Abstract
Alzheimer’s disease (AD) is the most common form of dementia, with no means of cure or prevention. The presence of abnormal disease-related proteins in the population is, in turn, much more common than the incidence of dementia. In this context, the cognitive reserve (CR) hypothesis has been proposed to explain the discontinuity between pathophysiological and clinical expression of AD, suggesting that CR mitigates the effects of pathology on clinical expression and cognition. fMRI studies of the human connectome have recently reported that AD patients present diminished functional efficiency in resting-state networks, leading to a loss in information flow and cognitive processing. No study has investigated, however, whether CR modifies the effects of the pathology in functional network efficiency in AD patients. We analyzed the relationship between CR, pathophysiology and network efficiency, and whether CR modifies the relationship between them. Fourteen mild AD, 28 amnestic mild cognitive impairment (aMCI) due to AD, and 28 controls were enrolled. We used education to measure CR, cerebrospinal fluid (CSF) biomarkers to evaluate pathophysiology, and graph metrics to measure network efficiency. We found no relationship between CR and CSF biomarkers; CR was related to higher network efficiency in all groups; and abnormal levels of CSF protein biomarkers were related to more efficient networks in the AD group. Education modified the effects of tau-related pathology in the aMCI and mild AD groups. Although higher CR might not protect individuals from developing AD pathophysiology, AD patients with higher CR are better able to cope with the effects of pathology—presenting more efficient networks despite pathology burden. The present study highlights that interventions focusing on cognitive stimulation might be useful to slow age-related cognitive decline or dementia and lengthen healthy aging.
Keywords: fMRI, graph theory, mild cognitive impairment, neuropathology, educational measurement, network efficiency
Introduction
In 2014, the movie “Still Alice,” interpreted by the actress Julianne Moore, brought to the public the dramatic experience of someone living with Alzheimer’s disease (AD), the most common form of dementia worldwide (and yet with no means of cure or even prevention). More interesting, however, was the fact that the onset of the disease’s clinical expression was delayed by modifiable factors, such as her high intellectual level. The cognitive reserve (CR) hypothesis, in this context, has been proposed to account for the discontinuity between pathophysiological and clinical expression of AD. This hypothesis claims that individuals with higher CR can better maintain cognitive functions despite AD pathology (Stern, 2012), thus consisting in an important factor in the fight against the disease. In fact, innumerable epidemiological studies report that greater CR may postpone the clinical expression of AD (Amieva et al., 2014; Osone et al., 2015), yet accelerate the cognitive decline after its onset (Scarmeas et al., 2006; Bruandet et al., 2008).
Neuroimaging studies (Scarmeas et al., 2003; Perneczky et al., 2006; Serra et al., 2011; Liu et al., 2012; Boots et al., 2015) have consistently shown a positive relationship between brain structure/metabolism/perfusion and CR in the healthy elderly, whereas in AD patients there is an inverse relationship between them. These findings suggest that patients with higher CR are better able to cope with the brain pathology than patients with lower CR, and more pathology is needed in the former group to reach the dementia level (thus mitigating the clinical impact of the disease). Studies involving fMRI analysis (Solé-Padullés et al., 2009; Bosch et al., 2010; Arenaza-Urquijo et al., 2013a; Bozzali et al., 2015; Marques et al., 2015; Serra et al., 2017), in turn, found that healthy elders, mild cognitive impairment (MCI) and AD patients with higher CR present more activation/connectivity in brain regions than the individuals with lower CR. These interesting results have shed some light onto the issue, proposing that the healthy elderly with higher education level present increased brain efficiency, while AD patients are able to recruit compensatory mechanisms to maintain cognitive function. Because of the lack of pathophysiological data for the subjects, however, these results only support the CR hypothesis, and preclude any further assumptions on it.
The role of CR in attenuating the prejudicial effects of AD pathology in cognition has been explored in several studies involving both healthy controls (Bennett et al., 2003; Yaffe et al., 2011; Almeida et al., 2015) and patients (Roe et al., 2008; Rentz et al., 2010). Individuals with higher CR who are still cognitively normal have less chance to develop dementia, despite presenting abnormal pathological protein levels such as amyloid β (Aβ) and phospho-tau (p-tau; Soldan et al., 2013). Although the neurobiological mechanism behind this process is not known, some studies posit that a greater exposure to a cognitively stimulating environment is associated with reduced levels of the pathological proteins, protecting the individuals from developing AD-related pathology (Lazarov et al., 2005; Landau et al., 2012; Harris et al., 2015).
Among the negative impacts of the disease in a subject’s brain, is the disruption in the topological organization of the functional connectome, leading to a loss of global information integration in disease due to the randomization of the brain functional networks (Sanz-Arigita et al., 2010). Although disruption in resting-state networks has already been related to abnormal levels of cerebrospinal fluid (CSF) proteins (Aβ and 181Thr-phosphorylated tau, p-tau) in older adults (Jiang et al., 2016), amnestic mild cognitive impairment (aMCI) subjects (Canuet et al., 2015) and mild AD patients (Li et al., 2013; Celebi et al., 2016), no study has investigated whether CR is able to mitigate the effects of the pathology in network efficiency through graph theory analysis. Thus, in the present study we mainly sought to investigate the moderator effect of CR (if any) on the association between pathophysiology and functional network efficiency in aMCI and mild AD patients (i.e., can CR modify the effects of pathology on network topology?) as a primary outcome. As secondary outcomes, we analyzed the relationship between CR proxies and functional network efficiency (resting-state fMRI graph metrics) in healthy controls, aMCI and mild AD patients; the relationship between pathophysiology (CSF Aβ, total tau (t-tau) and p-tau levels) and functional network efficiency in aMCI and mild AD patients; and the relationship between pathophysiology and CR proxies in aMCI and mild AD patients.
Materials and Methods
Participants
This study included 70 participants: 14 mild AD patients, 28 aMCI subjects, and 28 healthy controls. To indicate dementia severity, we used ratings on the Clinical Dementia Rating (Morris, 1993) scale and a semi-structured interview about the patients’ functional level in their daily activities (Pfeffer et al., 1982). The diagnosis of probable AD patients fulfilled the standards of the National Institute of Aging and Alzheimer’s Association (NIA/AA; McKhann, 2011), and patients had a Clinical Dementia Rating score of 1. aMCI patients were diagnosed using the core criteria of the NIA/AA for MCI (Albert et al., 2011) and had a Clinical Dementia Rating score of 0.5 (with an obligatory memory score of 0.5). All aMCI subjects had memory complaints confirmed by a full range of neuropsychological testing, absence of dementia, and pathophysiological evidence of AD, characterized by low CSF Aβ1–42 (<416 pg/mL) and/or low Aβ1–42/p-tau (<9.53 pg/mL; Forlenza et al., 2015).
Controls were identified as cognitively normal: they did not exhibit any neurological or psychiatric disorders or require psychoactive medication; they demonstrated Mini Mental State Examination (MMSE) scores within the normal limits, considering scores corrected for educational level (Brucki et al., 2003); and their structural images were without any abnormalities. The control group had no memory complaints nor neurological deficits, and they also had a Clinical Dementia Rating score of zero. Exclusion criteria for all participants included: history of other neurological or psychiatric diseases or head injury with loss of consciousness, use of sedative drugs in the last 24 h before the neuropsychological assessment, drug or alcohol addiction, prior chronic exposure to neurotoxic substances, Hachinski ischemeic score (Hachinski et al., 1975) >4, and Fazekas Scale (Fazekas et al., 1987) >1.
Pre-diagnostic procedures also comprised laboratory tests including Vitamin B12, folate and thyroid hormones. The Medical Research Ethics Committee of University of Campinas (UNICAMP) approved this study and written informed consent (either from the subjects or from their responsible guardians, if incapable) was obtained from all participants before study initiation, according to the Declaration of Helsinki.
Neuropsychological Assessment
Experienced neuropsychologists, blinded to the magnetic resonance imaging (MRI) data, performed the neuropsychological evaluations. These evaluations helped with the clinical assessment and diagnosis of patients. Detailed information for the neuropsychological testing can be found at Supplementary Materials.
Cognitive Reserve
In the present study, we used years of education as a measure of CR (measured as the total number of years of schooling). Our study used a wide education range for all three groups, extending its low end to include no years of formal schooling (range of education in controls: 0–19 years; in aMCI: 0–23 years; in mild AD: 0–16 years). We did not dichotomize the subjects into high or low CR groups and treated the data as continuous variables in all analyses.
Cerebrospinal Fluid Assessment
CSF samples from aMCI and AD patients were collected by lumbar puncture and stored in a polypropylene tube of 1 ml. Then the samples were centrifuged at 800 rpm for 10 min and stored at −80°C until analysis. Aβ1–42, t-tau protein and p-tau protein were measured using Luminex xMAP plataform (Inno-Bia Alzbio3 immunoassay reagents, Innogenetics, Ghent, Belgium).
