Abstract
Purpose
The purpose of this study was to describe the linguistic environment of phonological paraphasias in 3 variants of primary progressive aphasia (semantic, logopenic, and nonfluent) and to describe the profiles of paraphasia production for each of these variants.
Method
Discourse samples of 26 individuals diagnosed with primary progressive aphasia were investigated for phonological paraphasias using the criteria established for the Philadelphia Naming Test (Moss Rehabilitation Research Institute, 2013). Phonological paraphasias were coded for paraphasia type, part of speech of the target word, target word frequency, type of segment in error, word position of consonant errors, type of error, and degree of change in consonant errors.
Results
Eighteen individuals across the 3 variants produced phonological paraphasias. Most paraphasias were nonword, followed by formal, and then mixed, with errors primarily occurring on nouns and verbs, with relatively few on function words. Most errors were substitutions, followed by addition and deletion errors, and few sequencing errors. Errors were evenly distributed across vowels, consonant singletons, and clusters, with more errors occurring in initial and medial positions of words than in the final position of words. Most consonant errors consisted of only a single-feature change, with few 2- or 3-feature changes. Importantly, paraphasia productions by variant differed from these aggregate results, with unique production patterns for each variant.
Conclusions
These results suggest that a system where paraphasias are coded as present versus absent may be insufficient to adequately distinguish between the 3 subtypes of PPA. The 3 variants demonstrate patterns that may be used to improve phenotyping and diagnostic sensitivity. These results should be integrated with recent findings on phonological processing and speech rate. Future research should attempt to replicate these results in a larger sample of participants with longer speech samples and varied elicitation tasks.
Supplemental Materials
Primary progressive aphasia (PPA) was first described by Dejerine and Serieux in 1879 (as cited in Mesulam, 1982). More recently, Mesulam (1982, p. 592) described six cases of “slowly progressing aphasia,” and this diagnosis began to receive further attention from the medical and rehabilitation communities. Additional research has been conducted, leading to a better understanding of PPA and its effects on communication (see the chapter by Gorno-Tempini & Pressman, 2016). Although PPA is distinct from the better understood stroke-induced aphasias, one common characteristic is the production of phonological paraphasias (production errors where phonemes are omitted, added, or substituted in a target word). Investigations of these errors have focused primarily on the presence and frequency of occurrence of phonological paraphasias in individuals with PPA (e.g., Ash et al., 2013; Croot, Ballard, Leyton, & Hodges, 2012; Gorno-Tempini et al., 2011; Gunawardena et al., 2010; Leyton, Ballard, Piguet, & Hodges, 2014; Patterson & MacDonald, 2006; Wilson et al., 2010). A more detailed analysis of the production of phonological paraphasias in individuals with PPA may provide useful insights into this disorder.
PPA
PPA is a neurodegenerative syndrome characterized by progressive impairment of speech and language function (Mesulam, 2001). Accurate diagnosis is hampered because several distinct underlying disease processes can cause PPA, including Alzheimer's pathology, tauopathy, and transactive response DNA-binding protein 43 proteinopathy (Gorno-Tempini et al., 2011). However, PPA can now generally be diagnosed through a combination of neuroimaging, behavioral testing, and medical history (Gorno-Tempini et al., 2011). The requirements for diagnosis of PPA include a primary and initial difficulty with language while other cognitive functions remain intact or are better preserved, and neuroimaging results indicating degeneration predominantly within speech–language networks in the brain (Botha et al., 2015; Gorno-Tempini et al., 2011 Gorno-Tempini & Pressman, 2016; Jung, Duffy, & Josephs, 2013).
Individuals with PPA have relatively intact reasoning, problem solving, and memory skills until later stages of the disease, with communication impairments ranging from altered speech production to impaired single-word comprehension, as well as reading and writing difficulties (Gorno-Tempini et al., 2008, Henry & Gorno-Tempini, 2010; Jung et al., 2013; Leyton et al., 2014; Mesulam, 1982, 1987, 2001). Three types of PPA have consistently been described, namely, semantic variant (SvPPA), logopenic variant (LvPPA), and nonfluent, agrammatic variant (NFvPPA); see Gorno-Tempini et al. (2004, 2008), Hodges and Patterson (1996), Neary et al. (1998), and Wilson et al. (2010). For a discussion of other proposed variants, see Botha et al. (2015).
Speech and Language Characteristics of PPA Variants
SvPPA is characterized by fluent speech production (Wilson et al., 2010) and impaired word comprehension (Gorno-Tempini et al., 2011; Patterson & MacDonald, 2006). Vocabulary use is characterized by increased pronouns, verbs, and high-frequency nouns compared with controls (Bird, Ralph, Patterson, & Hodges, 2000; Patterson & MacDonald, 2006; Wilson et al., 2010). This paucity of nouns leads to utterances filled with general or nonspecific words (e.g., this, that, and thing) such as the following utterance for dock: “that may be where you take your boat to get onto the boat there” (Wilson et al., 2010, p. 2079). Individuals with SvPPA may also use utterances with reduced syntactic complexity compared with controls (although these differences have not been well-defined; Patterson & MacDonald, 2006). Individuals with SvPPA do not typically have difficulty producing the correct phonological forms for words (e.g., Patterson & MacDonald, 2006).
The speech of individuals with LvPPA is characterized by a poverty of words. Speech rate is slightly slower in LvPPA than in SvPPA (but faster than in NFvPPA), and high-frequency nouns are not preferentially produced as in SvPPA (Wilson et al., 2010). Like individuals with SvPPA, individuals with LvPPA also show differences in syntax usage relative to controls, although there are few outright errors of syntax (Wilson et al., 2010). Compared with individuals with SvPPA and compared with controls, those with LvPPA produce more revisions, fillers, and false starts. A characteristic that may distinguish individuals with LvPPA from those with SvPPA is the presence of impaired phonology in LvPPA, such that words are produced with omitted, added, or substituted (but not distorted) phonemes (Botha et al., 2015; Gorno-Tempini et al., 2011; Jung et al., 2013; Leyton et al., 2014; Wilson et al., 2010). Individuals with LvPPA have impaired sentence repetition, evidence of an underlying impairment in phonological working memory (Sepelyak et al., 2011).
Individuals with NFvPPA are characterized by severely reduced speech rate and word production in connected speech, leading to a nonfluent classification (Wilson et al., 2010). In addition, impaired syntax resulting in agrammatism is common (Gorno-Tempini et al., 2011). Individuals with NFvPPA may produce utterances containing phonological errors (Wilson et al., 2010); however, unlike those with LvPPA, they often produce phonetic distortions due to motor speech impairment (apraxia of speech [AOS] and, in some cases, dysarthria; Henry et al., 2016). It is important to note that some research groups distinguish between NFvPPA and primary progressive AOS, which denotes progressive impairment of motor speech with only very mild or no aphasia (Botha et al., 2015; Josephs et al., 2012).
Phonological Paraphasias
There is a rich history of research on phonological paraphasias in individuals with stroke-induced aphasia (e.g., Berg, 2006; Caramazza, Berndt, & Basili, 1983; Dell, Schwartz, Martin, Saffran, & Gagnon, 1997). Persons with fluent aphasia (conduction and Wernicke's) produce phonological paraphasias characterized by more errors on consonants and consonant clusters in word-final and word-medial positions than in the word-initial position, increased errors for targets with greater articulatory complexity (e.g., fewer vowel errors and more consonant cluster errors), large numbers of substitution errors (along with addition and deletion errors), and errors that result in increased articulatory complexity compared with the target (e.g., [strɪs] for /ʃɪp/; Burns & Canter, 1977; Canter, Trost, & Burns, 1985; Caramazza et al., 1983; Gagnon, Schwartz, Martin, Dell & Saffran, 1997; Schwartz, Wilshire, Gagnon, & Polansky, 2004). When substitution errors occur on consonants in this group, primarily single-feature change errors are observed (e.g., either voice, place, or manner), though two- and three-feature changes may be present (Burns & Canter, 1977; Canter et al., 1985). Few phonological paraphasias occur on high-frequency (e.g., closed-class) words (Caramazza et al., 1983; Ellis, Miller, & Sin, 1983; Kay & Ellis, 1987; Schwartz et al., 2004), although most studies have examined picture naming, which excludes closed-class words. Gagnon et al. (1997) reported that phonological paraphasias resulting in productions of different real words occurred above the chance level, showed a frequency effect, and were more likely to be nouns than other word classes.
