Abstract
Purpose
The effect of repeated naming on both object and action picture naming in individuals with anomic aphasia is explored. We asked whether repeatedly naming the same items leads to improved accuracy and reduced response latency.
Method
Ten individuals with anomic aphasia and 6 healthy adults, 3 young and 3 old, named a set of 27 object pictures and a set of 27 action pictures presented 1 at a time on a computer screen. We examined accuracy and response times (RTs) across the 2 blocks of 10 repeated trials.
Results
Results demonstrated higher accuracy and faster RTs for object than for action naming for all participants, with lower accuracy rates and slower RTs for the people with aphasia (PWA) compared with the healthy individuals, and diverging patterns of change across trials. Unlike the healthy participants, whose RTs decreased across trials, PWA continued to demonstrate variability in response latencies across the trials.
Conclusions
Our preliminary results suggest that measuring RT may be useful in characterizing retrieval difficulty in anomic aphasia and that the retrieval processes in PWA, even in those who experience mild anomia, may be less efficient or different from those processes in neurologically healthy individuals.
Models of word production for picture naming consider several stages, typically including, at the very least, the activation of a concept, of the related lexical item, and of the phonologic form of that word (Francis, 2014; Rapp & Goldrick, 2006). Existing models vary in whether they postulate discrete stages (e.g., Levelt et al., 1991) or a parallel, interactive activation of these stages (e.g., Dell, 1986). The process of word retrieval may also include the inhibition of nontarget competitors (e.g., Rapp & Goldrick, 2006). Given the complexity of the mental lexicon, it is plausible to hypothesize that the same word can be retrieved via different routes within the lexical network, that is, that divergent processes can be engaged in the retrieval of the same word each time it is retrieved, depending on variables such as the context and previously activated words.
Anomic aphasia can be considered as a mild form of aphasia, characterized by the persistent inability to produce content words. Anomia is thought to be due to difficulty with accessing language representations, as opposed to a loss of language (e.g., Butterworth, 1992; Silkes, McNeil, & Drton, 2004), and inconsistency in naming ability is a hallmark feature of anomia (e.g., Howard, Patterson, Franklin, Morton, & Orchard-Lisle, 1984). Although people with anomic aphasia experience frequent word retrieval difficulty, accuracy rates on picture naming tests may not be sensitive enough to detect the impairment. In fact, people with anomic aphasia may exhibit accuracy rates on language tests of word retrieval comparable to those observed for healthy older adults (Grima & Franklin, 2017; Jaecks, Hielscher-Fastabend, & Stenneken, 2012). However, people with anomic aphasia are often inconsistent in their word production, perhaps completing all the stages for successful production in some, but not all, attempts. Furthermore, they do so with varying latencies for response times (RTs). For those individuals with anomic aphasia who achieve high accuracy rates but vary in their response latencies, measuring RT may be valuable.
Relatively little has been published in the literature concerning RT in picture naming in anomic aphasia. Wingfield, Brownell, and Hoyte (2006) found a decrease in RT followed by a plateau for both young and older healthy participants across five trials of naming the same lists of items. In contrast, three persons with aphasia (PWA) did not show this pattern, and their RTs continued to vary considerably across trials. It is possible that, with a greater number of trials, PWA may also benefit from the repeated naming. Nevertheless, to account for their findings, Wingfield et al. (2006) suggested that different underlying processes might be involved in word retrieval for PWA compared with adults without brain injuries. Specifically, they hypothesized that the ability to reuse the same pathway from a concept to its name when a picture is named repeatedly is reduced in aphasia. It can be hypothesized that when neurologically healthy individuals repeatedly name the same picture, the network responsible for the word retrieved remains active, such that when that word is produced again, the sustained activation reduces the latency required for completing the retrieval process of that word (e.g., Prince, Bucher, & Marder, 2004). In contrast, if PWA resort to using a new pathway in each attempt at retrieving the same word, repeated naming may not result in speeded retrieval. Alternatively, retrieval inconsistencies associated with anomia may be due to inefficiencies in the activation mechanisms (e.g., Schwartz, Saffran, Bloch, & Dell, 1994) or the faster decay rate of the activated networks.
Repetition priming effects in picture naming are consistent with the findings reported in Wingfield et al. (2006). Healthy individuals who are engaged in picture naming demonstrate a facilitation effect—measured by reduced RT—when they encounter the same item a second time (e.g., Mitchell & Brown, 1988; Vitkovitch, Rutter, & Read, 2001). Evidence suggests that PWA benefit from immediate repetition or when the number of intervening items is small (short lag), but that the benefit may not last when the time between repetitions increases (long lag; Soni, Lambon Ralph, & Woollams, 2012). Researchers have attempted to employ priming in speech-language therapy for anomia (e.g., Howard, Patterson, Franklin, Orchard-Lisle, & Morton, 1985; Leonard, Rochon, & Laird, 2008; Martin & Laine, 2000; Silkes, Dierkes, & Kendall, 2013), but how precisely priming may be beneficial to anomia treatment is not well understood.
