Abstract
Berberine-containing plants have been traditionally used in different parts of the world for the treatment of inflammatory disorders, skin diseases, wound healing, reducing fevers, affections of eyes, treatment of tumors, digestive and respiratory diseases, and microbial pathologies. The physico-chemical properties of berberine contribute to the high diversity of extraction and detection methods. Considering its particularities this review describes various methods mentioned in the literature so far with reference to the most important factors influencing berberine extraction. Further, the common separation and detection methods like thin layer chromatography, high performance liquid chromatography, and mass spectrometry are discussed in order to give a complex overview of the existing methods. Additionally, many clinical and experimental studies suggest that berberine has several pharmacological properties, such as immunomodulatory, antioxidative, cardioprotective, hepatoprotective, and renoprotective effects. This review summarizes the main information about botanical occurrence, traditional uses, extraction methods, and pharmacological effects of berberine and berberine-containing plants.
Keywords: berberine, botanical occurrence, traditional uses, extraction methods, biological activities
Introduction
Berberine
Berberine(5,6-dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a] quinolizinium) Figure 1, is a nonbasic and quaternary benzylisoquinoline alkaloid, a relevant molecule in pharmacology and medicinal chemistry. Indeed, it is known as a very important natural alkaloid for the synthesis of several bioactive derivatives by means of condensation, modification, and substitution of functional groups in strategic positions for the design of new, selective, and powerful drugs (Chen et al., 2005).
Figure 1.
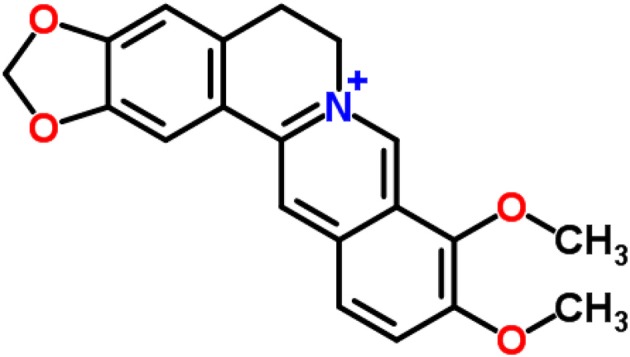
Berberine structure (according to ChemSpider database).
Traditional use of berberine-containing species
In the Berberidaceae family, the genus Berberis comprises of ~450–500 species, which represent the main natural source of berberine. Plants of this genus are used against inflammation, infectious diseases, diabetes, constipation, and other pathologies (Singh A. et al., 2010). The oldest evidence of using barberry fruit (Berberis vulgaris) as a blood purifying agent was written on the clay tablets in the library of Assyrian emperor Asurbanipal during 650 BC (Karimov, 1993). In Asia, the extensive use of the stem, stem bark, roots, and root bark of plants rich in berberine, particularly Berberis species, has more than 3000 years of history. Moreover, they have been used as raw material or as an important ingredient in Ayurvedic and traditional Chinese medicine (Birdsall, 1997; Kirtikar and Basu, 1998; Gupta and Tandon, 2004; Kulkarni and Dhir, 2010). In Ayurveda, Berberis species have been traditionally used for the treatment of a wide range of infections of the ear, eye, and mouth, for quick healing of wounds, curing hemorrhoids, indigestion and dysentery, or treatment of uterine and vaginal disorders. It has also been used to reduce obesity, and as an antidote for the treatment of scorpion sting or snakebite (Dev, 2006). Berberine extracts and decoctions are traditionally used for their activities against a variety of microorganisms including bacteria, viruses, fungi, protozoa, helminthes, in Ayurvedic, Chinese, and Middle-Eastern folk medicines (Tang et al., 2009; Gu et al., 2010).
In Yunani medicine, Berberis asiatica has multiple uses, such as for the treatment of asthma, eye sores, jaundice, skin pigmentation, and toothache, as well as for favoring the elimination of inflammation and swelling, and for drying ulcers (Kirtikar and Basu, 1998). Decoction of the roots, and stem barks originating from Berberis aristata, B. chitria, and B. lycium (Indian Berberis species), have been used as domestic treatment of conjunctivitis or other ophthalmic diseases, enlarged liver and spleen, hemorrhages, jaundice, and skin diseases like ulcers (Rajasekaran and Kumar, 2009). On the other hand, the use of decoction of Indian barberry mixed with honey has also been reported for the treatment of jaundice. Additionally, it has been reported the use of decoction of Indian barberry and Emblic myrobalan mixed with honey in the cure of urinary disorders as painful micturition (Kirtikar and Basu, 1998). Numerous studies dealing with its antimicrobial and antiprotozoal activities against different types of infectious organisms (Vennerstrom et al., 1990; Stermitz et al., 2000; Bahar et al., 2011) have been assessed so far. Moreover, it has been used to treat diarrhea (Chen et al., 2014) and intestinal parasites since ancient times in China (Singh and Mahajan, 2013), and the Eastern hemisphere, while in China it is also used for treating diabetes (Li et al., 2004).
Nowadays, a significant number of dietary supplements based on plants containing berberine (Kataoka et al., 2008) are used for reducing fever, common cold, respiratory infections, and influenza (Fabricant and Farnsworth, 2001). Another reported use for berberine-containing plants is their application as an astringent agent to lower the tone of the skin. Also, positive effects were observed on the mucous membranes of the upper respiratory tract and gastrointestinal system with effects on the associated ailments (Chen et al., 2014; Yu et al., 2016).
In southern South America leaves and bark of species of the genus Berberis are used in traditional medicine administered for mountain sickness, infections, and fever (San Martín, 1983; Houghton and Manby, 1985; Anesini and Perez, 1993).
Furthermore, there are other genera which contain berberine. The genus Mahonia comprises of several species that contain berberine. Within them, M. aquifolium has been traditionally used for various skin conditions. Due to its main alkaloid (berberine), is known to be used in Asian medicine for its antimicrobial activity. Coptidis rhizoma (rhizomes of Coptis chinensis), another plant which contains berberine, is a famous herb very frequently used in traditional Chinese medicine for the elimination of toxins, “damp-heat syndromes”, “purge fire”, and to “clear heat in the liver” (Tang et al., 2009). Table 1 gathers a synthesis of the main traditional uses of species containing berberine.
Table 1.
Traditional uses of berberine-containing species.
| Family | Scientific name | Traditional uses | References |
|---|---|---|---|
| Annonaceae | Annickia chlorantha (Oliv.) Setten & Maas (ex-Enantia chlorantha Oliv.) | Treat jaundice, hepatitis A, B, C, and D, conjunctivitis, leishmaniasis, medicine for cuts and infected wounds, sores and ulcers, antipyretic for various fevers, tuberculosis, vomiting of blood, urinary tract infections, treatment of fatigue, rheumatism, treat malaria symptoms, aches, wounds, boils, vomiting, yellow bitter, chills, sore, spleen in children and body pains, skin ailments, intercostal pain and to promote conception, intestinal worms, intestinal spasms, malaria and sexual asthenia, treat coughs and wounds; rickettsia fever, treat of sleeping sickness and dysentery, hemostatic and rickettsia, treat yellow fever and typhoid fever, treat diabetes, treat syphilis, and other infectious diseases, poliomyelitis, treat hypertension, treat HIV and prostate cancer | Oliver, 1960; Sandberg, 1965; Bouquet, 1969; Hamonniere et al., 1975; Onwuanibe, 1979; Burkill, 1985; Gill and Akinwumi, 1986; Gbile et al., 1988; Vennerstrom and Klayman, 1988; Vennerstrom et al., 1990; Adjanohoun et al., 1996; Nguimatsia et al., 1998; Kayode, 2006; Odugbemi et al., 2007; Ehiagbonare and Onyibe, 2008; Jiofack et al., 2008, 2009; Kadiri, 2008; Ogbonna et al., 2008; Olowokudejo et al., 2008; Betti and Lejoly, 2009; Ndenecho, 2009; Adeyemi et al., 2010; Noumi, 2010; Noumi and Anguessin, 2010; Noumi and Yumdinguetmun, 2010; Bele et al., 2011; Din et al., 2011; Ngono Ngane et al., 2011; Oladunmoye and Kehinde, 2011; Gbolade, 2012; Musuyu Muganza et al., 2012; Tsabang et al., 2012; Betti et al., 2013; Borokini et al., 2013; Fongod, 2014; Ishola et al., 2014; Ohemu et al., 2014 |
| Annickia pilosa (Exell) Setten & Maas (ex-Enantia pilosa Exell) | Medicine for cuts | Versteegh and Sosef, 2007 | |
| Annickia polycarpa (DC.) Setten & Maas ex I.M.Turner (ex-Enantia polycarpa (DC.) Engl. & Diels) | Treat cuts, antiseptic to treat sores, stomach ulcers, leprosy and ophthalmia, treatment of skin infections and sores, treat jaundice, and treat fever including malaria and to promote wound healing, against intestinal problems | Irvine, 1961; Bouquet and Debray, 1974; Ajali, 2000; Govindasamy et al., 2007; Versteegh and Sosef, 2007 | |
| Rollinia mucosa (Jacq.) Baill. | Treat of tumors | Hartwell, 1982 | |
| Xylopia polycarpa (DC.) Oliv. | Treat wounds, ulcers, leprosy, rheumatism, stomach and gall-bladder problems, eye diseases, for conception, diarrhea, malaria, fevers and sleeping disorders | Neuwinger, 1996 | |
| Berberidaceae | Berberis actinacantha Mart. | Antipyretic | San Martín, 1983 |
| Berberis aquifolium Pursh | Skin conditions, treat eczema, acne, conjunctivitis and herpes, alleviate the symptoms of psoriasis, treat diarrhea and in higher doses to treat constipation, improvement of blood flow to the liver, stimulate intestinal secretions and bile flow, treat jaundice, hepatitis, cirrhosis and general digestive problems, treatment of gall bladder disease, hemorrhages and a few forms of cancer, fungal infections, dysentery, anti-inflammatory properties, stomach problems, sore womb following childbirth and/or menstruation | King, 1898; Ritch-Krc et al., 1996 | |
| Berberis aristata DC. | Treat allergies, metabolic disorders, ophthalmia, and other eye diseases, treat bleeding piles, anti-osteoporosis, treat skin diseases, menorrhagia, fever, diarrhea, dysentery, cholera, jaundice, ear and urinary tract infections, anti-bacterial, anti-fungal, anti-inflammatory, analgesic, anti-pyretic, jaundice, piles, malaria, laxative, anti-scorbutic, anti-diabetic, and anti-hepatopathic | Bhattacharjee et al., 1980; Duke and Beckstrom-Sternberg, 1994; Küpeli et al., 2002; Acharya and Rokaya, 2005; Chhetri et al., 2005; Kunwar and Adhikari, 2005; Sharma et al., 2005; Joshi and Joshi, 2007; Meena et al., 2009; Shahid et al., 2009; Phondani et al., 2010; Saraf et al., 2010; Tiwary et al., 2010; Sati and Joshi, 2011; Yogesh et al., 2011 | |
| Berberis asiatica Roxb. ex DC. | Jaundice, diabetes mellitus, wound healing, asthma; drying unhealthy ulcers, anti-inflammatory, swelling, treat pneumococcal infections, eye (conjunctivitis) and ear diseases, rheumatism, fever, stomach disorders, skin disease (hyperpigmentation), malarial fever, laxative, teeth problems (toothache), and headache | Watt, 1883; Kirtikar and Basu, 1933; Samhita, 1963; Hashmi and Hafiz, 1986; Bhandari et al., 2000; Shah and Khan, 2006; Uniyal et al., 2006; Uprety et al., 2010; Maithani et al., 2014 | |
| Berberis buxifolia Lam. | Treat infections | Anesini and Perez, 1993; Mølgaard et al., 2011 | |
| Berberis chitria Buch.-Ham. ex Lindl. | Treat skin disease, jaundice, rheumatism, affection of eyes (household treatment for conjunctivitis, ophthalmic, bleeding piles), ulcers, skin diseases, enlarged liver and spleen | Watt, 1883; Kirtikar and Basu, 1933; Sir and Chopra, 1958 | |
| Berberis darwinii Hook. | Antipyrectic, anti-inflammatory, treat stomach pains, indigestion, and colitis | Montes and Wilkomirsky, 1987 | |
| Berberis empetrifolia Lam. | Treat mountain sickness | San Martín, 1983 | |
| Berberis integerrima Bunge. | Antipyretic, treat diabetes, bone fractures, rheumatism, radiculitis, heart pain, stomach aches, kidney stones, tuberculosis, chest pain, headaches, constipation, and wound | Khalmatov, 1964; Khodzhimatov, 1989; Baharvand-Ahmadi et al., 2016 | |
| Berberis jaeschkeana C. K. Schneid. | Treat eye diseases | Kala, 2006 | |
| Berberis koreana Palib. | Antipyretic, treat gastroenteritis, sore throats, and conjunctivitis | Ahn, 2003 | |
| Berberis leschenaultia Wall. ex Wight & Arn. | Antipyretic, cold and complications during post-natal period | Rajan and Sethuraman, 1992 | |
| Berberis libanotica Ehrenb. ex C. K. Schneid. | Treat rheumatic and neuralgic diseases, anti-inflammatory, treat arthritis and muscular pain | El Beyrouthy et al., 2008; Esseily et al., 2012 | |
| Berberis lycium Royle | Treat eye diseases, febrifuge, jaundice, diarrhoea, menorrhagia, piles, backache, dysentery, earache, fracture, eye ache, pimples, boils, wound healing, cough and throat pain, intestinal colic, diabetes, throat pain, scabies, bone fractures, sun blindness, against stomachache and intestinal problems | Zaman and Khan, 1970; ul Haq and Hussain, 1993; Bushra et al., 2000; Kaur and Miani, 2001; Hamayun et al., 2003; Ahmed et al., 2004; Abbasi et al., 2005, 2009, 2010; Shah and Khan, 2006; Zabihullah et al., 2006; Hussain et al., 2008; Sood et al., 2010 | |
| Berberis microphylla G. Forst. (ex-Berberis heterophylla Juss. ex Poir.) | Febrifuge, anti-inflammatory and treat diarrhea | Muñoz, 2001 | |
| Berberis oblonga (Regel) C. K. Schneid | Heart tonic, treat neurasthenia, antipyretic, antidiarrheal, treat rheumatism, eye diseases and wounds of the mouth, jaundice, stomach aches, back pain and arthralgia | Khalmatov, 1964; Sezik et al., 2004; Pak, 2005 | |
| Berberis petiolaris Wall. ex G. Don | Treat malarial fever, diarrhea, conjunctivitis, and jaundice | Karimov, 1993 | |
| Berberis pseudumbellata R. Parker | Diuretic, treat jaundice, intestinal disorders, eye diseases, oxytocic and throat ache, stomach problems and ulcers | Kala, 2006; Khan and Khatoon, 2007; Singh et al., 2009; Khan et al., 2016 | |
| Berberis thunbergii DC. | Anti-inflammatory | Küpeli et al., 2002 | |
| Berberis tinctoria Lesch. | Antimicrobial for skin disease, jaundice, affection of eyes, treat menorrhagia, diarrhea, and rheumatism | Fyson, 1975; Satyavati et al., 1987 | |
| Berberis umbellata Wall. ex G. Don | Treating fever, jaundice, nausea, eye disorders and skin problems, tonic | Singh et al., 2012 | |
| Berberis vulgaris L. | Antiarrhythmic, sedative, anticancer, heal internal injuries, remove kidney stones, treat sore throat and fever | Tantaquidgeon, 1928; Chaudhury et al., 1980; Zovko Koncić et al., 2010 | |
| Caulophyllum thalictroides (L.) Michaux | Menstrual cramps, relieve the pain of childbirth, promote prompt delivery, treat colics, cramps, hysteria, rheumatism, uterine stimulant, inducer of menstruation, and antispasmodic | Castleman, 1991; Hutchens, 1992 | |
| Jeffersonia diphylla (L.) Pers. | Antispasmodic, diuretic, emetic, expectorant, treat diarrhea, dropsy, gravel and urinary problems, emetic, expectorant, treat sores, ulcers and inflamed parts | Uphof, 1959; Duke and Ayensu, 1985; Foster and Duke, 1990; Coffey, 1993; Moerman, 1998; Lust, 2014 | |
| Mahonia fortunei (Lindl.) Fedde | Anticancer, febrifuge, antiodontalgic, treat testicular swelling and arthritic pain | Duke and Ayensu, 1985; He and Mu, 2015 | |
| Mahonia napaulensis DC. | Diuretic, demulcent, treat dysentery and inflammations of the eyes | Chopra et al., 1986; Manandhar, 2002 | |
| Nandina domestica Thunb. | Antitussive, astringent, febrifuge, stomachic and tonic, treat of fever in influenza, acute bronchitis, whooping cough, indigestion, acute gastro-enteritis, tooth abscess, pain in the bones, muscles and traumatic injuries, and antirheumatic | Kariyone and Koiso, 1971; Duke and Ayensu, 1985; Fogarty, 1990 | |
| Sinopodophyllum hexandrum (Royle) T. S. Ying | Regulate menstruation, promote the circulation of blood, treat amenorrhea, difficult labor and retention of dead fetus or placenta | Kong et al., 2010 | |
| Menispermaceae | Tinospora sinensis (Lour.) Merr (ex-Tinospora cordifolia (Willd.) Miers) | Tonic, antiperiodic, anti-spasmodic, anti-inflammatory, antiarthritic, anti-allergic, anti-diabetic, improve the immune system, antistress, anti-leprotic and anti-malarial activities | Singh et al., 2003 |
| Papaveraceae | Argemone albiflora Hornem (ex-Argemone alba F. Lestib.) | Anthydropic, cathartic, diaphoretic, diuretic, demulcent, emetic, purgative, treat jaundice, skin ailments, colds, colics and wounds | Smyth, 1903; Foster and Duke, 1990 |
| Argemone mexicana L. | Analgesic, antispasmodic, sedative, treat warts, cold sores, cutaneous affections, skin diseases, itches, treat cataracts, treat dropsy, jaundice, treat chronic skin diseases, expectorant, treat coughs and chest complaints, demulcent, emetic, expectorant, laxative and antidote to snake poisoning | Uphof, 1959; Pesman, 1962; Usher, 1974; Stuart and Smith, 1977; Emboden, 1979; Chopra et al., 1986; Coffey, 1993; Chevallier, 1996 | |
| Argemone platyceras L. | Treat respiratory ailments as asthma, cough, bronchitis and pneumonia | Emes et al., 1994 | |
| Bocconia frutescens L. | Treat skin conditions (ulcers and eruptions) and respiratory tract infections (bronchistis and tuberculosis) | Martinez, 1977, 1984 | |
| Chelidonium majus L. | Treat ophthalmic diseases (remove films from the cornea of the eye), mild sedative, antispasmodic, relaxing the muscles of the bronchial tubes and intestines, treat warts, alterative, anodyne, antispasmodic, cholagogue, diaphoretic, diuretic, hydrogogue, narcotic, purgative, treat bronchitis, whooping cough, asthma, jaundice, gallstones and gallbladder pains, anticancer, analgesic, treat stomach ulcer, treat get rid of warts, ringworm and corns | Launert, 1981; Grieve, 1984; Phillips and Foy, 1990; Phillips and Rix, 1991; Chevallier, 1996; Lust, 2014 | |
| Corydalis solida subsp. brachylova | Anodyne, antibacterial, antispasmodic, hallucinogenic, calm the nerves, sedative for insomnia, CNS stimulant, painkiller, treat painful menstruation, lowering the blood pressure, traumatic injury and lumbago | Launert, 1981; Bown, 1995 | |
| Corydalis solida subsp. slivenensis (Velen.) Hayek (ex-Corydalis slivenensis Velen.) | |||
| Corydalis solida subsp. tauri cola | |||
| Corydalis turtschaninovii Besser (ex-Corydalis ternata (Nakai) Nakai) | Treat memory dysfunction, treat gastric, duodenal ulcer, cardiac arrhythmia disease, rheumatism and dysmenorrhea | Tang and Eisenbrand, 1992; Kamigauchi and Iwasa, 1994; Orhan et al., 2004; Houghton et al., 2006 | |
| Eschscholzia californica Cham. | Sedative, diuretic, relieve pain, relax spasms, promote perspiration, treat nervous tension, anxiety, insomnia, urinary incontinence (especially in children), narcotic, relieve toothache, antispasmodic, analgesic and suppress the flow of milk in lactating women | Coffey, 1993; Bown, 1995; Chevallier, 1996; Moerman, 1998 | |
| Glaucium corniculatum (L.) Rud. subsp. corniculatum | Reduce warts, antitusive, treat CNS disturbances, sedative, cooling, and mild laxative | Al-Douri, 2000; Al-Qura'n, 2009; Hayta et al., 2014 | |
| Macleaya cordata (Willd.) R.Br. | Analgesic, antioedemic, carminative, depurative, diuretic, treat insect bites, and ringworm | Grieve, 1984; Duke and Ayensu, 1985 | |
| Macleaya microcarpa (Maxim.) Fedde | Treat some skin diseases and inflammation | Deng and Qin, 2010 | |
| Papaver dubium L. | Sudorific, diuretic, expectorant and ophthalmia | Chopra et al., 1986 | |
| Papaver dubium var. lecoquii | |||
| Papaver rhoeas L. var. chelidonioides | Ailments in the elderly and children, mild pain reliever, treat irritable coughs, reduce nervous over-activity, anodyne, emollient, emmenagogue, expectorant, hypnotic, slightly narcotic, sedative, treat bronchial complaints and coughs, insomnia, poor digestion, nervous digestive disorders and minor painful conditions, treat jaundice, fevers, and anticancer | Uphof, 1959; Launert, 1981; Grieve, 1984; Duke and Ayensu, 1985; Phillips and Foy, 1990; Bown, 1995; Chevallier, 1996 | |
| Papaver hybridum L. | Treat dermatologic diseases, anti-infective, diuretic, sedative, and antitussive | Rivera Núñez and Obon de Castro, 1996; Ali et al., 2018 | |
| Ranunculaceae | Coptis chinensis Franch. | Control of bacterial and viral infections, relax spasms, lower fevers, stimulate the circulation, treat diabetes mellitus, analgesic, locally anaesthetic, antibacterial, antipyretic, bitter, blood tonic, carminative, cholagogue, digestive, sedative, stomachic, vasodilator, treat diarrhoea, acute enteritis and dysentery, treat insomnia, fidget, delirium due to high fever, leukaemia and otitis media, treat conjunctivitis, skin problems (acne, boils, abscesses and burns whilst), mouth, tongue ulcers, swollen gums, and toothache | Uphof, 1959; Usher, 1974; Duke and Ayensu, 1985; Yeung, 1985; Bown, 1995 |
| Coptis japonica (Thunb.) Makino | Control of bacterial and viral infections, relax spasms, lower fevers, stimulate the circulation, locally analgesic and anaesthetic, anti-inflammatory, stomachic, treat conjunctivitis, intestinal catarrh, dysentery, enteritis, high fevers, inflamed mouth and tongue | Kariyone and Koiso, 1971; Usher, 1974; Grieve, 1984; Bown, 1995 | |
| Coptis teeta Wall. | Control of bacterial and viral infections, relaxes spasms, lowers fevers and stimulate the circulation, locally analgesic, anaesthetic, ophthalmic and pectoral diseases, effective antibacterial, treat dysentery | Stuart and Smith, 1977; Duke and Ayensu, 1985; Bown, 1995 | |
| Hydrastis canadensis L. | Treat disorders of the digestive system and mucous membranes, treat constipation, antiperiodic, antiseptic, astringent, cholagogue, diuretic, laxative, stomachic, tonic, treat disorders affecting the ears, eyes, throat, nose, stomach, intestines, and vagina | Uphof, 1959; Weiner, 1980; Grieve, 1984; Mills, 1985; Foster and Duke, 1990; Coffey, 1993; Bown, 1995; Chevallier, 1996; Lust, 2014 | |
| Xanthorhiza simplicissima Marshall | Treat mouth ulcers, stomach ulcers, colds, jaundice, treat piles, and digestive disorders | Weiner, 1980; Foster and Duke, 1990; Moerman, 1998 | |
| Rutaceae | Phellodendron amurense Rupr. | Treat gastroenteritis, abdominal pain and diarrhea, antiinflammator, immunostimulator and treat cancer (antitumor activities) | Uchiyama et al., 1989; Park et al., 1999 |
|
Act strongly on the kidneys, detoxicant for hot damp conditions, treat meningitis, conjunctivitis, antibacterial, antirheumatic, aphrodisiac, bitter stomachic, cholagogue, diuretic, expectorant, febrifuge, hypoglycaemic, treat ophtalmia, skin, vasodilator and tonic, treat acute diarrhoea, dysentery, jaundice, vaginal infections (with Trichomonas vaginalis), acute urinary tract infections, enteritis, boils, abscesses, night sweats and skin diseases, and expectorant | Kariyone and Koiso, 1971; Usher, 1974; Stuart and Smith, 1977; Grieve, 1984; Yeung, 1985; Bown, 1995; Chevallier, 1996 | |
| Zanthoxylum monophyllum Tul. | Treat eye infections and dark vomitus | Hirschhorn, 1981; Eric Brussell, 2004 |
Botanical sources of berberine
Berberine has been detected, isolated, and quantified from various plant families and genera including Annonaceae (Annickia, Coelocline, Rollinia, and Xylopia), Berberidaceae (Berberis, Caulophyllum, Jeffersonia, Mahonia, Nandina, and Sinopodophyllum), Menispermaceae (Tinospora), Papaveraceae (Argemone, Bocconia, Chelidonium, Corydalis, Eschscholzia, Glaucium, Hunnemannia, Macleaya, Papaver, and Sanguinaria), Ranunculaceae (Coptis, Hydrastis, and Xanthorhiza), and Rutaceae (Evodia, Phellodendron, and Zanthoxyllum) (Table 2). The genus Berberis is well-known as the most widely distributed natural source of berberine. The bark of B. vulgaris contains more than 8% of alkaloids, berberine being the major alkaloid (about 5%) (Arayne et al., 2007).