Phonological (caused by phonological impairment) and/or phonetic (caused by motor speech impairment) errors are present in PPA, in both naming and connected speech (Ash et al., 2013; Croot et al., 2012; Gorno-Tempini et al., 2011; Gunawardena et al., 2010; Leyton et al., 2014; Patterson & MacDonald, 2006; Petroi, Duffy, Strand & Josephs, 2014; Wilson et al., 2010). Two studies have reported rates of phonological paraphasia production for all three variants. In one study, phonemic errors (defined as insertion, deletion, or substitution of phonemes) per 100 words were counted during connected speech, and individuals diagnosed with SvPPA produced 3.3 phonemic errors per 100 words, individuals with LvPPA produced 1.6 per 100 words, and individuals with NFvPPA produced 7.3 per 100 words (Ash et al., 2013). In contrast, another study reported 0.2, 0.7, and 1.4 phonological paraphasias per 100 words for individuals with SvPPA, LvPPA, and NFvPPA, respectively (also in a connected speech task; Wilson et al., 2010). Given these contradictory results, it is difficult to draw conclusions about the rate of occurrence or characteristics of phonological paraphasias in the three variants.
Little attention has been paid to the linguistic environment in which phonological paraphasias are observed in PPA or to linguistic factors that may promote phonological paraphasias. Two studies have examined the impact of elicitation task on the frequency of paraphasia production. One examined multisyllabic word repetition and speech samples derived from clinical interviews in individuals with NFvPPA and LvPPA (Croot et al., 2012). Another examined nine tasks, including reading (word and nonword), repetition (monosyllabic and multisyllabic), verbal fluency (animal, action, and letter), naming, and picture description in individuals with LvPPA (Petroi et al., 2014). These studies found that reading, repetition, and naming tasks elicited higher proportions of phonological paraphasias than connected speech tasks. Petroi et al. (2014) also reported that for persons with LvPPA, substitutions, omissions, additions, and sequencing errors (from most to least common) were more likely to appear on multisyllabic words than on monosyllabic words. This type of analysis has not been conducted for SvPPA and NFvPPA.
Information regarding phonological and phonetic errors across the three variants may play an important role in phenotyping PPA. The presence of phonetic errors is a diagnostic feature of NFvPPA; the presence of phonological errors, although not required, is considered supportive of the diagnosis of LvPPA, and spared speech production (neither phonetic nor phonological errors) is diagnostic of SvPPA. However, phonological paraphasias have been documented in individuals with NFvPPA, presenting a diagnostic conundrum. The “inconsistent speech sound errors” in individuals with NFvPPA, as noted in Gorno-Tempini et al. (2011, p. 1009), may derive from either phonological or motoric mechanisms. Detailed information regarding phonological paraphasias may lead to identification of more specific diagnostic features for PPA variants, especially for NFvPPA and LvPPA. A better understanding of these errors may offer speech-language pathologists and neurologists insights into the specific language breakdowns experienced by individuals with different variants, which could further assist in selecting or designing interventions to provide individualized care for persons with PPA.
We sought to characterize phonological paraphasias in individuals with PPA and to describe production profiles for each variant during a picture description task. Although picture description may not elicit as many phonological paraphasias as other tasks (Croot et al., 2012; Petroi et al., 2014), it was selected because it is an ecologically valid task that allows for production of words across classes. Understanding how phonological paraphasias may affect everyday communication is important for treatment planning and family education. Phonological paraphasias were identified and coded for selected variables based on a study conducted by Burns and Canter (1977), who investigated phonological paraphasias in poststroke aphasia.
Method
Participants
A convenience sample of de-identified audio recordings of 26 individuals with PPA were provided by Authors 3 (n = 5) and 4 (n = 21). These recordings were collected as part of unrelated grants awarded to those authors. Participants were asked to retell the Cinderella story or complete a sequential picture description of the Cinderella story. Transcripts included nine individuals with NFvPPA, nine with LvPPA, and eight with SvPPA (see Tables 1 and 2). Participants included 16 women and 10 men, ages 57–87 (M = 71.3, SD = 6.5). Symptom onset ranged from 12 to 120 months (M = 43.8, SD = 24.1). All individuals were diagnosed with PPA following a comprehensive evaluation using consensus criteria (Gorno-Tempini et al., 2011).
Table 1.
Demographic information.
| Participant | Gender | Age (years) | Education (years) | Race | TPO (months) | Variant | Paraphasia present |
|---|---|---|---|---|---|---|---|
| Lv1 | Woman | 69 | 18 | AA | 36 | Logopenic | Yes |
| Lv2 | Woman | 70 | 18 | W/EA | 66 | Logopenic | Yes |
| Lv3 | Woman | 72 | 14 | W/EA | 48 | Logopenic | No |
| Lv4 | Woman | 80 | 16 | W/EA | 18 | Logopenic | No |
| Lv5 | Woman | 72 | 20 | W/EA | 36 | Logopenic | Yes |
| LV6 | Woman | 75 | 18 | W/EA | 36 | Logopenic | No |
| Lv7 | Man | 58 | 18 | W/EA | 12 | Logopenic | Yes |
| Lv8 | Man | 76 | 20 | W/EA | 38 | Logopenic | Yes |
| Lv9 | Woman | 80 | 12 | W/EA | 24 | Logopenic | No |
| NFv1 | Man | 68 | 19 | W/EA | 36 | Nonfluent | No |
| NFv2 | Woman | 76 | 18 | W/EA | 48 | Nonfluent | Yes |
| NFv3 | Woman | 71 | 16 | AA | 68 | Nonfluent | Yes |
| NFv4 | Man | 87 | 12 | AA | 12 | Nonfluent | Yes |
| NFv5 | Man | 68 | 18 | W/EA | 24 | Nonfluent | Yes |
| NFv6 | Man | 71 | 14 | W/EA | 48 | Nonfluent | Yes |
| NFv7 | Man | 57 | 18 | W/EA | 48 | Nonfluent | Yes |
| NFv8 | Woman | 72 | 16 | W/EA | 60 | Nonfluent | Yes |
| NFv9 | Woman | 71 | 14 | W/EA | 12 | Nonfluent | Yes |
| Sv1 | Woman | 66 | 16 | W/EA | 70 | Semantic | Yes |
| Sv2 | Woman | 76 | 12 | W/EA | 48 | Semantic | Yes |
| Sv3 | Woman | 78 | 16 | W/EA | 120 | Semantic | No |
| Sv4 | Man | 74 | 20 | W/EA | 42 | Semantic | No |
| Sv5 | Woman | 67 | 13 | W/EA | 48 | Semantic | Yes |
| Sv6 | Man | 68 | 16 | W/EA | 45 | Semantic | No |
| Sv7 | Man | 64 | 18 | W/EA | 18 | Semantic | Yes |
| Sv8 | Woman | 68 | 12 | W/EA | 78 | Semantic | Yes |
| Mean | 71.3 | 16.2 | 43.8 | ||||
| SD | 6.5 | 2.6 | 24.1 | ||||
| Range | 57–87 | 12–20 | 12–120 | ||||
Note. Participants in bold produced paraphasias. TPO = time post onset; AA = African American; W/EA = White/European American.