Another possible factor that may affect picture naming performance involves grammatical class. Studies on object and action naming in aphasia have reported differing levels of retrieval difficulty (e.g., Druks, 2002) for nouns and verbs (e.g., Jonkers, & Bastiaanse, 2007; Williams & Canter, 1982), and it has been suggested that verbs may be fundamentally more difficult to produce than nouns across all individuals with aphasia (Mätzig, Druks, Masterson, & Vigliocco, 2009). In addition, noun–verb differences have been attributed to imageability differences and visual complexity differences (Bird, Howard, & Franklin, 2000), as well as to grammatical complexity (Black & Chiat, 2003). Effects of repeated naming of action pictures on RT have not been reported for PWA.
In summary, relatively little is known about word retrieval RTs in individuals with anomic aphasia and about the effects of repeated activation of the same words on RTs. Thus, in our study, we aimed to further consider the effect of repeated naming on both object and action picture naming in individuals with anomic aphasia whose aphasia severity was in the mild range. For PWA who demonstrate retrieval difficulty but high accuracy rates on picture naming tests, measuring RT may be an effective way to assess their retrieval difficulties. The following research questions guided the current investigation:
Research Question 1: Do people with anomic aphasia differ from young and older healthy individuals in naming accuracy and latency of objects and actions?
Research Question 2: Do people with anomic aphasia show practice effects (i.e., increased accuracy and decreased response times) in naming given multiple trials? And, if so, do object naming and action naming show the same practice effects?
Method
Research Design
This was a between-subjects group design in which PWA were compared with healthy older and healthy young adult controls. All participants participated in all conditions. The variables examined were accuracy and RT for naming pictures of objects and actions.
Participants
Institutional review board approval was provided through the City University of New York. Participants were recruited via posting fliers. Participants signed an informed consent on the first contact prior to the initial assessment. Ten individuals (three male, seven female) with anomic aphasia based on the Western Aphasia Battery–Revised (Kertesz, 2006) participated. Aphasia severity based on the Western Aphasia Battery–Aphasia Quotient was in the mild range, yet degree of anomia varied among participants. None of the participants had concomitant motor speech disorders. In addition, three healthy young and three healthy older controls were included in this the study. Participants' information is presented in Table 1.
Table 1.
Participants' information.
| Participant | Age | Sex | Education (years) | Handedness | Years post onset | WAB-AQ | % Objects accuracy, M (SD) | Objects RT in seconds, M (SD) | No. of items | % Actions accuracy, M (SD) | Actions RT in seconds, M (SD) | No. of items |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HY1 | 25 | F | 18 | Right | 100 | 0.62 (0.09) | 27 | 99.26 (1.56) | 0.97 (0.37) | 26 | ||
| HY2 | 24 | M | 18 | Right | 100 | 0.69 (0.15) | 27 | 92.22 (1.17) | 0.90 (0.32) | 23 | ||
| HY3 | 23 | F | 18 | Right | 100 | 0.52 (0.08) | 27 | 95.19 (1.79) | 0.77 (0.30) | 23 | ||
| M | 24 | 18 | 100 | 0.61 | 95.56 | 0.88 | ||||||
| HO1 | 78 | F | 18 | Left | 99.63 (1.17) | 0.68 (0.14) | 25 | 99.26 (1.56) | 0.86 (0.23) | 26 | ||
| HO2 | 68 | F | 18 | Right | 100 | 0.68 (0.14) | 27 | 94.44 (3.59) | 0.97 (0.57) | 23 | ||
| HO3 | 69 | F | 13 | Right | 100 | 0.60 (0.10) | 27 | 92.59 (3.02) | 1.02 (0.35) | 23 | ||
| M | 71.67 | 16.33 | 99.88 | 0.65 | 95.43 | 0.95 | ||||||
| A1 | 38 | F | 18 | Right | 1 | 88 | 89.63 (1.56) | 1.29 (0.66) | 16 | 65.93 (2.34) | 2.32 (1.55) | 8 |
| A2 | 46 | M | 16 | Right | 4 | 82 | 93.70 (3.05) | 1.94 (1.69) | 6 | 71.85 (4.68) | 1.78 (1.04) | 6 |
| A3 | 75 | F | 12 | Right | 3 | 95 | 98.52 (1.91) | 0.93 (0.45) | 24 | 78.52 (5.74) | 1.30 (0.43) | 11 |
| A4 | 45 | F | 16 | Right | 5 | 81 | 92.59 (3.90) | 2.10 (1.67) | 10 | 78.89 (7.42) | 3.21 (2.82) | 3 |
| A5 | 67 | M | 19 | Left | 6 | 94 | 93.70 (4.64) | 0.78 (0.32) | 15 | 87.41 (4.68) | 1.15 (0.62) | 11 |
| A6 | 62 | F | 12 | Right | 2 | 89 | 95.56 (1.56) | 1.19 (0.73) | 21 | 72.22 (6.36) | 1.49 (0.56) | 13 |
| A7 | 57 | F | 12 | Right | 2 | 92 | 91.85 (6.72) | 1.92 (1.46) | 14 | 85.93 (7.57) | 2.20 (1.47) | 9 |