MRI Acquisition
All MR images were acquired on a 3.0 T MRI Philips Achieva® scanner. The following acquisition protocol was applied to each subject: (a) sagittal high-resolution T1-weighted (isotropic voxels of 1 × 1 × 1 mm3, TR/TE = 7/3.2 ms, FOV = 240 × 240 mm, 180 slices); (b) functional acquisitions (EPI) with TR/TE = 2000/30 ms, FOV = 240 × 240, isotropic voxels set to 3 × 3 × 3 mm3, and no gap with a total scan time of 6 min, resulting in 180 full brain volumes with 40 axial slices each. All participants were instructed to keep their eyes closed, to relax, to move as little as possible, and to not fall asleep. The total scan time was 30 min.
Image Processing and Statistical Analysis
We performed the resting-state functional connectivity preprocessing and analysis using an in-house toolbox (UF2C1; de Campos et al., 2016) that runs in the MATLAB platform (2014b, The MathWorks Inc., Natick, MA, USA) with SPM122. We performed the T1-weighted image coregistration with the fMRI mean image, tissue segmentation, and normalization. We preprocessed the functional images based on volumes realignment, normalization (unified segmentation method—MNI152), and smoothing (6 × 6 × 6 mm3 FWHM). Additionally, we performed regressions for head motion, white matter, and CSF global signals and band-pass filtering (0.008–0.1 Hz). No subjects in the present study exceeded the mean framewise displacement threshold (0.5 mm), and there were no significant differences in head movement among the groups (analysis of variance, ANOVA, p = 0.187).
We used the UF2C toolbox to construct functional connectivity matrices (i.e., the input for graph analysis), based on 70 functional regions-of-interest (ROIs)—corresponding to the 12 resting-state networks established elsewhere (Shirer et al., 2012; regions available at http://findlab.stanford.edu/functional_ROIs.html (Supplementary Table S1). Functional connectivities of all ROI pairs were estimated as Pearson’s correlation coefficients and then transformed to Fisher’s Z estimates using Fisher’s r-to-z transformation. Due to local atrophy present in aMCI and mild AD patients, the local average of time-series per ROI could include signal derived from non-gray matter voxels, thereby introducing artificial differences between patients and controls. To overcome this issue, time-series were extracted only from voxels that were included in the subject’s gray matter mask; furthermore, UF2C correlates each single ROI voxel time series with the average ROI time series (gray matter-masked). The voxel was included (into the average) if its correlation value was within the average + standard deviation (SD) of all correlations between the ROI-masked voxels.
Because the arbitrary thresholding and binarization processes in graph theoretical analysis often lead to loss of information (Rubinov and Sporns, 2011), we chose weighted correlation matrices (in which the links contain information about the connection strength). Negative weights were considered zero. The following 10 graph metrics were obtained for each subject to estimate network efficiency: local betweenness centrality (Bc) and local eigenvector centrality (v) as measures of centrality; global and local clustering coefficient (Cglob and Cloc, respectively) and global transitivity (T) as measures of segregation; global (λ) and local (λ(v)) characteristic path length, and global and local efficiency (Eglob and Eloc, respectively) as measures of integration. In addition to all these metrics, we also calculated small-worldness (σ), a global measure that combines path length and clustering coefficient. To give a brief idea, higher betweenness centrality, eigenvector centrality, clustering coefficient, transitivity, efficiency and small-worldness values are features of more efficient networks. On the other hand, lower values of characteristic path length represent more efficient networks.
To see the relationship between CSF values and graph metrics (secondary outcome), for each group separately (i.e., controlling for clinical dementia severity), we added CSF values as independent variables and graph metrics as dependent variables in the GraphVar toolbox (version 1.023; Kruschwitz et al., 2015). We then did the same for education and graph metrics. In the next step, to test whether education modifies the relationship between CSF biomarker levels and the topological organization of the functional connectome (primary outcome), we used regression models in which we included an interaction term between education and the CSF biomarker levels. We then verified whether including this interaction term added significantly to the model.
For each graph parameter analyzed, we created a random distribution of the parameter value (5,000 permutations). We then employed a network-based statistic—NBS (Zalesky et al., 2010a), a process that tests whether a set of multiple pairwise connections forms ROI–ROI pairs that would be highly unlikely to occur randomly. Finally, as we used the same general linear models to test the relationships between CR, pathophysiological levels (CSF proteins) and network topology (graph metrics) for all three groups (MCI, AD and controls), we employed a Bonferroni correction to further account for the multiplicity of tests.
Non-imaging Statistical Analysis
We first tested the normal distribution with Kolmogorov-Smirnov test for all non-imaging variables. The chi-square test was used for categorical variables comparison, such as sex. Differences between groups for demographic and years of education were tested with ANOVAs, Bonferroni post hoc. Group comparison for CSF variables was performed with t-tests (aMCI vs. mild AD group). Pearson’s correlation analyses were used to investigate the associations among demographic data (age and sex) with education. To investigate the relationship between CR and CSF biomarker levels in both aMCI and mild AD patients (secondary outcome), we used linear regression models, including the CSF data as dependent variables, and sex, age and education as independent variables. All statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) program, version 22. The level of significance accepted was α = 0.05.
Results
Participants Characteristics
There was no difference in sex (p = 0.871) and educational level among the groups; and aMCI and mild AD groups did not statistically differ in CSF biomarker levels (Table 1). Controls were younger than AD patients (p = 0.009), and this variable was included as a confounding factor throughout the analysis. There was no statistically significant relationship between education and age or sex in controls, aMCI nor mild AD patients (data not shown). Neuropsychological performance results can be found at Supplementary Table S2.
Table 1.
Group comparison for demographic and cerebrospinal fluid (CSF) proteins data.
| Controls | aMCI | Mild Alzheimer’s disease | Controls vs. aMCI | Controls vs. mild Alzheimer’s disease | aMCI vs. mild Alzheimer’s disease | |
|---|---|---|---|---|---|---|
| N (female) | 28 (19) | 28 (17) | 14 (9) | |||
| Age | 66.36 (5.6) | 70.38 (6.9) | 72.67 (6.8) | 0.097 | 0.019 | 0.93 |
| MMSE | 28 (1.3) | 25.34 (2.9) | 19.8 (5.1) | 0.013 | <0.001 | <0.001 |
| Education | 10.37 (5.2) | 7.59 (5.5) | 8.93 (5.2) | 0.242 | 1 | 1 |
| t-tau | NA | 101.73 (66.1) | 132.07 (76.2) | NA | NA | 0.465 |
| p-tau | NA | 50.15 (32.3) | 54.9 (32.2) | NA | NA | 1 |
| Aβ1–42 | NA | 428.83 (151.9) | 355.89 (121.1) | NA | NA | 0.316 |
Notes: mean (sd). aMCI, amnestic mild cognitively impaired subjects; MMSE, Mini mental state examination; NA, not available.
Primary Outcome: The Interplay Between CR and Pathophysiology in Network Efficiency
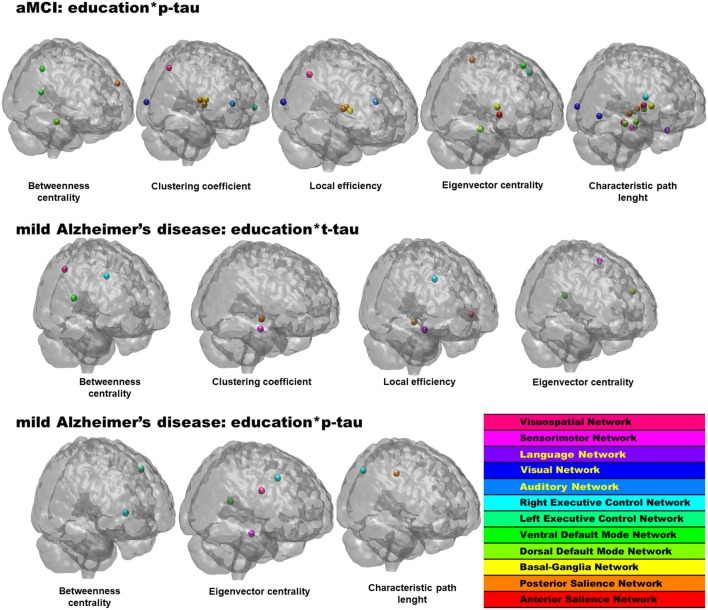
At this level, an interaction term between CSF proteins and education was added to the models, and when significant, the interaction term indicated an interplay between pathophysiological level and CR on network topology. As shown in Table 2, Figure 1 the interaction term between years of education and CSF p-tau level was a statistically significant predictor of graph metrics for the aMCI group. Similarly, interaction terms between education and both CSF t-tau and p-tau added significantly to models in the mild AD group, indicating that these interactions affect network topology. No results were observed for interaction terms involving CSF Aβ1–42.
Table 2.