Table 2.
Demographic information for participants who produced paraphasias.
| Demographic variables | All (N = 18) | Logopenic (n = 5) | Nonfluent (n = 8) | Semantic (n = 5) | |
|---|---|---|---|---|---|
| Gender | 11 Women | 3 Women | 4 Women | 4 Women | |
| 7 Men | 2 Men | 4 Men | 1 Man | ||
| Age (years) | Mean (SD) | 69.9 (6.8) | 69.0 (6.7) | 71.6 (8.3) | 68.2 (4.6) |
| Median | 70.5 | 70 | 71 | 67 | |
| Range | 57–87 | 58–76 | 57–87 | 64–76 | |
| Education (years) | Mean (SD) | 16.2 (2.7) | 18.8 (1.1) | 15.8 (2.3) | 14.2 (2.7) |
| Median | 17 | 18 | 16 | 13 | |
| Range | 12–20 | 18–20 | 12–18 | 12–18 | |
| Race | 15 W/EA | 4 W/EA | 6 W/EA | 5 W/EA | |
| 3 AA | 1 AA | 2 AA | |||
| TPO (months) | Mean (SD) | 42.7 (21.0) | 37.6 (19.2) | 40.0 (21.4) | 52.4 (23.4) |
| Median | 48 | 36 | 48 | 48.0 | |
| Range | 12–78 | 12–66 | 12–68 | 18–78 |
Note. TPO = time post onset; AA = African American; W/EA = White/European American.
Identifying Paraphasias
Two certified speech-language pathologists (Authors 1 and 2) transcribed the audio recordings, identified phonological paraphasias, and recorded the participant's error and the target word. Phonological paraphasias were identified using the criteria established for the Philadelphia Naming Test (PNT; Moss Rehabilitation Research Institute, 2013). To be coded as a phonological paraphasia, the actual production must share one or more phonemes with the target word—referred to as the phonological similarity rule. This rule is met if the first or last phonemes or the stressed vowel are the same, if two or more phonemes in any position of the words are the same, or if one phoneme in the same syllable and word position is the same. After phonological similarity is determined, the paraphasia is classified as follows (for all examples, [] indicates the actual production and // the target production): (a) nonword ([tɪd]–/kɪd/); (b) formal, where the production is an unrelated real word ([laɪf]–/naɪf/); and (c) mixed, where the production is semantically and phonologically related to the target ([kæt]–/kaʊ/; Schwartz et al., 2004).
In this study, we use the term phonological paraphasia whenever an error met the above criteria, even when it could have been to be due to a motor programming deficit, because these perceptual judgments do not reliably lend themselves to elucidating underlying mechanisms (Haley, Jacks, Richardson, & Wambaugh, 2017). Distortions were not considered phonological paraphasias unless they crossed phoneme boundaries and the resulting production met the criteria for phonological similarity. This is consistent with recent research that has shown that there is a high degree of overlap between persons with poststroke aphasia and AOS in production of distorted errors that crossed phoneme boundaries, such that these types of errors cannot reliably indicate group membership (Haley et al., 2017).
Only those errors for which the target word was clear (based on context, self-corrections, or preserved word form) were included because targets were not prespecified. Often, a combination of context, self-correction, and word form was used to ensure that the target word was known. When an error affected an initial or final consonant cluster, it counted as an error in that position, regardless of which member was affected (e.g., [slɛpsɪstɚ] for /stɛpsɪstɚ/), consistent with PNT rules. Self-interrupted productions (i.e., false starts such as glo– glo– globe) were not considered, consistent with PNT coding rules, where only complete productions are scored. Because the PNT contains only object pictures, there are no rules for errors in verb inflection. We scored self-corrected verb inflections as paraphasias (e.g., if the participant produced had and then self-corrected to have) if they met the phonological similarity rule (see Kellogg, 1994; Stark & Dressler, 1990).
Coding Variables
Phonological paraphasias were coded for variables used in a study conducted by Burns and Canter (1977): (a) paraphasia type: nonword, formal, or mixed; (b) part of speech of the target; (c) target word frequency in words per million, taken from spoken word frequency in the Corpus of Contemporary American English database (Davies, 2008); (d) type of segment(s) in error: vowel, consonant singleton, or consonant cluster; (e) type of error: addition (sound or syllable), deletion (sound or syllable), substitution, or sequencing; (f) word position of substituted consonants; (g) degree of change (consonants only): measured from one to three using the phonological features voice, place, and manner of articulation; and (h) number of errors for which a target could not be determined or that did not meet the phonological similarity rule. The frequency of paraphasia production (e.g., paraphasias per 100 words) was calculated to aid in comparison with prior research on phonological paraphasias in PPA.
To improve coding reliability, several scoring rules were established. Schwar (ɚ) was treated as a single phoneme and classified as a vowel, consistent with its description as a rhotic vowel. The errors he for she and prince for princess were considered semantic paraphasias and therefore not examined for this paper. Distortions in the absence of a co-occurring addition or deletion error were not counted as phonological paraphasias unless they crossed phoneme boundaries (resulting in a distorted substitution error).
Data Analysis and Reliability
Due to the exploratory nature of the analysis (and the limited sample size), quantitative statistical analyses were not utilized. Instead, descriptive statistics (for target frequency) and frequency counts (for all other variables) were used to build a profile of errors for each variant. To ensure reliability of paraphasia identification and coding, Authors 1 and 2 reviewed all audio files and jointly identified paraphasias. Forced choice agreement was utilized to resolve disagreements whenever paraphasia identification or coding differed, resulting in 100% agreement.