| A8 | 48 | F | 18 | Right | 5 | 90 | 94.81 (2.59) | 1.64 (1.23) | 11 | 65.93 (4.20) | 2.16 (1.52) | 6 |
| A9 | 66 | M | 16 | Right | 5 | 87 | 95.93 (2.73) | 1.25 (0.99) | 18 | 64.81 (4.00) | 1.60 (0.89) | 9 |
| A10 | 48 | F | 12 | Right | 3 | 90 | 87.04 (3.60) | 0.89 (0.12) | 20 | 54.44 (6.77) | 1.36 (0.74) | 7 |
| M | 55.22 | 15.10 | 3.6 | 88.75 | 93.33 | 1.39 | 72.59 | 1.86 | ||||
Note. WAB-AQ = Western Aphasia Battery–Aphasia Quotient; RT = response time; HY = healthy young; HO = healthy older; A = aphasia.
Material and Procedures
Participants were tested individually on two separate days within a week, on a set of 27 object pictures (selected from Gollan, Weissberger, Runnqvist, Montoya, & Cera, 2012) on Day 1 and a set of 27 action pictures (selected from Bastiaanse, Mass, & Rispens, 2003) on Day 2, presented one at a time on a computer screen. We note that two sets were selected from the larger inventory with the aim of having high name agreement, but were not matched for other psycholinguistic variables (e.g., word length or frequency). All participants were tested by the first author. The participants were instructed to name each picture as quickly as possible as soon as each picture appeared on the screen. Once a participant responded, the examiner waited 2 s before presenting the next picture. If a participant did not respond within 30 s from the initial presentation of the picture (measured manually by the examiner), the examiner advanced to the next item.
Testing began with a practice set of three items to ensure that the participant had learned the task. When the first trial of 27 items was completed, the next trial began after a brief delay. There were 10 trials of each set of pictures, with different random orders for each trial. The order of administration of the object and action picture sets alternated across participants, such that Day 1 and Day 2 were counterbalanced regarding which word type was administered first.
Scoring and Data Analysis
Naming responses were recorded to computer sound files for later measurements of RTs. A brief tone was presented simultaneously with the picture onset, allowing later measurement of RTs from the onset of picture presentation to the onset of the participant's correct response. All responses were recorded using the computerized recording program Audacity (The Audacity Team, 2017). All scores were noted manually on an electronic spreadsheet. First, responses were scored as correct or incorrect. Responses with phonemic paraphasic errors were scored correct. There were no pluralization errors. For items that were correct, RT was measured manually for each response. For these measurements, the cursor was placed at the onset of the tone that occurred concurrently with picture presentation. The cursor was then dragged to the onset of the participant's correct response. Once cursors were placed in these locations, the RT measurement (located on the bottom of the Audacity application) was noted and recorded on the spreadsheet used for data recording.
One senior research assistant (RA) was trained to oversee the RT measurements and to train additional RAs as needed. The manual process was time-consuming but necessary because using voice-activated, automatic measurements has drawbacks when participants have false starts and self-correct initial productions (Conroy, Sage, & Lambon Ralph, 2009). Ten percent of responses were scored by two RAs for interrater reliability purposes. Point-by-point interrater reliability among the measurement team was 99% for responses that were within 3 ms.
For each participant for each list, a mean RT was calculated. A correct response was considered the target response or an alternative appropriate to the pictures, for example, birthday cake for cake when the picture depicted a cake with candles on it. Hesitations and self-corrections were noted. We further calculated the mean RT only for items that were named immediately and consistently correct, as well as for corrected responses that followed self-corrections and hesitations (see, e.g., Schwartz, Middleton, Brecher, Gagliardi, & Garvey, 2015). There was no significant difference in the results between obtained mean overall RTs when calculating the mean using items that were immediately correct compared with obtained mean overall RTs when calculating the mean using items that were immediately correct as well as items that were self-corrected responses, which we report here.
