magnetic resonance imaging (MRI), the soft tissue component showed (a) mildly hypointense signal on T2-weighted images and (b) hyperintense signal on T1-weighted images. Mass effect upon the underlying left temporo-parietal lobes was observed with mild vasogenic oedema within the left temporal lobe.
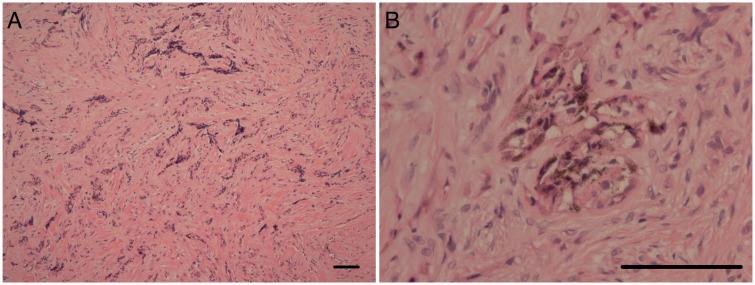
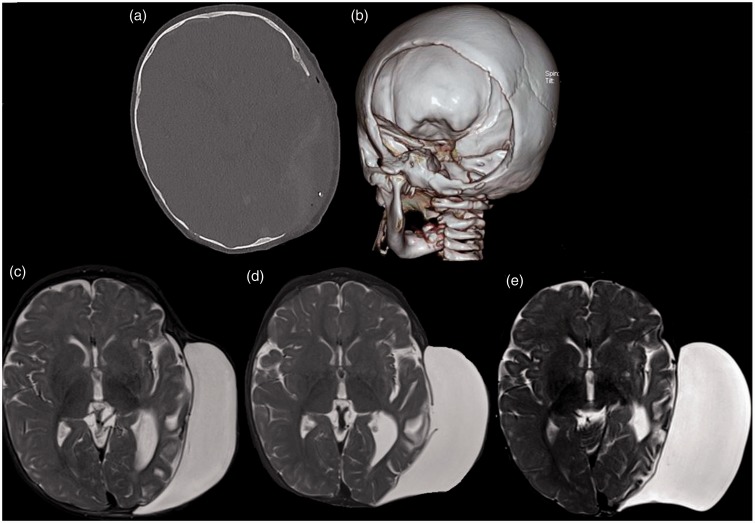
The patient was referred to the neurosurgical team with view for biopsy of the left calvarial lesion. Biopsy was performed and a histopathological diagnosis of MNTI was made (Figure 3). The patient was then scheduled for surgical excision of the tumour. The surgery was uneventful and patient recovered well with no focal neurological deficits. A large calvarial defect was present after the surgery due to the large size of the lesion (Figure 4(a) and (b)). A few weeks after surgery, a large fluctuant mass was seen at the surgical site, confirmed to be a pseudomeningocele on MRI. The pseudomeningocele continued to grow in size over a period of six months despite compression bandaging (Figure 4(c)–(e)). A decision was hence made for surgical intervention (aspiration of pseudomeningocele followed by duraplasty and cranioplasty).
Figure 3.
The histology showed a tumour composed of three elements; small neuroblastic cells (a), a dense fibrous stroma ((a) and (b)) and islands of epithelial cells containing pigment (b). Scale bars – 100 µm.
Figure 4.
A large calvarial defect was present after the surgery due to the large size of the lesion, (a) and (b). A few weeks after surgery, a large fluctuant mass was seen at the surgical site, confirmed to be a pseudomeningocele on magnetic resonance imaging (MRI). The pseudomeningocele continued to grow in size over a duration of six months despite compression bandaging, (c)–(e).
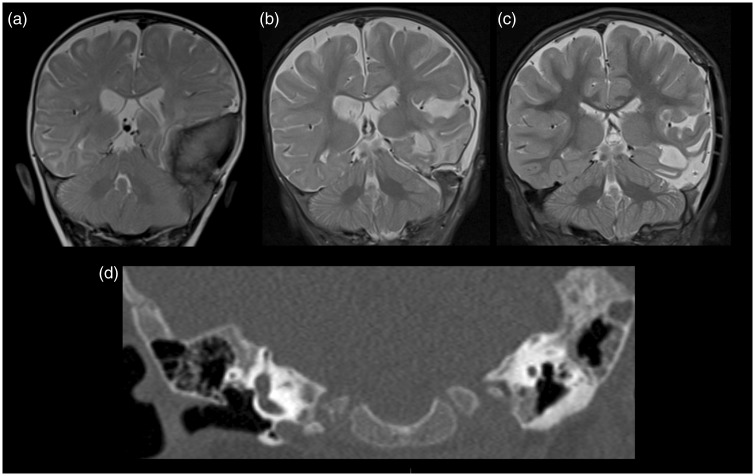
In the follow-up MRI of the brain one month after surgery, a small residual area of sclerosis was seen within the left petrous temporal bone (Figure 5(b)), raising the diagnostic dilemma of reactive osseous sclerosis versus direct tumour infiltration, both of which can occur in MNTI. Follow-up scan one year later showed interval reduction in the extent of bony sclerosis within the left petrous temporal bone (Figure 5(c)). This interval change is more suggestive of reactive sclerosis than residual tumour.