Results
All Variants
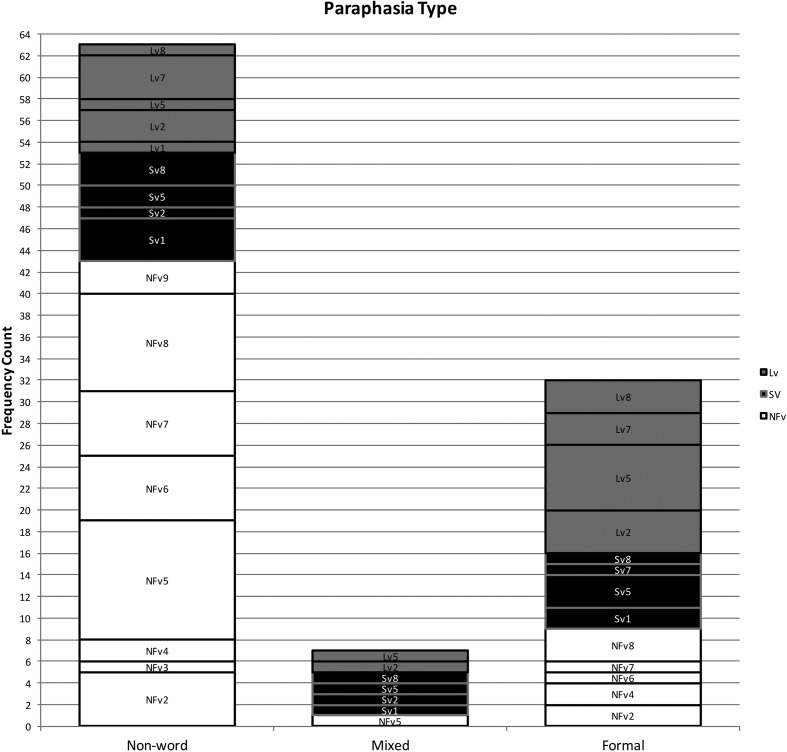
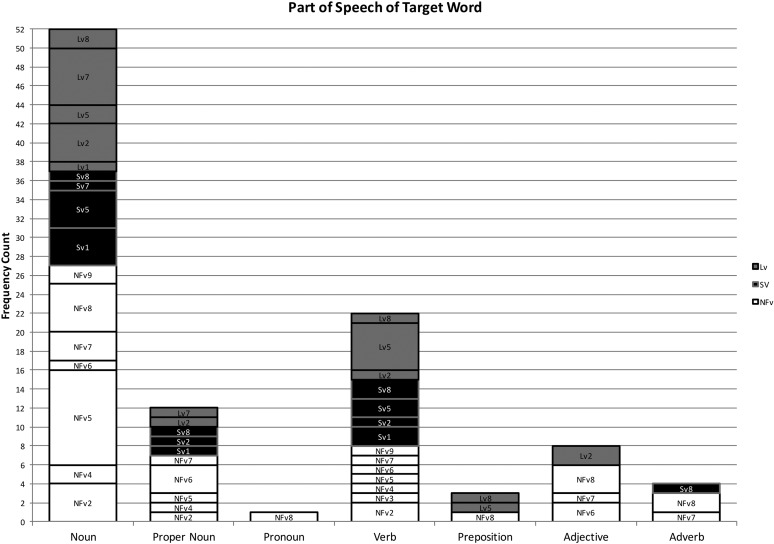
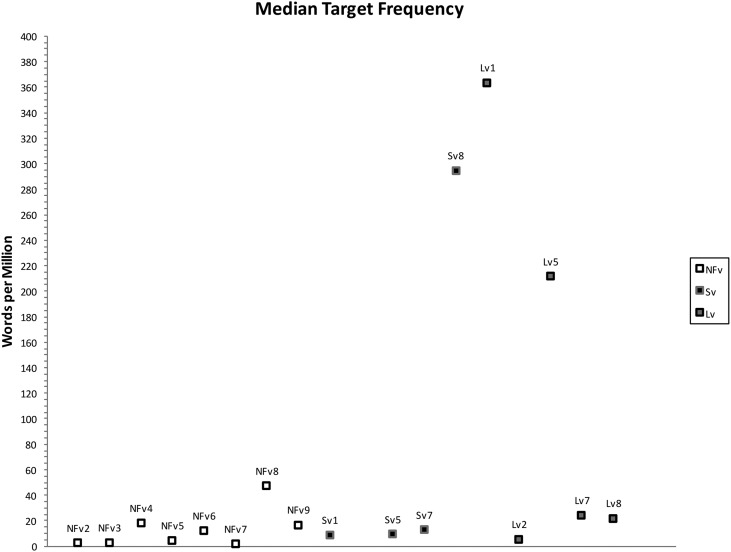
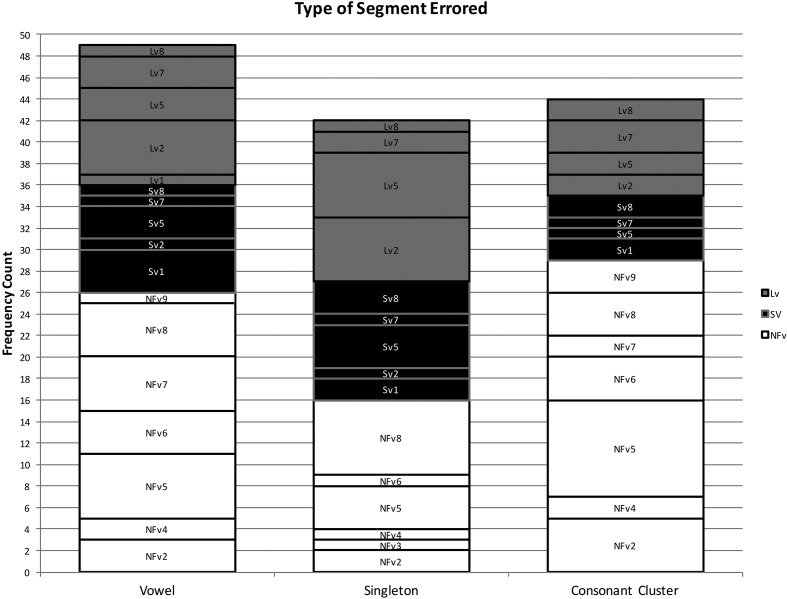
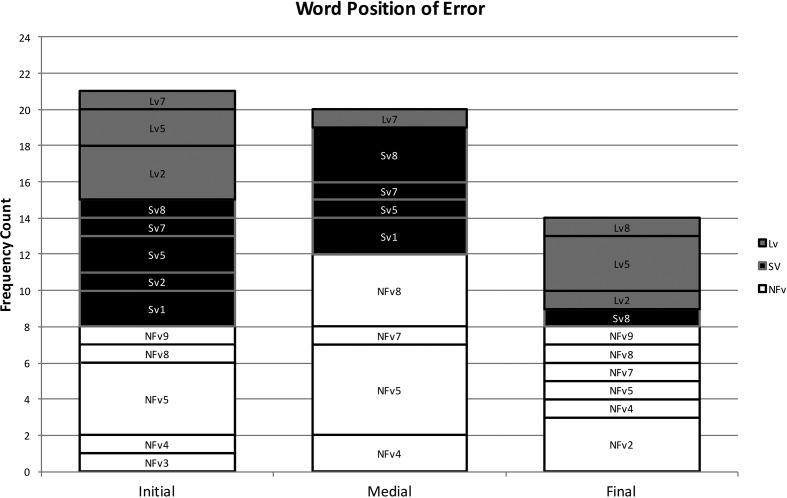
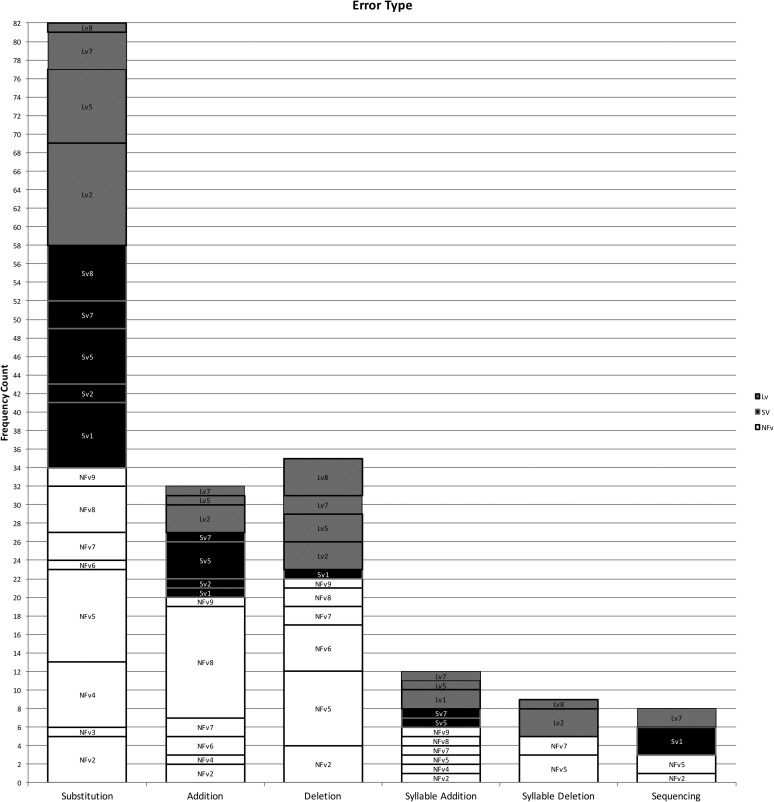
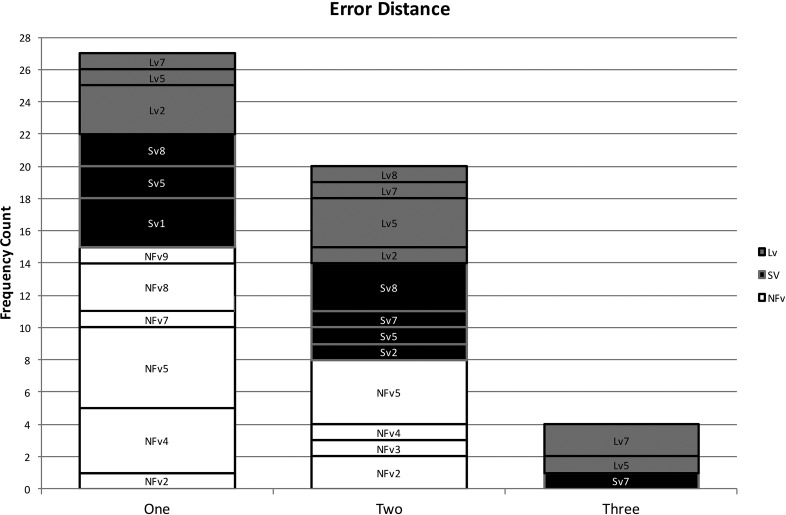
Using the coding system described above, 102 phonological paraphasias were produced by 18 individuals with PPA out of 4,561 words produced (see Supplemental Material S1). There were seven mixed, 32 formal, and 63 nonword paraphasias (see Figure 1). Over half of the paraphasias occurred on nouns (52 regular nouns, 12 proper nouns [Cinderella]), 22 occurred on verbs, eight on adjectives, four on adverbs, three on prepositions, and one on a pronoun (see Figure 2). Paraphasias occurred across a range of target word frequencies (0.03–7,438.32 words per million; median = 16.56; see Figure 3 and Supplemental Materials S2–S4) 1 and segments: 49 vowels, 42 singleton consonants, and 44 consonant clusters were produced in error (see Figure 4). Errored segments (consonant substitutions) were word-initial 21 times, word-medial 17 times, and word-final 10 times (see Figure 5). Types of errors included 44 addition errors (32 sound, 12 syllable), 44 deletion errors (35 sound, nine syllable), 82 substitution errors, and eight sequencing errors (see Figure 6). For errored segments and error types, the sum is greater than the total number of paraphasias because more than one segment in a word could be produced in error. Regarding substitution consonant errors, 21 involved a single-feature change (i.e., voice, place, or manner), 17 involved a two-feature change, and three involved a three-feature change (see Figure 7).