Table 2.
Botanical sources of berberine.
| Family | Scientific name | Common name | Used part | References |
|---|---|---|---|---|
| Annonaceae | Annickia chlorantha (Oliv.) Setten & Maas (ex-Enantia chlorantha Oliv.) | African whitewood, african yellow wood Epfoué, Péyé, Nfol, Poyo | Bark | Mell, 1929 |
| Annickia pilosa (Exell) Setten & Maas (ex-Enantia pilosa Exell) | – | Bark | Buzas and Egnell, 1965 | |
| Annickia polycarpa (DC.) Setten & Maas ex I. M. Turner (ex-Enantia polycarpa (DC.) Engl. & Diels) | African yellow wood | Bark | Buzas and Egnell, 1965 | |
| Coelocline polycarpa A.DC. | Yellow-dye tree of Soudan | Bark | Henry, 1949 | |
| Rollinia mucosa (Jacq.) Baill. | Biriba, wild sweet sop, wild cashina | Fruit | Chen et al., 1996 | |
| Xylopia macrocarpa A.Chev. | Jangkang | Stem bark | Willaman and Schubert, 1961 | |
| Xylopia polycarpa (DC.) Oliv. | – | Stem bark | Willaman and Schubert, 1961 | |
| Berberidaceae | Berberis aetnensis C.Presl | – | Roots | Bonesi et al., 2013 |
| Leaves | Musumeci et al., 2003 | |||
| Root | Henry, 1949 | |||
| Berberis amurensis Rupr. | Barberry | Stem & roots | Tomita and Kugo, 1956 | |
| Berberis aquifolium Pursh | Oregon grape | Roots | Parsons, 1882 | |
| Berberis aristata DC. | Tree turmeric | Bark | Chakravarti et al., 1950 | |
| Roots | Singh A. et al., 2010 | |||
| Stem | ||||
| Raw herb | Singh R. et al., 2010 | |||
| Extract | ||||
| Fruit | Kamal et al., 2011 | |||
| Roots | Andola et al., 2010a,c | |||
| Roots | Rashmi et al., 2009 | |||
| Roots | Singh and Kakkar, 2009 | |||
| Roots | Srivastava et al., 2004 | |||
| Roots | Srivastava et al., 2001 | |||
| Bark | Willaman and Schubert, 1961 | |||
| Berberis asiatica Roxb. ex DC. | Chutro, rasanjan (Nep); marpyashi (Newa); daruharidra, darbi (Sans) | Roots | Andola et al., 2010b | |
| Roots | Andola et al., 2010c | |||
| Roots | Srivastava et al., 2004 | |||
| Roots, stem, bark | Willaman and Schubert, 1961 | |||
| Berberis barandana Vidal. | – | ND | Willaman and Schubert, 1961 | |
| Berberis beaniana C. K. Schneid. | Kang song xiao bo (pinyin, China) | – | Steffens et al., 1985 | |
| Berberis chitria Buch.-Ham. ex Lindl. | Chitra, indian barberry | Whole plant | Hussaini and Shoeb, 1985 | |
| Roots | Srivastava et al., 2006a,b,c | |||
| Berberis concinna Hook.f. | Barberry | Stem bark | Tiwari and Masood, 1979 | |
| Berberis congestiflora Gay | Michay | Leaves and stem | Torres et al., 1992 | |
| Berberis coriaria Royle ex Lindl. | – | Stem bark | Tiwari and Masood, 1979 | |
| Berberis croatica Mart. ex Schult. & Schult.f. | Croatian barberry | Roots | Končić et al., 2010 | |
| Roots | Kosalec et al., 2009 | |||
| Berberis darwinii Hook. | Michai, calafate | Roots | Richert, 1918 | |
| Leaves | Urzúa et al., 1984 | |||
| Stem-bark | Habtemariam, 2011 | |||
| Berberis densiflora Raf. | – | Leaves | Khamidov et al., 1997b | |
| Berberis floribunda Wall. ex G.Don | Nepal barberry | Roots | Chatterjee, 1951 | |
| Berberis fortunei Lindl. | Fortune's Mahonia | Wood | Willaman and Schubert, 1961 | |
| Berberis guimpelii K. Koch & C. D. Bouché | – | Roots | Petcu, 1965a | |
| Berberis heteropoda Schrank | – | Root bark | Willaman and Schubert, 1961 | |
| Berberis himalaica Ahrendt | – | Stem-bark | Chatterjee et al., 1952 | |
| Berberis horrida Gay | – | Leaves and stem | Torres et al., 1992 | |
| Berberis iliensis Popov | – | Young shoots | Karimov and Shakirov, 1993 | |
| Roots | Dzhalilov et al., 1963 | |||
| Berberis integerrima Bunge. | – | Root | Karimov et al., 1993 | |
| Leaves | Karimov et al., 1993; Khamidov et al., 1996c, 1997b | |||
| Berberis jaeschkeana C. K. Schneid. | Jaeschke's Barberry | – | Rashid and Malik, 1972 | |
| Berberis jamesonii Lindl (ex-Berberis glauca Benth) | – | – | Willaman and Schubert, 1961 | |
| Berberis japonica R.Br | Japanese Mahonia | Wood, root | Willaman and Schubert, 1961 | |
| Berberis kawakamii Hayata | – | Roots | Yang and Lu, 1960a | |
| Berberis koreana Palib. | Korean barberry | Bark of the stem | Petcu, 1965b | |
| Bark of the roots | ||||
| Seeds | ||||
| Stem | ||||
| Roots | ||||
| – | Kostalova et al., 1982 | |||
| Roots | Yoo et al., 1986 | |||
| Leaves | ||||
| Berberis lambertii R. Parker | – | Roots | Chatterjee and Banerjee, 1953 | |
| Berberis laurina Thunb | Laurel barberry | Roots | Gurguel et al., 1934; Willaman and Schubert, 1961 | |
| Berberis leschenaultii Wall. ex Wight & Arn (ex-Mahonia leschenaultii (Wall. ex Wight & Arn.) Takeda) | – | Bark | Willaman and Schubert, 1961 | |
| Berberis libanotica Ehrenb. ex C. K. Schneid. | – | Root | Bonesi et al., 2013 | |
| Berberis lycium Royle | Boxthorn barberry | Roots | Andola et al., 2010c | |
| Berberis microphylla G. Forst. (ex-Berberis heterophylla Juss. ex Poir. Berberis buxifolia Lam.) | Patagonian barberry, magellan barberry, calafate | Roots | Freile et al., 2006 | |
| – | Rashid and Malik, 1972 | |||
| Berberis mingetsensis Hayata | – | Roots | Yang and Lu, 1960b | |
| Berberis nummularia Bunge | Nummular barberry | Young shoots | Karimov et al., 1993 | |
| Berberis morrisonensis Hayata | – | Roots | Yang, 1960a,b | |
| Stem | ||||
| – | ||||
| Berberis nepalensis Spreng. (ex-Mahonia acanthifolia Wall. ex G.Don) | – | – | Willaman and Schubert, 1961 | |
| Berberis nervosa Pursh | Dwarf Oregon-grape | – | Willaman and Schubert, 1961 | |
| Berberis oblonga (Regel) C. K. Schneid | Oblong barberry | Stem | Karimov and Lutfullin, 1986; Gorval' and Grishkovets, 1999 | |
| Leaves | Khamidov et al., 2003 | |||
| Roots | Tadzhibaev et al., 1974 | |||
| Berberis petiolaris Wall. ex G. Don | Chochar | Roots | Huq and Ikram, 1968 | |
| Berberis pseudumbellata R. Parker | – | Roots | Andola et al., 2010b | |
| Stem bark | ||||
| – | Pant et al., 1986 | |||
| Berberis repens Lindl. | Creeping mahonia, creeping Oregon grape, creeping barberry, or prostrate barberry | – | Willaman and Schubert, 1961 | |
| Berberis sargentiana C. K. Schneid. | Sankezhen | – | Liu, 1992 | |
| Berberis swaseyi Buckley | – | – | Willaman and Schubert, 1961 | |
| Berberis thunbergii DC. | Japanese barberry | Stem | Khamidov et al., 1997a | |
| Leaves | Khamidov et al., 1997a | |||
| Berberis tinctoria Lesch. | Nilgiri barberry | Roots | Srivastava and Rawat, 2007 | |
| Berberis trifolia (Cham. & Schltdl.) Schult. & Schult.f. | – | Root, stem | Willaman and Schubert, 1961 | |
| Berberis turcomanica Kar. ex Ledeb. | – | Leaves | Khamidov et al., 1996a,b,c | |
| Berberis umbellata Wall. ex G.Don | Himalayan barberry | Roots | Singh et al., 2012 | |
| Berberis vulgaris L. | Barberry | Stems and roots | Imanshahidi and Hosseinzadeh, 2008 | |
| Roots | Končić et al., 2010 | |||
| Roots | Kosalec et al., 2009 | |||
| Berberis waziristanica Hieron. | – | Root bark | Atta-ur-Rahma and Ahmad, 1992 | |
| Caulophyllum thalictroides (L.) Michaux (ex-Leontice thalictroides L.) | Blue cohosh | – | Willaman and Schubert, 1961 | |
| Jeffersonia diphylla (L.) Pers. | Twinleaf | – | Willaman and Schubert, 1961 | |
| Mahonia borealis Takeda | – | – | Willaman and Schubert, 1961 | |
| Mahonia fortunei (Lindl.) Fedde | Fortune's Mahonia | wood | Willaman and Schubert, 1961 | |
| Mahonia napaulensis DC. (ex- Mahonia griffithii; ex-Mahonia manipurensis Takeda; Mahonia sikkimensis Takeda) | Nepal Barberry | bark | Willaman and Schubert, 1961 | |
| Mahonia simonsii Takeda | – | – | Willaman and Schubert, 1961 | |
| Nandina domestica Thunb. | Nandina, heavenly bamboo or sacred bamboo | bark, root | Willaman and Schubert, 1961 | |
| Sinopodophyllum hexandrum (Royle) T.S.Ying | Himalayan May Apple, Indian may apple | Root, rhizome | Willaman and Schubert, 1961 | |
| Menispermaceae | Tinospora sinensis (Lour.) Merr. (ex-Tinospora cordifolia) (Willd.) Miers | Gulbel, indian tinospora | Stem | Srinivasan et al., 2008 |
| – | Singh et al., 2003 | |||
| Papaveraceae | Argemone albiflora Hornem. (ex-Argemone alba F.Lestib.) | White prickly poppy, Bluestem pricklypoppy | Aerial part and roots | Slavikova et al., 1960 |
| Foote, 1932 | ||||
| Israilov and Yunusov, 1986 | ||||
| Argemone hybrida R.Otto & Verloove | – | Leaves and stem | Israilov and Yunusov, 1986 | |
| Argemone mexicana L. | Prickly poppy | Apigeal parts, seeds | Haisova and Slavik, 1975; Israilov and Yunusov, 1986; Fletcher et al., 1993 | |
| Leaves | Bapna et al., 2015 | |||
| Seeds | Fletcher et al., 1993 | |||
| – | Singh, 2014 | |||
| – | Majumder et al., 1956; Hakim et al., 1961; Misra et al., 1961 | |||
| Superterranean parts | Slavikova and Slavik, 1955 | |||
| Roots | ||||
| – | Santos and Adkilen, 1932; de Almeida Costa, 1935; Misra et al., 1961; Doepke et al., 1976; Abou-Donia and El-Din, 1986; Monforte-Gonzalez et al., 2012 | |||
| Roots | Pathak et al., 1985; Kukula-Koch and Mroczek, 2015 | |||
| Leaves and capsules | Schlotterbeck, 1902 | |||
| Whole plant | Bose et al., 1963; Haisova and Slavik, 1975 | |||
| Latex | Santra and Saoji, 1971 | |||
| Argemone ochroleuca Sweet | Chicalote | Seeds | Fletcher et al., 1993 | |
| Argemone platyceras L. | Chicalote poppy, crested poppy | Leaves and stem | Israilov and Yunusov, 1986 | |
| Argemone subintegrifolia Ownbey | – | Aerial part | Stermitz et al., 1974 | |
| Argemone squarrosa Greene | Hedgehog pricklypoppy | Aerial part | Stermitz, 1967 | |
| Bocconia frutescens L. | Plume poppy, tree poppy, tree celandine, parrotweed, sea oxeye daisy, john crow bush | Leaves | Slavik and Slavikova, 1975 | |
| Roots, stalks, leaves | Taborska et al., 1980 | |||
| Chelidonium majus L. | Celandine poppy | Roots | Jusiak, 1967 | |
| Corydalis chaerophylla DC. | Fitweed | Roots | Jha et al., 2009 | |
| Corydalis ophiocarpa Hook. f. et Thoms | Fitweed | Manske, 1939 | ||
| Corydalis solida subsp. brachyloba | Fitweed | Aerial parts | Sener and Temizer, 1988, 1991 | |
| Corydalis solida subsp. slivenensis (Velen.) Hayek (ex-Corydalis slivenensis Velen.) | Fitweed | – | Kiryakov et al., 1982a,b | |
| Corydalis solida subsp. tauricola | Fitweed | – | Kiryakov et al., 1982b | |
| Rhizome | Sener and Temizer, 1990 | |||
| Corydalis turtschaninovii Besser. (ex-Corydalis ternata (Nakai) Nakai) | Fitweed | Tubers | Lee and Kim, 1999 | |
| Eschscholzia californica Cham. | Californian poppy | Roots | Gertig, 1964 | |
| Glaucium corniculatum (L.) Rud. subsp. corniculatum | Blackspot Hornpoppy | Aerial parts | Doncheva et al., 2014 | |
| – | Slavik and Slavikova, 1957 | |||
| Glaucium grandiflorum Boiss. & A.Huet | Red Horned Poppy, Grand-flowered Horned Poppy | Aerial part | Phillipson et al., 1981 | |
| Hunnemannia fumariifolia Sweet | Mexican Tulip Poppy, Golden Cup | Roots | Slavikova and Slavik, 1966 | |
| Macleaya cordata (Willd.) R.Br. | Plume poppy | – | Kosina et al., 2010 | |
| Macleaya microcarpa (Maxim.) Fedde | Poppy | Roots | Pěnčíková et al., 2011 | |
| Papaver dubium L. | Long-Head Poppy | Roots | Slavik et al., 1989 | |
| Papaver dubium var. lecoquii | Long-Head Poppy | Latex | Egels, 1959 | |
| Papaver rhoeas L. var. chelidonioides | Corn Poppy | Roots | Slavík, 1978 | |
| Papaver hybridum L. | Poppy | Aerial part | Phillipson et al., 1981 | |
| Sanguinaria canadensis L. | Bloodroot | Greathouse, 1939 | ||
| Ranunculaceae | Coptis chinensis Franch. | Chinese goldthread | Roots | Jin and Shan, 1982 |
| Roots | Lou et al., 1982 | |||
| Coptis japonica (Thunb.) Makino | Japanese goldthread | Rhizome | Kubota et al., 1980 | |
| Coptis teeta Wall. | Gold thread | Rhizome | Chen and Chen, 1988 | |
| Rhizome | Zhang et al., 2008 | |||
| Roots | ||||
| Hydrastis canadensis L. | Goldenseal | – | Baldazzi et al., 1998 | |
| – | Leone et al., 1996 | |||
| Xanthorhiza simplicissima Marshall | Yellowroot | Root, stem, and leaves | Okunade et al., 1994 | |
| Rutaceae | Evodia meliaefolia (Hance ex Walp.) Benth. | – | Bark | Perkin and Hummel, 1895 |
| Phellodendron amurense Rupr. | Amur cork tree | Bark | Chiang et al., 2006 | |
| Root bark | Zhang et al., 2008 | |||
| Trunk bark | ||||
| Perennial Branch bark | ||||
| Annual branches | ||||
| Leaves | ||||
| Phellodendron chinense C. K. Schneid. | Chinese cork tree | Bark | Chan et al., 2007 | |
| Phellodendron chinense var. glabriusculum C. K. Schneid. (ex-Phellodendron wilsonii Hayata & Kaneh.) | Chinese cork tree | Bark, branch, leaf and heartwood | Chen, 1981 | |
| – | Tan et al., 2013 | |||
| Bark | Chen, 1982 | |||
| Phellodendron lavallei Dode | Lavalle corktree | Bark | Yavich et al., 1993 | |
| Zanthoxylum monophyllum (Lam.) P. Wilson | Palo rubio | Stem and branches | Stermitz and Sharifi, 1977 | |
| Zanthoxylum quinduense Tul. | – | – | Ladino and Suárez, 2010 |
Berberine is also widely present in barks, leaves, twigs, rhizomes, roots, and stems of several medicinal plants species, including Argemone mexicana (Etminan et al., 2005), Berberis aristata, B. aquifolium, B. heterophylla, B. beaniana, Coscinium fenestratum (Rojsanga and Gritsanapan, 2005), C. chinensis, C. japonica, C. rhizome, Hydratis canadensis (Imanshahidi and Hosseinzadeh, 2008), Phellodendron amurense, P. chinense, Tinospora cordifolia (Khan et al., 2011), Xanthorhiza simplicissima (Bose et al., 1963; Knapp et al., 1967; Sato and Yamada, 1984; Steffens et al., 1985; Inbaraj et al., 2001; Liu et al., 2008a; Srinivasan et al., 2008; Vuddanda et al., 2010). Several researches found that berberine is widely distributed in the barks, roots, and stems of plants, nevertheless, bark and roots are richer in berberine compared to other plant parts (Andola et al., 2010a,b). In the Papaveraceae family, Chelidonium majus is another important herbal source of berberine (Tomè and Colombo, 1995). An important number of plants for medicinal use, such as Coptidis rhizoma and barberry, are the natural sources with the highest concentration of berberine. Barberries, such as Berberis aristata, B. aquifolium, B. asiatica, B. croatica, B. thunbergii, and B. vulgaris, are shrubs grown mainly in Asia and Europe, and their barks, fruits, leaves, and roots are often widely used as folk medicines (Imanshahidi and Hosseinzadeh, 2008; Kosalec et al., 2009; Andola et al., 2010c; Kulkarni and Dhir, 2010). Different research groups have reported that maximum berberine concentration accumulates in root (1.6–4.3%) and in most of the Berberis species, plants that grow at low altitude contain more berberine compared to higher altitude plants (Chandra and Purohit, 1980; Mikage and Mouri, 1999; Andola et al., 2010a). However, a correlation could not be established within the results of berberine concentration regarding to species and season of the year (Srivastava et al., 2006a,c; Andola et al., 2010c; Singh et al., 2012). Comparative studies of berberine concentration contained in different species of the same genus have been reported, e.g., higher berberine content in B. asiatica (4.3%) in comparison to B. lycium (4.0%), and B. aristata (3.8%). Meanwhile, Srivastava et al. (2004) documented a higher berberine content in root of B. aristata (2.8%) compared with B. asiatica (2.4%) (Andola et al., 2010a). Seasonal variation of berberine concentration has been reported, e.g., the maximum yield of berberine for B. pseudumbellata was obtained in the summer harvest, and was 2.8% in the roots and 1.8% for the stem bark, contrary to that reported in the roots of B. aristata, where the berberine concentration (1.9%) is higher for the winter harvest (Rashmi et al., 2009). These variations may be caused to multiple factors, among which stand out: (i) the intraspecific differences, (ii) location and/or, (iii) the analytical techniques used. Table 2 gathers a synthesis of the main species containing berberine.
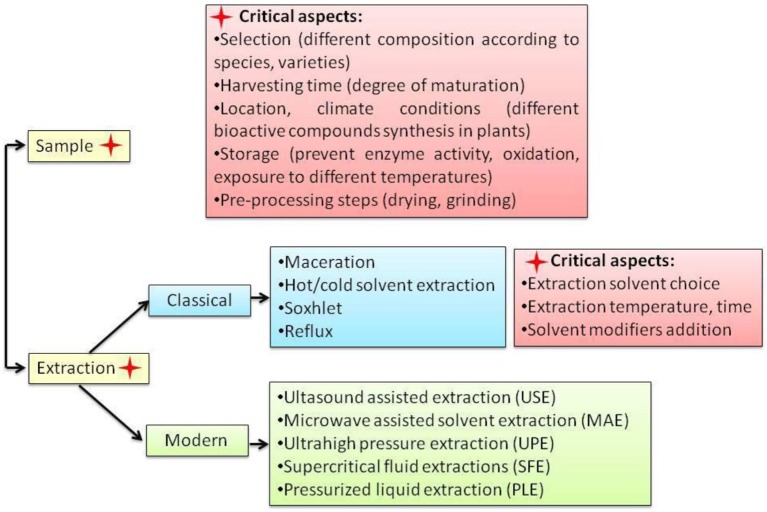
Extraction methods
Berberine, a quaternary protoberberine alkaloid (QPA) is one of the most widely distributed alkaloid of its class. Current studies suggest that isolation of the QPA alkaloids from their matrix can be performed using several methods. The principles behind these methods consist of the interconversion reaction between the protoberberine salt and the base. The salts are soluble in water, stable in acidic, and neutral media, while the base is soluble in organic solvents. Thus during the extraction procedure, the protoberberine salts are converted in their specific bases and further extracted in the organic solvents (Marek et al., 2003; Grycová et al., 2007).