Statistically significant linear contents regression models examining interaction terms in network topology measures.
| Interaction term | Graph metric | Anatomical region (network) | p value |
|---|---|---|---|
| aMCI | |||
| Education*p-tau | Characteristic path length | NA | 0.023 |
| Betweenness centrality | Left middle frontal gyrus (pSN) | 0.003 | |
| Right hippocampus (dDMN) | 0.011 | ||
| Left middle occipital gyrus (vDMN) | 0.006 | ||
| Right retrosplenial cortex, posterior cingulate cortex (vDMN) | 0.004 | ||
| Clustering coefficient | Right thalamus (pSN) | 0.008 | |
| Left thalamus (pSN) | 0.002 | ||
| Right brainstem/midbrain (BGN) | 0.004 | ||
| Left inferior frontal gyrus, orbitofrontal gyrus (lECN) | 0.011 | ||
| Left superior temporal gyrus (AN) | 0.009 | ||
| Right middle occipital gyrus (VN) | 0.019 | ||
| Right inferior parietal gyrus (VSN) | 0.018 | ||
| Local efficiency | Right thalamus (pSN) | 0.010 | |
| Left thalamus (pSN) | 0.004 | ||
| Right brainstem/midbrain (BGN) | 0.001 | ||
| Left superior temporal gyrus (AN) | 0.024 | ||
| Right middle occipital gyrus (VN) | 0.005 | ||
| Right inferior parietal gyrus (VSN) | 0.017 | ||
| Eigenvector centrality | Right insula (aSN) | 0.002 | |
| Left precuneus (pSN) | 0.006 | ||
| Right brainstem/midbrain (BGN) | 0.023 | ||
| Right hippocampus (dDMN) | <0.001 | ||
| Left middle frontal gyrus (vDMN) | 0.010 | ||
| Left middle frontal gyrus, superior frontal gyrus (lECN) | <0.001 | ||
| Characteristic path length | Right insula (aSN) | 0.022 | |
| Right posterior insula (pSN) | 0.008 | ||
| Left thalamus (pSN) | 0.006 | ||
| Right brainstem/midbrain (BGN) | <0.001 | ||
| Left and right thalamus (dDMN) | 0.016 | ||
| Left hippocampus (dDMN) | <0.001 | ||
| Right hippocampus (dDMN) | <0.001 | ||
| Left parahipocampal gyrus (vDMN) | 0.011 | ||
| Left thalamus (lECN) | <0.001 | ||
| Right caudate (rECN) | 0.002 | ||
| Left middle occipital gyrus (VN) | <0.001 | ||
| Right middle occipital gyrus (VN) | <0.001 | ||
| Left middle temporal gyrus (LN) | 0.002 | ||
| Cerebellum (SMN) | 0.004 | ||
| Left inferior temporal gyrus (VSN) | <0.001 | ||
| Mild Alzheimer’s disease | |||
| Education*t-tau | Betweenness centrality | Right retrosplenial cortex, posterior cingulate cortex (vDMN) | 0.004 |
| Right middle frontal gyrus, superior frontal gyrus (rECN) | 0.002 | ||
| Right inferior parietal gyrus (VSN) | 0.014 | ||
| Clustering coefficient | Right posterior insula (pSN) | 0.007 | |
| Cerebellum (SMN) | 0.015 | ||
| Local efficiency | Left insula (aSN) | 0.024 | |
| Right posterior insula (pSN) | 0.006 | ||
| Right middle frontal gyrus, superior frontal gyrus (rECN) | 0.022 | ||
| Cerebellum (SMN) | 0.013 | ||
| Eigenvector centrality | Left inferior frontal gyrus (BGN) | 0.002 | |
| Left retrosplenial cortex, posterior cingulate cortex (vDMN) | 0.004 | ||
| Left precentral gyrus (SMN) | 0.003 | ||
| Education*p-tau | Betweenness centrality | Left middle frontal gyrus, superior frontal gyrus (lECN) | <0.001 |
| Right middle frontal gyrus (rECN) | 0.001 | ||
| Eigenvector centrality | Left retrosplenial cortex, posterior cingulate cortex (vDMN) | 0.013 | |
| Right middle frontal gyrus, superior frontal gyrus (rECN) | 0.002 | ||
| Cerebellum (SMN) | 0.015 | ||
| Right frontal operculum, inferior frontal gyrus (VSN) | 0.024 | ||
| Characteristic path length | Right middle cingulate cortex (pSN) | 0.007 | |
| Right inferior parietal gyrus, supramarginal gyrus, angular gyrus (rECN) | 0.021 |
Notes: aMCI, amnestic mild cognitively impaired subjects; CSF, cerebrospinal fluid; dDMN, dorsal Default Mode Network; vDMN, ventral Default Mode Network; lECN, left Executive Control Network; AN, Auditory Network; VSN, Visuospatial Network; aSN, anterior Salience Network; rECN, right Executive Control Network; SMN, Sensorimotor Network; pSN, posterior Salience Network; LN, Language Network; BGN, Basal Ganglia Network.
Figure 1.
Statistically significant regions-of-interest (ROIs) for the interplay between educational level and pathophysiology in the network topology in amnestic mild cognitive impairment (aMCI) and mild Alzheimer’s disease (AD) groups. p values and anatomical brain regions in Table 2.
Secondary Outcomes
Group Comparison for Network Efficiency
Compared to controls, the aMCI group presented increased betweenness centrality (Bc), local clustering coefficient (Cloc), local efficiency (Eloc) and eigenvector centrality (v) in several regions belonging to the Default Mode Network (DMN), Executive Control Network (ECN), Auditory Network (AN), Salience Network (SN), Visuospatial Network (VSN) and Sensorimotor Network (SMN); whereas diminished betweenness centrality (Bc) and eigenvector centrality (v) were observed in regions belonging to the DMN, ECN and AN. Interestingly, although belonging to a variety of different networks, the regions that presented features of increased network efficiency in the aMCI were predominantly located in the frontal region. These findings suggest that predementia subjects present frontal nodes that work more efficiently than the cognitively healthy.
Compared to controls, mild AD patients presented increased local characteristic path length (λ(v)) in three regions belonging to the SN, DMN and language network (LN), indicating a possible disruption in these network’s information processing. aMCI subjects presented increased centrality values (Bc and v) in regions of the DMN, SN, basal ganglia network (BGN) and VSN compared to mild AD patients; whereas decreased local characteristic path length (λ(v)) and centrality metrics (Bc and v) were present in regions of the ECN, DMN, SMN and VSN (Supplementary Table S3).
Relationship Between Education and Pathophysiology
Multiple linear regression models failed to detect any statistically significant relationship between CSF biomarker levels and education in aMCI or mild AD groups, suggesting that the level of CR has no influence on brain AD-related pathology levels (Supplementary Table S4).
Relationship Between Education and Network Efficiency
In the control group, years of education had a positive correlation with local clustering coefficient (Cloc) and local efficiency (Eloc) in areas belonging to the SN, DMN, AN, VSN, LN and AN (the higher the education, the higher the Cloc and Eloc in these areas); whereas, there was negative correlation with local characteristic path length (λ(v)) in regions belonging to the DMN, AN, LN and VSN (the higher the education, the lower the λ(v)). In the aMCI group, the level of education had a positive relationship with the betweenness centrality (Bc), local clustering coefficient (Cloc) and local efficiency (Eloc) of regions belonging to the SN, ECN, VSN and DMN. A negative relationship was observed between education and betweenness centrality (Bc) at a region belonging to the VSN. In the mild AD group, years of education positively correlated with betweenness centrality (Bc), local clustering coefficient (Cloc), eigenvector centrality (v) and local efficiency (Eloc) of regions belonging to the ECN, LN and DMN (Table 3). These findings indicate that healthy controls, aMCI and mild AD patients with higher level of CR present characteristics of more efficient networks.
Table 3.
Statistically significant linear contents regression models examining relation of educational level and graph metrics in controls, aMCI and mild Alzheimer’s disease groups.