Figure 1.
Frequency count of each type of paraphasia produced. NFv = nonfluent agrammatic variant; Lv = logopenic variant; Sv = semantic variant.
Figure 2.
Frequency count of the part of speech of the target word. NFv = nonfluent agrammatic variant; Lv = logopenic variant; Sv = semantic variant.
Figure 3.
Median target word frequency by participants. NFv = nonfluent agrammatic variant; Lv = logopenic variant; Sv = semantic variant.
Figure 4.
Frequency count of the type of segment produced in error. NFv = nonfluent agrammatic variant; Lv = logopenic variant; Sv = semantic variant.
Figure 5.
Frequency count of the word position of errored consonants. NFv = nonfluent agrammatic variant; Lv = logopenic variant; Sv = semantic variant.
Figure 6.
Frequency count of the type of error. NFv = nonfluent agrammatic variant; Lv = logopenic variant; Sv = semantic variant.
Figure 7.
Frequency count of the degree of change of errored consonants. NFv = nonfluent agrammatic variant; Lv = logopenic variant; Sv = semantic variant.
Five (62.5%) of eight individuals with SvPPA, five (55.6%) of nine individuals with LvPPA, and eight (88.9%) of nine individuals with NFvPPA produced phonological paraphasias according to the methods described above (see Supplemental Material S5). Whereas all individuals with NFvPPA exhibited motor speech deficits, individuals with LvPPA and SvPPA did not. Five of the eight participants with NFvPPA who produced phonological paraphasias exhibited both dysarthria and AOS, whereas three exhibited only AOS. According to the consensus criteria in Gorno-Tempini et al. (2011), individuals with NFvPPA may exhibit a primary deficit in either AOS or agrammatism, or they may have deficits in both. In this sample, all demonstrated both motor speech and agrammatic deficits except participant NFv7, who demonstrated primarily a motor speech deficit (AOS and dysarthria). To better characterize the sample of participants who contributed phonological paraphasias to the analysis, several additional variables are reported in Supplemental Material S5, including the length of the sample (in seconds), number of words, number of paraphasias, words per minute, and proportion of paraphasias to total words produced.
SvPPA
Individuals with SvPPA produced 21 phonological paraphasias (1.45 paraphasias per 100 words), including four mixed, seven formal, and 10 nonword. Ten nouns, three proper nouns, seven verbs, and one adverb were produced in error and ranged in frequency from 1.98 to 7,438.32 words per million (M = 684.22, median = 43.66). Errors involved 10 vowels, 11 singleton consonants, and six consonant clusters. Substituted consonants were word-initial seven times and word-medial six times. Error types included nine addition (seven sound, two syllable), one sound deletion, 24 substitution, and three sequencing errors. Regarding substitution consonant errors, six involved a single-feature change (i.e., voice, place, or manner), five involved a two-feature change, and one involved a three-feature change.
LvPPA
Individuals with LvPPA produced 28 phonological paraphasias (2.14 paraphasias per 100 words), including two mixed, 16 formal, and 10 nonword. Fifteen nouns, two proper nouns, seven verbs, two adjectives, and two prepositions were produced in error and ranged in frequency from 1.15 to 4,252.59 words per million (M = 441.17, median = 27.13). Errors involved 13 vowels, 15 singleton consonants, and nine consonant clusters. Substituted consonants were word-initial six times, word-medial one time, and word-final five times. Error types included nine addition (five sound, four syllable), 16 deletion (12 sound, four syllable), 24 substitution, and two sequencing errors. Of the substitution consonant errors, five involved a single-feature change (i.e., voice, place, or manner), five involved a two-feature change, and one involved a three-feature change.
NFvPPA
Individuals with NFvPPA produced 53 phonological paraphasias (2.93 paraphasias per 100 words), including one mixed, nine formal, and 43 nonword. Twenty-seven nouns, seven proper nouns, one pronoun, eight verbs, six adjectives, three adverbs, and one preposition were produced in error and ranged in frequency from 0.03 to 4,367.07 words per million (M = 261.3, median = 11.71). Errors involved 26 vowels, 16 singleton consonants, and 29 consonant clusters. Substituted consonants were word-initial eight times, word-medial 10 times, and word-final five times. Error types included 26 addition (20 sound, six syllable), 27 deletion (22 sound, five syllable), 34 substitution, and three sequencing errors. Regarding the substitution consonant errors, 10 involved a single-feature change (i.e., voice, place, or manner), seven involved a two-feature change, and none involved a three-feature change.
Paraphasia Profiles by Variant
SvPPA
Individuals with SvPPA who produced phonological paraphasias did so on relatively high frequency words (e.g., people) compared with the other variants, although this could be an effect of higher word frequency use throughout the sample. Errored target words were mostly content words (e.g., nouns and verbs), with only a single paraphasia on a function word (away). Not surprisingly, individuals with SvPPA (who have impaired conceptual knowledge) produced a greater proportion of mixed paraphasias than the other groups (e.g., [mʌŋkis] for /maɪs/); however, as a group, they produced more formal and nonword paraphasias than mixed. This effect has also been observed in an immediate serial word recall task, suggesting that phonological forms may deteriorate in the absence or degradation of semantic information (e.g., Patterson, Graham, & Hodges, 1994). The distribution of error types was weighted primarily toward substitution errors, with a large number of addition errors and relatively few deletion or sequencing errors. Errors were evenly distributed across vowels, consonant singletons, and clusters, but consonant substitution errors occurred only in initial and medial positions.