Nonparametric tests were used to compare accuracy and RT among the participant groups (independent-samples Kruskal–Wallis test) and between noun and verb naming (Wilcoxon signed-ranks test). Regression analyses were used to examine change in accuracy and RT across trials.
Results
Means and standard deviations for accuracy and for RT by participants and groups are presented in Table 1 and in Figure 1.
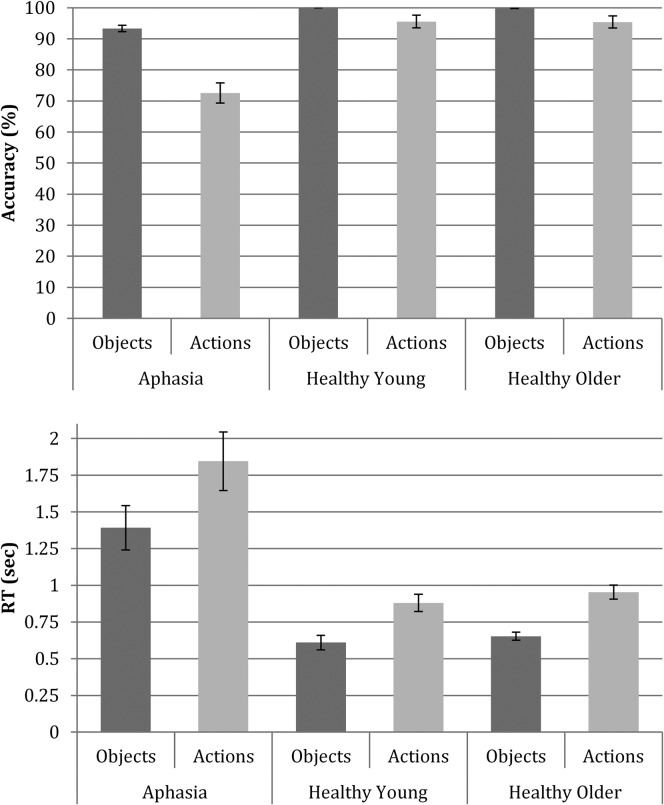
Figure 1.
Average accuracy rates (top) and response times (RT; bottom) for object and action naming. Means calculated across 10 lists per person and then per group.
Naming Accuracy and RT Across Groups
Response accuracy for object naming was high for all participants. An independent-samples Kruskal–Wallis test revealed a significant difference among the groups, χ2(2) = 10.95, p = .004; paired comparisons adjusted with the Bonferroni correction revealed that PWA were significantly less accurate than were the healthy younger (t = −8.50, p = .018) and the healthy older (t = −7.50, p = .045) individuals; there was no difference between the healthy groups (t = 1.00, p = 1.0). A Wilcoxon signed-ranks test showed that the response accuracy for action naming was lower than for object naming across all participants (Z = −3.52, p < .001). An independent-samples Kruskal–Wallis test confirmed that the accuracy for action naming was significantly different among the three groups, χ2(2) = 10.62, p = .005. Paired comparisons showed lower accuracy for the aphasia group than for the healthy young (t = −8.00, p = .032) and older (t = −8.00, p = .032) participants (Bonferroni corrected).
A similar pattern of results was observed for the RT data, with a significant difference among the groups as revealed by an independent-samples Kruskal–Wallis test for object naming, χ2(2) = 10.61, p = .005, and for action naming, χ2(2) = 10.77, p = .005. Paired comparisons showed that PWA had significantly slower RTs for object naming than the healthy young (t = 8.17, p = .027) and older (t = 7.83, p = .037) participants (Bonferroni corrected), as well as slower RTs for action naming than the young (t = 8.83, p = .014) and marginally slower than the older (t = 7.17, p = .067) participants (Bonferroni corrected). A Wilcoxon signed-ranks test revealed slower RT for action naming than for object naming across participants (Z = 3.47, p = .001; see Figure 1).