| CR proxy | Graph metric | Anatomical region (network) | p value | β |
|---|---|---|---|---|
| Controls | ||||
| Education | Clustering coefficient | Right posterior insula (pSN) | 0.016 | 0.414 |
| Left and right thalamus (dDMN) | 0.002 | 0.564 | ||
| Left superior temporal gyrus (AN) | 0.004 | 0.469 | ||
| Right inferior temporal gyrus (VSN) | 0.011 | 0.363 | ||
| Local efficiency | Left and right thalamus (dDMN) | 0.001 | 0.580 | |
| Left superior temporal gyrus (AN) | 0.001 | 0.516 | ||
| Left middle temporal gyrus, angular gyrus (LN) | 0.010 | 0.409 | ||
| Right inferior temporal gyrus (VSN) | 0.013 | 0.363 | ||
| Characteristic path length | Left and right thalamus (dDMN) | 0.004 | −0.382 | |
| Left superior temporal gyrus (AN) | 0.014 | −0.404 | ||
| Left middle temporal gyrus, angular gyrus (LN) | 0.007 | −0.448 | ||
| Right inferior temporal gyrus (VSN) | 0.005 | −0.354 | ||
| aMCI | ||||
| Education | Betweenness centrality | Anterior cingulate cortex, medial prefrontal cortex, supplementary motor area (aSN) | 0.011 | 0.433 |
| Right inferior parietal gyrus, supramarginal gyrus, angular gyrus (rECN) | 0.004 | 0.481 | ||
| Left inferior parietal gyrus (VSN) | 0.007 | −0.323 | ||
| Clustering coefficient | Right middle frontal gyrus (rECN) | 0.014 | 0.410 | |
| Local efficiency | Posterior cingulate cortex, precuneus (dDMN) | 0.011 | 0.467 | |
| Right middle frontal gyrus (rECN) | 0.012 | 0.442 | ||
| Mild Alzheimer’s disease | ||||
| Education | Betweenness centrality | Left middle frontal gyrus, superior frontal gyrus (lECN) | <0.001 | 0.675 |
| Left superior parietal gyrus, inferior parietal gyrus, precuneus, angular gyrus (lECN) | 0.001 | 0.501 | ||
| Left middle temporal gyrus (LN) | 0.001 | 0.728 | ||
| Clustering coefficient | Medial prefrontal cortex, anterior cingulate cortex, orbitofrontal cortex (dDMN) | 0.008 | 0.685 | |
| Eigenvector centrality | Right superior frontal gyrus (dDMN) | 0.001 | 0.565 | |
| Local efficiency | Medial prefrontal cortex, anterior cingulate cortex, orbitofrontal cortex (dDMN) | 0.002 | 0.715 |
Notes: aMCI, amnestic mild cognitively impaired subjects; dDMN, dorsal Default Mode Network; vDMN, ventral Default Mode Network; lECN, left Executive Control Network; AN, Auditory Network; VSN, Visuospatial Network; aSN, anterior Salience Network; rECN, right Executive Control Network; SMN, Sensorimotor Network; pSN, posterior Salience Network; LN, Language Network; BGN, Basal Ganglia Network; β, linear regression coefficient. In bold, expected direction for the relationship.
Relationship Between Pathophysiology and Network Efficiency
Before hand, we think it is helpful to describe the expected concentration of t-tau, p-tau and Aβ1–42 extracted from the CSF of patients. Because molecules of Aβ1–42 are composing amyloid plaques in the brain, low quantities diffuse to the CSF in AD (Blennow and Zetterberg, 2009), in which CSF Aβ1–42 correlates inversely with total Aβ load in the brain (Tapiola et al., 2009). In contrast, high levels of CSF t-tau and p-tau are correlated with the presence of neocortical neurofibrillary tangles (Tapiola et al., 2009). Hence, AD patients are expected to present lower levels of CSF Aβ1–42, but higher levels of both CSF t-tau and p-tau than cognitively healthy elderlies.
Overall, the relationship between CSF biomarkers and graph metrics did not follow an expected pattern for the aMCI group, in which both positive and negative correlations were observed for t-tau, p-tau and Aβ1–42 levels. Interestingly, the relationship between CSF protein levels and graph metrics in the mild AD group was mostly unexpected: with few exceptions, the brain pathological features (high levels of t-tau and p-tau, and low levels of Aβ1–42) were related to features of more efficient networks. For example, higher levels of both t-tau and p-tau were related to increased betweenness (Bc), clustering coefficient (Cloc), local efficiency (Eloc) and eigenvector centrality (v) and decreased characteristic path length (λ(v)) in several regions. Likewise, lower levels of Aβ1–42 were related to increased betweenness (Bc) and eigenvector centrality (v) in some regions belonging to the SMN and VSN (Table 4).
Table 4.
Statistically significant linear contents regression models examining the relationship of CSF biomarkers and graph metrics in aMCI and mild Alzheimer’s disease groups.
| CSF protein | Graph metric | Anatomical region (network) | p value | β |
|---|---|---|---|---|
| aMCI | ||||
| t-tau | Betweenness centrality | Right inferior frontal gyrus (BGN) | 0.023 | −0.460 |
| Eigenvector centrality | Right brainstem/midbrain (BGN) | 0.008 | 0.407 | |
| Right frontal operculum, inferior frontal gyrus (VSN) | 0.006 | 0.493 | ||
| p-tau | Betweenness centrality | Left superior parietal gyrus, inferior parietal gyrus, precuneus, angular gyrus (lECN) | 0.015 | 0.519 |
| Left middle temporal gyrus, angular gyrus (LN) | 0.022 | 0.615 | ||
| Local efficiency | Left superior temporal gyrus (AN) | 0.013 | −0.387 | |
| Eigenvector centrality | Left parahipocampal gyrus (vDMN) | 0.014 | −0.413 | |
| Right angular gyrus, middle occipital gyrus (vDMN) | 0.023 | 0.483 | ||
| Right middle frontal gyrus, superior frontal gyrus (rECN) | 0.002 | 0.443 | ||
| Right frontal operculum, inferior frontal gyrus (VSN) | 0.011 | 0.459 | ||
| Characteristic path length | Left superior temporal gyrus (AN) | 0.016 | 0.510 | |
| Aβ1–42 | Betweenness centrality | Left thalamus (lECN) | 0.021 | 0.406 |
| Right frontal operculum, inferior frontal gyrus (VSN) | 0.009 | 0.340 | ||
| Clustering coefficient | Right angular gyrus (dDMN) | 0.008 | −0.511 | |
| Local efficiency | Posterior cingulate cortex, precuneus (dDMN) | 0.021 | −0.428 | |
| Right angular gyrus (dDMN) | 0.008 | −0.513 | ||
| Eigenvector centrality | Left precuneus (pSN) | 0.005 | −0.480 | |
| Left inferior frontal gyrus (BGN) | 0.009 | 0.514 | ||
| Right superior frontal gyrus, middle frontal gyrus (vDMN) | 0.020 | 0.431 | ||
| Right supplementary motor area (SMN) | 0.020 | −0.388 | ||
| Mild Alzheimer’s disease | ||||
| t-tau | Global efficiency | NA | 0.024 | 0.605 |
| Smallworldness | NA | 0.005 | −0.697 | |
| Betweenness centrality | Posterior cingulate cortex, precuneus (dDMN) | 0.008 | 0.582 | |
| Right middle frontal gyrus (rECN) | 0.004 | 0.609 | ||
| Left inferior parietal gyrus (VSN) | 0.002 | −0.593 | ||
| Right frontal operculum, inferior frontal gyrus (VSN) | 0.019 | −0.513 | ||
| Clustering coefficient | Left precuneus (pSN) | 0.006 | 0.535 | |
| Left hippocampus (dDMN) | 0.017 | 0.719 | ||
| Right hippocampus (dDMN) | 0.008 | 0.626 | ||
| Left parahipocampal gyrus (vDMN) | 0.009 | 0.647 | ||
| Right superior frontal gyrus, middle frontal gyrus (vDMN) | 0.004 | 0.579 | ||
| Right middle frontal gyrus, superior frontal gyrus (rECN) | 0.013 | 0.500 | ||
| Right superior frontal gyrus (rECN) | 0.001 | 0.601 | ||
| Right caudate (rECN) | 0.020 | 0.545 | ||
| Left middle frontal gyrus, superior frontal gyrus, precentral gyrus (VSN) | 0.007 | 0.545 | ||
| Left frontal operculum, inferior frontal gyrus (VSN) | 0.022 | 0.522 | ||
| Right frontal operculum, inferior frontal gyrus (VSN) | 0.013 | 0.555 | ||
| Local efficiency | Left precuneus (pSN) | 0.011 | 0.500 | |
| Right brainstem/midbrain (BGN) | 0.020 | 0.582 | ||
| Midcingulate cortex (dDMN) | 0.204 | 0.350 | ||
| Right hippocampus (dDMN) | 0.006 | 0.583 | ||
| Left parahipocampal gyrus (vDMN) | 0.008 | 0.622 | ||
| Right superior frontal gyrus, middle frontal gyrus (vDMN) | 0.003 | 0.593 | ||
| Right superior frontal gyrus (rECN) | 0.001 | 0.592 | ||
| Right caudate (rECN) | 0.020 | 0.535 | ||
| Left middle frontal gyrus, superior frontal gyrus, precentral gyrus (VSN) | 0.017 | 0.524 | ||
| Left frontal operculum, inferior frontal gyrus (VSN) | 0.019 | 0.529 | ||
| Eigenvector centrality | Left precuneus (pSN) | <0.001 | 0.735 | |
| Right middle frontal gyrus, superior frontal gyrus (rECN) | 0.001 | 0.670 | ||
| Right precentral gyrus (SMN) | 0.010 | 0.589 | ||
| Characteristic path length | Right middle frontal gyrus (aSN) | 0.015 | −0.657 | |
| Left precuneus (pSN) | 0.018 | −0.590 | ||
| Right superior frontal gyrus (dDMN) | 0.019 | −0.642 | ||
| Left and right thalamus (dDMN) | 0.010 | −0.613 | ||
| Right superior frontal gyrus (rECN) | <0.001 | −0.719 | ||
| Left middle occipital gyrus (VN) | 0.003 | −0.449 | ||
| Right middle occipital gyrus (VN) | 0.015 | −0.482 | ||
| p-tau | Betweenness centrality | Posterior cingulate cortex, precuneus (dDMN) | 0.011 | 0.574 |
| Left hippocampus (dDMN) | 0.003 | 0.695 | ||
| Left inferior parietal gyrus (VSN) | 0.001 | −0.611 | ||
| Right frontal operculum, inferior frontal gyrus (VSN) | 0.019 | −0.527 | ||
| Clustering coefficient | Left precuneus (pSN) | 0.012 | 0.520 | |
| Posterior cingulate cortex, precuneus (dDMN) | 0.007 | 0.610 | ||
| Right caudate (rECN) | 0.017 | 0.572 | ||
| Local efficiency | Left precuneus (pSN) | 0.006 | 0.532 | |
| Posterior cingulate cortex, precuneus (dDMN) | 0.002 | 0.634 | ||
| Right hippocampus (dDMN) | 0.010 | 0.563 | ||