LvPPA
The word frequency of targets produced in error and the rate of paraphasia production by individuals with LvPPA were midway between the other variants. Target words were distributed across both content and function words, with more errors on content words. Individuals with LvPPA produced the greatest proportion of formal paraphasias and the lowest proportion of nonword paraphasias of the three groups, with a low proportion of mixed paraphasias (although greater than those with NFvPPA). Participants with LvPPA also produced primarily substitution errors but had more deletion than addition errors. These were relatively evenly distributed across vowels, consonant singletons, and clusters, but consonant substitution errors occurred primarily in initial and final word positions, with only a single medial error.
NFvPPA
Errors by participants with NFvPPA occurred on the lowest frequency target words, although again, this could be an effect of a sample with overall lower frequency words. Although most errors occurred on content words, this group produced a greater proportion of paraphasias on function words (e.g., at or as) than the other two groups. They also produced a greater proportion of nonword paraphasias than the other two variants and comparatively few formal and mixed paraphasias. By frequency count, most errors were substitutions, but this group also produced proportionately more addition and deletion errors (and proportionately fewer substitution errors) than those with LvPPA or SvPPA. Errors were relatively evenly distributed across vowels, consonant singletons, and clusters, although more errors involved vowels and clusters than singletons. Consonant substitution errors were relatively evenly distributed across word position, with more initial and medial errors than final. This group was the only group that did not produce consonant substitution errors where all three features changed.
Discussion
Previous research has demonstrated that individuals with PPA produce phonological paraphasias, that paraphasia rates differ according to variant (Ash et al., 2013; Wilson et al., 2010), and that the elicitation task affects the frequency of these errors (Croot et al., 2012; Petroi et al., 2014). We extend this work with an in-depth description of the linguistic characteristics of these errors and provide details about how these characteristics differed for each variant, beyond frequency of words produced in error. We found that all three subtypes produced phonological paraphasias, but a greater number of individuals with NFvPPA produced phonological paraphasias, and more frequently, than individuals with LvPPA or SvPPA.
We replicated findings on phonological paraphasias in individuals with fluent poststroke aphasia and LvPPA that indicated substitution errors are the most common error type (Ardila & Rosselli, 1993; Burns & Canter, 1977; Petroi et al., 2014). Research has also demonstrated that errors tend to occur more frequently on word-final and word-medial consonants and clusters than on word-initial clusters (Burns & Canter, 1977; Canter et al., 1985; Caramazza et al., 1983; Gagnon et al., 1997; Schwartz et al., 2004). Interestingly, our results show that individuals with SvPPA produced consonant substitution errors on word-initial and word-medial consonants, and no substitution errors on word-final consonants, whereas individuals with LvPPA and NFvPPA had substitution errors in all three word positions (although with different ratios). These results, replicated in a larger sample of participants with more extensive speech samples, could provide useful diagnostic information regarding the three variants, because the ratio of errors in each word position differed across groups. For example, a high ratio of word-initial and word-medial errors to word-final errors could suggest diagnosis of SvPPA, a high ratio of word-initial and word-final errors to word-medial errors could suggest diagnosis of LvPPA, and a relatively even distribution of errors across word position could suggest diagnosis of NFvPPA. Similarly, those with SvPPA only produced a single phonological paraphasia on a closed-class word, whereas those with LvPPA and NFvPPA produced phonological paraphasias on several different types of closed-class words. This could be combined with recent research investigating phonological processing and speech rate (Cordella, Dickerson, Quimby, Yunusova, & Green, 2017; Henry et al., 2016) to yield sensitive, accurate, and easy-to-use diagnostic indicators.
In addition to the word position of consonant substitution errors, several other results contrasted with those reported in the poststroke aphasia literature. Within each PPA variant, errors were relatively evenly distributed across segment types, whereas in poststroke aphasia, errors occur in an increasing gradient from vowels to consonant singletons to clusters (Burns & Canter, 1977; Canter et al., 1985). Also, errors are rarely reported on high-frequency or closed-class words in individuals with poststroke aphasia (Caramazza et al., 1983; Ellis et al., 1983; Kay & Ellis, 1987; Schwartz et al., 2004). Although our participants committed most errors on nouns and verbs, errors were also observed across closed-class words, including pronouns, prepositions, adjectives, and adverbs. Other results do coincide with the poststroke aphasia literature: Each variant produced mostly substitution errors, errors did not necessarily result in decreased phonological complexity, and consonant errors tended to change only a single feature (voice, place, or manner), with fewer instances of two- and three-feature changes. These results suggest that the translation of knowledge of phonological paraphasias in poststroke aphasia to individuals with PPA may not be straightforward. Population-specific investigations are needed to check assumptions regarding characteristic speech features (especially as error production relates to treatment design and goal setting) of individuals with PPA.
Individuals with LvPPA produced few mixed paraphasias (although proportionately more than individuals with NFvPPA) and the greatest proportion of formal paraphasias of the three groups. The high proportion of formal paraphasias indicates that phonological errors were most likely to result in the production of a real English word (with nonword errors produced at a similar proportion to those with SvPPA). It is possible that, in this group, when an error occurs at the level of phonological encoding, the phonological system receives input from the semantic system that biases productions to be real words in the language. Previous research investigating phonological paraphasias in adults with fluent poststroke aphasia found that adults with Wernicke's and conduction aphasia produce formal paraphasias at a higher rate than expected given the probability of an error causing a nonword versus real-word production (Gagnon et al., 1997). These aphasia subtypes are also associated with phonological impairments in the absence of motor speech deficits.
Whereas those with LvPPA produced more formal paraphasias, individuals with NFvPPA produced proportionately more nonword phonological paraphasias, and errors occurred on lower frequency target words than for the other variants. These results are somewhat paradoxical, as more errors on low-frequency words suggest a relatively intact semantic system, whereas primarily nonword paraphasias may indicate less support from the semantic system, as discussed above. However, this discrepancy may be resolved by considering the level at which the error arises. It is commonly acknowledged that LvPPA is characterized by phonological processing and phonological working memory deficits (Gorno-Tempini et al., 2008; Henry et al., 2016; Rohrer et al., 2010) and that NFvPPA is more commonly associated with motor speech deficits (e.g., Gorno-Tempini et al., 2011). In this group, if processing is intact through the level of phonological encoding, and an error occurs during motor planning or production, the semantic system would not be expected to bias productions toward real words. If this explanation is accurate, it may indicate that, even though distortions were not coded for this study, the substitution, addition, and deletion errors identified here are more consistent with a motor speech impairment than a phonological impairment. Further evidence for this account may be that, when consonant substitutions occurred in NFvPPA, changes in all three features never occurred, suggesting that phonological encoding was accurate, and the error arose during motor planning or production.
Prior research has reported varying rates of production of phonological paraphasias across the three variants (Ash et al., 2013; Wilson et al., 2010). Consistent with Wilson et al. (2010), we found that individuals with LvPPA produced phonological paraphasias more frequently than individuals with SvPPA (2.14 versus 1.45 paraphasias per 100 words) and that individuals with NFvPPA produced phonological paraphasias at the highest rate (2.93 per 100 words). However, we also found that all three groups produced higher rates of phonological paraphasias than those reported by Wilson and colleagues. It is important to note that our results examine only the rate of phonological paraphasia production in individuals who produced at least one phonological paraphasia. It is possible that our results would more closely mirror the results of Wilson and colleagues if calculations included all participants examined, rather than just those who produced paraphasias. An additional coding difference likely contributing to magnitude differences in results involves exclusion of distortions by Wilson et al. (2010), whereas we included speech sound errors that were distorted, as long as there was a substitution, addition, deletion, or sequencing error.