In the case of berberine, the classical extraction techniques like maceration, percolation, Soxhlet, cold or hot continuous extraction are using different solvent systems like methanol, ethanol, chloroform, aqueous, and/or acidified mixtures. Berberine's sensitivity to light and heat is the major challenge for its extraction. Hence, exposure to high temperature and light could lead to berberine degradation and thus influencing its matrix recovery. In his study Babu et al. (2012) demonstrated that temperature represent a crucial factor in both extraction and drying treatments prior extraction. The yield of berberine content in C. fenestratum stem tissue samples was higher in case of samples dried under the constant shade with 4.6% weight/weight (w/w) as compared to samples dried in oven at 65°C (1.32% w/w) or sun drying (3.21% w/w). As well hot extraction procedure with methanol or ethanol at 50°C gave lower extraction yields when compared with methanol or ethanol cold extraction at −20°C. Thus, berberine content in the shade-dried samples was 4.6% (w/w) for methanolic cold extraction and 1.29% (w/w) for methanolic hot extraction (Babu et al., 2012).
Along with extraction temperature, the choice of solvents is considered a critical step in berberine extraction as well (Figure 2). As seen in Table 3, methanol, ethanol, aqueous or acidified methanol or ethanol are the most used extraction solvents. The acidified solvents (usually with the addition of 0.5% of inorganic or organic acids) are used to combine with free base organic alkaloids and transform them in alkaloid salts with higher solubility (Teng and Choi, 2013). The effect of different inorganic acids like hydrochloric acid, phosphoric acid, nitric acid, and sulfuric acid as well as the effect of an organic acid like acetic acid were tested on berberine content and other alkaloids in rhizomes of Coptis chinensis Franch by Teng and Choi (2013). In this case, 0.34% phosphoric acid concentration was considered optimal. Moreover, when compared to other classical extraction techniques like reflux and Soxhlet extraction, the cold acid assisted extraction gave 1.1 times higher berberine yields.
Figure 2.
Short view on berberine extraction methods.
Table 3.
Extraction and detection methods for berberine in different herbal and biological matrixes.
| Sample (weight) | Extraction method | Detection method | References |
|---|---|---|---|
| Dried stem powder Coscinium fenestratum (Gaertn.) (1 g) |
Extraction solvents (ES): water, methanol–water (1:1. v/v), and methanol Sonication (15 min, room temperature) Centrifugation (2,800 rpm, 15 min) Filtration and evaporation Extracts resolubilization (methanol:water, 9:1 v/v) |
HPLC - DAD Column: ODS, Chromolith, RP-18e,100 × 4.6 mm Mobile Phases: Methanol/Deionized Water (90:10, v/v) Flow: 0.5 mL/min, Temperature: 25°C UV Spectrophotometric Analysis | Akowuah et al., 2014 |
| C. fenestratum (Gaertn.) (10 g) |
ES: methanol Hot extraction: sample refluxed with ES for 3 h Filtration and evaporation. Extracts resolubilization (methanol) |
TLC
Adsorbent: Silica Gel GF 254 Solvent system: n-Butanol: Ethyl acetate: Acetic acid (2.5:1.5:1, v/v/v) Detection: 254 and 366 nm |
Arawwawala and Wickramaar, 2012 |
|
Cold extraction: sample extraction with ES for 24 h Filtration and evaporation. Extracts resolubilization (methanol) |
|||
| Dried C. fenestratum (0.1 g) |
ES: absolute methanol Cold extraction: sample extraction at −20°C Hot extraction: water bath sample extraction at 50°C ES: absolute ethanol Cold extraction sample extraction at −20°C Hot extraction: water bath sample extraction at 50°C Samples centrifugation (10 min at 10°C after cooling down) Samples filtration |
HPLC Column: C18, 250 × 4.6 mm, 5 μm Mobile Phases: Acetonitrile/0.1% Trifluro-acetic acid (50:50, v/v) Detection: 344 nm Flow: 0.8 mL/min |
Babu et al., 2012 |
|
C. fenestratum (1,000g) Capsules (containing 62.5 mg C. fenestratum) |
ES: petroleum ether, chloroform, methanol (1L each) Soxhlet extraction: with each ES for 3 days at (30–40°C) ES: methanol (10 mL) Extraction for 1 h Filtration and evaporation Resolubilisation in methanol (5 mL) |
HPLC
Column: Luna C18, 150 × 4.6 mm, 5 μm, Phenomenex Mobile Phases: (A) Potassium dihydrogen phosphate (pH −2.5) and (B) Acetonitrile Detection: 220 nm Flow: 1 mL/min HPTLC Adsorbent: Silica Gel 60F 254 Solvent system: n-Butanol: Glacial acetic acid: Water (8:1:1, v/v/v) Detection: 350 nm for all measurements |
Jayaprakasam and Ravi, 2014 |
| Tinospora cordifolia (Willd.), Tribulus terrestris (L.), Emblica officinalis (Gaertn.) (3 g) |
ES: chloroform Dried sample trituration with ammonia solution Drying at room temperature Extraction with ES for 1h Chloroform phase extraction with 5% sulfuric acid (x 3) Basification of acid extract with sodium carbonate (pH −9) Extraction of basified solution with chloroform (X 3) Evaporation of chloroform phase (temperature under 50°C) Residue solubilization with methanol |
UV-VIS
UV absorbance: 348 nm |
Joshi and Kanaki, 2013 |
| Cortex phellodendri (2 g) |
Ultrahigh pressure extraction (UPE) Optimal parameters: ES: ethanol (69.1%), liquid-solid ratio−31.3, extracting pressure−243.30 MPa, extraction time−2 min |
HPLC
Column: Hypersil ODS C18, 250 × 4.6 mm, 5 μm Mobile Phases: (A) 0.3% triethanolamine aqueous solution (pH − 3.5) Detection: 265 nm Temperature: 30°C Flow: 1 mL/min |
Guoping et al., 2012 |
| Rhizome of Coptis chinensis Franch (1 g) |
Supercritical fluid extraction Extraction time: up to 3 h Temperature: 60°C Pressure: from 200 to 500 bar Flow-rate of carbon dioxide (gaseous state): 1 L/min Flow-rate of modifier: 0.4 mL/min. Organic solvent modifier systems: ethanol-modified supercritical carbon dioxide, methanol-modified supercritical carbon dioxide, 1,2-propanediol-modified supercritical carbon dioxide, 5% Tween 80 in methanol-modified supercritical carbon dioxide, 5% Tween in ethanol-modified supercritical carbon dioxide |
HPLC
Column: Diamonsil C18, 250 × 4.6 mm, 5 μm Mobile Phases: 33 mM Potassium dihydrogen phosphate : acetonitrile (70:30, v/v) Detection: 345 nm Flow: 1 mL/min |
Liu et al., 2006 |
|
Soxhlet extraction ES: hydrochloric acid: methanol (1: 100, v/v) Time: 8 h |
|||
| Cortex pellodendri amurensis (1 g) |
Ultrahigh pressure extraction ES: ethanol (50 %), liquid-solid ratio −30: 1, extracting pressure −400 MPa, extraction time −4 min, extraction temperaturte −40°C Ultrasonic extraction ES: 70% ethanol Sample soaking for 24 h in 40 ml ES Sonic extraction for 60 min at 30°C |
HPLC- DAD
Column: Daisopak SP-120-5-ODS_BP, 250 × 4.6 mm, 5 μm Mobile Phases: (A) acetonitrile and (B) phosphoric acid: water (0.7:100, v/v) Detection: 345 nm Temperature: 25°C Flow: 1 mL/min |
Liu et al., 2013 |
|
Heat reflux extraction ES: 70% ethanol Sample soaking for 24 h in 40 ml ES Sample extraction for 4 h at boiling state |
|||
|
Soxhlet extraction ES: 70% ethanol Sample soaking for 24 h in 40 ml ES Sample extraction: 4 h |
|||
| Goldenseal (Hydrastis canadensis L.) (2, 5, 5 g) |
Pressurized hot water extraction ES: water at 140°C, Optimal parameters: pressure: 50 bars and flow rate: 1 mL/min, Time: 15 min Reflux extraction ES: methanol (200 mL) Sonication: 4 h at 80°C Ultrasonic extraction ES: methanol (50 mL) Reflux: 6 h with continuous stirring |
HPLC-DAD Column: Zorbax eclipse Plus C 18, 75 x 4.6 mm, 3.5 μm Mobile phases: (A) 0.1 % Formic Acid (pH 2.7) and (B) methanol Detection: 242 nm Temperature: 35°C Flow: 1 mL/min MS Detection: ESI (+) Capillary temperature: 200°C, Sheath gas: 80, Capillary voltage: 20 V, Tube lens voltage: 5V |
Mokgadi et al., 2013 |
| Berberis aristata DC (1.5 g), Berberis aristata herb extract (0.1 g), Ayurvedic form (6 g) |
Crude herb reflux extraction ES: methanol (100 mL) for 1 h in a water bath Filtratio Reextraction with ES (50 mL) for 30 min (× 2) Filtrates combination and concentration to 50 mL Herb extracts ultrasonic extraction ES: methanol (up to 10 mL) Sonication Filtration |
HPLC Column: Zorbax ODS II, 250 x 4.6 mm, 5 μm Mobile phase: potassium hydrogen phosphate buffer (pH 2.5)/ acetonitrile Detection: 346 nm Temperature: 40°C Flow: 1 mL/min |
Singh R. et al., 2010 |
|
Ayrvedic form ultrasonic extraction ES: methanol (up to 25 mL) Sonication |
|||
| Berberis aristata DC root |
Soxhlet extraction ES: ethanol Berberine isolation Ethanolic extract concentration to obtain a syrup mass Dissolvation in hot water and filtration Acidification (36.5% w/v hydrochloric acid) Cool: ice bath - 30 min, overnight in refrigerator |
HPTLC Stationary phase: precoated silica gel 60GF254 Mobile phases: n-butanol: glacial acetic acid: water (12:3:4 v/v/v) Temperature: 33 ± 5°C Detection: 350 nm |
Patel, 2013 |
| Mahonia manipurensis (Takeda) stem bark (100 g) |
Cold extraction ES: 80% methanol (1,000 mL) Stirring at room temperature Extract concentration |
TLC Stationary phase: precoated silica gel G F254 Mobile phase: hexane: ethyl acetate: methanol (56:20:5) Fraction purification: positive test using Dragendroff's reagent Further analysis of purified fraction Mobile phase: chloroform: ethyl acetate: diethylamine: methanol: 20% ammonium hidroxide (6:24:1.5:6:0.3) |
Pfoze et al., 2014 |
|
HPLC
Column: Water Symmetry C18, 250 x 4.6 mm, 5 μm Mobile phase: methanol/ formic acid buffer (0.1%, v/v) Detection: 346 nm Flow: 1 mL/min |
|||
|
UV-VIS UV spectra: 200–500 nm |
|||
| ESI-MS | |||
| Coscinium fenestratum (100 g) |
Maceration ES: 80% ethanol (500 ml), 160 h Shaken: 80 h (200 rpm), stand: 80 h Reextraction: 48 h, shaken: 24 h, stand: 24 h Combined extracts concentration Evaporation to dryness (dry extract) Resolubilisation in 80% ethanol (10 mg dry extract/mL) |
TLC Stationary phase: Silica gel GF254 Mobile phase: ethyl acetate : butanol : formic acid : water (50:30:12:10); Detection: 366 nm |
Rojsanga and Gritsanapan, 2005 |
| Argemone mexicana |
Soxhlet extraction ES: methanol Evaporation to dryness Resolubilisation in methanol (known concentration) |
HPTLC Stationary phase: precoated silica gel 60F254 Mobile phases: toluene: ethyl acetate (9:3, v/v). Detection: 266 nm |
Samal, 2013 |
| Tinospora cordifolia (20 g) |
Microwave assisted extraction (MAE) ES: 80% ethanol Irradiation power: 60%, Extraction time: 3 min Soxhlet extraction ES: ethanol, for 3 h Filtration Concentration |
HPTLC Mobile phases: methanol: acetic acid: water (8: 1: 1, v/v/v). Detection: 366 nm |
Satija et al., 2015 |
|
Maceration ES: ethanol (200 mL), 7 days, occasional stirring |
|||
| Berberis aristata, Berberis tinctoria (800 g) |
Hot extraction ES: methanol (2.5 L) (X2) Extraction time: 3 h Temperature: 50°C Extract concentration under vacuum |
HPLC Column: Unisphere C18, 150 x 4.6 mm, 5 μm Mobile phase: (A) 0.1% trifluoroacetic acid and (B) acetonitrile (60:40, v/v) Detection: 350 nm Temperature: 30°C Flow: 1 mL/min |
Shigwan et al., 2013 |
| Coptis chinensis Franch. (1g) |
Acid assisted extraction ES: several inorganic acids (hydrochloric acid, phosphoric acid, nitric acid, and sulfuric acid) and one organic acid (acetic acid) Extraction time:1–8 h, Acid concentrations: 0–1% Solvent to sample ratios: 20–60 mL/g Maceration at 25°C Filtration Dilution to 100 mL final volume |
HPLC Column: XTerra C18, 250 x 4.6 mm Mobile phase: (A) acetonitrile and (B) 25 mmol/L potassium dihydrogen phosphate,(27:75, v/v) Detection: 345 nm Temperature: 30°C |
Teng and Choi, 2013 |
|
Soxhlet extraction ES: 50% ethanol (100 mL), 4 h at 70°C Extract evaporation to dryness Resolubilization in ES (up to 100 mL final volume) |
|||
|
Heating reflux extraction ES: 50% ethanol Soaked for 1 h Extraction: 4 h at 70°C (heated water bath) Filtration Dilution (up to 100 mL final volume) |
|||
| Rabbit plasma (100 μl) | Mixing 100 μl sample with 3% formic acid in acetonitrile (200 μl) Vortex: 30 s Centrifugation: 10 min at 4°C Evaporation of supernatant: under nitrogen stream at 40°C |
LC-ESI-MS HPLC system Column: Capcell Pakc18 MG, 100 × 2.1 mm, 5 μm with Security Guard C18, 4 × 2 mm, 5 μm Mobile Phases: (A) 0.4% formic acid solution and (B) 0.2 % formic acid solution in methanol (60:40, v/v) |
Liu et al., 2011 |
| Residue solubilization: in 100 μl of 20% methanol | Temperature: 25°C Flow: 0.4 mL/min MS detection: Source: ESI (+) Quantification: MRM mode |
||
| Rat plasma |
Solid phase extraction (SPE) Cartridges: Oasis HLB (1 cc, 30 mg) Pre-conditioning: 2 mL methanol Equilibrtating: |
UPLC-MS/MS UPLC system Column: 120 EC-C18, 50 × 4.6 mm, 2.7 μm with Security Guard C18, 4 × 2 mm, 5 μm Mobile Phases: (A) 10 mM ammonium acetate in water (pH- 4.5) and (B) acetonitrile Temperature: 35°C Flow: 0.8 mL/min MS detection: Source: ESI (+) Quantification: MRM mode |
Liu M. et al., 2015 |
| Rat plasma Rat tissue |
Rat plasma ES: methanol Mixing sample (200 μl) with internal standard (40 μl) and ES (560 μl) Vortex: 20 s Centrifugation: 10 min, 12,000x g Filtration |
UPLC-MS/MS UPLC system Column: Acquity BEH C18, 50 × 2.1 mm, 1.7 μm Mobile Phases: (A) acetonitrile and (B) formic acid: water (0.1:99.9, v/v) Flow: 0.25 mL/min MS detection: Source: ESI (+) Quantification: MRM mode |
Wang et al., 2016 |
|
Rat tissue Grinding: 3 mL physiological saline with 600 mg tissue Centrifugation: 10 min, 12,000x g, 4°C Mixing supernatant (200 μl) with internal standard (40 μl) and ES (560 μl) Vortex: 20 s Centrifugation: 10 min, 12,000x g Filtration |
|||
| Rat plasma | Evaporation of 10 ul IS in the working tube Mixing sample (200 μl) with internal evaporated standard Vortex: 1 min Mixing sample with 10 μl 1% formic acid and 200 μl acetone Vortex: 2 min Centrifugation: 10 min, 10,000 rpm Mixing supernatant with 200 μl methanol Vortexing, centrifugation Mixing supernatant wit 400 μl acetonitrile Vortexing, centrifugation Evaporation to dryness (37°C, under nitrogen stream) Resolubilization in methanol |
LC-MS/MS LC system Column: Zorbax Eclipse XDB-C18, 150 × 2.1 mm, 3.5 μm Mobile Phases: (A) acetonitrile and (B) water with 1% acetic acid and 0.001 mol/L ammonium acetate Flow: 0.2 mL/min MS detection: Source: ESI (+) Quantification: MRM mode |
Xu et al., 2015 |
| Rat plasma |
ES: 90% methanol Mixing sample (100 μl) with internal standard (10 μl) and ES (100 μl) Vortex: 1 min Centrifugation: 10 min, 12,000 rpm, 4°C Supernatant evaporation to dryness under nitrogen stream Resolubilization (100 μl ES) |
UPLC-MS/MS UPLC system Column: Acquity UPLC BEH C18, 50 × 2.1 mm, 1.7 μm Mobile Phases: (A) formic acid: water (0.1:99.9, v/v) and (B) acetonitrile Flow: 0.4 mL/min MS detection: Source: ESI Quantification: MRM mode |
Yang et al., 2017 |
Large solvent volumes and long extraction time represent other drawbacks of conventional extraction methods (Mokgadi et al., 2013). For example, Rojsanga and Gritsanapan (2005) used maceration process to extract 100 g of C. fenestratum plant material with a total volume of 3,200 mL solvent (80% ethanol) over a period of 416 h. Furthermore, in a different study, Rojsanga et al. (2006) used several classical extraction techniques like maceration, percolation, and Soxhlet extraction to extract the berberine from C. fenestratum stems. This time even if the extracted plant material was in a lower amount than the previous study (30 vs. 100 g), large solvent volumes (2,000 mL for maceration, 5,000 mL for percolation, and 600 mL for Soxhlet extraction) over long time periods (7 days for maceration and 72 h for Soxhlet extraction) were employed (Rojsanga and Gritsanapan, 2005; Rojsanga et al., 2006).
Large solvent volumes are characteristic for other conventional methods too. Shigwan et al. (2013) extracted berberine from Berberis aristata and B. tinctoria powdered stem bark (800 g) using hot extraction (50°C for 3 h) with 2,500 mL methanol (Shigwan et al., 2013).
Even though conventional methods are widely used in berberine extraction, a number of other different methods have been developed lately. This led to an improved extraction efficiency, a decreased extraction time and solvents' volumes used in the extraction. Thus, ultrasound assisted solvent extraction (USE), microwave-assisted solvent extraction (MAE), ultrahigh pressure extraction (UPE), and supercritical fluid extractions (SFE), pressurized liquid extraction (PLE) have been successfully used as alternative extraction techniques with better results when compared with classical extraction methods.
Ultrasonically and microwave-assisted extraction are considered green, simple, efficient, and inexpensive techniques (Alupului et al., 2009).
Teng and Choi (2013) extracted berberine from Rhizome coptidis by optimized USE. Using response surface methodology, they identified that the optimal extraction conditions were 59% ethanol concentration, at 66.22°C within 46.57 min. A decrease in the extraction time (39.81 min) was obtained by Chang (2013). He used the combination of ionic liquids solutions as green solvents with USE to extract berberine from Coptis chinensis in order to apply an environmentally friendly approach (Chang, 2013). Moreover, in their study, Xu et al. (2017) compared several extraction tehniques like USE, distillation, and Soxhlet extraction in order to establish an high-efficient method for phellodendrine, berberine, and palmatine extraction from fresh Phellodendron bark (Cortex phellodendri). In the case of berberine, the combination of simple or acidified solvent (water, ethanol, and methanol) with the adjustment of the specific setting characteristics to each extraction type enabled them to determine the highest extraction yield. They concluded that the use of USE and hydrochloric acid-acidified methanol were the most efficient in extracting berberine. The USE extraction yield was significantly higher when compared to distillation and Soxhlet extraction, with values of ~100 mg/g toward 50 and 40 mg/g berberine, respectively (Xu et al., 2017).
The important reduction in organic solvent and extraction time determined the increasing interest in MAE, too. Lately, MAE was used as a green and cost-effective alternative to conventional methods. Using central composite design, Satija et al. (2015) successfully optimized the MAE parameters in terms of irradiation power, time, and solvent concentration to extract berberine form Tinospora cardifolia. They compared two classical extraction techniques like maceration and Soxhlet extraction with MAE under optimized conditions (60% irradiation power, 80% ethanol concentration, and 3 min extraction time). The results showed that MAE extraction had the highest yield of berberine content with 1.66% (w/w) while Soxhlet and maceration had 1.04 and 0.28% (w/w), respectively. Their study is emphasizing the dramatic time reduction in case of MAE (3 min) when compared with Soxhlet extraction (3 h) and maceration (7 days) together with solvent and energy consumption (Satija et al., 2015).
Another novel extraction technique considered to be environmentally friendly is UPE. The interest toward this extraction technique is increasing because it presents several advantages toward classical extraction techniques like increased extraction yields, higher quality of extracts, less extraction time, and decreased solvent consumption (Xi, 2015). These are achieved at room temperature by applying different pressure levels (from 100 to 600 MPa) between the interior (higher values) and the exterior of cells (lower values) in order to facilitate the transfer of the bioactive compounds through the plant matrices in the extraction solvent (Liu et al., 2006, 2013). In the study regarding berberine content in Cortex phellodendri, Guoping et al. (2012) made a comparison between UPE, MAE, USE, and heat reflux extraction techniques. They observed that the higher extraction yield and the lower extraction time was obtained in case of UPE with 7.7 mg/g and 2 min extraction time toward reflux, USE and MAE with 5.35 mg/g and 2 h, 5.61 mg/g and 1 h. and 6 mg/g and 15 min, respectively (Guoping et al., 2012).
Super critical fluid extraction is another environmentally friendly efficient technique used in phytochemical extraction. Because the extraction is performed in the absence of light and oxygen, the degradation of bioactive compounds is reduced. Also, the inert and non-toxic carbon dioxide used as a main extraction solvent in combination with various modifiers (e.g., methanol) and surfactants (e.g., Tween 80) at lower temperatures and relatively low pressure, allows the efficient extraction of bioactive compounds (Liu et al., 2006; Farías-Campomanes et al., 2015). In case of berberine extraction from the powdered rhizome of Coptis chinensis Franch, the highest recovery of berberine was obtained when 1,2-propanediol was used as a modifier of supercritical CO2 (Liu et al., 2006).