Response Across Trials
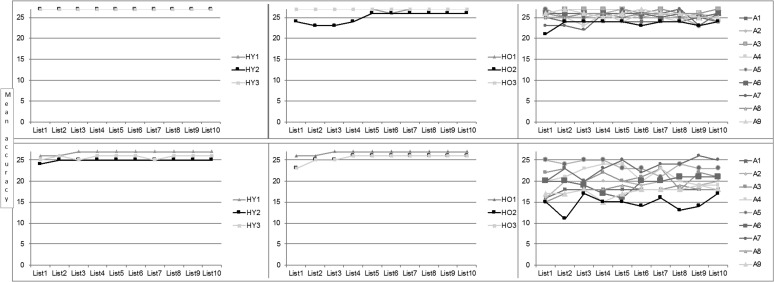
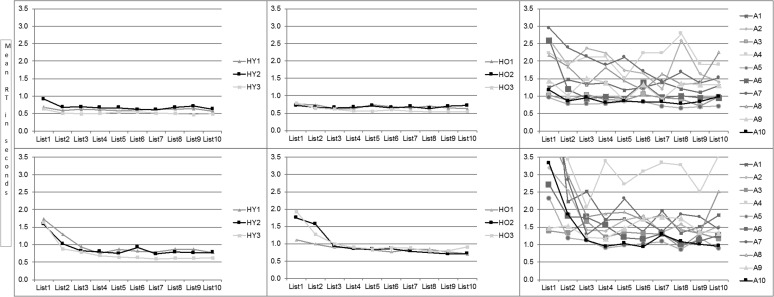
There was a minimal change in response accuracy across trials (see Figure 2), and a regression analysis confirmed no significant effect of Trial (B = −.001, t = −.36; B = .004, t = 1.00, for object and action naming, respectively, both p > .1). Regression analyses for RTs for object naming and action naming revealed similar results, as follows. There was a significant effect of Trial (B = −.059, t = −4.08; B = −.174, t = −7.79, for object and action naming, respectively, both p < .001), demonstrating a decrease in RT across trials; a significant effect of Group (B = −.583, t = −11.45; B = −.795, t = −10.27, for object and action naming, respectively, both p < .001), confirming the slower RT for the PWA than for the healthy participants; and a significant Trial × Group interaction (B = .019, t = 2.34, p = .019; B = .038, t = 3.06, p = .002, for object and action, respectively). The time course of the decrease in RT across trials is depicted in Figure 3. As can be seen in the figure, PWA overall did not demonstrate the plateau following a decrease in RT that characterized responses of healthy participants. Instead, most of the individuals in the aphasia group demonstrated continued variability in RT across the trials. Nevertheless, we note that four of the PWA (A5, A6, A7, and A10) did show some decrease in RT, thus approaching the pattern noted in the neurologically healthy participants.
Figure 2.
Accuracy rates (maximum score = 27) among healthy young (left) and older (middle) individuals and people with aphasia (right) for object naming (top) and action naming (bottom) over 10 trials. HY = healthy young; HO = healthy old; A = aphasia.
Figure 3.
Response time (RT, in seconds) among healthy young (left) and older (middle) individuals and people with aphasia (right) for object naming (top) and action naming (bottom) over 10 trials. HY = healthy young; HO = healthy old; A = aphasia.
Discussion
Ten people with anomic aphasia completed two picture naming tests, one comprising 27 pictures of objects and one comprising 27 pictures of actions, and their accuracy and RT performance were compared with that of six healthy individuals, three young and three old. Results demonstrated higher accuracy and faster RTs for object than for action naming for all participants, lower accuracy rates and slower RTs for the PWA compared with the healthy individuals, and diverging patterns of change across trials. Thus, the answer to our first research question, whether people with anomic aphasia differ from healthy individuals in their performance on the naming tests, is “yes.” Regarding our second research question, whether people with anomia benefit from naming repetition, our data suggest that, at least for repeatedly naming the same set of pictures at long lags, most of our participants with anomia showed no measurable benefit in either accuracy or RT.
Our 10 participants with aphasia demonstrated variable difficulty in consistently correctly naming the objects and actions depicted in the pictures, and when they did produce the target word, they took longer to initiate their response than did the neurologically typical individuals. This was the case even when additional trials were included in the study, beyond the five trials studied in Wingfield et al. (2006). We hypothesized that PWA might show the repetition benefit when given additional trials, and this was not the case. Because we asked our participants to name the sets of items 10 times in a single session, PWA might have experienced fatigue that may have countered any potential repetition benefit. It is also possible that the additional trials we provided in this study were still not sufficient and that, with additional trials, PWA's RT would eventually plateau.