| Right caudate (rECN) | 0.016 | 0.535 | ||
| Right inferior parietal gyrus (VSN) | 0.022 | 0.501 | ||
| Eigenvector centrality | Right middle frontal gyrus, superior frontal gyrus (rECN) | 0.011 | 0.562 | |
| characteristic path length | Left precuneus (pSN) | <0.001 | −0.768 | |
| Posterior cingulate cortex, precuneus (dDMN) | 0.018 | −0.662 | ||
| Left and right thalamus (dDMN) | 0.005 | −0.655 | ||
| Left hippocampus (dDMN) | 0.007 | −0.509 | ||
| Right hippocampus (dDMN) | 0.003 | −0.580 | ||
| Precuneus (vDMN) | 0.022 | −0.671 | ||
| Left middle occipital gyrus (VN) | 0.007 | −0.412 | ||
| Right middle temporal gyrus, superior temporal gyrus, supramarginal gyrus, angular gyrus (LN) | 0.020 | −0.506 | ||
| Right inferior parietal gyrus (VSN) | 0.023 | −0.717 | ||
| Right inferior temporal gyrus (VSN) | 0.007 | 0.530 | ||
| Aβ1–42 | Betweenness centrality | Left precentral gyrus (SMN) | 0.016 | −0.603 |
| Eigenvector centrality | Left inferior temporal gyrus (VSN) | 0.017 | −0.595 | |
| Right middle frontal gyrus (VSN) | 0.009 | 0.527 |
Notes: aMCI, amnestic mild cognitively impaired subjects; CSF, cerebrospinal fluid; dDMN, dorsal Default Mode Network; vDMN, ventral Default Mode Network, lECN, left Executive Control Network; AN, Auditory Network; VSN, Visuospatial Network; aSN, anterior Salience Network; rECN, right Executive Control Network; SMN, Sensorimotor Network; pSN, posterior Salience Network; LN, Language Network; BGN, Basal Ganglia Network; β, linear regression coefficient. In bold, expected direction for the relationship.
Discussion
Previous studies have suggested that CR could explain why nearly one third of the cognitively healthy population met criteria for AD at necropsies without clinically expressing it (Bennett et al., 2006; Morris et al., 2010). In this context, we aimed to analyze the relationship between a CR proxy, CSF biomarker levels (pathology burden) and graph metrics (network topology), as well as whether CR could modify the relationship between pathology burden and network topology (i.e., the CR hypothesis). According to our findings, although higher levels of CR did not seem to protect individuals from developing the pathophysiological features of AD, cognitively healthy controls, aMCI and mild AD patients with higher level of CR presented features of more efficient networks. Altered pathological levels of CSF protein biomarkers were related to a dual pattern of network efficiency in aMCI, whereas they were related to more efficient networks in the mild AD group. We suppose that educational level could modify the effects of p-tau in network topology for the aMCI group, and the effects of t-tau and p-tau in network topology for the mild AD group. In what follows, we will discuss these points in turn.
The Interplay Between CR and Pathophysiology in Network Efficiency
The analysis undertaken in the present work demonstrated that educational level—a proxy for CR—, has modifying effects on the relationship between evidence of AD pathophysiology and network topology in both aMCI and mild AD groups. While CR did not seem to protect individuals from developing the pathophysiological features of AD, it did seem to modify the association between pathology and resting-state network topology for both groups. A previous study claimed that CR can only compensate for pathology up to an initial phase and does not act in a certain stage of dementia (Serra et al., 2017). To test this hypothesis, however, the authors analyzed differences in graph metrics between high/low CR aMCI and mild AD patients and could only find differences for the aMCI subjects. In the present work, we have directly tested whether CR has any modifying effect in network topology, and we extend previous findings claiming that CR acts as a modifying factor even in the dementia phase. The relationship between higher burden of abnormal proteins and graph metrics for AD has been interpreted previously in the light of a compensatory mechanism. We could extend these results hypothesizing that at the dementia stage, this compensatory mechanism (i.e., enhanced network efficiency) is further increased by a high CR level. The findings with interaction terms give supplementary evidence for that, pointing that educational level can, indeed, modify the effect of pathology on network topology.
Individuals with higher educational level might use brain networks more efficiently. The biological underpinnings behind this association are yet to be further elucidated. In vivo studies have shown that animals living in an enriched environment restored neurogenesis (Ihunwo et al., 2016) and increased the number of surviving newborn progenitor-derived cells in the hippocampal dentate gyrus (Nilsson et al., 1999). The human brain also retains its ability to generate neurons throughout life (Johansson et al., 1999) and engagement in more cognitively stimulating activities might increase neurogenesis, synaptogenesis, levels of brain derived neurotrophic factor and related neurotrophins (van Praag et al., 2000), as well as change astroglial morphology and volume (Beauquis et al., 2013). CR may also involve upregulation of the noradrenergic system—which is depleted with age and AD (Robertson, 2013).
Group Comparison for Network Efficiency
In the present study, we found that mild AD patients presented longer characteristic path length (λ(v)) than healthy controls, aligning with previous research (Stam et al., 2007; Sanz-Arigita et al., 2010; Supplementary Table S3). Some other studies have also found a decrease in the eigenvector centrality (v; Binnewijzend et al., 2014) and clustering coefficient (Cloc; Supekar et al., 2008), and a diminished number of hubs (Khazaee et al., 2017) in patients, suggesting a randomization of the brain networks and a loss of information flow and integration. In the present work, the regions that presented longer characteristic path length (λ(v)) in mild AD (the right middle cingulate cortex, the left hippocampus and the left middle temporal gyrus) are the ones more commonly hit by the pathophysiological alterations, markedly presenting metabolic dysfunctions and atrophy. Thus, it is not surprising that these regions presented longer local path lengths (λ(v)) and lower capacity to combine information from their neighbors.
Studies involving aMCI subjects, in turn, are much less frequent. Some authors have reported a loss in “small-worldness” features in the aMCI group, such as an increased path length (λ(v); Wang et al., 2013) and decreased eigenvector centrality (v; Qiu et al., 2016) when compared to controls. Our aMCI subjects presented diminished measures of centrality in some regions, whereas other regions showed increased measurements of centrality, segregation and integration when compared to controls (Supplementary Table S3). Although unexpected, previous results (Qiu et al., 2016) can shed some light onto this issue regarding increases in “small-worldness” properties predominantly in frontal regions in our predementia subjects. The authors performed a longitudinal analysis in a MCI group and, interestingly, the ones that converted to dementia presented increased eigenvector centrality (v) in frontal regions when compared to the stable ones. The authors interpreted that the frontal regions became more important facing the alterations within the hippocampal network, with increased eigenvector centrality in those regions consisting of a mechanism of functional compensation.
Relationship Between Education and Pathophysiology
Consistent to previous studies (Brayne et al., 2010), we did not find any associations between pathophysiological measurements and CR proxies (Supplementary Table S4). Our results suggest that educational level does not protect individuals from developing pathology. The divergence between our results and others could be explained by other factors associated to higher CR proxies, which could act as confounding factors (Del Ser et al., 1999). Additionally, cognitively stimulating activities could play different roles during pre- and post-amyloid plaque stages, diminishing Aβ1–42 production in pre-amyloid plaque stages (Jagust and Mormino, 2011).
Relationship Between Education and Network Efficiency
Converging evidence suggests that healthy individuals with higher CR proxies present greater volume and metabolism in some brain regions (Arenaza-Urquijo et al., 2013a; Rzezak et al., 2015). The same relationship is true for resting-state fMRI (Song et al., 2008) and graph analysis studies (van den Heuvel et al., 2009; Fischer et al., 2014; Santarnecchi et al., 2015), suggesting that healthy individuals with higher CR proxies present a greater processing capacity and network efficiency. Our results align with these previous findings: healthy controls with higher level of education presented higher clustering coefficient (Cloc) and local efficiency (Eloc) in areas belonging to the posterior Salience Network (pSN), dDMN, AN, VSN, LN and AN and lower characteristic path length (λ(v)) in regions belonging to the dDMN, AN, LN and VSN (Table 3).