Figure 5.
Coronal T2-weighted images (a) before surgery, (b) one month after surgery and (c) one year after surgery demonstrated an area of residual sclerosis within the left petrous temporal bone which subsequently showed interval reduction in extent on follow-up at one year. Coronal computed tomography (CT) image (d) showed the area of residual sclerosis within the left petrous temporal bone one month after surgery.
Discussion
MNTI is an uncommon pigmented neoplasm of neural crest origin, most commonly seen in patients below the age of one year. The majority of these cases occur within the maxilla and its occurrence in the cranial vault represents approximately 15.6% of cases.2,5 Within the cranial vault, most of these lesions are seen closely related to the cranial sutures and anterior fontanelle. Although it is generally agreed to be a benign entity, it is locally aggressive and has a rapid growth rate. Even after surgical resection, radiological follow-up is recommended due to its significant recurrence rate, reported to be between 10–15%. Regional nodal metastasis is uncommon, and seen in only approximately 7% of cases.6
On plain radiographs, MNTI is usually seen as a well circumscribed, expansile radiolucent lesion. Spiculated periosteal reaction can sometimes be observed.6 In our case, the lesion appeared as an ill-defined sclerotic lesion due to the predominant osseous changes of cranial vault thickening and reactive sclerosis. The presence of melanin in MNTI is important as it defines the typical radiological appearances on CT and MRI. Due to the presence of melanin, the soft tissue component is typically mildly hyperdense on CT.7 Enhancement is often present, as seen in our case. The bony changes range from cranial vault thickening with reactive sclerosis to spiculated periosteal reaction and osseous destruction. In our case, the soft tissue component is indeed mildly hyperdense on CT. Both cranial vault thickening with reactive sclerosis and spiculated periosteal reaction were seen. On MRI, hyperintense signal on T1-weighted images is characteristic of MNTI due to the T1 shortening effect of melanin, which enhances T1 relaxation.8 Melanin also accelerates T2 decay, resulting in a hypointense signal on T2-weighted images. These typical MRI signal characteristics are however not always present due to a multitude of factors, such as the density of melanin pigment within the tumour, presence of tumoral calcifications and reactive osseous sclerosis, both of which result in areas of decreased signal on T1- and T2-weighted images. In our case, the soft tissue component of the mass demonstrates the typical signal pattern of melanin-containing tumours, possibly related to the high density of melanin pigment.
Cranial vault involvement in metastatic neuroblastoma is not uncommon, seen in up to 25% of cases.9 Neuroblastoma is the most common malignant metastasis to the skull in children.10 Typical imaging findings include spiculated periosteal reaction with or without associated osseous destruction. The soft tissue component often shows heterogeneous avid enhancement. These imaging features are similar to those of MNTI. However, metastatic neuroblastoma usually shows a predominant hypointense signal on T1-weighted images. Occasionally, a hyperintense signal may be seen on T1-weighted images due to the presence of haemorrhage or calcifications. Abdominal imaging is crucial to identify a primary tumour site. Meta-iodobenzylguanidine (MIBG) scan has a specificity of up to 99% for neuroblastoma although up to 30% of neuroblastomas are not MIBG-avid. Urine homovanillic acid (HVA) and vanillymandelic acid (VMA) are elevated in more than 90% of cases. It is also important to note that cranial involvement of neuroblastoma rarely occurs in isolation.