The neurologically healthy individuals in our study, as in Wingfield et al.'s (2006) study, were close to ceiling in their accuracy rates and showed a consistent pattern of behavior in their RTs. Namely, RTs in the picture naming tests decreased after the initial trials and then plateaued. This pattern is consistent with the idea that once the picture is recognized and the lexical item is retrieved, there is sustained activation that results in the facilitation of retrieval and production of the same word upon subsequent trials (e.g., Hillis & Caramazza, 1994). In contrast, and again consistent with Wingfield et al. (2006), most of our participants with anomic aphasia did not show the same benefit from repetition that the control participants showed. Several of our participants did show a decrease in RT after the initial trials, perhaps indicating a faster recognition of the object or action depicted in the picture, and possibly faster access to semantic representations of repeated target items, and four of the PWA showed a decrease in RT followed by little RT variability, approaching the typical pattern. An examination of the participants' demographics did not point to any systematic difference in aphasia severity, age, or time post onset that could explain why these individuals approached the typical pattern. The variability in RT observed among the PWA persisted in both object and action naming and appeared to be evident for the majority of the individuals with aphasia.
It is possible that, as suggested by Wingfield et al. (2006), PWA employ divergent routes or activate divergent networks in multiple retrieval attempts of the same word. That is, as suggested above, in an interactive, interconnected lexical network, naming the same picture may be accomplished via several pathways between activated concepts and the target name, which could account for the absence of consistent reduction in RT. Alternatively, PWA's lexical networks may be less efficient than those of healthy adults. It is also possible that the activation decay in people with aphasia is faster than the decay in healthy individuals, and therefore, the repeated activation did not facilitate faster RTs among the individuals with aphasia. This may be particularly true in the present study, because, whereas the items were named repeatedly, they were embedded in a list, rather than in an immediate repetition paradigm. Soni et al. (2012) demonstrated a repetition priming effect for PWA only at short lags. In the present study, however, participants named 27 items per list, in randomized order. That is, whereas it was possible that a participant received a given item as the last item on list n and the first item on list n + 1, in the majority of the cases, there were varying numbers of intervening items (up to 52 items), resulting in long lags for the majority of items across lists, which may have reduced any potential effects of repetition priming.
We note that the classic repetition priming effect may be explained by sustained activation of the lexical item, with gradual decay over time, which corresponds to the time course or number of intervening items in a typical priming paradigm. In contrast, the design of our study aimed to uncover a repeated naming effect that is more in line with the practice of aphasia treatment often employed in the clinic, namely, the repeated production of lexical items within the course of a therapy session, with varying intervals (and intervening words) between repeated production. Future studies could examine whether repeated naming in intervals that begin with immediate repetition and gradually increase, with increasing numbers of intervening items, could be used for effective naming therapy (e.g., Fridriksson, Holland, Beeson, & Morrow, 2005).
In addition, although we did not attempt to equate the two naming tests for difficulty levels, we note that visual inspection suggested that our neurologically healthy individuals (and especially the older participants) demonstrated a somewhat greater and more gradual decrease in RT in the action naming compared with the object naming. This could be attributed to the visual processing associated with the picture stimuli or to differences in the words that needed to be retrieved. Visual processing differences may be related to the level of abstractness of the action pictures compared with that of the objects (Bird et al., 2000) or to the visual complexity of the pictures. In addition, pictures of actions often include an action and a subject or an object (e.g., a man kicking a ball), whereas pictures of objects typically include only the object (e.g., a ball). Furthermore, the retrieval of verbs may be more difficult than the retrieval of nouns due to inherent differences between the two parts of speech, including selection restriction and argument structure associated with verbs but not with nouns (e.g., Black & Chiat, 2003; Thompson & Shapiro, 2005). Whereas we found overall lower accuracy rates and slower RTs to the action naming than the object naming, we cannot be sure that this difference is not a result of difficulty differences between the two sets. Thus, the finding that we deem relevant here is that the same general pattern of repetition effects (for healthy participants and its absence for PWA) was observed for both object and action naming, despite the differences for nouns and verbs reported in the aphasia literature.
In summary, our preliminary results demonstrate that people with aphasia differ from controls with respect to their RTs to pictures of objects and actions, even when they are successful in retrieving the target word and even when given the opportunity to name the same item repeatedly, across 10 lists. While PWA varied in their RTs, healthy controls showed slightly longer RT initially, then plateaued and did not vary. We conclude that measuring RT may be useful in characterizing retrieval difficulty in anomic aphasia and that the retrieval processes in people with aphasia, even in those who experience mild anomia, may differ from those processes in neurologically typical individuals.
Clinical Implications
Current assessment tools for aphasia are often less successful than RT measures in capturing subtle deficits associated with mild aphasia. Measuring RTs for naming in mild aphasia could be a useful measure, especially for individuals who perform at near ceiling levels in terms of response accuracy yet experience and report mild to severe word retrieval difficulty in conversation. In order to make decisions regarding treatment recommendations, documenting varied RTs could support the experience of word retrieval difficulty reported by the patient. Exploring the time course of activation and decay in PWA as they relate to the use of repeated production in treatment for anomia (Howard et al., 1984; Nickels, 2002; Silkes et al., 2013) presents fertile ground for further research.