By contrast, the interpretation of the protective effects of CR in AD patients could be tricky. For example, structural MRI studies have consistently found that MCI and AD patients with higher CR proxies present reduced brain volumes and/or thickness (Solé-Padullés et al., 2009; Arenaza-Urquijo et al., 2013b), suggesting that they can tolerate a more advanced neurodegenerative process when a certain clinical condition is reached. In fMRI studies, though, patients with higher CR present higher brain activation during task performance (Bosch et al., 2010; Colangeli et al., 2016), and higher DMN functional connectivity (Bozzali et al., 2015) and network efficiency (Franzmeier et al., 2017) during resting-state. Consistent with these previous results, we found that both aMCI and mild AD patients with higher levels of education generally presented graph metrics suggestive of more efficient network topologies in regions belonging to several networks. These findings will be discussed in the light of two complementary facets to the neural implementation of the CR hypothesis: neural reserve and neural compensation (Stern, 2002).
Neural reserve may represent innate occurring individual differences in brain networks that may be modulated through life events (Stern et al., 2005). In this context, the positive relationship between efficiency of networks and educational level in both aMCI and mild AD patients may be explained by the pre-existing high levels of network efficiency before the development of the disease. Alternatively, it could reflect a compensatory increase in the efficiency of networks facing the development of pathological features (i.e., neural compensation; Stern et al., 2005). Given that healthy individuals with higher CR proxies also presented higher network efficiency (i.e., the beneficial effects of education were observed in the absence of the disease), it is tempting to say that our results point towards the neural reserve explanation. However, we did not obtain pathophysiological measurements for healthy controls, and it is known that the pathological burden starts even decades before the onset of dementia, and cognitively healthy subjects could present amyloidosis even without cognitive symptoms. Thus, we cannot discard the possibility that some examples of our healthy sample presented AD pathophysiological features, and it could be that a “neural compensation-like” mechanism is already taking place in some cognitively normal subjects.
The present work brings evidence of an association between educational level and network efficiency not only in the healthy elderly, but also among the aMCI and the demented. It is yet to be found, however, whether possessing higher CR proxies promotes network efficiency or that those subjects with more efficient networks tend to engage more in stimulating activities (Scarmeas and Stern, 2003).
Relationship Between Pathophysiology and Network Efficiency
Overall, we found that aMCI CSF biomarker levels were associated with a dual pattern of network topology (Table 4), similarly to previous research reporting that CSF markers of amyloid deposition and neuronal injury in MCI were associated with both increased and decreased functional connectivity of resting-state networks (Canuet et al., 2015). In the mild AD sample, in turn, we could observe some more notorious results: generally, pathological levels of CSF proteins were related to features of more efficient networks (mostly, but not limited to, the DMN). Divergent results from our and previous work (such as in Binnewijzend et al., 2014), in which the authors report no significant correlations of eigenvector centrality (v) with CSF biomarker) may have arisen from different methodologies adopted.
Since the pathophysiological process is known to start during a preclinical phase, protein levels have been related to alterations in functional connectivity (Wang L. et al., 2013) and graph metrics (Brier et al., 2014) in cognitively normal older individuals with evidence of preclinical AD. At a further stage, such as in MCI subjects for example, there could be an inconsistent adaptation process arising as a result of higher protein abnormalities and structural brain damage. Such speculation could possibly explain the lack of relationship pattern between CSF protein levels and graph metrics found here. Mild AD patients, in turn, might have already reached a plateau for protein biomarkers (Jack et al., 2010) when a process of compensation takes place, explaining the relationship between higher pathological levels of CSF proteins and network efficiency. However, such interpretation is speculative and longitudinal studies would be necessary to confirm it.
Limitations and Conclusion
This work has some limitations that must be acknowledged. First, its relative small sample size and the lack of CSF data for the control sample. Second, its cross-sectional nature does not allow for understanding the effect CR may have on neuropathological accumulation and network topology over time. Third, it is possible that the relationship between CR proxies and network topology, as well as the modifying effects of education are not directly or linearly related to CR. For instance, higher educational level is usually related to higher levels of indexes of socioeconomic status and lifestyle that have been shown to mitigate the risk of AD (Lu et al., 2016). Relatedly, it has been shown that being engaged in intellectual leisure, social and physical activities reduces the risk of developing dementia (Scarmeas et al., 2001). In the present study, we did not evaluate personal engagement in such activities, nor cardiovascular risk factors or unhealthy lifestyles. Brain volume/size was also claimed to protect against clinical deterioration (Guo et al., 2013), and this variable was not accounted for.
We should not forget to mention the contribution of some confounding factors to the graph analysis approach as well. Whereas most studies have used the AAL template for defining nodes, we chose our nodes based on a functional atlas of resting-state networks; and it has been demonstrated that both local and global topological properties of networks exhibit strong dependence on the choice of parcellation scale (Zalesky et al., 2010b). Furthermore, some studies have used thresholds to produce binary adjacency matrices, which generate graphs of different sparsity or connection density (Bullmore and Sporns, 2009). Lastly, unanalyzed biological factors such as the presence of APOE4 gene have been associated with disrupted graph topologies (Daianu et al., 2015; Wang et al., 2015).
Over the last few decades, many authors have committed to studying the relationships and effects of pursuing higher CR levels for the brain. Such studies are of utmost importance because not only do they provide evidence of the beneficial effects of being immersed in a cognitively stimulating environment, but also highlight that interventions might be useful for slow age-related cognitive decline or dementia and to lengthen healthy aging. The current study has the strength of combining a wide range of educational levels that are not often seen in European or North-American studies, and could potentially lead to new insights into the mechanisms of CR. In summary, our findings suggest that although higher levels of CR did not seem to protect individuals from developing the pathophysiological features of AD, cognitively healthy controls, aMCI and demented patients with higher level of CR presented features of more efficient networks. Moreover, educational level could modify the effects of p-tau in network topology in the aMCI group, and the effects of t-tau and p-tau in network topology in the mild AD group; meaning that subjects with higher CR are better able to cope with the effects of pathology in terms of network efficiency.
Author Contributions
MW: conception and design, data collection, data analysis and interpretation, article drafting and critical revision, final approval of version to be published. RFC and MLFB: conception and design, data analysis and interpretation, article drafting and critical revision, final approval of version to be published. BMC: data analysis and interpretation, article drafting, final approval of version to be published. CVLT, AFMKC-C, JEV, TNCM, DQA, LLT and OVF: data collection, article drafting, final approval of version to be published. GC: conception and design of the work, data analysis and interpretation, article drafting and critical revision, final approval of version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer CS declared a shared affiliation, though no other collaboration, with several of the authors LLT and OVF to the handling Editor at the time of the review.
Acknowledgments
We thank the GraphVar group for all the help with statistical analyses.