Pressurized liquid extraction, also known as pressurized fluid extraction, pressurized solvent extraction, and accelerated solvent extraction (ASE) is considered a green technology used for compounds extraction from plants (Mustafa and Turner, 2011). Compared with conventional methods, PLE increases the extraction yield, decreases time and solvent consumption, and protects sensitive compounds. In their study, Schieffer and Pfeiffer (2001) compared different extraction techniques like PLE, multiple USE, single USE, and Soxhlet extraction in order to extract berberine from goldenseal (Hydrastis canadensis). When compared in terms of extraction yield the results are comparable, ~42 mg/g berberine, except single USE with slightly lower content (37 mg/g berberine). Big differences were observed in the extraction time, PLE requiring only 30 min for a single sample extraction compared to 2 h for multiple extraction techniques or 6 h for Soxhlet extraction (Schieffer and Pfeiffer, 2001).
When referring to berberine extraction from biological samples, the extraction process is relatively simple and involves several steps like sample mixing with extraction solvents (e.g., methanol, acetone, acetonitrile), vortex, centrifugation followed by supernatant evaporation under nitrogen stream (Table 3). Other extraction techniques like solid phase extraction (SPE) can also be applied.
Analytical techniques
After extraction and purification, the separation and quantification of berberine are commonly resolved by chromatographic methods. According to literature studies, berberine determination in plants was predominantly performed using methods like UV spectrophotometry (Joshi and Kanaki, 2013), HPLC (Babu et al., 2012; Akowuah et al., 2014), HPTLC and TLC (Rojsanga and Gritsanapan, 2005; Arawwawala and Wickramaar, 2012; Samal, 2013), capillary electrophoresis (Du and Wang, 2010), while berberine content in biological fluids was mainly achieved by using LC-MS (Deng et al., 2008; Feng et al., 2010), UPLC-MS (Liu M. et al., 2015; Liu L. et al., 2016), UHPLC/Q-TOF-MS (Wu et al., 2015).
UV-Vis spectrophotometry can be considered as one of the most rapid detection methods for berberine quantitative analysis from plant extracts. Based on the Beer-Lambert law, berberine concentration can be determined according to its absorption maxima at 348 nm. Joshi and Kanaki (2013) quantified berberine in Rasayana churna samples in the range of 2–20 μg/mL, the interference with other compounds being avoided by the specific isolation of the alkaloid fraction (Joshi and Kanaki, 2013).
Next, high-performance liquid chromatography (HPLC) is a versatile, robust, and widely used technique for the qualitative and quantitative analysis of natural products (Sasidharan et al., 2011). This approach is widely used in berberine identification and quantification. Generally, the choices of stationary phase in berberine separation are variants of C18-based silica column (Table 3) with a mobile phase consisting of simple or acidified solvents like water, methanol, or acetonitrile, used as such or in combination with phosphate buffers. Normally, the identification and separation of berberine can be accomplished using either isocratic or gradient elution system. Berberine identification is further accomplished using high sensitivity UV or DAD (diode array detectors) detectors. For example, Shigwan et al. (2013) developed in his study a reverse phase HPLC method with photodiode array detection (PDA) to quantify berberine from Berberis aristata and B. tinctoria. They used a Unisphere-C18 column (5 μm, 4.6 × 150 mm) with an isocratic gradient of acidified water (with 0.1% trifluoroacetic acid) and acetonitrile (60:40, v/v) to elute berberine within 5 min. The developed method was reproducible, validated, precise, and specific for berberine quantification (with a concentration range between 0.2 and 150 μg/mL; Shigwan et al., 2013).
Two other commonly used techniques in berberine quantification are thin layer chromatography (TLC) and high performance thin layer chromatography (HTPLC). Sometimes, these methods are preferred over HPLC, offering the possibility of running several samples simultaneously along with the use of small amount of both samples and mobile phases (Samal, 2013). For these reasons, Samal (2013) used an HPTLC method to quantify berberine from A. mexicana L. using toluene and ethyl acetate (9:3, v/v) as mobile phases, and a silica gel plate as stationary phase, they developed a simple, rapid, and cost-effective method for berberine quantification. The LOD (0.120 μg) and LOQ (0.362 μg) of the method are in accordance with high-quality requirements.
Following the same principles (small sample volume, high separation efficiency, and short analysis time), capillary electrophoresis (CE) was successfully used in berberine analysis. Du and Wang (2010) used CE with end-column electrochemiluminescence (ECL) detection for berberine analysis in both tablets and Rhizoma coptidis. Using a 4 min analysis time, a small sample volume (3.3 nL) and a LOD of (5 × 10−9 g/mL), the developed method proved to be highly sensitive and with good resolution (Du and Wang, 2010).
Besides UV, HPLC, HTPLC, TLC, and CE, other detection methods like liquid chromatography coupled with mass spectrometry (LC/MS) are currently employed to quantify berberine in biological fluids. Generally, it is considered a powerful technique for the analysis of complex samples because it offers rapid and accurate information about the structural composition of the compounds, especially when tandem mass spectrometry (MSn) is applied. For example, Xu et al. (2015) developed a sensitive an accurate LC-MS/MS method to determine berberine and other seven components in rat plasma using multiple reactions monitoring (MRM) mode. Compounds separation was optimized using six different types of reverse-phase columns, and two different mobile phases (methanol–water and acetonitrile–water with different additives). Additives like formic acid, acetic acid, and ammonium acetate were added in different concentrations as follows: 0.1, 0.5, 1, and 2% for formic acid, 0.1, 0.5, 1, and 2% for acetic acid and 0.0001, 0.001, 0.01 mol/L for ammonium acetate. The method was also tested in terms of specificity, linearity, lower limit of quantification (LLOQ), precision, accuracy, and stability (Xu et al., 2015).
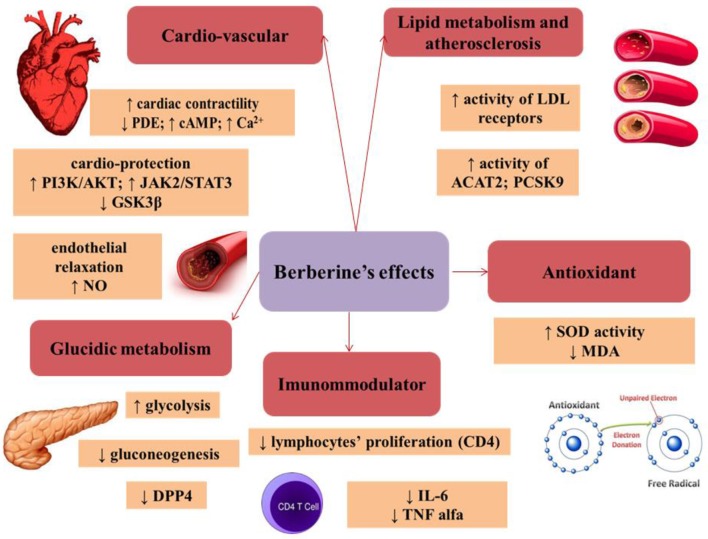
Antioxidant effect
Under normal conditions, the body maintains a balance between the antioxidant and pro-oxidant agents (reactive oxygen species—ROS and reactive nitrogen species—RNS; Rahal et al., 2014).
The imbalance between pro and antioxidants occurs in case of increased oxidative stress (Bhattacharyya et al., 2014).
The oxidative stress builds up through several mechanisms: an increase in the production of reactive species, a decrease in the levels of enzymes involved in blocking the actions of pro-oxidant compounds, and/or the decrease in free radical scavengers (Pilch et al., 2014).
An experimental study demonstrated the effect of berberine on lipid peroxidation after inducing chemical carcinogenesis in small animals (rats). An increase in LPO (lipid peroxidation) was observed after carcinogenesis induction, but also its significant reversal after berberine administration (30 mg/kg). Berberine shows therefore at least partial antioxidant properties, due to its effect on lipid peroxidation (Thirupurasundari et al., 2009).
Other mechanisms involved in the antioxidant role of berberine are: ROS/RNS scavenging, binding of metals leading to the transformation/oxidation of certain substances, free-oxygen removal, reducing the destructiveness of superoxide ions and nitric oxide, or increasing the antioxidant effect of some endogenous substances. The antioxidant effect of berberine was comparable with that of vitamin C, a highly-potent antioxidant (Shirwaikar et al., 2006; Ahmed et al., 2015).
The increase in blood sugar leads to oxidative stress not by generating oxygen reactive species but by impairing the antioxidant mechanisms. Administration of berberine to rats with diabetes mellitus increased the SOD (superoxide dismutase) activity and decreased the MDA (malondialdehyde) level (marker of lipid peroxidation). This antioxidant effect of berberine could explain the renal function improvement in diabetic nephropathy (Liu et al., 2008b).
The oxidative stress plays an important role in the pathogenesis of many diseases. The beneficial effect of berberine is presumed to reside mostly in its antioxidant role.
Cardiovascular effects of berberine
Effect on cardiac contractility
The beneficial effect of berberine in cardiac failure was demonstrated in a study on 51 patients diagnosed with NYHA (New York Heart Association) III/IV cardiac failure with low left ventricular ejection fraction (LVEF) and premature ventricular contractions and/or ventricular tachycardia. These patients received tablets containing 1.2 g berberine/day, together with conventional therapy (diuretics, ACEI—angiotensin-converting-enzyme inhibitors, digoxin, nitrates) for 2 weeks. An increase in LVEF was observed in all patients after this period, but also a decrease in the frequency and complexity of premature ventricular contractions. The magnitude of the beneficial effect was in direct proportion with the plasma concentration of berberine (Zeng, 1999).
The cardioprotective effect during ischemia
Berberine can provide cardio-protection in ischemic conditions by playing various roles at different levels: modulation of AMPK (AMP—activated kinase) activity, AKT (protein kinase B) phosphorylation, modulation of the JAK/STAT (Janus kinase/signal transducers and activators of transcription) pathway and of GSK3β (glycogen synthase kinase 3β; Chang et al., 2016). AMPK is an important enzyme playing an essential role in cellular metabolism and offering protection in ischemic conditions by adjusting the carbohydrate and lipid metabolism, the function of cell organelles (mitochondria, endoplasmic reticulum) and the apoptosis (Zaha et al., 2016).
Berberine activates the PI3K (phosphoinositide 3-kinase)/AKT pathway which is considered a compensatory mechanism limiting the pro-inflammatory processes and apoptotic events in the presence of aggressive factors. The activation of this pathway is associated with a reduction of the ischemic injury through the modulation of the TLR4 (toll-like receptor 4)-mediated signal transduction (Hua et al., 2007).
Several supporting data indicate that the JAK2/STAT3 signaling plays an important role in cardioprotection against ischemia-reperfusion injury (Mascareno et al., 2001).
GSK3β is a serine/threonine protein-kinase, an enzyme involved in reactions associated to important processes at the cellular level: metabolization, differentiation, proliferation, and apoptosis. Berberine inhibits this kinase, thereby exercising its cardioprotective effect (Park et al., 2014).
Effects on the endothelium
Berberine induces endothelial relaxation by increasing NO production from arginine through the activity of eNOS (endothelial nitric oxide synthase) which is considered a key element in the vasodilation process. Besides increasing the NO level, it also up-regulates eNOS mRNA. Furthermore, berberine facilitates the phosphorylation of eNOS and its coupling to HSP 90 (heat shock proteins), which consequently increases NO production (Wang et al., 2009).
Moreover, berberine reduces endothelial contraction by reducing COX-2 expression. Any imbalance in COX 1 or 2 activity may alter the ratio between prothrombotic/antithrombotic and vasodilator/vasoconstrictor effects (Liu L. et al., 2015).
The beneficial effect of berberine on the TNFα-induced endothelial contraction was also recorded, as well as an increase in the level of PI3K/AKT/eNOS mRNA (Xiao et al., 2014).
The role of berberine in atherosclerosis
Atherogenesis is a consequence of high blood lipid levels and is associated with inflammatory changes in the vascular wall. Berberine interferes with this process by up-regulating the expression of SIRT1 (silent information regulator T1) and by inhibiting the expression of PPARγ (peroxisome proliferator-activated receptor-γ). SIRT1 is a NAD-dependent deacetylase. The SIRT1 enzyme has many targets (PPARγ, p53), all playing different roles in atherogenesis (Chi et al., 2014).
The role of berberine in lipid metabolism
The effects of berberine on lipid metabolism are also the consequence of its effects on LDL cholesterol receptors. On one hand, these receptors are stabilized by an extracellular signal-regulated kinase (ERK)-dependent pathway, and on the other, berberine increases the activity of LDL receptors through the JNK pathway (Cicero and Ertek, 2009).
Moreover, berberine has an effect on ACAT (cholesterol acyltransferases), a class of enzymes that transform cholesterol into esters, thus playing an essential role in maintaining cholesterol homeostasis in different tissues. There are two types of ACAT enzymes, ACAT1, and ACAT2. ACAT1 is a ubiquitous enzyme, while ACAT2 can be found only in hepatic cells and enterocytes. Berberine influences the activity of ACAT2 without an effect on ACAT1, therefore reducing the intestinal absorption of cholesterol and decreasing its plasmatic level (Chang et al., 2009; Wang et al., 2014).
The hypolipidemic effect of berberine is also a result of its action on PCSK9 (proprotein convertase subtilisin kexin 9). This enzyme can attach itself to LDL receptors, leading to a decrease in LDL metabolization and an increase in its blood level (Xiao et al., 2012).
In a clinical trial, 63 patients with dyslipidemia were randomly divided in three groups. The first group was treated with berberine (1,000 mg/day), the second with simvastatin (20 mg/day) and the third with a combination of berberine and simvastatin. The authors reported a 23.8% reduction in LDL-C levels in patients treated with berberine, a 14.3% reduction in those treated with simvastatin and a 31.8% LDL-C reduction in the group treated with both simvastatin and berberine. This result demonstrates that berberine can be used alone or in association with simvastatin in the treatment of dyslipidemia (Kong et al., 2008).
The role of berberine in glucose metabolism
Many studies demonstrated that berberine lowers blood sugar, through the following mechanisms:
- Inhibition of mitochondrial glucose oxidation and stimulation of glycolysis, and subsequently increased glucose metabolization (Yin et al., 2008a).
- Decreased ATP level through the inhibition of mitochondrial function in the liver, which may be the probable explanation of gluconeogenesis inhibition by berberine (Xia et al., 2011).
- Inhibition of DPP 4 (dipeptidyl peptidase-4), a ubiquitous serine protease responsible for cleaving certain peptides, such as the incretins GLP1 (glucagon-like peptide-1) and GIP (gastric inhibitory polypeptide); their role is to raise the insulin level in the context of hyperglycemia. The DPP4 inhibition will prolong the duration of action for these peptides, therefore improving overall glucose tolerance (Al-masri et al., 2009; Seino et al., 2010).
Berberine has a beneficial effect in improving insulin resistance and glucose utilization in tissues by lowering the lipid (especially triglyceride) and plasma free fatty acids levels (Chen et al., 2011).
The effect of berberine (1,500 mg day) on glucose metabolism was also demonstrated in a pilot study enrolling 84 patients with type 2 diabetes mellitus. The effect, including on HbA1c, was comparable to that of metformin (1,500 mg/day), one of the most widely used hypoglycemic drugs. In addition, berberine has a favorable influence on the lipid profile, unlike metformin, which has barely any effect (Yin et al., 2008b).
Hepatoprotective effect of berberine
The hepatoprotective effect of berberine was demonstrated on lab animals (mice), in which hepatotoxicity was induced by doxorubicin. Pretreatment with berberine significantly reduced both functional hepatic tests and histological damage (inflammatory cellular infiltrate, hepatocyte necrosis; Zhao et al., 2012).
The mechanism by which berberine reduces hepatotoxicity was also studied on CCl4 (carbon tetrachloride)-induced hepatotoxicity. Berberine lowers the oxidative and nitrosamine stress and also modulates the inflammatory response in the liver, with favorable effects on the changes occurring in the liver. Berberine prevents the decrease in SOD activity and the increase in lipid peroxidation and contributes to the reduction in TNF-α, COX-2, and iNOS (inducible nitric oxide synthase) levels. The decrease in transaminase levels supports the hypothesis according to which berberine helps maintain the integrity of the hepatocellular membrane (Domitrović et al., 2011).
Nephroprotective effect of berberine
The chronic kidney damage occurring in time in patients with HT (hypertension) and DM (diabetes mellitus) is well known; it is mainly due to the atherosclerosis of the renal artery, caused by inflammation and oxidative stress. The protective effect of berberine on kidneys was studied on 69 patients suffering from both HT and DM, with blood pressure and blood sugar levels controlled with conventional medication. The patients received 300 mg berberine/day for 24 months, with 2-week interruptions every 5 months. The authors recorded lower CRP (C-reactive protein), MDA and SOD levels after treatment, but without significant changes in creatinine, arterial pressure, or glycaemia levels. These results support the renal protective effect of berberine through its anti-inflammatory and antioxidant effects (Dai et al., 2015).
Another animal study tested the renoprotective effect of berberine after administration of HgCl2 (mercury chloride). This substance induces hepato-renal damage by increasing the oxidative stress (increases lipid peroxidation and NO levels, and lowers glutathione and SOD levels as well as the activity of other protective enzymes). Administration of HgCl2 increased the AST (aspartate aminotransferase), ALT (alanine aminotransferase), and ALP (alkaline phosphatase) levels, compared to the control group. However, pretreatment with berberine lowered these enzymes significantly. In addition, both urea and creatinine levels were significantly increased in the HgCl2 group vs. the control group, and again pretreatment with berberine prevented these changes. Additionally, the authors recorded higher pro-oxidant and lower antioxidant levels in the intervention group. These data support the hepatic and renal protective effects of berberine. Other studies performed on animal models with CCl4−induced hepatotoxicity demonstrated the same effect (Othman et al., 2014).
In addition, berberine can lower the nephrotoxicity caused by cisplatine. In an animal study, berberine was administered in progressive doses of 1, 2, 3 mg/kg, orally, for 2 successive days, starting 2 days after cisplatine administration. After the last doses of berberine, the animals were sacrificed and the kidneys were examined by the pathologist. The results showed significant histological improvement and a reduction in NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells), TNF α, COX2 an iNOS levels, all of which support the anti-inflammatory effect of berberine (Domitrović et al., 2013).
Immunomodulatory effect of berberine
The immunomodulatory effect of berberine was demonstrated in many experimental and clinical contexts.
In an experimental autoimmune myocarditis model, berberine contributed to mitigate the cardiac damage by: limiting the rise in anticardiac myosin antibodies, modulating the activity of certain STATs and blocking Th1 and Th2 cell differentiation, which play an important role in the pathogenesis of myocarditis (Liu X. et al., 2016).
Experimental autoimmune neuritis is an experimental animal model equivalent to the Guillain-Barre syndrome in humans. This neurologic syndrome is characterized by autoimmune injury of the peripheral nervous system. The beneficial effect of berberine on this animal model resided in its influence on cellular and humoral immunity through the inhibition of lymphocyte proliferation (especially CD4), and the decrease in pro-inflammatory cytokines (IL-6 and TNF α; Li et al., 2014).
Experimental autoimmune encephalomyelitis is an established model of multiple sclerosis. Multiple sclerosis is a one of the most common diseases of the central nervous system (CNS) and involves neurodegenerative and inflammatory processes, and autoimmune demyelination (Ransohoff et al., 2015). The blood-brain barrier permeability and changes in matrix metalloproteinase (MMP) levels in the cerebrospinal fluid and brain were studied using this model (Ma et al., 2010). MMPs may be involved in demyelination and their activity in tissues depends on the balance between their level and their tissue inhibitors. MMP2 and MMP9 are the main endoproteinases involved in the migration of lymphocytes in CNS and in altering the BBB (blood brain barrier) (Avolio et al., 2003). Berberine has a beneficial effect in experimental autoimmune encephalomyelitis by inhibiting the activity of MMP9, reducing BBB permeability and, consecutively, by decreasing the inflammatory cellular infiltration of the CNS (Ma et al., 2010).
The current therapy used for inflammatory bowel diseases, including glucocorticoids and immunosuppressive agents, has a low level of safety. The effect of berberine was studied in combination with 5-ASA (5-aminosalicylic acid) vs. 5-ASA alone using an experimental animal model with DSS (dextran sulfate sodium)-induced colitis. The authors analyzed the level of proinflammatory cytokines in the animal gut. A decrease in COX2, IL6, and IL23 mRNA levels was observed in animals treated only with 5-ASA, whereas animals treated with both 5-ASA and berberine had a reduction in mRNA levels for COX2, IL6, IL23 as well as for TNF alfa and IL12b. This beneficial effect could partially be attributed to the inhibition of NF-kB and the reduction in JAK2 phosphorylation (through the influence on the JAK/STAT pathway) by both 5-ASA and berberine (Li et al., 2015; Figure 3).
Figure 3.
Therapeutic effects of berberine in vivo. Mechanisms of berberine in regulation of metabolism, immunity and oxidative reactions. Phosphodiesterase (PDE), cyclic 3′,5′-adenosine monophosphate (cAMP), phosphoinositide 3-kinase/protein kinase B (PI3K/AKT), Janus kinase/signal transducers and activators of transcription (JAK/STAT), glycogen synthase kinase 3β (GSK3β), superoxide dismutase (SOD), malondialdehyde (MDA), nitric oxide (NO), cholesterol acyltransferases (ACAT2), dipeptidyl peptidase-4 (DPP 4), proprotein convertase subtilisin kexin 9 (PCSK9).
Another study demonstrated that berberine increases the corticosteroid level in rats with experimentally-induced colitis. This engendered the theory that its beneficial effect may also be attributed to the increase in endogenous glucocorticoid levels, compounds with well-known therapeutic effect in inflammatory bowel disease (Minaiyan et al., 2011).
Conclusion
A review of the available scientific literature shows that the traditional medical uses of berberine-containing plants have been evaluated by modern pharmacological studies. Different species of berberine-rich plants have multiple pharmacological and therapeutic actions, such as antioxidant and immunomodulatory effects, protective action on the cardiovascular system, liver and kidney, endothelial relaxation, regulator on glucose metabolism and atherosclerosis, which can all be explained by the presence of berberine as well as other phyto constituents (when dealing with berberine-containing plant extracts). Moreover, the effects of berberine vary according to its origin (different plants or pharmaceutical products) and its concentration, depending on the very diverse extraction and detection techniques already described. Over time, modern extraction techniques were increasingly preferred to classical ones. Since classical methods are generally time- and solvent-consuming processes, modern extraction techniques such as USE, MAE, UPE, SFE, and PLE are seen as better alternatives to overcome these limitations. Furthermore, berberine, due to its antioxidant and anti-inflammatory effects, has several clinical applications in many disorders, from inflammatory conditions to the metabolic syndrome. However, there are some traditional uses that have not yet been completely elucidated, and further studies are needed. Therefore, extensive studies on the potential of plants containing berberine that have shown aforementioned pharmacological activities should go through additional in vitro and in vivo studies.
