Abstract
A 50‐year‐old woman visited the emergency department with a high fever due to pneumonia. Incessant monomorphic ventricular tachycardia occurred and was terminated by intravenous lidocaine. Her ECG during sinus rhythm demonstrated ST segment elevation suggestive of Brugada syndrome (BS). An intensive examination could not detect any structural disease, and typical coved‐type ST elevation was unmasked by a pilsicainide injection leading to a diagnosis of BS. An ICD was implanted for secondary prevention of ventricular arrhythmia. The patient has been free from any recurrences of arrhythmia for 3 years.
Keywords: Brugada syndrome, fever, implantable cardioverter defibrillator, ventricular tachycardia
1. INTRODUCTION
Brugada syndrome (BS) is characterized by ST elevation in the right precordial leads and is associated with sudden cardiac death.1, 2 Symptoms including syncope, nocturnal agonal respiration, ventricular fibrillation (VF), and sudden cardiac death often occur during nocturnal period, at rest or sleep, during febrile state, or with parasympathetic activation.2
2. CASE REPORT
A 50‐year‐old woman visited our hospital by ambulance with a fever and chest pain. She visited the outpatient clinic due to a fever and cough and was prescribed with an antipyretic 5 days before admission. Her body temperature was 40 degrees Celsius, and the rapid influenza diagnostic tests were negative. The blood tests (Table 1), chest X‐ray (Figure 1A), and chest‐CT confirmed a diagnosis of pneumonia. The causative bacteria were confirmed to be Streptococcus pneumoniae by blood cultures and sputum cultures later. Monomorphic ventricular tachycardia (VT) incessantly developed in the emergency department (Figure 1B). She did not experience any palpitations or faintness before or during the VT. An intravenous lidocaine injection was effective in terminating the VT. From the ECG taken after termination of the VT, ST elevation in lead V2 became evident (Figure 1C,D). Although we could not perform cardiac catheterization due to acute respiratory failure, acute coronary syndrome was ruled out by serial blood tests for the cardiac enzymes. She was admitted to the intensive care unit, and the pneumonia was treated with intravenous antibiotics. A continuous lidocaine injection was terminated 3 days after admission, and the ST elevation gradually normalized along with her body temperature. The cardiac echocardiography and coronary CT were all normal. Her past history was insignificant with no familial members believed to have died from sudden cardiac death. A pilsicainide injection test was performed after the complete remission of her pneumonia, which unmasked a typical coved‐type ST elevation (Figure 2A,B). Late potentials were negative on signal‐averaged electrocardiography. No ventricular arrhythmias were induced by an electrophysiological study in which burst pacing and up to 3 extra‐stimuli from RV apex and RV outflow tract with minimum coupling interval of 180 ms. Programmed ventricular stimulation was repeated under continuous infusion of isoproterenol. A genetic analysis was performed by exome sequencing, but no pathogenic mutations of the genes previously linked to BS were detected. She was transferred to our affiliated hospital for an implantation of an implantable cardioverter defibrillator (ICD). After obtaining written informed consent, a single‐chamber ICD was implanted (Evera MRI XT SureScan, Medtronic, Figure 2C). During the ICD implantation, programmed ventricular stimulation from the RV ICD lead, burst, single, double, triple extra‐stimulus, could not induce any VT or VF. A defibrillation threshold test could not be performed because the additional induction of VF by a shock on T could induce only nonsustained VF. Following the device implantation, her clinical course was uneventful, and she was discharged without any remarkable events.
Table 1.