LCH is a spectrum of disease characterised by pathological proliferation of Langerhans-type histiocytes. The discovery of somatic activating BRAF mutations in a significant proportion of patients with LCH has led to the possibility of targeted inhibitor therapy.11 Cranial vault lesions of LCH are often seen as ‘punched-out’ or geographic lytic bone lesions with a bevelled edge and associated heterogeneously enhancing soft-tissue component. Periosteal reaction and matrix calcifications are almost always absent in LCH, a useful imaging clue to differentiate it from MNTI and metastatic neuroblastoma. The presence of additional intracranial findings such as an absent posterior pituitary ‘bright spot’ and thickened pituitary stalk should also be sought for. A thickened enhancing pituitary stalk is the most common intracranial manifestation of LCH. If LCH is suspected, skeletal survey or radionuclide bone scan should be carried out to exclude multifocal disease, which has a worse prognosis. A unifocal lesion is treated with local curettage and intralesional corticosteroid injection. Radiation therapy is primarily reserved for recurrent disease.
Primary Ewing's sarcoma of the cranial vault is extremely rare, accounting for less than 1% of cases.12 The peak incidence is between the age of 5 and 13, slightly older compared to those with MNTI. Within the cranial vault, the temporal bone is the most common site of occurrence. On imaging, it is often seen as a destructive osseous lesion, with erosion of both the inner and outer tables. An associated soft tissue mass is usually seen, predominantly within the epidural compartment. The presence of tumour matrix calcification is also a feature of Ewing's sarcoma. Breaching of the dura and cerebral parenchymal invasion are rare.13 Primary cranial Ewing's sarcoma has a relatively good prognosis, especially if diagnosed in the early stages and treated adequately.
Intraosseous hemangiomas are slow-growing vascular tumours, commonly seen within the vertebral body and occasionally in the cranial vault (frequently within the parietal and frontal bones).14–16 Patients typically present in adulthood with only a few reported cases in infancy.15 On plain radiographs and CT, the tumour is seen as a sharply marginated expansile lesion, with a sclerotic rim in one-third of cases. Osteoblastic remodelling with trabecular bone deposition following osteoclastic activity of the tumour gives rise to the classical sunburst appearance on imaging.17 Unfortunately, this is not seen in all cases and its absence does not exclude the diagnosis of cavernous hemangioma. Associated bony erosion may be seen, especially in large lesions, more commonly affecting the outer table.15 On MRI, intraosseous hemangioma displays variable signal intensities, depending upon the quantity of slow-flow venous blood, trabecular bone and ratio of red marrow to converted fatty marrow.18 It is however usually heterogeneously hyperintense on T2-weighted images (described as ‘light bulb bright’) and hypointense on T1-weighted images. Post-contrast administration, diffuse heterogenous enhancement is seen with ‘filling-in’ on delayed imaging.
Solitary fibrous tumour (SFT)/HPCs are rare tumours that in most cases harbour a NAB2-STAT6 gene fusion.19 Intracranial infantile HPCs are extremely rare, accounting for less than 1% of all intracranial tumours and little is known about their prognosis or course. Infantile types, both peripheral and intracranial, have been reported to have a less aggressive course compared to their adult counterpart.20 They are usually seen as well-demarcated multilobulated masses with heterogenous signal on MR imaging. They demonstrate a predominant isointense signal on T1W and T2W images.21 On unenhanced CT, they are slightly hyperdense and may show osseous erosion, although matrix calcifications, hyperostosis and spiculated periosteal reaction are rare. Heterogeneous contrast enhancement is usually demonstrated on both CT and MRI.21
Conclusion
Although rare, MNTI should be in the list of differential diagnoses for a rapidly growing cranial vault mass in infancy. Timely and accurate radiological diagnosis is crucial due to the rapid growth and locally invasive nature of the tumour. It is not always possible to make a definitive diagnosis on radiological grounds due to the variable imaging appearances of MNTI. However, when typical imaging features are present, a diagnosis can often be made. Hence, awareness of this rare entity and familiarity with its imaging features are crucial. This rare case of MNTI with concomitant involvement of the left cranial vault and petrous temporal bone also illustrates the diagnostic dilemma in differentiating reactive osseous sclerosis in surrounding bone (in this case, the left petrous temporal bone) from direct tumour infiltration, both of which can occur in the context of MNTI.
Acknowledgements
Ai P Tan: conceptualised the study, drafted the initial manuscript, and approved the final manuscript as submitted. Thomas S Jacques: contributed the pathology and review the manuscript. Gregory James: reviewed and revised the manuscript. Owase Jeelani: reviewed and revised the manuscript. Kshitij Mankad: critically reviewed the manuscript, and approved the final manuscript as submitted. Olga Slater: Reviewed and revised the manuscript. Felice D’Arco: conceptualized the study, critically reviewed the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
No funding was secured for this study. Thomas S Jacques has funding from the Brain Tumour Charity, Great Ormond Street Children’s Charity, Children with Cancer UK and National Institute for Health Research (NIHR).