Author contributions
MN, AM, JE, and RP have conceived and designed the structure of the manuscript, data collection, and drafting, as well as its revision. CB, GC, and AB have critically reviewed the manuscript. All authors have seen and agreed on the final version of the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This article was published under the frame of the internal grant no. 4945/15/08.03.2016 of the Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
References
- Abbasi A. M., Dastagir G., Hussain F., Sanaullah P. (2005). Ethnobotany and marketing of crude drug plants in district Haripur, Pakistan. Pak. J. Plant Sci. 11, 103–114. [Google Scholar]
- Abbasi A. M., Khan M. A., Ahmad M., Zafar M., Jahan S., Sultana S. (2010). Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. J. Ethnopharmacol. 128, 322–335. 10.1016/j.jep.2010.01.052 [DOI] [PubMed] [Google Scholar]
- Abbasi A. M., Khan M. A., Ahmad M., Zafar M., Khan H., Muhammad N., et al. (2009). Medicinal plants used for the treatment of jaundice and hepatitis based on socio-economic documentation. Afr. J. Biotechnol. 8, 1643–1650. [Google Scholar]
- Abou-Donia A. H. A., El-Din A. A. S. (1986). Phytochemical study of Argemone mexicana L. grown in Egypt. Egypt. J. Pharm. Sci. 25, 1–5. [Google Scholar]
- Acharya K. P., Rokaya M. B. (2005). Ethnobotanical survey of medicinal plants traded in the streets of Kathmandu valley. Sci. World 3, 44–48. [Google Scholar]
- Adeyemi A. A., Gbolade A. A., Moody J. O., Ogbole O. O., Fasanya M. T. (2010). Traditional anti-fever phytotherapies in Sagamu and Remo north districts in Ogun State, Nigeria. J. Herbs. Spices Med. Plants 16, 203–218. 10.1080/10496475.2010.511075 [DOI] [Google Scholar]
- Adjanohoun J. E., Aboobakar N., Dramane K. (1996). Traditional Medicine and Pharmacopoeia: Contribution to Ethnobotanical and Floristic Studies in Cameroon. Porto-Novo: Technical and Research Commission (STRC) of the Organization of African Unity. [Google Scholar]
- Ahmed E., Arshad M., Ahmad M., Saeed M., Ishaque M. (2004). Ethnopharmacological survey of some medicinally important plants of Galliyat Areas of NWFP, Pakistan. Asian J. Plant Sci. 3, 410–415. 10.3923/ajps.2004.410.415 [DOI] [Google Scholar]
- Ahmed T., Gilani A. U., Abdollahi M., Daglia M., Nabavi S. F., Nabavi S. M. (2015). Berberine and neurodegeneration: a review of literature. Pharmacol. Rep. 67, 970–979. 10.1016/j.pharep.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Ahn D. K. (2003). Illustrated Book of Korean Medicinal Herbs. Seoul: Kyo-Hak Publishing, Kyohaksa. [Google Scholar]
- Ajali U. (2000). Antibacterial activity of Enantia polycarpa bark. Fitoterapia 71, 315–316. 10.1016/S0367-326X(99)00153-7 [DOI] [PubMed] [Google Scholar]
- Akowuah G. A., Okechukwu P. N., Chiam N. C. (2014). Evaluation of HPLC and spectrophotometric methods for analysis of bioactive constituent berberine in stem extracts of Coscinium fenestratum. Acta Chromatogr. 26, 243–254. 10.1556/AChrom.26.2014.2.4 [DOI] [Google Scholar]
- Al-Douri N. A. (2000). A survey of medicinal plants and their traditional uses in Iraq. Pharm. Biol. 38, 74–79. 10.1076/1388-0209(200001)3811-BFT074 [DOI] [PubMed] [Google Scholar]
- Ali M., Shah S. Z., Khan M. S., Naz M. F. R., Zafar A. (2018). Ethnobotanical study on the weeds of wheat crop in district Swabi, Khyber Pakhtunkhwa, Pakistan. Int. J. Biosci. 12, 363–374. 10.12692/ijb/12.1.363-374 [DOI] [Google Scholar]
- Al-masri I. M., Mohammad M. K., Tahaa M. O. (2009). Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J. Enzyme Inhib. Med. Chem. 24, 1061–1066. 10.1080/14756360802610761 [DOI] [PubMed] [Google Scholar]
- Al-Qura'n S. (2009). Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 123, 45–50. 10.1016/j.jep.2009.02.031 [DOI] [PubMed] [Google Scholar]
- Alupului A., Calinescu I., Lavric V. (2009). Ultrasonic vs. microwave extraction intensification of active principles from medicinal plants. Chem. Eng. Trans. 17, 1023–1028. 10.3303/cet0917171 [DOI] [Google Scholar]
- Andola H. C., Gaira K. S., Rawal R. S., Rawat M. S., Bhatt I. D. (2010a). Habitat-dependent variations in berberine content of Berberis asiatica Roxb. ex. DC. in Kumaon, Western Himalaya. Chem. Biodivers. 7, 415–420. 10.1002/cbdv.200900041 [DOI] [PubMed] [Google Scholar]
- Andola H. C., Rawal R. S., Rawat M. S. M., Bhatt I. D., Purohit V. K. (2010b). Variations of berberine contents in Berberis pseudumbellata: a high value medicinal shrub of west Himalaya, India. Med. Plants Int. J. Phytomed. Relat. Ind. 2, 111–115. 10.5958/j.0975-4261.2.2.017 [DOI] [Google Scholar]
- Andola H. C., Rawal R. S., Rawat M. S. M., Bhatt I. D., Purohit V. K. (2010c). Analysis of berberine content using HPTLC fingerprinting of root and bark of three Himalayan Berberis species. Asian J. Biotechnol. 2, 239–245. 10.3923/ajbkr.2010.239.245 [DOI] [Google Scholar]
- Anesini C., Perez C. (1993). Screening of plants used in Argentine folk medicine for antimicrobial activity. J. Ethnopharmacol. 39, 119–128. 10.1016/0378-8741(93)90027-3 [DOI] [PubMed] [Google Scholar]
- Arawwawala L. D. A. M., Wickramaar W. A. N. (2012). Berberine content in Coscinium fenestratum (Gaertn.) Colebr grown in Sri Lanka. Pharmacologia 3, 679–682. 10.5567/pharmacologia.2012.679.682 [DOI] [Google Scholar]
- Arayne M. S., Sultana N., Bahadur S. S. (2007). The berberis story: Berberis vulgaris in therapeutics. Pak. J. Pharm. Sci. 20, 83–92. [PubMed] [Google Scholar]
- Atta-ur-Rahma, Ahmad H. (1992). An aporphine-benzylisoquinoline alkaloid from Berberis waziristanica. Phytochemistry 31, 1835–1836. 10.1016/0031-9422(92)83163-S [DOI] [Google Scholar]
- Avolio C., Ruggieri M., Giuliani F., Liuzzi G. M., Leante R., Riccio P., et al. (2003). Serum MMP-2 and MMP-9 are elevated in different multiple sclerosis subtypes. J. Neuroimmunol. 136, 46–53. 10.1016/S0165-5728(03)00006-7 [DOI] [PubMed] [Google Scholar]
- Babu N. H. R., Thriveni H. N., Vasudeva R. (2012). Influence of drying methods and extraction procedures on the recovery of berberine content in Coscinium fenestratum. J. Nat. Prod. Plant Resour. 2, 540–544. [Google Scholar]
- Bahar M., Deng Y., Zhu X., He S., Pandharkar T., Drew M. E., et al. (2011). Potent antiprotozoal activity of a novel semi-synthetic berberine derivative. Bioorg. Med. Chem. Lett. 21, 2606–2610. 10.1016/j.bmcl.2011.01.101 [DOI] [PubMed] [Google Scholar]
- Baharvand-Ahmadi B., Bahmani M., Tajeddini P., Naghdi N., Rafieian-Kopaei M. (2016). An ethno-medicinal study of medicinal plants used for the treatment of diabetes. J. Nephropathol. 5, 44–50. 10.15171/jnp.2016.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldazzi C., Leone M. G., Casini M. L., Tita B. (1998). Effects of the major alkaloid of Hydrastis canadensis L., berberine, on rabbit prostate strips. Phyther. Res. 12, 589–591. [DOI] [Google Scholar]
- Bapna S., Choudhary P. K., Ramaiya M., Chowdhary A. (2015). Antiplasmodial activity of Argemone mexicana: an in vivo and in vitro study. World J. Pharm. Res. 4, 1653–1663. [Google Scholar]
- Bele M. Y., Focho D. A., Egbe E. A., Chuyong B. G. (2011). Ethnobotanical survey of the uses Annonaceae around mount Cameroon. Afr. J. Plant Sci. 5, 237–247. [Google Scholar]
- Betti J. L., Caspa R., Ambara J., Kourogue R. L. (2013). Ethno-botanical study of plants used for treating malaria in a forest: savanna margin area, East region, Cameroon. Glob. J. Res. Med. Plants Indig. Med. 2, 692. [Google Scholar]
- Betti J. L., Lejoly J. (2009). Contribution to the knowledge of medicinal plants of the Dja Biosphere Reserve, Cameroon: plants used for treating jaundice. J. Med. Plants Res. 3, 1056–1065. [Google Scholar]
- Bhandari D. K., Nath G., Ray A. B., Tewari P. V. (2000). Antimicrobial activity of crude extracts from Berberis asiatica stem bark. Pharm. Biol. 38, 254–257. 10.1076/1388-0209(200009)3841-AFT254 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S., Tiwari K. C., Majumdar R., Misra A. K. (1980). Folklore medicine from district Kamrup (Assam). Bull. Medic. Ethno. Bot. Res. 1, 447–460. [Google Scholar]
- Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S. E. (2014). Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 94, 329–354. 10.1152/physrev.00040.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall T. C. (1997). Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Altern. Med. Rev. 2, 94–103. [Google Scholar]
- Bonesi M., Loizzo M. R., Conforti F., Passalacqua N. G., Saab A., Menichini F., et al. (2013). Berberis aetnensis and B. libanotica: A comparative study on the chemical composition, inhibitory effect on key enzymes linked to Alzheimer's disease and antioxidant activity. J. Pharm. Pharmacol. 65, 1726–1735. 10.1111/jphp.12172 [DOI] [PubMed] [Google Scholar]
- Borokini T. I., Clement M., Dickson N. J., Edagbo D. E. (2013). Ethnobiological survey of traditional medicine practice for fevers and headaches in Oyo State, Nigeria. Topclass J. Herb. Med. 2, 121–130. [Google Scholar]
- Bose B. C., Vijayvargiya R., Saifi A. Q., Sharma S. K. (1963). Chemical and pharmacological studies on Argemone mexicana. J. Pharm. Sci. 52, 1172–1175. 10.1002/jps.2600521216 [DOI] [PubMed] [Google Scholar]
- Bouquet A. (1969). Féticheurs et Médecines Traditionnelles du Congo (Brazzaville). Mém. O.R.S.T.O.M. (Paris: Office la Rech. Sci. Tech. outre-mer; ) 36, 282. [Google Scholar]
- Bouquet A., Debray M. (1974). Plantes Médicinales de la Côte d'Ivoire. Paris Off. la Rech. Sci. Tech. Paris: Outre Mer 231p. (Travaux Doc. l'ORSTOM no. 32) Illus., col. illus. Geog 5. [Google Scholar]
- Bown D. (1995). Encyclopaedia of Herbs and Their Uses. London: Dorling Kindersley London. [Google Scholar]
- Burkill H. M. (1985). The Useful Plants of West Tropical Africa. London: Royal Botanic Gardens, Kew. [Google Scholar]
- Bushra I., Kishwar S., Qureshi R. A., Saddiqa M. (2000). A checklist of plants of Bhogarmang, Siran Valley NWFP, Pakistan. Hamdard Med. 43, 62–76. [Google Scholar]
- Buzas A., Egnell C. (1965). On the presence of quinidine in addition to berberine alkaloids in the barks of Enantia pilosa and Enantia polycarpa (Annonaceae). Ann. Pharm. Fr. 23, 351. [PubMed] [Google Scholar]
- Castleman M. (1991). The Healing Herbs: The Ultimate Guide to the Curative Powers of Nature's Medicine. Emmaus: Rodale Press. [Google Scholar]
- Chakravarti K. K., Dhar D. C., Siddiqui S. (1950). Alkaloidal constituents of the bark of Berberis aristata. J. Sci. Ind. Res. 9, 161–164. [Google Scholar]
- Chan C.-O., Chu C.-C., Mok D. K., Chau F.-T. (2007). Analysis of berberine and total alkaloid content in Cortex phellodendri by near infrared spectroscopy (NIRS) compared with high-performance liquid chromatography coupled with ultra-visible spectrometric detection. Anal. Chim. Acta 592, 121–131. 10.1016/j.aca.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Chandra P., Purohit A. N. (1980). Berberine contents and alkaloid profile of Berberis species from different altitudes. Biochem. Syst. Ecol. 8, 379–380. 10.1016/0305-1978(80)90040-X [DOI] [Google Scholar]
- Chang T.-Y., Li B.-L., Chang C. C., Urano Y. (2009). Acyl-coenzyme A:cholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 297, E1–E9. 10.1152/ajpendo.90926.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W., Li K., Guan F., Yao F., Yu Y., Zhang M., et al. (2016). Berberine pretreatment confers cardioprotection against ischemia-reperfusion injury in a rat model of type 2 diabetes. J. Cardiovasc. Pharmacol. Ther. 21, 486–494. 10.1177/1074248415627873 [DOI] [PubMed] [Google Scholar]
- Chang Y. (2013). Ultrasonic-assisted extraction of berberine in ionic liquid. Pharm. Eng. 33, 1–4. [Google Scholar]
- Chatterjee D. R. (1951). Plant alkaloids. I. Berberis floribunda. J. Indian Chem. Soc. 28, 225–228. [Google Scholar]
- Chatterjee R., Banerjee A. (1953). Plant alkaloids. V. Berberis lambertii. J. Indian Chem. Soc. 30, 705–707. [Google Scholar]
- Chatterjee R., Guha M. P., Das Gupta A. K. (1952). Plant alkaloids. IV. Berberis himalaica and B. tinctoria. J. Indian Chem. Soc. 29, 921–924. [Google Scholar]
- Chaudhury R. H. N., Guha A., Chaudhury R., Pal D. C. (1980). Ethnobotanical uses of herbaria-2. J. Econ. Taxon. Bot. 1, 163–168. [Google Scholar]
- Chen A. H. (1981). Studies on the analysis of alkaloids of Phellodendron wilsonii Hay. et Kaneh. Kaneh. Kexue Fazhan Yuekan 9, 398–411. [Google Scholar]
- Chen A. H. (1982). Applied studies on the alkaloids of Phellodendron wilsonii Hay. et Kaneh. II. the alkaloid contents in Taiwan plants. Kexue Fazhan Yuekan 10, 279–286. [Google Scholar]
- Chen C., Yu Z., Li Y., Fichna J., Storr M. (2014). Effects of berberine in the gastrointestinal tract — a review of actions and therapeutic implications. Am. J. Chin. Med. 42, 1053–1070. 10.1142/S0192415X14500669 [DOI] [PubMed] [Google Scholar]
- Chen H. F., Chen C. M. (1988). Determination of berberine in crude and processed Chinese herb: Coptidis rhizoma and Phellodendri cortex. Zhonghua Yaoxue Zazhi 40, 259–264. [Google Scholar]
- Chen W. H., Pang J. Y., Qin Y., Peng Q., Cai Z., Jiang Z. H. (2005). Synthesis of linked berberine dimers and their remarkably enhanced DNA-binding affinities. Bioorg. Med. Chem. Lett. 15, 2689–2692. 10.1016/j.bmcl.2004.10.098 [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang Y., Zhang J., Sun C., Lopez A. (2011). Berberine improves glucose homeostasis in streptozotocin-induced diabetic rats in association with multiple factors of insulin resistance. ISRN Endocrinol. 2011, 1–8. 10.5402/2011/519371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Y., Chang F. R., Wu Y. C. (1996). Isoquinoline alkaloids and lignans from Rollinia mucosa. J. Nat. Prod. 59, 904–906. 10.1021/np960414z [DOI] [Google Scholar]
- Chevallier A. (1996). The Encyclopedia of Medicinal Plants. London: Dorling Kindersley. [Google Scholar]
- Chhetri D. R., Parajuli P., Subba G. C. (2005). Antidiabetic plants used by Sikkim and Darjeeling Himalayan tribes, India. J. Ethnopharmacol. 99, 199–202. 10.1016/j.jep.2005.01.058 [DOI] [PubMed] [Google Scholar]
- Chi L., Peng L., Pan N., Hu X., Zhang Y. (2014). The anti-atherogenic effects of berberine on foam cell formation are mediated through the upregulation of sirtuin 1. Int. J. Mol. Med. 34, 1087–1093. 10.3892/ijmm.2014.1868 [DOI] [PubMed] [Google Scholar]
- Chiang Y. L., Su C. R., Kuo P. C., Damu A. G., Wu T. S. (2006). Two isoquinolones from the roots of Phellodendron amurense var. Wilsonii. Heterocycles 68, 339–345. 10.3987/COM-05-10598 [DOI] [Google Scholar]
- Chopra R. N., Nayar S. I., Chopra I. C. (1986). Glossary of Indian Medicinal Plants (Including the Supplement). New Delhi: Canal of Scientific and Industrial Research. [Google Scholar]
- Cicero A., Ertek S. (2009). Berberine: metabolic and cardiovascular effects in preclinical and clinical trials. Nutr. Diet Suppl. 1, 1–10. 10.2147/NDS.S6084 [DOI] [Google Scholar]
- Coffey T. (1993). The History and Folklore of North American Wildflowers. New York, NY: Facts on File Limited. [Google Scholar]
- Dai P., Wang J., Lin L., Zhang Y., Wang Z. (2015). Renoprotective effects of berberine as adjuvant therapy for hypertensive patients with type 2 diabetes mellitus: evaluation via biochemical markers and color Doppler ultrasonography. Exp. Ther. Med. 10, 869–876. 10.3892/etm.2015.2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Costa O. (1935). (Mexican poppy) Argemone mexicana L. Rev. Flora Med 1, 271–282. [Google Scholar]
- Deng A. J., Qin H. L. (2010). Cytotoxic dihydrobenzophenanthridine alkaloids from the roots of Macleaya microcarpa. Phytochemistry 71, 816–822. 10.1016/j.phytochem.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Deng Y., Liao Q., Li S., Bi K., Pan B., Xie Z. (2008). Simultaneous determination of berberine, palmatine and jatrorrhizine by liquid chromatography-tandem mass spectrometry in rat plasma and its application in a pharmacokinetic study after oral administration of coptis-evodia herb couple. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 863, 195–205. 10.1016/j.jchromb.2007.12.028 [DOI] [PubMed] [Google Scholar]
- Dev S. (2006). A Selection of Prime Ayurvedic Plants Drugsancient- Modern Concordance. New Delhi: Anamaya Publishers. [Google Scholar]
- Din N., Dibong S. D., Mpondo E. M., Priso R. J., Kwin N. F., Ngoye A. (2011). Inventory and identification of plants used in the treatment of diabetes in douala town (Cameroon). Eur. J. Med. Plants 1, 60–73. 10.9734/EJMP/2011/273 [DOI] [Google Scholar]
- Doepke W., Ulrich H., Jimenez V. (1976). On the structure of a new alkaloid from Argemone mexicana. Z. Chem. 16, 54–55. [Google Scholar]
- Domitrović R., Cvijanović O., Pernjak-Pugel E., Škoda M., Mikelić L., Crnčević-Orlić Ž. (2013). Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 62, 397–406. 10.1016/j.fct.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Domitrović R., Jakovac H., Blagojević G. (2011). Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl4-intoxicated mice. Toxicology 280, 33–43. 10.1016/j.tox.2010.11.005 [DOI] [PubMed] [Google Scholar]
- Doncheva T., Kostova N., Yordanova G., Saadi H., Akrib F., Dimitrov D., et al. (2014). Comparison of alkaloid profile from Glaucium corniculatum (Papaveraceae) of Algerian and Bulgarian origin. Biochem. Syst. Ecol. 56, 278–280. 10.1016/j.bse.2014.07.007 [DOI] [Google Scholar]
- Du J. X., Wang M. (2010). Capillary electrophoresis determination of berberine in pharmaceuticals with end-column electrochemiluminescence detection. J. Chinese Chem. Soc. 57, 696–700. 10.1002/jccs.201000097 [DOI] [Google Scholar]
- Duke J. A., Ayensu E. S. (1985). Medicinal Plants of China. Algonac, MI: Reference Publications. [Google Scholar]
- Duke J. A., Beckstrom-Sternberg S. M. (1994). Dr. Duke's phytochemical and ethnobotanical databases. Available online at: http://www.ars-grin.gov/duke/plants.html (Accessed January 15, 2017).