Funding. The study was supported by Fundacão de Amparo à Pesquisa do Estado de São Paulo (FAPESP; São Paulo Research Foundation), grants #2015/06163-4, #2013/07559-3 and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, National Council for Scientific and Technological Development) #304442/2015-1.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00255/full#supplementary-material
References
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida R. P., Schultz S. A., Austin B. P., Boots E. A., Dowling N. M., Gleason C. E., et al. (2015). Effect of cognitive reserve on age-related changes in cerebrospinal fluid biomarkers of Alzheimer disease. JAMA Neurol. 72, 699–706. 10.1001/jamaneurol.2015.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H., Mokri H., Le Goff M., Meillon C., Jacqmin-Gadda H., Foubert-Samier A., et al. (2014). Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: a study of 20 years of cognitive decline. Brain 137, 1167–1175. 10.1093/brain/awu035 [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo E. M., Landeau B., La Joie R., Mevel K., Mézenge F., Perrotin A., et al. (2013a). Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage 83, 450–457. 10.1016/j.neuroimage.2013.06.053 [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo E. M., Molinuevo J. L., Sala-Llonch R., Solé-Padullés C., Balasa M., Bosch B., et al. (2013b). Cognitive reserve proxies relate to gray matter loss in cognitively healthy elderly with abnormal cerebrospinal fluid amyloid-β levels. J. Alzheimers Dis. 35, 715–726. 10.3233/JAD-121906 [DOI] [PubMed] [Google Scholar]
- Beauquis J., Pavia P., Pomilio C., Vinuesa A., Podlutskaya N., Galvan V., et al. (2013). Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of Alzheimer’s disease. Exp. Neurol. 239, 28–37. 10.1016/j.expneurol.2012.09.009 [DOI] [PubMed] [Google Scholar]
- Bennett D. A., Schneider J. A., Arvanitakis Z., Kelly J. F., Aggarwal N. T., Shah R. C., et al. (2006). Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66, 1837–1844. 10.1212/01.wnl.0000219668.47116.e6 [DOI] [PubMed] [Google Scholar]
- Bennett D. A., Wilson R. S., Schneider J. A., Evans D. A., Mendes de Leon C. F., Arnold S. E., et al. (2003). Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 60, 1909–1915. 10.1212/01.wnl.0000069923.64550.9f [DOI] [PubMed] [Google Scholar]
- Binnewijzend M. A., Adriaanse S. M., Van der Flier W. M., Teunissen C. E., de Munck J. C., Stam C. J., et al. (2014). Brain network alterations in Alzheimer’s disease measured by eigenvector centrality in fMRI are related to cognition and CSF biomarkers. Hum. Brain Mapp. 35, 2383–2393. 10.1002/hbm.22335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K., Zetterberg H. (2009). Cerebrospinal fluid biomarkers for Alzheimer’s disease. J. Alzheimers Dis. 18, 413–417. 10.3233/JAD-2009-1177 [DOI] [PubMed] [Google Scholar]
- Boots E. A., Schultz S. A., Almeida R. P., Oh J. M., Koscik R. L., Dowling M. N., et al. (2015). Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Arch. Clin. Neuropsychol. 30, 634–642. 10.1093/arclin/acv041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B., Bartrés-Faz D., Rami L., Arenaza-Urquijo E. M., Fernández-Espejo D., Junque C., et al. (2010). Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex 46, 451–461. 10.1016/j.cortex.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Bozzali M., Dowling C., Serra L., Spanò B., Torso M., Marra C., et al. (2015). The impact of cognitive reserve on brain functional connectivity in Alzheimer’s disease. J. Alzheimers Dis. 44, 243–250. 10.3233/JAD-141824 [DOI] [PubMed] [Google Scholar]
- Brayne C., Ince P. G., Keage H. A., McKeith I. G., Matthews F. E., Polvikoski T., et al. (2010). Education, the brain and dementia: neuroprotection or compensation? Brain 133, 2210–2216. 10.1093/brain/awq185 [DOI] [PubMed] [Google Scholar]
- Brier M. R., Thomas J. B., Fagan A. M., Hassenstab J., Holtzman D. M., Benzinger T. L., et al. (2014). Functional connectivity and graph theory in preclinical Alzheimer’s disease. Neurobiol. Aging 35, 757–768. 10.1016/j.neurobiolaging.2013.10.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruandet A., Richard F., Bombois S., Maurage C. A., Masse I., Amouyel P., et al. (2008). Cognitive decline and survival in Alzheimer’s disease according to education level. Dement. Geriatr. Cogn. Disord. 25, 74–80. 10.1159/000111693 [DOI] [PubMed] [Google Scholar]
- Brucki S. M., Nitrini R., Caramelli P., Bertolucci P. H., Okamoto I. H. (2003). Suggestions for utilization of the mini-mental state examination in Brazil. Arq. Neuropsiquiatr. 61, 777–781. 10.1590/S0004-282X2003000500014 [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- Canuet L., Pusil S., López M. E., Bajo R., Pineda-Pardo J. A., Cuesta P., et al. (2015). Network disruption and cerebrospinal fluid amyloid-β and phospho-tau levels in mild cognitive impairment. J. Neurosci. 35, 10325–10330. 10.1523/JNEUROSCI.0704-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebi O., Uzdogan A., Oguz K. K., Has A. C., Dolgun A., Cakmakli G. Y., et al. (2016). Default mode network connectivity is linked to cognitive functioning and CSF Aβ1–42 levels in Alzheimer’s disease. Arch. Gerontol. Geriatr. 62, 125–132. 10.1016/j.archger.2015.09.010 [DOI] [PubMed] [Google Scholar]
- Colangeli S., Boccia M., Verde P., Guariglia P., Bianchini F., Piccardi L. (2016). Cognitive reserve in healthy aging and Alzheimer’s disease: a meta-analysis of fMRI studies. Am. J. Alzheimers Dis. Other Demen. 31, 443–449. 10.1177/1533317516653826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daianu M., Mezher A., Jahanshad N., Hibar D. P., Nir T. M., Jack C. R., Jr., et al. (2015). Spectral graph theory and graph energy metrics show evidence For The Alzheimer’s disease disconnection syndrome in Apoe-4 risk gene carriers. Proc. IEEE Int. Symp. Biomed. Imaging 2015, 458–461. 10.1109/isbi.2015.7163910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos B. M., Coan A. C., Lin Yasuda C., Casseb R. F., Cendes F. (2016). Large-scale brain networks are distinctly affected in right and left mesial temporal lobe epilepsy. Hum. Brain Mapp. 37, 3137–3152. 10.1002/hbm.23231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Ser T., Hachinski V., Merskey H., Munoz D. G. (1999). An autopsy-verified study of the effect of education on degenerative dementia. Brain 122, 2309–2319. 10.1093/brain/122.12.2309 [DOI] [PubMed] [Google Scholar]
- Fazekas F., Chawluk J., Alavi A., Hurtig H., Zimmerman R. (1987). MRI signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJNR Am. J. Roentgenol. 149, 351–356. [DOI] [PubMed] [Google Scholar]
- Fischer F. U., Wolf D., Scheurich A., Fellgiebel A. (2014). Association of structural global brain network properties with intelligence in normal aging. PLoS One 9:e86258. 10.1371/journal.pone.0086258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza O. V., Radanovic M., Talib L. L., Aprahamian I., Diniz B. S., Zetterberg H., et al. (2015). Cerebrospinal fluid biomarkers in Alzheimer’s disease: diagnostic accuracy and prediction of dementia. Alzheimers Dement. 1, 455–463. 10.1016/j.dadm.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier N., Caballero M. A., Taylor A. N., Simon-Vermot L., Buerger K., Ertl-Wagner B., et al. (2017). Resting-state global functional connectivity as a biomarker of cognitive reserve in mild cognitive impairment. Brain Imaging Behav. 11, 368–382. 10.1007/s11682-016-9599-1 [DOI] [PubMed] [Google Scholar]
- Guo L. H., Alexopoulos P., Wagenpfeil S., Kurz A., Perneczky R. (2013). Brain size and the compensation of Alzheimer’s disease symptoms: a longitudinal cohort study. Alzheimers Dement. 9, 580–586. 10.1016/j.jalz.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Hachinski V. C., Iliff L. D., Zilhka E., Du Boulay G. H., McAllister V. L., Marshall J., et al. (1975). Cerebral blood flow in dementia. Arch. Neurol. 32, 632–637. 10.1001/archneur.1975.00490510088009 [DOI] [PubMed] [Google Scholar]
- Harris P., Fernandez Suarez M., Surace E. I., Chrem Méndez P., Martín M. E., Clarens M. F., et al. (2015). Cognitive reserve and Aβ1–42 in mild cognitive impairment (Argentina-Alzheimer’s disease neuroimaging Initiative). Neuropsychiatr. Dis. Treat. 11, 2599–2604. 10.2147/NDT.S84292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihunwo A. O., Tembo L. H., Dzamalala C. (2016). The dynamics of adult neurogenesis in human hippocampus. Neural Regen. Res. 11, 1869–1883. 10.4103/1673-5374.195278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R., Jr., Knopman D. S., Jagust W. J., Shaw L. M., Aisen P. S., Weiner M. W., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128. 10.1016/s1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. J., Mormino E. C. (2011). Lifespan brain activity, β-amyloid, and Alzheimer’s disease. Trends Cogn. Sci. 15, 520–526. 10.1016/j.tics.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Huang H., Abner E., Broster L. S., Jicha G. A., Schmitt F. A., et al. (2016). Alzheimer’s biomarkers are correlated with brain connectivity in older adults differentially during resting and task states. Front. Aging Neurosci. 8:15. 10.3389/fnagi.2016.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C. B., Svensson M., Wallstedt L., Janson A. M., Frisén J. (1999). Neural stem cells in the adult human brain. Exp. Cell Res. 253, 733–736. 10.1006/excr.1999.4678 [DOI] [PubMed] [Google Scholar]
- Khazaee A., Ebrahimzadeh A., Babajani-Feremi A. (2017). Classification of patients with MCI and AD from healthy controls using directed graph measures of resting-state fMRI. Behav. Brain Res. 322, 339–350. 10.1016/j.bbr.2016.06.043 [DOI] [PubMed] [Google Scholar]
- Kruschwitz J. D., List D., Waller L., Rubinov M., Walter H. (2015). GraphVar: a user-friendly toolbox for comprehensive graph analyses of functional brain connectivity. J. Neurosci. Methods 245, 107–115. 10.1016/j.jneumeth.2015.02.021 [DOI] [PubMed] [Google Scholar]