Limitations and Future Directions
Although the selection of a discourse task for this study was intentional, as it likely has the most validity for extrapolation to conversational speech, connected speech tasks such as storytelling and conversation have been reported to elicit fewer errors than repetition, naming, and reading tasks (Croot et al., 2012; Petroi et al., 2014). This finding is likely because repetition, naming, and reading tasks all require individuals to produce specific items in order to be judged correct. On the other hand, storytelling and conversation are more flexible, and the same message can be expressed in many different forms, allowing for circumlocution and online revisions. Although the use of discourse is a potential limitation in this study, we would expect the findings reported here (with perhaps the exception of the rate of paraphasia production) to hold true across different elicitation tasks. To confirm the findings reported here, these variables should be examined in the context of other elicitation tasks and with larger participant groups.
An additional limitation of the current study is the difficulty in differentiating between errors that arise from a motor deficit versus a phonological deficit. All but one participant with NFvPPA had motor speech deficits in addition to language difficulties (except NFv7). We excluded errors that consisted of only distortions to control for strictly motoric errors. However, it is not straightforward to determine whether nondistorted errors or distorted sound substitutions, insertions, deletions, and sequencing errors produced by individuals with NFvPPA were motoric, phonologic, or both. Additional research to determine whether errors produced by individuals with NFvPPA are better classified as motoric or phonological is warranted, but it is likely that the classification will neither be straightforward nor consistent across individuals. Evidence from stroke-induced conduction aphasia and AOS indicates that speech errors (distortions and word syllable duration) have a continuous distribution rather than the bimodal distribution that would be expected if errors arose from two completely distinct systems (Haley et al., 2017).
Given the progressive nature of this disorder, it is important to consider that the results we describe may be affected by participants' symptom duration. In this study, there does not appear to be a difference in average symptom duration between those who produced phonological paraphasias and those who did not (within each variant), suggesting that symptom duration alone cannot predict paraphasia production. However, the average symptom duration for individuals with semantic variant in this study was 12–15 months longer than for the other variants. Moving forward, care should be taken to ensure that comparative studies match participant subgroups as closely as possible for symptom duration.
Conclusions
In this preliminary study, we sought to extend our knowledge of phonological paraphasias in PPA by providing an in-depth evaluation of the linguistic environment in which they occur. We excluded semantic errors and errors that were clearly attributable to motor speech impairment (e.g., distortions in the absence of phoneme substitutions, insertions, or deletions), although these should be investigated more fully as well. The qualitative differences between groups that we report should be investigated to determine whether significant quantitative differences are observed between groups. This same level of scrutiny should also be applied to other segment and word-level errors (e.g., semantic paraphasias). This information, combined with information from previous research regarding elicitation task (Croot et al., 2012; Petroi et al., 2014) and concomitant AOS (Croot et al., 2012), may inform diagnostic procedures. Specifically, researchers and clinicians could then determine what motoric and linguistic features to consider when diagnosing PPA, and could potentially increase sensitivity for accurate phenotyping and early detection. Finally, it has been observed that surface dyslexia is often present in individuals with SvPPA, indicating a potential link between semantic degradation and phonological activation (Gorno-Tempini et al., 2004; Jefferies, Lambon Ralph, Jones, Bateman, & Patterson, 2004; Woollams, Ralph, Plaut, & Patterson, 2007). Investigating this relationship by examining the varying degrees of orthographic regularity of words that are produced as semantic and phonological paraphasias may provide additional insight into the types of paraphasias produced by individuals with SvPPA.
We report a production rate for phonological paraphasias by individuals with SvPPA that, although lower than the rates for those with LvPPA and NFvPPA, is higher than previously reported (e.g., Patterson & MacDonald, 2006; Wilson et al., 2010), indicating that the presence or absence of phonological paraphasias may not be sufficient for dissociating variant profiles or for the formulation of diagnostic criteria, as has been the norm (e.g., Botha et al., 2015; Gorno-Tempini et al., 2011; Gorno-Tempini & Pressman, 2016; Jung et al., 2013; but not Petroi et al., 2014). It is likely that detailed information regarding frequency and location of paraphasias (as captured here), in addition to information about the proportion of these errors relative to other error types (i.e., semantic and motor speech errors), would provide greater insight into the speech–language characteristics of the variants and improve diagnostic precision. This type of information would complement recent findings on phonological processing and speech rate (Cordella et al., 2017; Henry et al., 2016), which revealed differences among the three variants.
More nuanced information about phonological paraphasias between variants may be useful for early detection and appropriate assignment to a variant and may help clinicians to identify variant type for those whose disease state (and thus behavioral deficits) is advanced. These individuals are often difficult to assign to a variant and may be labeled as PPA “not specified.” However, even in later stages, diagnostic accuracy is important in considering treatment approaches and prognosis. Finally, almost all diagnostic criteria acknowledge that not all cases fit neatly into SvPPA, LvPPA, or NFvPPA bins, and other research groups have put forth criteria for additional variants, including primary progressive AOS, progressive fluent aphasia, and progressive agrammatic aphasia (Botha et al., 2015). More detailed information about paraphasias may assist with the identification of additional variants or subvariants of PPA.
Although strong conclusions cannot be made on the basis of this study alone, we have attempted to shed light on previously unexamined variables. This study suggests several fruitful avenues for continued research on phonological paraphasias in PPA. It is our hope that future studies, guided by the findings reported here, might address the limitations of this work and allow for more concrete conclusions to be drawn regarding the nature of phonological paraphasias across the three variants. Finally, extension of this work to investigate semantic paraphasias and motor errors would be helpful in deepening our understanding of speech–language errors in PPA.
Supplementary Material
Acknowledgments
The data collection for this research was supported in part by National Institute on Deafness and Other Communication Disorders Grants R01 DC011317, awarded to Argye E. Hillis, and R03 DC013403, awarded to Maya L. Henry. We are also grateful for access to data made available through National Institute on Deafness and Other Communication Disorders Grant K24 DC015544 and National Institute of Neurological Disorders and Stroke Grant R01 NS050915, both awarded to Maria Luisa Gorno-Tempini, and National Institute on Aging Grant P01 AG019724, awarded to Bruce Miller, at the University of California, San Francisco. Finally, we express gratitude to the individuals with PPA who are willing to share with us their stories.
Funding Statement
The data collection for this research was supported in part by National Institute on Deafness and Other Communication Disorders Grants R01 DC011317, awarded to Argye E. Hillis, and R03 DC013403, awarded to Maya L. Henry. We are also grateful for access to data made available through National Institute on Deafness and Other Communication Disorders Grant K24 DC015544 and National Institute of Neurological Disorders and Stroke Grant R01 NS050915, both awarded to Maria Luisa Gorno-Tempini, and National Institute on Aging Grant P01 AG019724, awarded to Bruce Miller, at the University of California, San Francisco.
Footnote
One outlier (Sv2) was removed from Figure 3 to better represent the data. Please see Supplemental Materials S2–S4 for the figures with all data points.