- Dzhalilov D. R., Goryaev M. I., Kruglykhina G. K. (1963). Alkaloids from Berberis iliensis. I Izv. Akad. Nauk Kaz. SSR, Ser. Tekhn. i Khim. Nauk 3, 15–19. [Google Scholar]
- Egels W. (1959). Papaver dubium var. lecoquii, a berberine-containing poppy. Planta Med. 7, 92–102. 10.1055/s-0028-1101592 [DOI] [Google Scholar]
- Ehiagbonare P. O., Onyibe J. (2008). Conservation studies on four medicinal taxa of Southern Nigeria. Sci. Res. Essays 3, 40–45. [Google Scholar]
- El Beyrouthy M., Arnold N., Delelis-Dusollier A., Dupont F. (2008). Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 120, 315–334. 10.1016/j.jep.2008.08.024 [DOI] [PubMed] [Google Scholar]
- Emboden W. (1979). Narcotic Plants. New York, NY: Collier. [Google Scholar]
- Emes M., Aguilar A., Argueta A., Cano L. (1994). Indigenous Medicinal Florae from México, Vol. II. [Google Scholar]
- Eric Brussell D. (2004). A medicinal plant collection from Montserrat, West Indies. Econ. Bot. 58, S203–S220. 10.1663/0013-0001(2004)58[S203:AMPCFM]2.0.CO;2 [DOI] [Google Scholar]
- Esseily F., El Ezzy M., Gali-Muhtasib H., Safi S., Esseily J., Diab-Assaf M., et al. (2012). The ethanol fraction from the stem of Berberis libanotica inhibits the viability of adult T cell leukemia. Minerva Biotecnol. 24, 129–133. [Google Scholar]
- Etminan M., Gill S. S., Samii A. (2005). Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis. Lancet. Neurol. 4, 362–365. 10.1016/S1474-4422(05)70097-1 [DOI] [PubMed] [Google Scholar]
- Fabricant D. S., Farnsworth N. R. (2001). The value of plants used in traditional medicine for drug discovery. Environ. Heal. Perspect. Suppl. 109:69. 10.1289/ehp.01109s169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías-Campomanes A. M., Rostagno M. A., Coaquira-Quispe J. J., Meireles M. A. A. (2015). Supercritical fluid extraction of polyphenols from lees: overall extraction curve, kinetic data and composition of the extracts. Bioresour. Bioprocess. 2, 45 10.1186/s40643-015-0073-5 [DOI] [Google Scholar]
- Feng J., Xu W., Tao X., Wei H., Cai F., Jiang B., et al. (2010). Simultaneous determination of baicalin, baicalein, wogonin, berberine, palmatine and jatrorrhizine in rat plasma by liquid chromatography-tandem mass spectrometry and application in pharmacokinetic studies after oral administration of traditional Chinese medicinal preparations containing Scutellaria-Coptis herb couple. J. Pharm. Biomed. Anal. 53, 591–598. 10.1016/j.jpba.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Fletcher M. T., Takken G., Blaney B. J., Alberts V. (1993). Isoquinoline alkaloids and keto-fatty acids of Argemone ochroleuca and A. mexicana (Mexican poppy) seed. I. An assay method and factors affecting their concentration. Aust. J. Agric. Res. 44, 265–275. 10.1071/AR9930265 [DOI] [Google Scholar]
- Fogarty J. E. (1990). A Barefoot Doctor's Manual: The American Translation of the Official Chinese Paramedical Manual. Philadelphia, PA: Running Press Book Publishers. [Google Scholar]
- Fongod A. G. (2014). Ethnobotany, indigenous knowledge and unconscious preservation of the environment: An evaluation of indigenous knowledge in South and Southwest Regions of Cameroon. Int. J. Biodivers. Conserv. 6, 85–99. 10.5897/IJBC2013.0637 [DOI] [Google Scholar]
- Foote P. A. (1932). The alkaloids of Argemone alba Lestib. J. Am. Pharm. Assoc. 21, 246–248. [Google Scholar]
- Foster S., Duke J. A. (1990). A Field Guide to Medicinal Plants: Eastern and Central North America. Boston, MA: Houghton Mifflin Company. [Google Scholar]
- Freile M., Giannini F., Sortino M., Zamora M., Juarez A., Zacchino S., et al. (2006). Antifungal activity of aqueous extracts and of berberine isolated from Berberis heterophylla. Acta Farm. Bonaer. 25, 83–88. [Google Scholar]
- Fyson P. F. (1975). Flora of the Nilgiri and Pulney Hill-Tops. Dehra Dun: Bishen Singh Mahendra Pal Singh and Periodical Experts. [Google Scholar]
- Gbile Z. O., Soladoye M. O., Adesina S. K. (1988). Plants in traditional medicine in West Africa. Monogr. Syst. Bot. Missouri Bot. Gard. 25, 343–349. [Google Scholar]
- Gbolade A. (2012). Ethnobotanical study of plants used in treating hypertension in Edo State of Nigeria. J. Ethnopharmacol. 144, 1–10. 10.1016/j.jep.2012.07.018 [DOI] [PubMed] [Google Scholar]
- Gertig H. (1964). Alkaloids of Eschscholtzia californica. I. Isolation and thin-layer chromatography of alkaloid fractions from roots. Acta Pol. Pharm. 21, 59–64. [PubMed] [Google Scholar]
- Gill L. S., Akinwumi C. (1986). Nigerian folk medicine: practices and beliefs of the Ondo people. J. Ethnopharmacol. 18, 257–266. 10.1016/0378-8741(86)90004-8 [DOI] [PubMed] [Google Scholar]
- Gorval' L. M., Grishkovets V. I. (1999). Alkaloids of some species of the genus Berberis introduced into the Crimea. Chem. Nat. Compd. 35, 223–224. 10.1007/BF02234944 [DOI] [Google Scholar]
- Govindasamy R., Simon J., Puduri V. S., Juliani H. R., Asante-Dartey J., Arthur H., et al. (2007). Retailers and Wholesalers of African Herbal and Natural Products: Case Studies from Ghana and Rwanda. Issues New Crop. New Uses. Virginia ASHP, 332–337. [Google Scholar]
- Greathouse G. A. (1939). Alkaloids from Sanguinaria canadensis and their influence on growth of Phymatotrichum omnivorum. Plant Physiol. 14, 377. 10.1104/pp.14.2.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve A. (1984). A Modern Herbal Penguin. Harmondsworth: Dover Publications Inc. [Google Scholar]
- Grycová L., Dostál J., Marek R. (2007). Quaternary protoberberine alkaloids. Phytochemistry 68, 150–175. 10.1016/j.phytochem.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Gu Y., Zhang Y., Shi X., Li X., Hong J., Chen J., et al. (2010). Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta 81, 766–772. 10.1016/j.talanta.2010.01.015 [DOI] [PubMed] [Google Scholar]
- Guoping L., Jinhong L., Shuai H., Jian C., Zhongyi Z. (2012). Optimization for ultrahigh pressure extraction of berberine from Cortex phellodendri by central composite design-response surface methodology. J. Med. Plants Res. 6, 3963–3970. 10.5897/JMPR11.1092 [DOI] [Google Scholar]
- Gupta A. K., Tandon N. (2004). Rev. Indian Med. Plants, Vol 4. Delhi: ICMR. [Google Scholar]
- Gurguel L., de Costa O. A., da Silva R. D. (1934). Berberis laurina. Anatomic, histologic and chemical study. Bol. Assoc. Bras. pharm. 15, 11–20. [Google Scholar]
- Habtemariam S. (2011). The therapeutic potential of Berberis darwinii stem-bark: quantification of berberine and in vitro evidence for Alzheimer's disease therapy. Nat. Prod. Commun. 6, 1089–1090. [PubMed] [Google Scholar]
- Haisova K., Slavik J. (1975). On the minor alkaloids from Argemone mexicana L. Collect Czech. Chem. Commun. 40, 1576–1578. 10.1135/cccc19751576 [DOI] [Google Scholar]
- Hakim S. A., Mijovic V., Walker J. (1961). Distribution of certain poppy-fumaria alkaloids and a possible link with the incidence of glaucoma. Nature 189, 198–201. 10.1038/189198a0 [DOI] [PubMed] [Google Scholar]
- Hamayun M., Khan A., Khan M. A. (2003). Common medicinal folk recipes of District Buner, NWFP, Pakistan. Ethnobot. Leafl. 2003, 14. [Google Scholar]
- Hamonniere M., Leboeuf A., Paris R. R. (1975). Alcaloïdes des annonacées: alcaloïdes de l'Enantia chlorantha. Plant. Med. Phytother. 9, 296–303. [Google Scholar]
- Hartwell J. L. (1982). Plants Used Against Cancer. Lawrence, MA: Quarterman Publications. Inc. [Google Scholar]
- Hashmi K., Hafiz A. (1986). In vivo antibacterial activity of Berberis asiatica. J. Pak. Med. Assoc. 36, 5. [PubMed] [Google Scholar]
- Hayta S., Polat R., Selvi S. (2014). Traditional uses of medicinal plants in Elazig (Turkey). J. Ethnopharmacol. 154, 613–623. 10.1016/j.jep.2014.04.026 [DOI] [PubMed] [Google Scholar]
- He J.-M., Mu Q. (2015). The medicinal uses of the genus Mahonia in traditional Chinese medicine: an ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 175, 668–683. 10.1016/j.jep.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Henry T. A. (1949). The Plant Alkaloids, 4th Edn. Philadelphia, PA: Blakiston. [Google Scholar]
- Hirschhorn H. H. (1981). Botanical remedies of South and Central America, and the Caribbean: an archival analysis. Part I. J. Ethnopharmacol. 4, 129–158. 10.1016/0378-8741(81)90032-5 [DOI] [PubMed] [Google Scholar]
- Houghton P. J., Manby J. (1985). Medicinal plants of the Mapuche. J. Ethnopharmacol. 13, 89–103. 10.1016/0378-8741(85)90063-7 [DOI] [PubMed] [Google Scholar]
- Houghton P. J., Ren Y., Howes M.-J. (2006). Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 23, 181–199. 10.1039/b508966m [DOI] [PubMed] [Google Scholar]
- Hua F., Ha T., Ma J., Li Y., Kelley J., Gao X., et al. (2007). Protection against myocardial ischemia/reperfusion injury in TLR4-deficient mice is mediated through a phosphoinositide 3-kinase-dependent mechanism. J. Immunol. 178, 7317–7324. 10.4049/jimmunol.178.11.7317 [DOI] [PubMed] [Google Scholar]
- Huq M. E., Ikram M. (1968). Alkaloids of Berberis petiolaris. Sci. Res. 5, 75–76. [Google Scholar]
- Hussain K., Shahazad A., Zia-ul-Hussnain S. (2008). An ethnobotanical survey of important wild medicinal plants of Hattar district Haripur, Pakistan. Ethnobot. Leafl. 2008, 5. [Google Scholar]
- Hussaini F. A., Shoeb A. (1985). Isoquinoline derived alkaloids from Berberis chitria. Phytochemistry 24, 633 10.1016/S0031-9422(00)80794-3 [DOI] [Google Scholar]
- Hutchens A. R. (1992). A Handbook of Native American Herbs: The Pocket Guide to 125 Medicinal Plants and Their Uses. Boston, MA: Shambhala Publications. [Google Scholar]
- Imanshahidi M., Hosseinzadeh H. (2008). Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phyther. Res. 22, 999–1012. 10.1002/ptr.2399 [DOI] [PubMed] [Google Scholar]
- Inbaraj J. J., Kukielczak B. M., Bilski P., Sandvik S. L., Chignell C. F. (2001). Photochemistry and photocytotoxicity of alkaloids from goldenseal (Hydrastis canadensis L.) 1. Berberine. Chem. Res. Toxicol. 14, 1529–1534. 10.1021/tx0155247 [DOI] [PubMed] [Google Scholar]
- Irvine F. R. (1961). Woody Plants of Ghana. London: Oxford University Press. [Google Scholar]
- Ishola I. O., Oreagba I. A., Adeneye A. A., Adirije C., Oshikoya K. A., Ogunleye O. O. (2014). Ethnopharmacological survey of herbal treatment of malaria in Lagos, Southwest Nigeria. J. Herb. Med. 4, 224–234. 10.1016/j.hermed.2014.08.001 [DOI] [Google Scholar]
- Israilov I. A., Yunusov S. (1986). Alkaloids of four species of Argemone. Chem. Nat. Compd. 22, 189–192. 10.1007/BF00598384 [DOI] [Google Scholar]
- Jayaprakasam R., Ravi T. K. (2014). Development and validation of HPTLC and RP-HPLC methods for the estimation of berberine in Coscinium fenestratum extract and its formulation. World J. Pharm. Res. 4, 206–218. [Google Scholar]
- Jha R. N., Pandey M. B., Singh A. K., Singh S., Singh V. P. (2009). New alkaloids from Corydalis species. Nat. Prod. Res. 23, 250–255. 10.1080/14786410801996390 [DOI] [PubMed] [Google Scholar]
- Jin C., Shan W. (1982). Quantitative determination of berberine in Coptis chinensis by TLC scanner method. Yaoxue Tongbao 17, 145–146. [Google Scholar]
- Jiofack T., Fokunang C., Guedje N., Kemeuze V. (2009). Ethnobotany and phytomedicine of the upper Nyong valley forest in Cameroon. Afr. J. Pharm. Pharmacol. 3, 144–150. [Google Scholar]
- Jiofack T., Fokunang C., Kemeuze V., Fongnzossie E., Tsabang N., Nkuinkeu R., et al. (2008). Ethnobotany and phytopharmacopoea of the South-West ethnoecological region of Cameroon. J. Med. Plants Res. 2, 197–206. [Google Scholar]
- Joshi A. R., Joshi K. (2007). Ethnomedicinal plants used against skin diseases in some villages of Kali Gandaki, Bagmati and Tadi Likhu watersheds of Nepal. Ethnobot. Leafl. 2007, 27. [Google Scholar]
- Joshi H. R., Kanaki N. (2013). Quantitative analysis of berberine in an ayurvedic formulation-Rasayana churna by UV spectrophotometry. J. Pharm. Sci. Biosci. Res. 3, 32–34. [Google Scholar]
- Jusiak L. (1967). Separation of Chelidonium majus alkaloids by countercurrent cascade extraction. II. Acta Pol. Pharm. 24, 65–70. [Google Scholar]
- Kadiri A. B. (2008). Evaluation of medicinal herbal trade (Paraga) in Lagos State of Nigeria. Ethnobot. Leafl. 2008, 90. [Google Scholar]
- Kala C. P. (2006). Medicinal plants of the high altitude cold desert in India: diversity, distribution and traditional uses. Int. J. Biodivers. Sci. Manage. 2, 43–56. 10.1080/17451590609618098 [DOI] [Google Scholar]
- Kamal Y. T., Singh M., Tamboli E. T., Parveen R., Ahmad S. (2011). Quantitative analysis of berberine in Berberis aristata fruits and in a traditional anti-inflammatory unani formulation by use of a validated HPLC method. Acta Chromatogr. 23, 157–168. 10.1556/AChrom.21.2013.1.11 [DOI] [Google Scholar]
- Kamigauchi M., Iwasa K. (1994). Corydalis spp.: in vitro culture and the biotransformation of protoberberines, in Medicinal and Aromatic Plants VI. Biotechnology in Agriculture and Forestry, Vol 26, ed Bajaj Y. P. S. (Berlin; Heidelberg: Springer; ), 93–105. [Google Scholar]
- Karimov A. (1993). Berberis alkaloids. Chem. Nat. Compd. 29, 415–438. 10.1007/BF00630564 [DOI] [Google Scholar]
- Karimov A., Lutfullin K. L. (1986). Berberis alkaloids. 2'-N-methylisotetrandrine from Berberis oblonga. Khimiya Prir. Soedin. 2, 249–251. [Google Scholar]
- Karimov A., Meliboev S., Olimov V., Shakirov R. (1993). Berberis alkaloids. XXX. Dynamics of alkaloid accumulation in Berberis integerrima and B. nummularia. Khimiya Prir. Soedin. 3, 472–473. [Google Scholar]
- Karimov A., Shakirov R. (1993). Berberis alkaloids. XX. Alkaloids of Berberis iliensis. Khimiya Prir. Soedin. 1, 83–84. 10.1007/BF00631020 [DOI] [Google Scholar]
- Kariyone T., Koiso R. (1971). Atlas of Medicinal Plants. Osaka: Takeda Chemical Industries. [Google Scholar]
- Kataoka M., Tokuyama E., Miyanaga Y., Uchida T. (2008). The taste sensory evaluation of medicinal plants and Chinese medicines. Int. J. Pharm. 351, 36–44. 10.1016/j.ijpharm.2007.09.017 [DOI] [PubMed] [Google Scholar]
- Kaur C., Miani S. (2001). Fruits and vegetables healthy foods for new millennium. Indian Hort. 45, 29–32. [Google Scholar]
- Kayode J. (2006). Conservation of indigenous medicinal botanicals in Ekiti State, Nigeria. J. Zhejiang Univ. Sci. B 7, 713–718. 10.1631/jzus.2006.B0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalmatov K. (1964). Khalmatov, Wild-Growing Medicinal Plants of Uzbekistan [in Russian] Tashkent. Meditsina. [Google Scholar]
- Khamidov I., Faskhutdinov M., Telezhenetskaya M. V., Karimov A., Levkovich M. G., Abdullaev N. D., et al. (1996a). Berberis alkaloids. XXXIV. Turcomanine, a new alkaloid from Berberis turcomanica. Khimiya Prir. Soedin. 1, 74–76. [Google Scholar]
- Khamidov I., Karimov A. K., Telezhenetskaya M. V., Tashkhodzhaev B. (1996b). Berberis alkaloids. XXXV. Berberis turcomanica. Khimiya Prir. Soedin. 1, 107–109. [Google Scholar]
- Khamidov I. I., Aripova S. F., Karimov A., Yusupov M. M. (1997a). Berberis alkaloids. XL. An investigation of the alkaloids of Berberis thunbergii. Chem. Nat. Compd. 33, 599–599. 10.1007/BF02254817 [DOI] [Google Scholar]
- Khamidov I. I., Aripova S. F., Karimov A. K. (2003). Berberis alkaloids. XLI. Alkaloids from leaves of cultivated Berberis oblonga. Chem. Nat. Compd. 39, 407 10.1023/B:CONC.0000003429.41497.b6 [DOI] [Google Scholar]
- Khamidov I. I., Aripova S. F., Telezhenetskaya M. V., Karimov A., Dzhenberov I. (1997b). Berberis alkaloids XXXIX. New alkaloids from B. densiflora. Chem. Nat. Comp. 33, 323–325. 10.1007/BF02234886 [DOI] [Google Scholar]
- Khamidov I. I., Tashkhodzhaev B., Aripova S. F., Telezhenetskaya M. V., Karimov A. K. (1996c). Berberis alkaloids. XXXVII. Study of the alkaloids of B. oblonga and B. integerrima. Crystal structure of 8-trichloromethyldihydroberberine. Khimiya Prir. Soedin. 6, 889–893. [Google Scholar]
- Khan I., Najeebullah S., Ali M., Shinwari Z. K. (2016). Phytopharmacological and ethnomedicinal uses of the Genus Berberis (Berberidaceae): a review. Trop. J. Pharm. Res. 15, 2047–2057. 10.4314/tjpr.v15i9.33 [DOI] [Google Scholar]
- Khan M. I., Sri Harsha P. S. C., Giridhar P., Ravishankar G. A. (2011). Berberine and lycopene profiling during the ontogeny of Tinospora cordifolia (Willd.) Miers ex Hook. F. & Thoms fruit. Curr. Sci. 100, 1225–1231. [DOI] [PubMed] [Google Scholar]
- Khan S. W., Khatoon S. (2007). Ethnobotanical studies on useful trees and shrubs of Haramosh and Bugrote valleys in Gilgit northern areas of Pakistan. Pak. J. Bot. 39, 699–710. [Google Scholar]
- Khodzhimatov M. (1989). Dikorastushchiye Lekarstvennuiye Rasteniya Tadzhikistana [Wild-Growing Medicinal Plants of Tadjikistan]. [Google Scholar]
- King J. (1898). King's American Dispensatory. Cincinatti, OH: Ohio Valley Company. [Google Scholar]
- Kirtikar K., Basu B. (1933). Indian Medicinal Plants, I. Allahabad: Lalit Mohan Basu and Co. [Google Scholar]
- Kirtikar K. R., Basu B. D. (1998). Indian Medicinal Plants, Vol 1. Allahabad: CSIR publication. [Google Scholar]
- Kiryakov H. G., Daskalova E., Georgieva A., Kuzmanov B., Evstatieva L. (1982a). Alkaloids from Corydalis solida (L.) Swarz. Folia Med. 24, 19–22. [PubMed] [Google Scholar]
- Kiryakov H. G., Iskrenova E., Daskalova E., Kuzmanov B., Evstatieva L. (1982b). Alkaloids of Corydalis slivenensis. Planta Med. 44, 168–170. 10.1055/s-2007-971432 [DOI] [PubMed] [Google Scholar]
- Knapp J. E., Hussein F. T., Beal J. L., Doskotch R. W., Tomimatsu T. (1967). Isolation of two bisbenzylisoquinoline alkaloids from the rhizomes and roots of Xanthorhiza simplicissima. J. Pharm. Sci. 56, 139–141. 10.1002/jps.2600560129 [DOI] [PubMed] [Google Scholar]
- Končić M. Z., Kremer D., Schühly W., Brantner A., Karlović K., Kalodera Z. (2010). Chemical differentiation of Berberis croatica and B. vulgaris using HPLC fingerprinting. Croat. Chem. Acta 83, 451–456. [Google Scholar]
- Kong W. J., Wei J., Zuo Z. Y., Wang Y. M., Song D. Q., You X. F., et al. (2008). Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism. 57, 1029–1037. 10.1016/j.metabol.2008.01.037 [DOI] [PubMed] [Google Scholar]
- Kong Y., Xiao J.-J., Meng S.-C., Dong X.-M., Ge Y.-W., Wang R.-F., et al. (2010). A new cytotoxic flavonoid from the fruit of Sinopodophyllum hexandrum. Fitoterapia 81, 367–370. 10.1016/j.fitote.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Kosalec I., Gregurek B., Kremer D., Zovko M., Sanković K., Karlović K. (2009). Croatian barberry (Berberis croatica Horvat): a new source of berberine? analysis and antimicrobial activity. World J. Microbiol. Biotechnol. 25, 145–150. 10.1007/s11274-008-9860-x [DOI] [Google Scholar]
- Kosina P., Gregorova J., Gruz J., Vacek J., Kolar M., Vogel M., et al. (2010). Phytochemical and antimicrobial characterization of Macleaya cordata herb. Fitoterapia 81, 1006–1012. 10.1016/j.fitote.2010.06.020 [DOI] [PubMed] [Google Scholar]
- Kostalova D., Brazdovicova B., Jin H. Y. (1982). Alkaloids from the aboveground parts of Berberis koreana Palib. Farm. Obz. 51, 213–216. [Google Scholar]
- Kubota M., Katsunori M., Miyazawa Y. (1980). Berberine contents in cultivated Coptis japonica Makino. Nagano-ken Eisei Kogai Kenkyusho Kenkyu Hokoku 2, 22–27. [Google Scholar]
- Kukula-Koch W., Mroczek T. (2015). Application of hydrostatic CCC–TLC–HPLC–ESI-TOF-MS for the bioguided fractionation of anticholinesterase alkaloids from Argemone mexicana L. roots. Anal. Bioanal. Chem. 407, 2581–2589. 10.1007/s00216-015-8468-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S. K., Dhir A. (2010). Berberine: a plant alkaloid with therapeutic potential for central nervous system disorders. Phyther. Res. 24, 317–324. 10.1002/ptr.2968 [DOI] [PubMed] [Google Scholar]
- Kunwar R. M., Adhikari N. (2005). Ethnomedicine of Dolpa district, Nepal: the plants, their vernacular names and uses. Lyonia 8, 43–49. 10.1186/1746-4269-2-27 [DOI] [Google Scholar]
- Küpeli E., Koşar M., Yeşilada E., Hüsnü K., Başer C. (2002). A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 72, 645–657. 10.1016/S0024-3205(02)02200-2 [DOI] [PubMed] [Google Scholar]
- Ladino O. J. P., Suárez L. E. C. (2010). Chemical constituents of the wood from Zanthoxylum quinduense Tul. (Rutaceae). Quim. Nova 33, 1019–1021. 10.1590/S0100-40422010000500002 [DOI] [Google Scholar]