Caveats and Future Studies
Several caveats need to be considered. First, in this study, we enrolled a relatively small number of control participants. Therefore, the lack of a significant effect of age (age was not a significant predictor in the regression analyses, nor was there a significant difference between the younger and older healthy participants in accuracy and RT on the naming tests) needs to be taken with caution. As well, our participants were relatively accurate in their naming performance, and our accuracy data did not reveal a facilitation effect. Nevertheless, the absence of the pattern of decrease in RT followed by plateau for most of the PWA may be, in part, due to the small numbers of items that were averaged. Further, in order to accommodate the tendency of PWA to hesitate and false start before producing responses, we did not opt for automatic measurement of RT; rather, we manually measured the lag between the appearance of each picture and the start of the verbal response (e.g., Soni et al., 2012). If researchers and clinicians were to routinely assess RT in people with anomia, automaticity of the measurements would be of great advantage over the laborious manual method. Finally, although we found differences in accuracy and RT for action versus object naming, we did not attempt to equate the two sets of pictures we used in terms of levels of difficulty. Future studies designed to explore differences between noun and verb retrieval in healthy individuals and in PWA would benefit from such considerations.
Acknowledgments
This research was supported in part by National Institutes of Health Grant DC 009792, awarded to Mira Goral. The authors would like to thank the people with aphasia and the participants with no brain damage who volunteered to participate in this study. The authors would also like to thank May Sofi, Amy Vogel-Eyny, and Olga Iukalo for help with the reaction time measurements and data entry, and Katy Borodkin for developing the naming tests.
Funding Statement
This research was supported in part by National Institutes of Health Grant DC 009792, awarded to Mira Goral.
References
- The Audacity Team. (2017). Audacity (Version 2) [Computer software]. Retrieved from http://www.downloadix.com/Audacity/
- Bastiaanse R., Mass E., & Rispens J. (2003). Assessing comprehension and production of verbs and sentences: The Verb and Sentence Test (VAST). Aphasiology, 17(1), 49–73. [Google Scholar]
- Bird H., Howard D., & Franklin S. (2000). Why is a verb like an inanimate object? Grammatical category and semantic category deficits. Brain Language, 72, 246–309. [DOI] [PubMed] [Google Scholar]
- Black M., & Chiat S. (2003). Noun–verb dissociations: A multi-faceted phenomenon. Journal of Neurolinguistics, 16(2–3), 231–250. https://doi.org/10.1016/S0911-6044(02)00017-9 [Google Scholar]
- Butterworth B. (1992). Disorders of phonological encoding. Cognition, 42, 261–286. [DOI] [PubMed] [Google Scholar]
- Conroy P., Sage K., & Lambon Ralph M. A. (2009). The effects of decreasing and increasing cue therapy on improving naming speed and accuracy for verbs and nouns in aphasia. Aphasiology, 23(6), 707–730. [Google Scholar]
- Dell G. S. (1986). A spreading activation theory of retrieval in language production. Psychological Review, 93, 283–321. [PubMed] [Google Scholar]
- Druks J. (2002). Verbs and nouns—A review of the literature. Journal of Neurolinguistics, 15, 289–315. [Google Scholar]
- Francis W. (2014). Repetition priming in picture naming: Sustained learning through the speeding of multiple processes. Psychonomic Bulletin & Review, 21(5), 1301–1308. [DOI] [PubMed] [Google Scholar]
- Fridriksson J., Holland H., Beeson P., & Morrow L. (2005). Spaced retrieval treatment of anomia. Aphasiology, 19(2), 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan T. H., Weissberger G., Runnqvist E., Montoya R. I., & Cera C. M. (2012). Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish–English bilinguals. Bilingualism: Language and Cognition, 15, 594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima R., & Franklin S. (2017). Usefulness of investigating error profiles in diagnosis of naming impairments. International Journal of Language & Communication Disorders, 52(2), 214–226. https://doi.org/10.1111/1460-6984.12266 [DOI] [PubMed] [Google Scholar]
- Hillis A., & Caramazza A. (1994). Theories of lexical processing and rehabilitation of lexical deficits. In Riddoch M. J. & Humphreys G. W. (Eds.), Cognitive neuropsychology and cognitive rehabilitation. Hove, United Kingdom: Erlbaum. [Google Scholar]