Laboratory data on admission
| WBC | 18 000/μL |
| Neu | 97.5% |
| Lym | 1.7% |
| Mono | 0.7% |
| Eos | 0.0% |
| Baso | 0.1% |
| Hb | 12.9 g/dL |
| PLT | 198 000/μL |
| CRP | 35.37 mg/dL |
| BNP | 84.9 pg/mL |
| PT | 15.7 s |
| INR | 1.53 |
| APTT | 51.3 s |
| %PT | 51.5% |
| AST | 38 IU/L |
| ALT | 24 IU/L |
| LDH | 326 IU/L |
| BUN | 17.7 mg/dL |
| Cr | 0.90 mg/dL |
| Na | 131.0 mEq/L |
| Cl | 98.3 mEq/L |
| K | 3.39 mEq/L |
| CPK | 16 IU/L |
| Troponin I | 0.013 IU/L |
Figure 1.
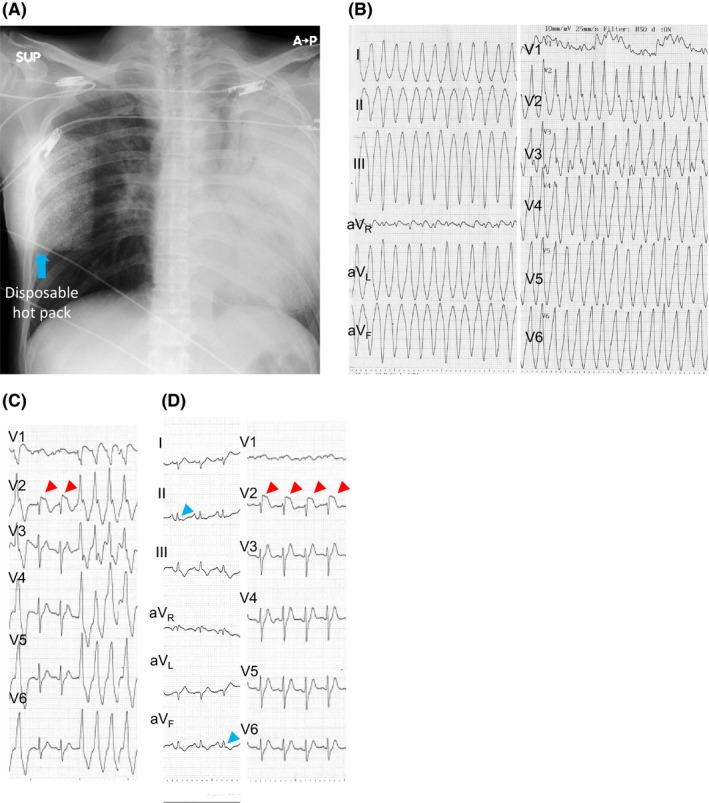
A, Chest X‐ray on admission. The chest X‐ray demonstrated a global consolidation of the left lung. The abnormal square shadow in the right mid‐lateral region was due to a disposable heat pack. B, 12‐lead ECG of the ventricular tachycardia. Monomorphic VT developed in the emergency department. The heart rate was 230 bpm. The QRS was negative in leads II, III, and aVF and positive in leads I, aVL, and V1‐6. C,D, ST elevation in lead V2. Marked ST elevation was observed during VT termination (D) and sinus rhythm (E) in lead V2. Notched type early repolarization pattern was observed in leads II and aVF during sinus rhythm
Figure 2.
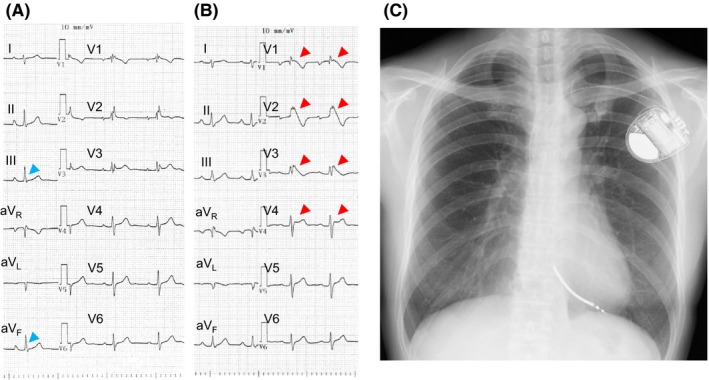
A,B, Unmasking a coved‐type ST elevation after a pilsicainide infusion. A, An rSR’ pattern was observed at baseline. B, After the pilsicainide infusion, a coved‐type ST elevation was observed in leads V1‐3 and a saddle‐back type ST elevation was observed in lead V4. Slurred type early repolarization pattern was observed in leads II and aVF and was diminished after the pilsicainide infusion. C, Chest X‐ray after the ICD implantation. A single‐chamber ICD was implanted for secondary prevention of VT
After the ICD implantation, she was followed up at the outpatient clinic. Periodical ICD checks demonstrated no VT/VF events. The patient has been free from any recurrences of VT/VF for 3 years.