- Launert E. (1981). Edible and Medicinal Plants. London: Hamlyn. [Google Scholar]
- Lee H. Y., Kim C. W. (1999). Isolation and quantitative determination of berberine and coptisine from tubers of Corydalis ternata. Saengyak Hakhoechi 30, 332–334. [Google Scholar]
- Leone M. G., Cometa M. F., Palmery M., Saso L. (1996). HPLC determination of the major alkaloids extracted from Hydrastis canadensis L. Phyther. Res. 10, S45–S46. [Google Scholar]
- Li H., Li X. L., Zhang M., Xu H., Wang C. C., Wang S., et al. (2014). Berberine ameliorates experimental autoimmune neuritis by suppressing both cellular and humoral immunity. Scand. J. Immunol. 79, 12–19. 10.1111/sji.12123 [DOI] [PubMed] [Google Scholar]
- Li W. L., Zheng H. C., Bukuru J., De Kimpe N. (2004). Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 92, 1–21. 10.1016/j.jep.2003.12.031 [DOI] [PubMed] [Google Scholar]
- Li Y.-H., Zhang M., Xiao H.-T., Fu H.-B., Ho A., Lin C.-Y., et al. (2015). Addition of berberine to 5-aminosalicylic acid for treatment of dextran sulfate sodium-induced chronic colitis in C57BL/6 Mice. PLoS ONE 10:e0144101. 10.1371/journal.pone.0144101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li W., Chang Y., Dong W., Ni L. (2006). Extraction of berberine from rhizome of Coptis chinensis Franch using supercritical fluid extraction. J. Pharm. Biomed. Anal. 41, 1056–1060. 10.1016/j.jpba.2006.01.034 [DOI] [PubMed] [Google Scholar]
- Liu F., Li Z., Shi X., Zhong M. (2011). Determination of berberine, palmatine and jatrorrhizine in rabbit plasma by liquid chromatography-electrospray ionization-mass spectrometry. J. Pharm. Biomed. Anal. 56, 1006–1015. 10.1016/j.jpba.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Liu J. (1992). Extraction of berbamine with water. Zhongguo Yaoxue Zazhi 27, 290–291. [Google Scholar]
- Liu L., Liu J., Huang Z., Yu X., Zhang X., Dou D., et al. (2015). Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 458, 796–801. 10.1016/j.bbrc.2015.02.028 [DOI] [PubMed] [Google Scholar]
- Liu L., Wang Z. B., Song Y., Yang J., Wu L. J., Yang B. Y., et al. (2016). Simultaneous determination of eight alkaloids in rat plasma by UHPLC-MS/MS after oral administration of Coptis deltoidea C.Y. Cheng et Hsiao and Coptis chinensis Franch. Molecules 21, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Su X., Li G., Zhao G., Zhao L. (2015). Validated UPLC-MS/MS method for simultaneous determination of simvastatin, simvastatin hydroxy acid and berberine in rat plasma: application to the drug-drug pharmacokinetic interaction study of simvastatin combined with berberine after oral administratio. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1006, 8–15. 10.1016/j.jchromb.2015.09.033 [DOI] [PubMed] [Google Scholar]
- Liu S., Chen Y., Gu L., Li Y., Wang B., Hao J., et al. (2013). Effects of ultrahigh pressure extraction conditions on yields of berberine and palmatine from Cortex phellodendri amurensis. Anal. Methods 5, 4506 10.1039/c3ay40784e [DOI] [Google Scholar]
- Liu W., Liu P., Tao S., Deng Y., Li X., Lan T., et al. (2008b). Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch. Biochem. Biophys. 475, 128–134. 10.1016/j.abb.2008.04.022 [DOI] [PubMed] [Google Scholar]
- Liu W. H., Hei Z. Q., Nie H., Tang F. T., Huang H. Q., Li X. J., et al. (2008a). Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin. Med. J. 121, 706–712. [PubMed] [Google Scholar]
- Liu X., Zhang X., Ye L., Yuan H. (2016). Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. Biomed. Pharmacother. 79, 222–230. 10.1016/j.biopha.2016.02.015 [DOI] [PubMed] [Google Scholar]
- Lou Y., Yuming W., Yanfen D., Jida S., Huang L. (1982). Extractive spectrophotometric determination of berberine. Yaowu Fenxi Zazhi 2, 82–85. [Google Scholar]
- Lust J. (2014). The Herb Book: The Most Complete Catalog of Herbs Ever Published. New York, NY: Courier Corporation. [Google Scholar]
- Ma X., Jiang Y., Wu A., Chen X., Pi R., Liu M., et al. (2010). Berberine attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. PLoS ONE 5:e13489. 10.1371/journal.pone.0013489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maithani A., Parcha V., Kumar D. (2014). Quantitative estimation of berberine content of Berberis asiatica from different altitude of Garhwal Himalaya. Asian J. Pharm. Clin. Res. 7, 165–167. [Google Scholar]
- Majumder B., Schindra S. N., Dutta P. C. (1956). Occurrence of ceryl alcohol in Argemone mexicana. J. Indian Chem. Soc. 33, 351–352. [Google Scholar]
- Manandhar N. P. (2002). Plants and People of Nepal. Portland, OR: Timber Press. [Google Scholar]
- Manske R. H. F. (1939). The alkaloids of fumariaceous plants. XIX. Corydalis ophiocarpa Hook. f. et Thoms. Can. J. Res. Sect. B Chem. Sci. 17, 51–56. 10.1139/cjr39b-009 [DOI] [Google Scholar]
- Marek R., Seckárová P., Hulová D., Marek J., Dostál J., Sklenár V. (2003). Palmatine and berberine isolation artifacts. J. Nat. Prod. 66, 481–486. 10.1021/np0204996 [DOI] [PubMed] [Google Scholar]
- Martinez M. (1984). Las Plantas Medicinales De México, 3rd Edn. Mexico City: CIESAS, Cuadernos de la Casa Chata. [Google Scholar]
- Martinez O. E. (1977). Flora de Veracruz, Fascículo 77 Riverside, CA: University of California. [Google Scholar]
- Mascareno E., El-Shafei M., Maulik N., Sato M., Guo Y., Das D. K., et al. (2001). JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation 104, 325–329. 10.1161/01.CIR.104.3.325 [DOI] [PubMed] [Google Scholar]
- Meena A. K., Bansal P., Kumar S. (2009). Plants-herbal wealth as a potential source of ayurvedic drugs. Asian J. Tradit. Med. 4, 152–170. [Google Scholar]
- Mell C. D. (1929). Interesting sources of natural dyestuffs. Color 51, 619–820. [Google Scholar]
- Mikage M., Mouri C. (1999). Pharmacognostical studies of Berberis plants (Berberidaceae) from Nepal (1). Altitudinal, interspecific, and partial variations of berberine content in the barks. Sect. Title Pharm. 53, 249–254. [Google Scholar]
- Mills S. (1985). The Dictionary of Modern Herbalism: A Comprehensive Guide to Practical Herbal Therapy. Wellingborough: Inner Traditions/Bear & Co. [Google Scholar]
- Minaiyan M., Ghannadi A., Mahzouni P., Jaffari-Shirazi E. (2011). Comparative study of Berberis vulgaris fruit extract and berberine chloride effects on acetic acid-induced colitis in rats. Iran. J. Pharm. Res. 10, 97–104. [PMC free article] [PubMed] [Google Scholar]
- Misra P. S., Bhakuni D. S., Sharma V. N., Kaul K. N. (1961). Chemical constituents of Argemone mexicana. J. Sci. Ind. Res. 20, 186. [Google Scholar]
- Moerman D. E. (1998). Native American Ethnobotany. Portland, OR: Timber Press. [Google Scholar]
- Mokgadi J., Turner C., Torto N. (2013). Pressurized hot water extraction of alkaloids in Goldenseal. Am. J. Anal. Chem. 4, 398–403. 10.4236/ajac.2013.48050 [DOI] [Google Scholar]
- Mølgaard P., Holler J. G., Asar B., Liberna I., Rosenbæk L. B., Jebjerg C. P., et al. (2011). Antimicrobial evaluation of Huilliche plant medicine used to treat wounds. J. Ethnopharmacol. 138, 219–227. 10.1016/j.jep.2011.09.006 [DOI] [PubMed] [Google Scholar]
- Monforte-Gonzalez M., Cecilia G. G., Jorge R. P., Mildred C. P., Vazquez-Flota F. (2012). Berberine and sanguinarine quantitation in Argemone mexicana L. (Papaveraceae) tissues by TLC-in situ fluorography. J. Planar Chromatogr. TLC 24, 358–360. 10.1556/JPC.25.2012.4.14 [DOI] [Google Scholar]
- Montes M., Wilkomirsky T. (1987). Medicina Tradicional Chilena. Concepción: Editiorial de la Universidad de Concepción. [Google Scholar]
- Muñoz O. (2001). Plantas Medicinales de uso en Chile: Química y Farmacología. Editorial Universitaria. [Google Scholar]
- Mustafa A., Turner C. (2011). Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal. Chim. Acta 703, 8–18. 10.1016/j.aca.2011.07.018 [DOI] [PubMed] [Google Scholar]
- Musumeci R., Speciale A., Costanzo R., Annino A., Ragusa S., Rapisarda A., et al. (2003). Berberis aetnensis C. Presl. extracts: antimicrobial properties and interaction with ciprofloxacin. Int. J. Antimicrob. Agents 22, 48–53. 10.1016/S0924-8579(03)00085-2 [DOI] [PubMed] [Google Scholar]
- Musuyu Muganza D., Fruth B. I., Nzunzu Lami J, Mesia G. K., Kambu O. K., Tona G. L., et al. (2012). In vitro antiprotozoal and cytotoxic activity of 33 ethonopharmacologically selected medicinal plants from Democratic Republic of Congo. J. Ethnopharmacol. 141, 301–308. 10.1016/j.jep.2012.02.035 [DOI] [PubMed] [Google Scholar]
- Ndenecho E. N. (2009). Herbalism and resources for the development of ethnopharmacology in Mount Cameroon region. Afr. J. Pharm. Pharmacol. 3, 78–86. [Google Scholar]
- Neuwinger H. D. (1996). African Ethnobotany: Poisons and Drugs: Chemistry, Pharmacology, Toxicology. London: CRC Press. [Google Scholar]
- Ngono Ngane R., Koanga Mogtomo M., Tchinda Tiabou A., Magnifouet Nana H., Motso Chieffo P. R., Mballa Bounou Z., et al. (2011). Ethnobotanical survey of some Cameroonian plants used for treatment of viral diseases. Afr. J. Plant Sci. 5, 15–21. [Google Scholar]
- Nguimatsia F., Boustie J., Baril F., Amoros M., Girre L. (1998). Les medicaments des pygmees Baka du Cameroun: moeurs therapeutiques, maladies et inventaire des plantes medicinales. Fitoterapia 69, 29–40. [Google Scholar]
- Noumi E. (2010). Ethno medicines used for treatment of prostatic disease in Foumban, Cameroon. Afr. J. Pharm. Pharmacol. 4, 793–805. [Google Scholar]
- Noumi E., Anguessin B. (2010). Insecticides and ethnomedicine of HIV/AIDS at Tokombere (Far North Cameroon). Int. J. Pharm. Biomed. Sci. 2, 20–28. [Google Scholar]
- Noumi E., Yumdinguetmun R. (2010). Plants and treatment of prostatic diseases in Foumban (West Region, Cameroon). Syllab. Rev. 2, 9–16. [Google Scholar]
- Odugbemi T. O., Akinsulire O. R., Aibinu I. E., Fabeku P. O. (2007). Medicinal plants useful for malaria therapy in Okeigbo, Ondo State, Southwest Nigeria. Afr. J. Tradit. Complement. Altern. Med. 4, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonna D. N., Sokari T. G., Agomuoh A. A. (2008). Antimalarial activities of some selected traditional herbs from Southeastern Nigeria against Plasmodium species. Res. J. Parasitol. 3, 25–31. 10.3923/jp.2008.25.31 [DOI] [Google Scholar]
- Ohemu T. L., Agunu A., Olotu P. N., Ajima U., Dafam D. G., Azila J. J. (2014). Ethnobotanical survey of medical plants used in the traditional treatment of viral infections in Jos, plateau state, Nigeria. Int. J. Med. Aromat. Plants 4, 74–81. [Google Scholar]
- Okunade A. L., Hufford C. D., Richardson M. D., Peterson J. R., Clar A. M. (1994). Antimicrobial Properties of Alkaloids from Xanthorhiza simplicissima. J. Pharm. Sci. 83, 404–406. 10.1002/jps.2600830327 [DOI] [PubMed] [Google Scholar]
- Oladunmoye M. K., Kehinde F. Y. (2011). Ethnobotanical survey of medicinal plants used in treating viral infections among Yoruba tribe of South Western Nigeria. Afr. J. Microbiol. Res. 5, 2991–3004. 10.5897/AJMR10.004 [DOI] [Google Scholar]
- Oliver B. E. P. (1960). Medicinal Plants in Nigeria: Being a Course of Four Lectures. Pharmacy Department of the Nigerian College of Arts, Science and Technology, Ibadan. [Google Scholar]
- Olowokudejo J. D., Kadiri A. B., Travih V. A. (2008). An ethnobotanical survey of herbal markets and medicinal plants in Lagos State of Nigeria. Ethnobot. Leafl. 2008, 116. [Google Scholar]
- Onwuanibe R. C. (1979). The philosophy of African medical practice. Afr. Issues 9, 25–28. 10.2307/1166259 [DOI] [Google Scholar]
- Orhan I., Sener B., Choudhary M. I., Khalid A. (2004). Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 91, 57–60. 10.1016/j.jep.2003.11.016 [DOI] [PubMed] [Google Scholar]
- Othman M. S., Safwat G., Aboulkhair M., Abdel Moneim A. E. (2014). The potential effect of berberine in mercury-induced hepatorenal toxicity in albino rats. Food Chem. Toxicol. 69, 175–181. 10.1016/j.fct.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Pak V. (2005). Medicine plants of folk medicine used for treatment of gastro-intestinal problems in Fergana valley. Korean Food Res. Inst. 18, 150–157. [Google Scholar]
- Pant N., Garg H. S., Bhakuni K. (1986). Chemical constituents of B. pseudoumbellata. Fitoterapia 51, 427–428. [Google Scholar]
- Park D. W., Jiang S., Liu Y., Siegal G. P., Inoki K., Abraham E., et al. (2014). GSK3β-Dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am. J. Physiol. 307, L735–L745. 10.1152/ajplung.00165.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-I., Shim J.-K., Do J.-W., Kim S.-Y., Seo E.-K., Kwon H.-J., et al. (1999). Immune-stimulating properties of polysaccharides from Phellodendri cortex (Hwangbek). Glycoconj. J. 16, 247–252. 10.1023/A:1007084506071 [DOI] [PubMed] [Google Scholar]
- Parsons H. B. (1882). Examination of the root of Berberis aquifolium, v. alpens, “oregon grape root.” Pharm. J. 13, 46–48. [Google Scholar]
- Patel M. C. (2013). Isolation of berberine from Berberis aristata by an acid dye method and optimization of parameters. Int. J. Pharm. Sci. Rev. Res. 20, 187–189. [Google Scholar]
- Pathak N. K. R., Biswas M., Seth K. K., Dwivedi S. P. D., Pandey V. B. (1985). Chemical investigation of Argemone mexicana. Pharmazie 40, 202.4023039 [Google Scholar]
- Pěnčíková K., Urbanová J., Musil P., Táborská E., Gregorová J. (2011). Seasonal Variation of Bioactive Alkaloid Contents in Macleaya microcarpa (Maxim.) Fedde. Molecules 16, 3391–3401. 10.3390/molecules16043391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkin A. G., Hummel J. J. (1895). XLV.—The colouring principle of Toddalia aculeata and Evodia meliaefolia. J. Chem. Soc. Trans. 67, 413–416. 10.1039/CT8956700413 [DOI] [Google Scholar]
- Pesman M. W. (1962). Meet Flora Mexicana. Globe, AZ: D.S. King. [Google Scholar]
- Petcu P. (1965a). Der gehalt an alkaloiden und vitamin C in Berberis guimpelii. Planta Med. 13, 178–181. 10.1055/s-0028-1100108 [DOI] [Google Scholar]
- Petcu P. (1965b). Phytochemical investigation of Berberis koreana. Farm. Bucharest, Rom. 13, 21–28. [Google Scholar]
- Pfoze N. L., Myrboh B., Kumar Y., Rohman R. (2014). Isolation of protoberberine alkaloids from stem bark of Mahonia manipurensis Takeda using RP-HPLC. J. Med. Plants Stud. 2, 48–57. [Google Scholar]
- Phillips R., Foy N. (1990). Herbs. London: Pan Books Ltd. [Google Scholar]
- Phillips R., Rix M. (1991). Perennials Vol. 1 and 2. London: Pan Books Ltd. [Google Scholar]
- Phillipson J. D., Gray A. I., Askari A. A. R., Khalil A. A. (1981). Alkaloids From Iraqi Species of Papaveraceae. J. Nat. Prod. 44, 296–307. 10.1021/np50015a011 [DOI] [Google Scholar]
- Phondani P. C., Maikhuri R. K., Rawat L. S., Farooquee N. A., Kala C. P., Vishvakarma S. C. R., et al. (2010). Ethnobotanical uses of plants among the Bhotiya tribal communities of Niti Valley in Central Himalaya, India. Ethnobot. Res. Appl. 8, 233–244. 10.17348/era.8.0.233-244 [DOI] [Google Scholar]
- Pilch W., Szygula Z., Tyka A. K., Palka T., Tyka A., Cison T., et al. (2014). Disturbances in pro-oxidant-antioxidant balance after passive body overheating and after exercise in elevated ambient temperatures in athletes and untrained men. PLoS ONE 9:e85320. 10.1371/journal.pone.0085320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., et al. (2014). Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res. Int. (2014). 10.1155/2014/761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S., Sethuraman M. (1992). Mahonia leschenaultii–a toda plant. Anc. Sci. Life 12, 242. [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran A., Kumar N. (2009). Rasont – A traditional crude drug prepared from Berberis sp and its uses. Indian, J. Tradit. Knowl. 8, 562–563. [Google Scholar]
- Ransohoff R. M., Hafler D. A., Lucchinetti C. F. (2015). Multiple sclerosis — a quiet revolution. Nat. Rev. Neurol. 11, 134–142. 10.1038/nrneurol.2015.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid M. H., Malik M. N. (1972). Composition of alkaloids in some Berberis species. Pakistan J. For. 22, 43–47. [Google Scholar]
- Rashmi R. A., Pokhriyal R., Singh Y. (2009). Quantitative Estimation of Berberine in Roots of Different provenances of Berberis aristata DC by HPLC and Study of their Antifungal Properties. Pharmacogn. Mag. 5, 355–358. 10.4103/0973-1296.58566 [DOI] [Google Scholar]
- Richert F. (1918). The extraction of berberine from “michai” (Berberis darwinii) and “calafate” (B. vuxifolia), in the Argentine. Rev. del Cent. Estud. Agron. y Vet. la Univ. Buenos Aires 11, 11–13. [Google Scholar]
- Ritch-Krc E. M., Thomas S., Turner N. J., Towers G. H. N. (1996). Carrier herbal medicine: traditional and contemporary plant use. J. Ethnopharmacol. 52, 85–94. 10.1016/0378-8741(96)01392-X [DOI] [PubMed] [Google Scholar]
- Rivera Núñez D., Obon de Castro C. (1996). Ethnopharmacology of Murcia, Actes du 2 a Colloque Européen d'Ethnopharmacologei et de la 11a Conférence internationale d'Ethnomédecine (Heidelberg), 24. [Google Scholar]
- Rojsanga P., Gritsanapan W. (2005). Variation of Berberine Content in Coscinium fenestratum Stem in Thailand Market. Mahidol Univ. J. Pharm. Sci. 32, 66–70. [Google Scholar]
- Rojsanga P., Gritsanapan W., Suntornsuk L. (2006). Determination of berberine content in the stem extracts of Coscinium fenestratum by TLC densitometry. Med. Princ. Pract. 15, 373–378. 10.1159/000094272 [DOI] [PubMed] [Google Scholar]
- Samal P. K. (2013). HPTLC analysis of berberine in Argemone mexicana, L. J. Glob. Trends Pharm. Sci. 4, 1073–1076. [Google Scholar]
- Samhita S. (1963). Sutrasthanam Lakshadi Group. Ed Bhishagratna K. K. Varanasi: Chaukhamba Sanskrit Sansthan. [Google Scholar]
- San Martín J. (1983). Medicinal plants in central Chile. Econ. Bot. 37, 216–227. 10.1007/BF02858788 [DOI] [Google Scholar]
- Sandberg F. (1965). Etude sur les plantes medicinales et toxiques d'Afrique equatoriale. 1. Premier inventaire des plantes medicinales et toxiques de la region sudouest de la Republique Centrafricaine et de la region nord de la Republique du Congo/Brazzaville. Cah. la Maboké 3, 5–49. [Google Scholar]
- Santos A. C., Adkilen P. (1932). The alkaloids of Argemone mexicana. J. Am. Chem. Soc. 54, 2923–2924. 10.1021/ja01346a037 [DOI] [Google Scholar]
- Santra D. K., Saoji A. N. (1971). Phytochemical study of Argemone mexicana latex. Curr. Sci. 40, 548–549. [Google Scholar]
- Saraf G., Mitra A., Kumar D., Mukherjee S., Basu A. (2010). Role of nonconventional remedies in rural India. Int. J. Pharm. Life Sci. 1, 141–159. [Google Scholar]
- Sasidharan S., Chen Y., Saravanan D., Sundram K. M., Yoga Latha L. (2011). Extraction, isolation and characterization of bioactive compounds from plants' extracts. Afr. J. Tradit. Complement. Altern. Med. 8, 1–10. [PMC free article] [PubMed] [Google Scholar]
- Sati S. C., Joshi S. (2011). Aspects of antifungal potential of ethnobotanically known medicinal plants. Res. J. Med. Plants 5, 377–391. 10.3923/rjmp.2011.377.391 [DOI] [Google Scholar]
- Satija S., Bansal P., Dureja H., Garg M. (2015). Microwave assisted extraction of Tinospora cordifolia and optimization through central composite design. J. Biol. Sci. 15, 106–115. 10.3923/jbs.2015.106.115 [DOI] [Google Scholar]
- Sato F., Yamada Y. (1984). High berberine-producing cultures of Coptis japonica cells. Phytochemistry 23, 281–285. 10.1016/S0031-9422(00)80318-0 [DOI] [Google Scholar]
- Satyavati G. V., Raina M. K., Sharma M. (1987). Medicinal plants of India. New Delhi: Indian Council of Medical Research. [Google Scholar]
- Schieffer G. W., Pfeiffer K. (2001). Pressurized liquid extraction and multiple, ultrasonically-assisted extraction of hydrastine and berberine from Goldenseal (Hydrastis canadensis) with susequent HPLC assay. J. Liq. Chromatogr. Relat. Technol. 24, 2415–2427. 10.1081/JLC-100105948 [DOI] [Google Scholar]
- Schlotterbeck J. O. (1902). Does Argemone mexicana contain morphine? J. Am. Chem. Soc. 24, 238–242. 10.1021/ja02017a006 [DOI] [Google Scholar]
