Abstract
Background:
Mounts of studies have shown that low estimated glomerular filtration rate (eGFR) is associated with increased risk of adverse outcomes in patients with coronary artery disease. However, high level of eGFR was less reported. In the study, we aimed to explore the relationship between the baseline eGFR, especially the high level, and contrast-induced acute kidney injury (CI-AKI) in a Chinese population who underwent an emergency percutaneous coronary intervention (PCI).
Methods:
Patients who underwent an emergency PCI from 2013 to 2015 were enrolled and divided into five groups as eGFR decreasing. Baseline characteristics were collected and analyzed. The rates of CI-AKI and the composite endpoint (including nonfatal myocardial infarction, revascularization, stroke, and all-cause death) at 6- and 12-month follow-up were compared. Logistic analysis for CI-AKI was performed.
Results:
A total of 1061 patients were included and the overall CI-AKI rate was 22.7% (241/1061). The separate rates were 77.8% (7/9) in Group 1 (eGFR ≥120 ml·min−1·1.73 m−2), 26.0% (118/454) in Group 2 (120 ml·min−1·1.73 m−2> eGFR ≥90 ml·min−1·1.73m−2), 18.3% (86/469) in Group 3 (90 ml·min−1·1.73 m−2> eGFR ≥60 ml·min−1·1.73 m−2), 21.8% (26/119) in Group 4 (60 ml·min−1·1.73 m−2> eGFR ≥30 ml·min−1·1.73 m−2), and 40.0% (4/10) in Group 5 (eGFR <30 ml·min−1·1.73 m−2), with statistical significance (χ2 = 25.19, P < 0.001). The rates of CI-AKI in five groups were 77.8%, 26.0%, 18.3%, 21.8%, and 40.0%, respectively, showing a U-typed curve as eGFR decreasing (the higher the level of eGFR, the higher the CI-AKI occurrence in case of eGFR ≥60 ml·min−1·1.73 m−2). The composite endpoint rates in five groups were 0, 0.9%, 2.1%, 6.7%, and 0 at 6-month follow-up, respectively, and 0, 3.3%, 3.4%, 16.0%, and 30.0% at 12-month follow-up, respectively, both with significant differences (χ2 = 16.26, P = 0.009 at 6-month follow-up, and χ2 = 49.05, P < 0.001 at 12-month follow-up). The logistic analysis confirmed that eGFR was one of independent risk factors of CI-AKI in emergency PCI patients.
Conclusions:
High level of eGFR might be associated with increased risk of CI-AKI in patients with emergency PCI, implying for future studies and risk stratification in clinical practice.
Keywords: Contrast-Induced Acute Kidney Injury, Emergency Percutaneous Coronary Intervention, Estimated Glomerular Filtration Rate
摘要
背景:
大量研究显示,在冠心病患者中低水平的估算肾小球滤过率(eGFR,estimated glomerular filtration rate)与不良临床结 局相关。然而,关于高水平的eGFR却鲜有报道。本研究目的是在行急诊经皮冠状动脉介入治疗(PCI,percutaneous coronary intervention)的中国人群中探索基线eGFR水平–尤其是高水平eGFR–与对比剂诱导的急性肾损伤(CI-AKI,contrast-induced acute kidney injury)之间的关系。
方法:
将2013-2015年间于我院行急诊PCI的患者纳入研究,并根据术前eGFR水平将患者分为5组。收集并分析患者基线资料, 对比各组CI-AKI的发生率和术后6月、12月复合终点事件(包括非致死性心肌梗死、再次血运重建、卒中和全因死亡)发生率的 差异,并对CI-AKI的危险因素进行Logistic分析。
结果:
本研究共纳入1061例患者,总CI -AKI发生率为22.7%(241/1061)。。随eGFR水平的降低, 各组CI - AKI发生率分别为77.8%(7/9)(组1: eGFR ≥ 120 ml·min-1·1.73m-2),26.0%(118/454)(组2: 120 ml·min-1·1.73m-2 > eGFR ≥ 90 ml·min-1·1.73m-2),18.3%(86/469)(组3:90 ml·min-1·1.73m-2 > eGFR ≥ 60 ml·min-1·1.73m-2),21.8%(26/119)(组4:60 ml·min-1·1.73m-2 > eGFR ≥ 30 ml·min-1·1.73m-2),和40.0%(4/10)(组5:eGFR < 30 ml·min-1·1.73m-2),具有显著的统计学差 异(χ2=25.19,P<0.001)。同时可见,随eGFR下降CI-AKI发生率呈U型,即在一定范围内(eGFR ≥ 60 ml·min-1·1.73m-2),eGFR 水平越高,CI-AKI发生率越高。术后6月5组患者复合终点事件发生率分别为0,0.9%,2.1%,6.7%,和 0,术后12月分别为 0,3.3%,3.4%,16.0%,和30.0%,均具有统计学差异(术后6月χ2=16.26,P=0.009,术后12月χ2=49.05,P<0.001)。Logistic分 析证实,在行急诊PCI的患者中eGFR是CI-AKI的独立危险因素。
结论:
在行急诊PCI 的患者中,高水平的eGFR可能增加术后CI-AKI的发生风险。
INTRODUCTION
With wide application of percutaneous coronary intervention (PCI) technology in patients with coronary artery disease (CAD), contrast-induced acute kidney injury (CI-AKI) has become one of serious complications, especially in those with acute coronary syndrome (ACS). CI-AKI is reported to be associated with increased morbidity and mortality[1,2,3,4,5] and has become the third leading cause of AKI in hospitalized patients.[6] The incidence of CI-AKI ranges from 2% to 30% due to different study populations and CI-AKI definitions.[7,8] The pathogenesis behind CI-AKI has not been completely understood,[1] and there is no effective treatment to CI-AKI, emphasizing the need for clinical prevention efforts.[3,9,10,11,12]
Mounts of studies have shown that a high serum creatinine (SCr) or a low estimated glomerular filtration rate (eGFR) indicates renal dysfunction and predicts increasing CI-AKI in patients with PCI.[13,14] However, several reports led to the conclusion that both a lower and higher eGFRs might be associated with increased risk of adverse outcomes comparing to the intermediate eGFR level.[15,16] There is no published study showing the exact relationship of high level of eGFR and CI-AKI development in CAD patients. In the study, we aimed to explore the relationship between the baseline eGFR, especially the high level, and CI-AKI incidence in a Chinese population who underwent an emergency PCI.