References
- Ardila A., & Rosselli M. (1993). Language deviations in aphasia: A frequency analysis. Brain and Language, 44(2), 165–180. [DOI] [PubMed] [Google Scholar]
- Ash S., Evans E., O'Shea J., Powers J., Boller A., Weinberg D., … Grossman M. (2013). Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology, 81(4), 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T. (2006). A structural account of phonological paraphasias. Brain and Language, 96(3), 331–356. [DOI] [PubMed] [Google Scholar]
- Bird H., Ralph M. A. L., Patterson K., & Hodges J. R. (2000). The rise and fall of frequency and imageability: Noun and verb production in semantic dementia. Brain and Language, 73(1), 17–49. [DOI] [PubMed] [Google Scholar]
- Botha H., Duffy J. R., Whitwell J. L., Strand E. A., Machulda M. M., Schwarz C. G., … Josephs K. A. (2015). Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex, 69, 220–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns M. S., & Canter G. J. (1977). Phonemic behavior of aphasic patients with posterior cerebral lesions. Brain and Language, 4(4), 492–507. [DOI] [PubMed] [Google Scholar]
- Canter G. J., Trost J. E., & Burns M. S. (1985). Contrasting speech patterns in apraxia of speech and phonemic paraphasia. Brain and Language, 24(2), 204–222. [DOI] [PubMed] [Google Scholar]
- Caramazza A., Berndt R. S., & Basili A. G. (1983). The selective impairment of phonological processing: A case study. Brain and Language, 18(1), 128–174. [DOI] [PubMed] [Google Scholar]
- Cordella C., Dickerson B. C., Quimby M., Yunusova Y., & Green J. R. (2017). Slowed articulation rate is a sensitive diagnostic marker for identifying non-fluent primary progressive aphasia. Aphasiology, 2, 241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croot K., Ballard K., Leyton C. E., & Hodges J. R. (2012). Apraxia of speech and phonological errors in the diagnosis of nonfluent/agrammatic and logopenic variants of primary progressive aphasia. Journal of Speech, Language, and Hearing Research, 55(5), S1562–S1572. [DOI] [PubMed] [Google Scholar]
- Davies M. (2008). The corpus of contemporary American English: 520 million words, 1990–present. Retrieved from http://corpus.byu.edu/coca/
- Dell G. S., Schwartz M. F., Martin N., Saffran E. M., & Gagnon D. A. (1997). Lexical access in aphasic and nonaphasic speakers. Psychological Review, 104(4), 801– 838. [DOI] [PubMed] [Google Scholar]
- Ellis A. W., Miller D., & Sin G. (1983). Wernicke's aphasia and normal language processing: A case study in cognitive neuropsychology. Cognition, 15(1), 111–144. [DOI] [PubMed] [Google Scholar]
- Gagnon D. A., Schwartz M. F., Martin N., Dell G. S., & Saffran E. M. (1997). The origins of formal paraphasias in aphasics' picture naming. Brain and Language, 59(3), 450–472. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Brambati S. M., Ginex V., Ogar J., Dronkers N. F., Marcone A., … Miller B. L. (2008). The logopenic/phonological variant of primary progressive aphasia. Neurology, 71(16), 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Dronkers N. F., Rankin K. P., Ogar J. M., Phengrasamy L., Rosen H. J., … Miller B. L. (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. E. E. A., … Manes F. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M. L., & Pressman P. (2016). Introduction to primary progressive aphasia. In Hickock G., & Small S. (Eds.), Neurobiology of language (pp. 935–952). Cambridge, MA: Academic Press. [Google Scholar]
- Gunawardena D., Ash S., McMillan C., Avants B., Gee J., & Grossman M. (2010). Why are patients with progressive nonfluent aphasia nonfluent? Neurology, 75(7), 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley K., Jacks A., Richardson J., & Wambaugh J. (2017). Perceptually salient sound distortions and apraxia of speech: A performance continuum. American Journal of Speech-Language Pathology, 31, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. L., & Gorno-Tempini M. L. (2010). The logopenic variant of primary progressive aphasia. Current Opinion in Neurology, 23(6), 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. L., Wilson S. M., Babiak M. C., Mandelli M. L., Beeson P. M., Miller Z. A., & Gorno-Tempini M. L. (2016). Phonological processing in primary progressive aphasia. Journal of Cognitive Neuroscience, 28(2), 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J. R., & Patterson K. (1996). Nonfluent progressive aphasia and semantic dementia: A comparative neuropsychological study. Journal of the International Neuropsychological Society, 2(6), 511–524. [DOI] [PubMed] [Google Scholar]
- Jefferies E., Lambon Ralph M. A., Jones R., Bateman D., & Patterson K. (2004). Surface dyslexia in semantic dementia: A comparison of the influence of consistency and regularity. Neurocase, 10(4), 290–299. [DOI] [PubMed] [Google Scholar]
- Josephs K. A., Duffy J. R., Strand E. A., Machulda M. M., Senjem M. L., Master A. V., … Whitwell J. L. (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135(Pt. 5), 1522–1536. https://doi.org/10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Duffy J. R., & Josephs K. A. (2013). Primary progressive aphasia and apraxia of speech. Seminars in Neurology, 33, 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J., & Ellis A. (1987). A cognitive neuropsychological case study of anomia: Implications for psychological models of word retrieval. Brain, 110(3), 613–629. [DOI] [PubMed] [Google Scholar]
- Kellogg M. K. (1994). Conceptual mechanisms underlying noun and verb categorization: Evidence from paraphasia. Proceedings of the Annual Meeting of the Berkeley Linguistics Society, 20(1), 300–309. [Google Scholar]
- Leyton C. E., Ballard K. J., Piguet O., & Hodges J. R. (2014). Phonologic errors as a clinical marker of the logopenic variant of PPA. Neurology, 82(18), 1620–1627. [DOI] [PubMed] [Google Scholar]
- Mesulam M. (1982). Slowly progressive aphasia without generalized dementia. Annals of Neurology, 11(6), 592–598. [DOI] [PubMed] [Google Scholar]
- Mesulam M. (1987). Primary progressive aphasia—Differentiation from Alzheimer's disease. Annals of Neurology, 22(4), 533–534. [DOI] [PubMed] [Google Scholar]
- Mesulam M. (2001). Primary progressive aphasia. Annals of Neurology, 49(4), 425–432. [PubMed] [Google Scholar]
- Moss Rehabilitation Research Institute. (2013). Philadelphia Naming Test (PNT) Scoring Protocol. Retrieved from http://www.mrri.org/philadelphia-naming-test (September 28, 2015).
- Neary D., Snowden J. S., Gustafson L., Passant U., Stuss D. T., Black S. E., … Benson D. F. (1998). Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–1554. [DOI] [PubMed] [Google Scholar]
- Patterson K., Graham N., & Hodges J. R. (1994). The impact of semantic memory loss on phonological representations. Journal of Cognitive Neuroscience, 6(1), 57–69. [DOI] [PubMed] [Google Scholar]
- Patterson K., & MacDonald M. C. (2006). Sweet nothings: Narrative speech in semantic dementia. In Andrews S. (Ed.), From inkmarks to ideas: Current issues in lexical processing (pp. 299–317). Hove, United Kingdom: Psychology Press. [Google Scholar]
- Petroi D., Duffy J. R., Strand E. A., & Josephs K. A. (2014). Phonologic errors in the logopenic variant of primary progressive aphasia. Aphasiology, 28(10), 1223–1243. [Google Scholar]
- Rohrer J. D., Ridgway G. R., Crutch S. J., Hailstone J., Goll J. C., Clarkson M. J., & Ourselin S. (2010). Progressive logopenic/phonological aphasia: Erosion of the language network. NeuroImage, 49(1), 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. F., Wilshire C. E., Gagnon D. A., & Polansky M. (2004). Origins of nonword phonological errors in aphasic picture naming. Cognitive Neuropsychology, 21, 159–186. [DOI] [PubMed] [Google Scholar]
- Sepelyak K., Crinion J., Molitoris J., Epstein-Peterson Z., Bann M., Davis C., … Hillis A. E. (2011). Patterns of breakdown in spelling in primary progressive aphasia. Cortex, 47, 342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark J. A., & Dressler W. U. (1990). Agrammatism in German: Two case studies. In Menn L. & Obler L. (Eds.), Agrammatic aphasia: A cross-language narrative sourcebook. Philadelphia, PA: John Benjamins. [Google Scholar]
- Wilson S. M., Henry M. L., Besbris M., Ogar J. M., Dronkers N. F., Jarrold W., … Gorno-Tempini M. L. (2010). Connected speech production in three variants of primary progressive aphasia. Brain, 133(7), 2069–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollams A. M., Ralph M. A. L., Plaut D. C., & Patterson K. (2007). SD-squared: On the association between semantic dementia and surface dyslexia. Psychological Review, 114(2), 316– 339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.