- Seino Y., Fukushima M., Yabe D. (2010). GIP and GLP-1, the two incretin hormones: similarities and differences. J. Diabetes Investig. 1, 8–23. 10.1111/j.2040-1124.2010.00022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener B., Temizer H. (1988). Pharmacognosic investigations on Corydalis solida (L.) Swartz ssp. brachyloba (Boiss.) Cullen & Davis. II. Alkaloids of Corydalis solida (L.) Swartz ssp. brachyloba (Boiss.) Cullen & Davis. Gazi Univ. Eczac. Fak. Derg. 5, 9–11. [Google Scholar]
- Sener B., Temizer H. (1990). Chemical Studies on the Alkaloids from Corydalis solida subsp. tauricola. Planta Med. 56, 510–510. 10.1055/s-2006-961052 [DOI] [PubMed] [Google Scholar]
- Sener B., Temizer H. (1991). Chemical studies on the minor isoquinoline alkaloids from Corydalis solida subsp. brachyloba. J. Chem. Soc. Pakistan 13, 63–66. [Google Scholar]
- Sezik E., Yesilada E., Shadidoyatov H., Kulivey Z., Nigmatullaev A. M., Aripov H. N., et al. (2004). Folk medicine in Uzbekistan: I. Toshkent, Djizzax, and Samarqand provinces. J. Ethnopharmacol. 92, 197–207. 10.1016/j.jep.2004.02.016 [DOI] [PubMed] [Google Scholar]
- Shah G. M., Khan M. A. (2006). Common medicinal folk recipes of Siran valley, Mansehra, Pakistan. Ethnobot. Leafl. 2006, 5. [Google Scholar]
- Shahid M., Rahim T., Shahzad A., Latif T. A., Fatma T., Rashid M., et al. (2009). Ethnobotanical studies on Berberis aristata DC. root extracts. African, J. Biotechnol. 8, 556–563. [Google Scholar]
- Sharma P. K., Chauhan N. S., Lal B. (2005). Studies on plant associated indigenous knowledge among Malanis of Kullu district, Himachal Pradesh. Indian J. Trad. Knowl. 4, 403–408. [Google Scholar]
- Shigwan H., Saklani A., Hamrapurkar P. D., Mane T., Bhatt P. (2013). HPLC method development and validation for quantification of berberine from Berberis aristata and Berberis tinctoria. Int. J. Appl. Sci. Eng. 11, 203–211. [Google Scholar]
- Shirwaikar A., Shirwaikar A., Rajendran K., Punitha I. S. R. (2006). In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol. Pharm. Bull. 29, 1906–1910. 10.1248/bpb.29.1906 [DOI] [PubMed] [Google Scholar]
- Singh A., Duggal S., Kaur N., Singh J. (2010). Berberine: Alkaloid with wide spectrum of pharmacological activities. J. Nat. Prod. 3, 64–75. [Google Scholar]
- Singh A., Lal M., Samant S. S. (2009). Diversity, indigenous uses and conservation prioritization of medicinal plants in Lahaul valley, proposed Cold Desert Biosphere Reserve, India. Int. J. Biodivers. Sci. Manag. 5, 132–154. 10.1080/17451590903230249 [DOI] [Google Scholar]
- Singh I. P., Mahajan S. (2013). Berberine and its derivatives: a patent review (2009-2012). Expert Opin. Ther. Pat. 23, 215–231. 10.1517/13543776.2013.746314 [DOI] [PubMed] [Google Scholar]
- Singh J., Kakkar P. (2009). Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J. Ethnopharmacol. 123, 22–26. 10.1016/j.jep.2009.02.038 [DOI] [PubMed] [Google Scholar]
- Singh R., Katiyar C., Pasrija A. (2010). Validated HPLC-UV method for the determination of berberine in raw herb Daruharidra (Berberis aristata DC), its extract, and in commercially marketed ayurvedic dosage forms. Int. J. Ayurveda Res. 1, 243. 10.4103/0974-7788.76789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Tiwari S. S., Srivastava S., Rawat A. K. S. (2012). Botanical and phytochemical studies on roots of Berberis umbellata Wall. ex G. Don. Indian J. Nat. Prod. Resour. 3, 55–60. [Google Scholar]
- Singh S. (2014). Quantitative analysis of Berberine in Argemone mexicana Linn. (Papaveraceae) using HPLC and HPTLC. Adv. Plant Sci. 27, 209–211. [Google Scholar]
- Singh S. S., Pandey S. C., Srivastava S., Gupta V. S., Patro B., Ghosh A. C. (2003). Chemistry and medicinal properties of Tinospora cordifolia (Guduchi). Indian J. Pharmacol. 35, 83–91. [Google Scholar]
- Sir C. C., Chopra I. C. (1958). Indigenous Drugs of India. Kolkata: U.N.Dhar and Sons Private Limted. [Google Scholar]
- Slavík J. (1978). Characterization of alkaloids from the roots of Papaver rhoeas L. Collect. Czechoslov. Chem. Commun. 43, 316–319. 10.1135/cccc19780316 [DOI] [Google Scholar]
- Slavik J., Slavikova L. (1957). Alkaloide der mohngewächse (Papaveraceae) VIII. Die alkaloide des roten hornmohns (Glaucium corniculatum CURT.). Collect. Czechoslov. Chem. Commun. 22, 279–285. 10.1135/cccc19570279 [DOI] [Google Scholar]
- Slavik J., Slavikova L. (1975). Alkaloids of Papaveraceae. LIX. Alkaloids from the leaves of Bocconia frutescens. Collect. Czechoslov. Chem. Commun. 40, 3206–3210. 10.1135/cccc19753206 [DOI] [Google Scholar]
- Slavik J., Slavikova L., Bochorakova J. (1989). Alkaloids of the Papaveraceae. Part LXXXVIII. Alkaloids from Papaver rhoeas var. chelidonioides O. Kuntze, P. confine Jord., and P. dubium L. Collect. Czechoslov. Chem. Commun. 54, 1118–1125. 10.1135/cccc19891118 [DOI] [Google Scholar]
- Slavikova L., Slavik J. (1955). Alkaloids of Papaveraceae. VII. Argemone mexicana. Chem. List. Pro Vedu a Prum. 49, 1546–1549. [Google Scholar]
- Slavikova L., Slavik J. (1966). Alkaloide der mohngewächse (Papaveraceae) XXXII. Über die alkaloide aus Hunnemannia fumariaefolia SWEET und über die konstitution des alkaloids HF 1. Collect. Czechoslov. Chem. Commun. 31, 1355–1362. 10.1135/cccc19661355 [DOI] [Google Scholar]
- Slavikova L., Tschu S., Slavik J. (1960). Alkaloids of Papaveraceae. XIV. Alkaloids of Argemone alba. Collect. Czechoslov. Chem. Commun. 25, 756–760. 10.1135/cccc19600756 [DOI] [Google Scholar]
- Smyth B. B. (1903). Preliminary list of medicinal and economic kansas plants, with their reputed therapeutic properties. Trans. Kansas Acad. Sci. 18, 191–209. 10.2307/3624794 [DOI] [Google Scholar]
- Sood P., Modgil R., Sood M. (2010). Physico-chemical and nutritional evaluation of indigenous wild fruit Kasmal, Berberis lycium Royle. Indian J. Nat. Prod. Resour. 1, 362–366. [Google Scholar]
- Srinivasan G. V., Unnikrishnan K. P., Rema Shree A. B., Balachandran I. (2008). HPLC estimation of berberine in Tinospora cordifolia and Tinospora sinensis. Indian J. Pharm. Sci. 70, 96–99. 10.4103/0250-474X.40341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. K., Rai V., Srivastava M., Rawat A. K. S., Mehrotra S. (2006a). Estimation of heavy metals in different Berberis species and its market samples. Environ. Monit. Assess. 116, 315–320. 10.1007/s10661-006-7395-x [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Rawat A. K. S., Manjoosha S., Mehrotra S. (2006c). Pharmacognostic Evaluation of the Roots of Berberis chitria Lindl. Nat. Prod. Sci. 12, 19–23. [Google Scholar]
- Srivastava S. K., Rawat A. K. S., Srivastava M. (2006b). Pharmacognostic evaluation of the roots of Berberis chitria. Nat. Prod. Sci. 12, 19–23. [Google Scholar]
- Srivastava S. K., Sayyada K., Singh Rawat A. K., Mehrotra S. (2001). Pharmacognostic evaluation of the root of Berberis aristata DC. Nat. Prod. Sci. 7, 102–106. [Google Scholar]
- Srivastava S. K., Singh Rawat A. K., Mehrotra S. (2004). Pharmacognostic evaluation of the root of Berberis asiatica. Pharm. Biol. 42, 467–473. 10.1080/13880200490886256 [DOI] [Google Scholar]
- Srivastava S. K., Rawat A. K. S. (2007). Pharmacognostic evaluation of the roots of Berberis tinctoria Lesch. Nat. Prod. Sci. 13, 27–32. [Google Scholar]
- Steffens P., Nagakura N., Zenk M. H. (1985). Purification and characterization of the berberine bridge enzyme from Berberis beaniana cell cultures. Phytochemistry 24, 2577–2583. 10.1016/S0031-9422(00)80672-X [DOI] [Google Scholar]
- Stermitz F. (1967). Alkaloids of the Papaveraceae. V. Muramine and berberine from Argemone squarrosa. J. Pharm. Sci. 55, 760–762. 10.1002/jps.2600560624 [DOI] [PubMed] [Google Scholar]
- Stermitz F. R., Lorenz P., Tawara J. N., Zenewicz L. A., Lewis K. (2000). Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5'-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. U.S.A. 97, 1433–1437. 10.1073/pnas.030540597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stermitz F. R., Sharifi I. A. (1977). Alkaloids of Zanthoxylum monophyllum and Z. punctatum. Phytochemistry 16, 2003–2006. 10.1016/0031-9422(77)80113-1 [DOI] [Google Scholar]
- Stermitz F. R., Stermitz J. R., Zanoni T. A., Gillespie J. (1974). Alkaloids of Argemone subintegrifolia and A. munita. Phytochemistry 13, 1151–1153. 10.1016/0031-9422(74)80089-0 [DOI] [Google Scholar]
- Stuart G. A., Smith F. P. (1977). Chinese Materia Medica: Vegetable Kingdom. Shanghai: Gordon Press Publishers. [Google Scholar]
- Taborska E., Frantisek V., Slavik J. (1980). Alkaloids of the Papaveraceae. LXXI. Alkaloids from Bocconia frutescens L. Collect. Czechoslov. Chem. Commun. 45, 1301–1304. 10.1135/cccc19801301 [DOI] [Google Scholar]
- Tadzhibaev M. M., Zatorskaya I. N., Lutfullin K. L., Shakirov T. T. (1974). Isolation of berberine. Khimiya Prir. Soedin. 10, 48–50. 10.1007/BF00568218 [DOI] [Google Scholar]
- Tan E., Luo S., Lin S., Tan R., Yu W., Yi Z., et al. (2013). Determination of five active ingredient in Phellodendron chinensis var. glabiusculum and P. chinense by HPLC. Zhongguo Shiyan Fangjixue Zazhi 19, 135–139. [Google Scholar]
- Tang J., Feng Y., Tsao S., Wang N., Curtain R., Wang Y. (2009). Berberine and Coptidis Rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J. Ethnopharmacol. 126, 5–17. 10.1016/j.jep.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Tang W., Eisenbrand G. (1992). Corydalis turtschaninovii Bess. f. yanhusuo YH Chou et CC Hsü, in Chinese Drugs of Plant Origin (Berlin; Heidelberg: Springer; ), 377–393. [Google Scholar]
- Tantaquidgeon G. (1928). Mohegan medicinal practices, weather-lore and superstitions. SI-BAE Annu. Rep. 43, 264–270. [Google Scholar]
- Teng H., Choi O. (2013). Optimum extraction of bioactive alkaloid compounds from Rhizome coptidis (Coptis chinensis Franch.) using response surface methodology. Solvent Extr. Res. Dev. 20, 91–104. 10.15261/serdj.20.91 [DOI] [PubMed] [Google Scholar]
- Thirupurasundari C. J., Padmini R., Devaraj S. N. (2009). Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem. Biol. Interact. 177, 190–195. 10.1016/j.cbi.2008.09.027 [DOI] [PubMed] [Google Scholar]
- Tiwari K. P., Masood M. (1979). Chemical constituents of Berberis coriaria Royle. J. Indian Chem. Soc. 56, 310–311. [Google Scholar]
- Tiwary J. K., Ballabha R., Tiwari P. (2010). Ethnopaediatrics in Garhwal Himalaya. Uttarakhand, India (Psychomedicine Medice). NY Sci. J. 3, 123–126. [Google Scholar]
- Tomè F., Colombo M. L. (1995). Distribution of alkaloids in Chelidonium majus and factors affecting their accumulation. Phytochemistry 40, 37–39. 10.1016/0031-9422(95)00055-C [DOI] [Google Scholar]
- Tomita M., Kugo T. (1956). Alkaloids of Berberidaceous plants - XIX: Alkaloids of B. tschonoskyana I. Isolation of bases. Yakugak Zasshi 79, 317–321. 10.1248/yakushi1947.79.3_317 [DOI] [Google Scholar]
- Torres R., Villarroel L., Urzua A., Fajardo V. (1992). Constituents of Berberis congestiflora and Berberis horrida. Fitoterapia 63:376. [Google Scholar]
- Tsabang N., Fokou P. V. T., Tchokouaha L. R. Y., Noguem B., Bakarnga-Via I., Nguepi M. S. D., et al. (2012). Ethnopharmacological survey of Annonaceae medicinal plants used to treat malaria in four areas of Cameroon. J. Ethnopharmacol. 139, 171–180. 10.1016/j.jep.2011.10.035 [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Kamikawa H., Ogita Z. (1989). Anti-ulcer effect of extract from Phellodendri cortex. Yakugaku zasshi J. Pharm. Soc. Japan 109, 672–676. 10.1248/yakushi1947.109.9_672 [DOI] [PubMed] [Google Scholar]
- ul Haq I., Hussain M. (1993). Medicinal plants of Mansehra. Hamdard Med. 36, 63–100. [Google Scholar]
- Uniyal S. K., Singh K. N., Jamwal P., Lal B. (2006). Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J. Ethnobiol. Ethnomed. 2:14. 10.1186/1746-4269-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphof J. C. (1959). Dictionary of Economic Plants, 2nd edn. Lehre. [Google Scholar]
- Uprety Y., Asselin H., Boon E. K., Yadav S., Shrestha K. K. (2010). Indigenous use and bio-efficacy of medicinal plants in the Rasuwa District, Central Nepal. J. Ethnobiol. Ethnomed. 6:3. 10.1186/1746-4269-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzúa A., Torres R., Villarroel L., Fajardo V. (1984). Secondary metabolites of Berberis darwinii. Rev. Latinoam. Quim. 15, 27–29. [Google Scholar]
- Usher G. (1974). A Dictionary of Plants Used by Man. London: Constable and Company Ltd. [Google Scholar]
- Vennerstrom J. L., Klayman D. L. (1988). Protoberberine alkaloids as antimalarials. J. Med. Chem. 31, 1084–1087. 10.1021/jm00401a006 [DOI] [PubMed] [Google Scholar]
- Vennerstrom J. L., Lovelace J. K., Waits V. B., Hanson W. L., Klayman D. L. (1990). Berberine derivatives as antileishmanial drugs. Antimicrob. Agents Chemother. 34, 918–921. 10.1128/AAC.34.5.918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteegh C. P. C., Sosef M. S. M. (2007). Revision of the African genus Annickia (Annonaceae). Syst. Geogr. Plants 77, 91–118. [Google Scholar]
- Vuddanda P. R., Chakraborty S., Singh S. (2010). Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs 19, 1297–1307. 10.1517/13543784.2010.517745 [DOI] [PubMed] [Google Scholar]
- Wang C., Li J., Lv X., Zhang M., Song Y., Chen L., et al. (2009). Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur. J. Pharmacol. 620, 131–137. 10.1016/j.ejphar.2009.07.027 [DOI] [PubMed] [Google Scholar]
- Wang W., Shen Q., Liang H., Hua C., Liu Y., Li F., et al. (2016). Pharmacokinetic studies of novel berberine derivatives with ultra-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1031, 172–180. 10.1016/j.jchromb.2016.07.038 [DOI] [PubMed] [Google Scholar]
- Wang Y., Yi X., Ghanam K., Zhang S., Zhao T., Zhu X. (2014). Berberine decreases cholesterol levels in rats through multiple mechanisms, including inhibition of cholesterol absorption. Metabolism 63, 1167–1177. 10.1016/j.metabol.2014.05.013 [DOI] [PubMed] [Google Scholar]
- Watt G. (1883). Economic Products of India, Calcutta International Exhibition. Calcuta: Medicinal Products, Superintendent of Government Print. [Google Scholar]
- Weiner M. A. (1980). Earth Medicine-Earth Food: Plant Remedies, Drugs, and Natural Foods of the North American Indians. New York, NY: Macmillan. [Google Scholar]
- Willaman J. J., Schubert B. G. (1961). Alkaloid-Bearing Plants and Their Contained Alkaloids (No. 1234). Agricultural Research Service, US Department of Agriculture. [Google Scholar]
- Wu X., Li Y., Wang Q., Li W., Feng Y. (2015). Effects of berberine and pomegranate seed oil on plasma phospholipid metabolites associated with risks of type 2 diabetes mellitus by U-HPLC/Q-TOF-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1007, 110–120. 10.1016/j.jchromb.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Xi J. (2015). Ultrahigh pressure extraction of bioactive compounds from plants-a review. Crit. Rev. Food Sci. Nutr. 57, 1097–1106. 10.1080/10408398.2013.874327 [DOI] [PubMed] [Google Scholar]
- Xia X., Yan J., Shen Y., Tang K., Yin J., Zhang Y., et al. (2011). Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE 6:e16556. 10.1371/journal.pone.0016556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H. B., Sun Z. L., Zhang H. B., Zhang D. S. (2012). Berberine inhibits dyslipidemia in C57BL/6 mice with lipopolysaccharide induced inflammation. Pharmacol. Rep. 64, 889–895. 10.1016/S1734-1140(12)70883-6 [DOI] [PubMed] [Google Scholar]
- Xiao L., Xu N., Guo M., Guo M., Lv B, Tao H., et al. (2014). Berberine protects endothelial progenitor cell from damage of TNF-alpha via the PI3K/AKT/eNOS signaling pathway. Eur. J. Pharmacol. 743, 11–16. 10.1016/j.ejphar.2014.09.024 [DOI] [PubMed] [Google Scholar]
- Xu B., Li P., Zhang G. (2015). Comparative pharmacokinetics of puerarin, daidzin, baicalin, glycyrrhizic acid, liquiritin, berberine, palmatine and jateorhizine by liquid chromatography-mass spectrometry after oral administration of Gegenqinlian decoction and active components alignmen. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 988, 33–44. 10.1016/j.jchromb.2015.01.039 [DOI] [PubMed] [Google Scholar]
- Xu K., He G., Qin J., Cheng X., He H., Zhang D., et al. (2017). High-efficient extraction of principal medicinal components from fresh Phellodendron bark (Cortex phellodendri). Saudi J. Biol. Sci. 25, 811–815. 10.1016/j.sjbs.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Meng X., Yu X., Kuang H. (2017). Simultaneous determination of anemoside B4, phellodendrine, berberine, palmatine, obakunone, esculin, esculetin in rat plasma by UPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study in normal and ulcerative colitis rats. J. Pharm. Biomed. Anal. 134, 43–52. 10.1016/j.jpba.2016.11.021 [DOI] [PubMed] [Google Scholar]
- Yang T.-H. (1960a). Alkaloids of Berberidaceae. XXIX. Alkaloids of Mahonia lomariifolia and M. morrisonensis. Yakugaku Zasshi 80, 1304–1307. 10.1248/yakushi1947.80.9_1304 [DOI] [Google Scholar]
- Yang T.-H. (1960b). Alkaloids of Berberidaceae. XXVIII. Alkaloids of Berberis morrisonensis. Yakugaku Zasshi 80, 1302–1304. 10.1248/yakushi1947.80.9_1302 [DOI] [Google Scholar]
- Yang T.-H., Lu S.-T. (1960a). Alkaloids of berberidaceous plants. XXV. Alkaloids of Berberis kawakamii. 1. Yakugaku Zasshi 80, 847–849. 10.1248/yakushi1947.80.6_847 [DOI] [Google Scholar]
- Yang T.-H., Lu S.-T. (1960b). Alkaloids of berberidaceous plants. XXVI. Alkaloids of Berberis mingetsensis. 1. Yakugaku Zasshi 80, 849–851. 10.1248/yakushi1947.80.6_849 [DOI] [Google Scholar]
- Yavich P. A., Kakhtelidze M. B., Sarabunovich A. G. (1993). Quantitative determination of berberine in Phellodendron lavallei bark. Farmatsiya 42, 49–50. [Google Scholar]
- Yeung H. (1985). Handbook of Chinese Herbs and Formulas, Vol. 1. Los Angeles, CA: Institute of Chinese Medicine. [Google Scholar]
- Yin J., Gao Z., Liu D., Liu Z., Ye J. (2008a). Berberine improves glucose metabolism through induction of glycolysis. Am. J. Physiol. Endocrinol. Metab. 294, E148–E156. 10.1152/ajpendo.00211.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Xing H., Ye J. (2008b). Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 57, 712–717. 10.1016/j.metabol.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogesh H. S., Chandrashekhar V. M., Katti H. R., Ganapaty S., Raghavendra H. L., Gowda G. K., et al. (2011). Anti-osteoporotic activity of aqueous-methanol extract of Berberis aristata in ovariectomized rats. J. Ethnopharmacol. 134, 334–338. 10.1016/j.jep.2010.12.013 [DOI] [PubMed] [Google Scholar]
- Yoo S. J., Lee K. B., Kwak J. H. (1986). Studies on the seasonal variation of berberine contents in Berberis koreana. Saengyak Hakhoechi 17, 123–128. [Google Scholar]
- Yu C., Tan S., Zhou C., Zhu C., Kang X., Liu S., et al. (2016). Berberine reduces uremia-associated intestinal mucosal barrier damage. Biol. Pharm. Bull. 39, 1787–1792. 10.1248/bpb.b16-00280 [DOI] [PubMed] [Google Scholar]
- Zabihullah Q., Rashid A., Akhtar N. (2006). Ethnobotanical survey in kot Manzaray Baba valley Malakand agency, Pakistan. Pak. J. Plant Sci. 12, 115–121. [Google Scholar]
- Zaha V. G., Qi D., Su K. N., Palmeri M., Lee H. Y., Hu X., et al. (2016). AMPK is critical for mitochondrial function during reperfusion after myocardial ischemia. J. Mol. Cell. Cardiol. 91, 104–113. 10.1016/j.yjmcc.2015.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman M. B., Khan M. S. (1970). Hundred drug plants of West Pakistan. Medicinal Plant Branch of Pakistan Forest Institute. [Google Scholar]
- Zeng X. (1999). Relationship between the clinical effects of berberine on severe congestive heart failure and its concentration in plasma studied by HPLC. Biomed. Chromatogr. 13, 442–444. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cai C. T., Cai Z. Q., Liu G. Z., Luo Y., Yang Z. X. (2008). Variation patterns of Coptis teeta biomass and its major active compounds along an altitude gradient. J. Appl. Ecol. 19, 1455–1461. [PubMed] [Google Scholar]
- Zhao X., Zhang J., Tong N., Chen Y., Luo Y. (2012). Protective effects of berberine on Doxorubicin-induced hepatotoxicity in mice. Biol. Pharm. Bull. 35, 796–800. 10.1248/bpb.35.796 [DOI] [PubMed] [Google Scholar]
- Zovko Koncić Z., Kremer D., Karlovć K., Kosalec I. (2010). Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem. Toxicol. 48, 2176–2180. 10.1016/j.fct.2010.05.025 [DOI] [PubMed] [Google Scholar]