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1.Borello ED, Gorlin RJ. Melanotic neuroectodermal tumor of infancy–a neoplasm of neural crest origin. Report of a case associated with high urinary excretion of vanilmandelic acid. Cancer 1966; 19: 196–206. [DOI] [PubMed] [Google Scholar]
- 2.Cutler LS, Chaudhry AP, Topazian R. Melanotic neuroectodermal tumor of infancy: An ultrastructural study, literature review, and reevaluation. Cancer 1981; 48: 257–270. [DOI] [PubMed] [Google Scholar]
- 3.Latchaw RE, L’Heureux PR, Young G, et al. Neuroblastoma presenting as central nervous system disease. AJNR Am J Neuroradiol 1982; 3: 623–630. [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman RA, Bilaniuk LT. CT of primary and secondary craniocerebral neuroblastoma. AJR Am J Roentgenol 1980; 135: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 5.Rachidi S, Sood AJ, Patel KG, et al. Melanotic neuroectodermal tumor of infancy: A systematic review. J Oral Maxillofac Surg 2015; 73: 1946–1956. [DOI] [PubMed] [Google Scholar]
- 6.Fowler DJ, Chisholm J, Roebuck D, et al. Melanotic neuroectodermal tumor of infancy: Clinical, radiological, and pathological features. Fetal Pediatr Pathol 2006; 25: 59–72. [DOI] [PubMed] [Google Scholar]
- 7.Chossegros C, Cheynet F, Gentet JC, et al. [Melanotic neuroectodermal tumor in an infant]. Arch Pediatr 1995; 2: 545–547. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson GO, Davis PC, Patrick LE, et al. Melanotic neuroectodermal tumor of infancy. MR findings and a review of the literature. Pediatr Radiol 1989; 20: 20–22. [DOI] [PubMed] [Google Scholar]
- 9.D’Ambrosio N, Lyo JK, Young RJ, et al. Imaging of metastatic CNS neuroblastoma. AJR Am J Roentgenol 2010; 194: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 10.Healy JF, Bishop J, Rosenkrantz H. Cranial computed tomography in the detection of dural, orbital, and skull involvement in metastatic neuroblastoma. J Comput Tomogr 1981; 5: 319–323. [DOI] [PubMed] [Google Scholar]
- 11.Nelson DS, Quispel W, Badalian-Very G, et al. Somatic activating ARAF mutations in Langerhans cell histiocytosis. Blood 2014; 123: 3152–3155. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Guo Z. Multiple primary Ewing’s sarcomas in cerebral cranium of a child: A case report and review of the literature. Int J Clin Exp Pathol 2015; 8: 7575–7582. [PMC free article] [PubMed] [Google Scholar]
- 13.Naama O, Ajja A, Gazzaz M, et al. [Primary Ewing sarcoma of the skull base with cerebral extension. A case report]. J Neuroradiol 2007; 34: 68–69. [DOI] [PubMed] [Google Scholar]
- 14.Honda M, Toda K, Baba H, et al. Congenital cavernous angioma of the temporal bone: Case report. Surg Neurol 2003; 59: 120–123. discussion 123. [DOI] [PubMed] [Google Scholar]
- 15.Koulouris G, Rao P. Multiple congenital cranial hemangiomas. Skeletal Radiol 2005; 34: 485–489. [DOI] [PubMed] [Google Scholar]
- 16.Heckl S, Aschoff A, Kunze S. Cavernomas of the skull: Review of the literature 1975-2000. Neurosurg Rev 2002; 25: 56–62. discussion 66. [DOI] [PubMed] [Google Scholar]
- 17.Liu JK, Burger PC, Harnsberger HR, et al. Primary intraosseous skull base cavernous hemangioma: Case report. Skull Base 2003; 13: 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yalçin O, Yildirim T, Kizilkiliç O, et al. CT and MRI findings in calvarial non-infectious lesions. Diagn Interv Radiol 2007; 13: 68–74. [PubMed] [Google Scholar]
- 19.Tai H-C, Chuang I-C, Chen T-C, et al. NAB2-STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol 2015; 28: 1324–1335. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Pineda I, Parida L, Jenkins JJ, et al. Childhood hemangiopericytoma: Review of St Jude Children’s Research Hospital. J Pediatr Hematol Oncol 2011; 33: 356–359. [DOI] [PubMed] [Google Scholar]
- 21.Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol 1996; 17: 1365–1371. [PMC free article] [PubMed] [Google Scholar]