3. DISCUSSION
Brugada syndrome (BS) is characterized by a coved‐type ST elevation in the right precordial leads and is associated with the risk of ventricular tachyarrhythmias and sudden cardiac death.1, 2 The incidence of BS is about 9 times more frequent in males than females.2 The occurrence of VF in BS patients typically develops during the nocturnal period.2 Most often, the presenting arrhythmia is VF or polymorphic VT but monomorphic VT is rare. However, some case reports have presented the appearance of monomorphic VT in BS patients.3 The morphology of monomorphic VT could be either an right bundle branch block pattern or left bundle branch block pattern.3 From previous studies reporting monomorphic VT in BS patients, VT can be induced by antiarrhythmic drugs or electrical stimulation and VT was associated with typical ECG pattern of BS. The mechanism of monomorphic VT developed in BS has not been clarified. The VT in our patient may originate from postero‐septum of RV or LV since rsr’ pattern in lead V1, positive QRS in leads V2‐4 and superior axis. We could not perform EP study during VT to confirm whether the mechanism was reentry or non‐reentry. It may share a common arrhythmogenic mechanism with BS, but further studies are required to confirm a true relationship. A fever, alcohol intake, full stomach, and glucose‐induced insulin secretion are known to trigger VF in BS patients.2 It is widely recognized that a fever triggers a typical BS‐ECG pattern and VF. From an experimental study, a reduction in the sodium current in a hypothermic state was found as the ionic basis of this phenomenon.4 Recent studies showed that the early repolarization (ER) pattern is associated with a high risk of arrhythmic events in patients with BS. In the presented case, ER pattern was observed in the inferior leads. We speculated the ER pattern in this patient is bystander because it did not show dynamic augmentation during arrhythmic state.
In summary, an ICD was implanted in a patient with fever‐induced BS who developed an incessant monomorphic VT. A typical coved‐type ST elevation was observed just after the VT termination and pilsicainide challenge test. Incessant monomorphic VT developed in this patient and lidocaine was effective in suppressing the VT. So far, no recurrence of the VT has occurred.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
ACKNOWLEDGEMENT
We are grateful to Mr. John Martin for editing this article. This work was supported by MEXT KAKENHI grant number 17K09524 and a research grant from the Japan Agency for Medical Research and Development (AMED) 17km0405109 h0005.
Sato Y, Aizawa Y, Fujisawa T, et al. Development of monomorphic ventricular tachycardia in a patient with fever‐induced Brugada syndrome. J Arrhythmia. 2018;34:465–468. 10.1002/joa3.12068
REFERENCES
- 1. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–6. [DOI] [PubMed] [Google Scholar]
- 2. Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–63. [DOI] [PubMed] [Google Scholar]
- 3. Dinckal MH, Davutoglu V, Akdemir I, Soydinc S, Kirilmaz A, Aksoy M. Incessant monomorphic ventricular tachycardia during febrile illness in a patient with Brugada syndrome: fatal electrical storm. Europace. 2003;5:257–61. [DOI] [PubMed] [Google Scholar]
- 4. Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–9. [DOI] [PubMed] [Google Scholar]