- Howard D., Patterson K., Franklin S., Morton J., & Orchard-Lisle V. (1984). Variability and consistency in picture naming by aphasic patients. Advances in Neurology, 42, 263–276. [PubMed] [Google Scholar]
- Howard D., Patterson K. E., Franklin S. E., Orchard-Lisle V. M., & Morton J. (1985). The treatment of word retrieval deficits in aphasia: A comparison of two therapy methods. Brain, 108, 817–829. [PubMed] [Google Scholar]
- Jaecks P., Hielscher-Fastabend M., & Stenneken P. (2012). Diagnosing residual aphasia using spontaneous speech analysis. Aphasiology, 26, 953–970. https://doi.org/10.1080/02687038.2012.663075 [Google Scholar]
- Jonkers R., & Bastiaanse R. (2007). Action naming in anomic aphasic speakers: Effects of instrumentality and name relation. Brain and Language, 102, 262–272. [DOI] [PubMed] [Google Scholar]
- Kertesz A. (2006). The Western Aphasia Battery–Revised (WAB-R). New York, NY: Grune & Stratton. [Google Scholar]
- Leonard C., Rochon E., & Laird L. (2008). Treating naming impairments in aphasia: Findings from a phonological components analysis treatment. Aphasiology, 22, 923–947. [Google Scholar]
- Levelt W. J. M., Schriefers H., Vorberg D., Meyer A. S., Pechmann T., & Havinga J. (1991). The time course of lexical access in speech production: A study of picture naming. Psychological Review, 98(1), 122–142. [Google Scholar]
- Martin N., & Laine M. (2000). Effects of contextual priming on impaired word retrieval. Aphasiology, 14(1), 53–70. [Google Scholar]
- Mätzig S., Druks J., Masterson J., & Vigliocco G. (2009). Noun and verb differences in picture naming: Past studies and new evidence. Cortex, 45, 738–758. [DOI] [PubMed] [Google Scholar]
- Mitchell D. B., & Brown A. S. (1988). Persistent repetition priming in picture naming and its dissociation from recognition memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 14(2), 213–222. [DOI] [PubMed] [Google Scholar]
- Nickels L. (2002). Improving word finding: Practice makes (closer to) perfect? Aphasiology, 16(10–11), 1047–1060. [Google Scholar]
- Prince A. A., Bucher D., & Marder E. (2004). Similar network activity from disparate circuit parameters. Nature Neuroscience, 7, 1345–1352. [DOI] [PubMed] [Google Scholar]
- Rapp B., & Goldrick M. (2006). Speaking words: Contributions of cognitive neuropsychological research. Cognitive Neuropsychology, 23, 39–73. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Middleton E. L., Brecher A., Gagliardi M., & Garvey K., (2015). Does naming accuracy improve through self-monitoring errors? Poster presented at the Society for the Neurobiology of Language, October 15–17, 2015 Chicago, IL. [Google Scholar]
- Schwartz M. F., Saffran E. M., Bloch D. E., & Dell G. S. (1994). Disordered speech production in aphasic and normal speakers. Brain and Language, 47(1), 52–88. [DOI] [PubMed] [Google Scholar]
- Silkes J. P., Dierkes K. E., & Kendall D. L. (2013). Masked repetition priming effects on naming in aphasia: A phase I treatment study. Aphasiology, 27(4), 381–397. https://doi.org/10.1080/02687038.2012.745475 [Google Scholar]
- Silkes J. P., McNeil M. R., & Drton M. (2004). Simulation of aphasic naming performance in non-brain damaged adults. Journal of Speech, Language, and Hearing Research, 47, 610–623. [DOI] [PubMed] [Google Scholar]
- Soni M., Lambon Ralph M., & Woollams A. M. (2012). Repetition priming of picture naming in semantic aphasia: The impact of intervening items. Aphasiology, 26(1), 44–63. [Google Scholar]
- Thompson C. K., & Shapiro L. P. (2005). Treating agrammatic aphasia within a linguistic framework: Treatment of underlying forms. Aphasiology, 19(10–11), 1021–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovitch M., Rutter C., & Read A. (2001). Inhibitory effects during object name retrieval: The effect of interval between prime and target on picture naming responses. British Journal of Psychology, 92, 483–506. [PubMed] [Google Scholar]
- Williams S. E., & Canter G. J. (1982). The influence of situational context on naming performance in aphasic syndromes. Brain and Language, 17, 92–106. [DOI] [PubMed] [Google Scholar]
- Wingfield A., Brownell H., & Hoyte K. (2006). Variable solutions to the same problem: Aberrant practice effects in object naming by three aphasic patients. Brain and Language, 97, 351–356. [DOI] [PubMed] [Google Scholar]