- Landau S. M., Marks S. M., Mormino E. C., Rabinovici G. D., Oh H., O’Neil J. P., et al. (2012). Association of lifetime cognitive engagement and low β-amyloid deposition. Arch. Neurol. 69, 623–629. 10.1001/archneurol.2011.2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O., Robinson J., Tang Y. P., Hairston I. S., Korade-Mirnics Z., Lee V. M., et al. (2005). Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell 120, 701–713. 10.1016/j.cell.2005.01.015 [DOI] [PubMed] [Google Scholar]
- Li X., Li T. Q., Andreasen N., Wiberg M. K., Westman E., Wahlund L. O. (2013). Ratio of Aβ42/P-tau181p in CSF is associated with aberrant default mode network in AD. Sci. Rep. 3:1339. 10.1038/srep01339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Julkunen V., Paajanen T., Westman E., Wahlund L. O., Aitken A., et al. (2012). Education increases reserve against Alzheimer’s disease—evidence from structural MRI analysis. Neuroradiology 54, 929–938. 10.1007/s00234-012-1005-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., An Y., Guo J., Zhang X., Wang H., Rong H., et al. (2016). Dietary intake of nutrients and lifestyle affect the risk of mild cognitive impairment in the chinese elderly population: a cross-sectional study. Front. Behav. Neurosci. 10:229. 10.3389/fnbeh.2016.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques P., Soares J. M., Magalhaes R., Santos N. C., Sousa N. (2015). The bounds of education in the human brain connectome. Sci. Rep. 5:12812. 10.1038/srep12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G. M. (2011). Changing concepts of Alzheimer disease. JAMA 305, 2458–2459. 10.1001/jama.2011.810 [DOI] [PubMed] [Google Scholar]
- Morris J. C. (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Morris J. C., Roe C. M., Xiong C., Fagan A. M., Goate A. M., Holtzman D. M., et al. (2010). APOE predicts amyloid-β but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 67, 122–131. 10.1002/ana.21843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M., Perfilieva E., Johansson U., Orwar O., Eriksson P. S. (1999). Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 39, 569–578. [DOI] [PubMed] [Google Scholar]
- Osone A., Arai R., Hakamada R., Shimoda K. (2015). Impact of cognitive reserve on the progression of mild cognitive impairment to Alzheimer’s disease in Japan. Geriatr. Gerontol. Int. 15, 428–434. 10.1111/ggi.12292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneczky R., Drzezga A., Diehl-Schmid J., Schmid G., Wohlschläger A., Kars S., et al. (2006). Schooling mediates brain reserve in Alzheimer’s disease: findings of fluoro-deoxy-glucose-positron emission tomography. J. Neurol. Neurosurg. Psychiatry 77, 1060–1063. 10.1136/jnnp.2006.094714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer R. I., Kurosaki T. T., Harrah C. H., Jr., Chance J. M., Filos S. (1982). Measurement of functional activities in older adults in the community. J. Gerontol. 37, 323–329. 10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- Qiu T., Luo X., Shen Z., Huang P., Xu X., Zhou J., et al. (2016). Disrupted brain network in progressive mild cognitive impairment measured by eigenvector centrality mapping is linked to cognition and cerebrospinal fluid biomarkers. J. Alzheimers Dis. 54, 1483–1493. 10.3233/jad-160403 [DOI] [PubMed] [Google Scholar]
- Rentz D. M., Locascio J. J., Becker J. A., Moran E. K., Eng E., Buckner R. L., et al. (2010). Cognition, reserve, and amyloid deposition in normal aging. Ann. Neurol. 67, 353–364. 10.1002/ana.21904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I. H. (2013). A noradrenergic theory of cognitive reserve: implications for Alzheimer’s disease. Neurobiol. Aging 34, 298–308. 10.1016/j.neurobiolaging.2012.05.019 [DOI] [PubMed] [Google Scholar]
- Roe C. M., Mintun M. A., D’Angelo G., Xiong C., Grant E. A., Morris J. C. (2008). Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch. Neurol. 65, 1467–1471. 10.1001/archneur.65.11.1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. (2011). Weight-conserving characterization of complex functional brain networks. Neuroimage 56, 2068–2079. 10.1016/j.neuroimage.2011.03.069 [DOI] [PubMed] [Google Scholar]
- Rzezak P., Squarzoni P., Duran F. L., de Toledo Ferraz Alves T., Tamashiro-Duran J., Bottino C. M., et al. (2015). Relationship between brain age-related reduction in gray matter and educational attainment. PLoS One 10:e0140945. 10.1371/journal.pone.0140945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E., Rossi S., Rossi A. (2015). The smarter, the stronger: intelligence level correlates with brain resilience to systematic insults. Cortex 64, 293–309. 10.1016/j.cortex.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Sanz-Arigita E. J., Schoonheim M. M., Damoiseaux J. S., Rombouts S. A., Maris E., Barkhof F., et al. (2010). Loss of ‘small-world’ networks in Alzheimer’s disease: graph analysis of fMRI resting-state functional connectivity. PLoS One 5:e13788. 10.1371/journal.pone.0013788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Albert S. M., Manly J. J., Stern Y. (2006). Education and rates of cognitive decline in incident Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 77, 308–316. 10.1136/jnnp.2005.072306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Levy G., Tang M. X., Manly J., Stern Y. (2001). Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 57, 2236–2242. 10.1212/WNL.57.12.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Stern Y. (2003). Cognitive reserve and lifestyle. J. Clin. Exp. Neuropsychol. 25, 625–633. 10.1076/jcen.25.5.625.14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Zarahn E., Anderson K. E., Habeck C. G., Hilton J., Flynn J., et al. (2003). Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch. Neurol. 60, 359–365. 10.1001/archneur.60.3.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra L., Cercignani M., Petrosini L., Basile B., Perri R., Fadda L., et al. (2011). Neuroanatomical correlates of cognitive reserve in Alzheimer disease. Rejuvenation Res. 14, 143–151. 10.1089/rej.2010.1103 [DOI] [PubMed] [Google Scholar]
- Serra L., Mancini M., Cercignani M., Di Domenico C., Spanò B., Giulietti G., et al. (2017). Network-based substrate of cognitive reserve in Alzheimer’s disease. J. Alzheimers Dis. 55, 421–430. 10.3233/jad-160735 [DOI] [PubMed] [Google Scholar]
- Shirer W. R., Ryali S., Rykhlevskaia E., Menon V., Greicius M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 22, 158–165. 10.1093/cercor/bhr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A., Pettigrew C., Li S., Wang M. C., Moghekar A., Selnes O. A., et al. (2013). Relationship of cognitive reserve and cerebrospinal fluid biomarkers to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Neurobiol. Aging 34, 2827–2834. 10.1016/j.neurobiolaging.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé-Padullés C., Bartrés-Faz D., Junqué C., Vendrell P., Rami L., Clemente I. C., et al. (2009). Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 30, 1114–1124. 10.1016/j.neurobiolaging.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Song M., Zhou Y., Li J., Liu Y., Tian L., Yu C., et al. (2008). Brain spontaneous functional connectivity and intelligence. Neuroimage 41, 1168–1176. 10.1016/j.neuroimage.2008.02.036 [DOI] [PubMed] [Google Scholar]
- Stam C. J., Jones B. F., Nolte G., Breakspear M., Scheltens P. (2007). Small-world networks and functional connectivity in Alzheimer’s disease. Cereb. Cortex 17, 92–99. 10.1093/cercor/bhj127 [DOI] [PubMed] [Google Scholar]
- Stern Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. 10.1017/s1355617702813248 [DOI] [PubMed] [Google Scholar]
- Stern Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 11, 1006–1012. 10.1016/s1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Habeck C., Moeller J., Scarmeas N., Anderson K. E., Hilton H. J., et al. (2005). Brain networks associated with cognitive reserve in healthy young and old adults. Cereb. Cortex 15, 394–402. 10.1093/cercor/bhh142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Menon V., Rubin D., Musen M., Greicius M. D. (2008). Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput. Biol. 4:e1000100. 10.1371/journal.pcbi.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiola T., Alafuzoff I., Herukka S. K., Parkkinen L., Hartikainen P., Soininen H., et al. (2009). Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 66, 382–389. 10.1001/archneurol.2008.596 [DOI] [PubMed] [Google Scholar]
- van den Heuvel M. P., Stam C. J., Kahn R. S., Hulshoff Pol H. E. (2009). Efficiency of functional brain networks and intellectual performance. J. Neurosci. 29, 7619–7624. 10.1523/JNEUROSCI.1443-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F. H. (2000). Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198. 10.1038/35044558 [DOI] [PubMed] [Google Scholar]
- Wang L., Brier M. R., Snyder A. Z., Thomas J. B., Fagan A. M., Xiong C., et al. (2013). Cerebrospinal fluid Aβ42, phosphorylated Tau181, and resting-state functional connectivity. JAMA Neurol. 70, 1242–1248. 10.1001/jamaneurol.2013.3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang X., He Y., Yu X., Wang H. (2015). Apolipoprotein E epsilon4 modulates functional brain connectome in Alzheimer’s disease. Hum. Brain Mapp. 36, 1828–1846. 10.1002/hbm.22740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zuo X., Dai Z., Xia M., Zhao Z., Zhao X., et al. (2013). Disrupted functional brain connectome in individuals at risk for Alzheimer’s disease. Biol. Psychiatry 73, 472–481. 10.1016/j.biopsych.2012.03.026 [DOI] [PubMed] [Google Scholar]
- Yaffe K., Weston A., Graff-Radford N. R., Satterfield S., Simonsick E. M., Younkin S. G., et al. (2011). Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA 305, 261–266. 10.1001/jama.2010.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E. T. (2010a). Network-based statistic: identifying differences in brain networks. Neuroimage 53, 1197–1207. 10.1016/j.neuroimage.2010.06.041 [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Harding I. H., Cocchi L., Yucel M., Pantelis C., et al. (2010b). Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage 50, 970–983. 10.1016/j.neuroimage.2009.12.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.