METHODS
Ethical approval
The study protocol conforms to the ethical guidelines of the 1964 Declaration of Helsinki and its later amendments. As a retrospective study, this study was exempt from the informed consent from patients.
Study population
From January 1, 2013, to June 30, 2015, patients who underwent an emergency PCI at Fuwai Hospital in Beijing, China, were consecutively enrolled in the study. The inclusion criterion was patients who underwent an emergency PCI, and the exclusion criteria were patients (1) who had contact with contrast medium <1 week before the procedure; (2) who were allergic to iodinated contrast medium; (3) who presented with severe heart failure (left ventricular ejection fraction [LVEF] <30%), cardiac shock, and serious valvular heart disease; (4) who had contact with any nephrotoxic medicine in 2 weeks before the procedure; and (5) who presented with severe hepatic disease, thyroid dysfunction, malignant carcinoma, or infectious disease.
Protocol and definition
Patients conforming to the inclusion and exclusion criteria were included in the study and divided into five groups according to the baseline eGFR: Group 1 including patients with eGFR ≥120 ml·min−1·1.73 m−2, Group 2 with 120 ml·min−1·1.73 m−2> eGFR ≥90 ml·min−1·1.73 m−2, Group 3 with 90 ml·min−1·1.73 m−2> eGFR ≥60 ml·min−1·1.73 m−2, Group 4 with 60 ml·min−1·1.73 m−2> eGFR ≥30 ml·min−1·1.73 m−2, and Group 5 with eGFR <30 ml·min−1·1.73 m−2. The clinical data of the patients were obtained through a review of the medical records. Blood and urine sampling measurements were operated by the clinical testing center of Fuwai Hospital. The data about intervention therapy were recorded and reported by operators and their assistants. The follow-up information was obtained from the follow-up team. The baseline clinical and procedural characteristics of the patients were collected and analyzed. The rates of CI-AKI and the composite endpoint events (including nonfatal myocardial infarction [MI], revascularization, stroke, and all-cause death) at 6- and 12-month follow-up of five groups were compared. Logistic analysis for CI-AKI was performed to confirm the effect of eGFR in the development of CI-AKI in emergency PCI patients. CI-AKI was defined as an increase in SCr concentration ≥44.2 μmol/L or ≥25% above baseline within 72 h after exposure to contrast medium.
Hydration therapy had begun to be carried out since patients’ admission to emergency department, which was performed with 1 ml·kg−1·h−1 of normal saline (0.9%) before procedure and 18–24 h after procedure. In those with reduced LVEF (<40%), presence of significant valvular disease, or overt heart failure upon presentation, the hydration rate was reduced to 0.5 ml·kg−1·h−1. Several kinds of nonionic, low, or iso-osmolar contrast medium, for example, iopromide (370 mg iodine/ml, Ultravist, Bayer, Guangzhou, China) and iodixanol (320 mg iodine/ml, Visipaque, GE Healthcare, Shanghai, China), were administered during the procedures. SCr concentration of each patient was routinely measured before procedure, and at least one postprocedural SCr testing was performed within 72 h after procedure. The baseline eGFR was calculated from baseline SCr using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation:[17,18] eGFR (ml·min−1·1.73 m−2) = 141 × min (SCr/k, 1)a × max (SCr/k, 1)−1.209 × 0.993age × 1.018 (if female) × 1.159 (if black) where SCr is serum creatinine (mg/dl), k is 0.7 for females and 0.9 for males, a is −0.329 for females and −0.411 for males, min indicates the minimum of SCr/k or 1, and max indicates the maximum of SCr/k or 1, which was performed by the CKD-EPI calculator on the website (http://www.qxmd.com/calculate-online/nephrology/ckd-epi-egfr).
Statistical analysis
Continuous variables were expressed as median and interquartile range, and Kruskal-Wallis test was used to evaluate differences among groups. Categorical variables were shown as count and percentage, and comparisons among groups were performed with Chi-square test or Fisher's exact test as appropriate. Univariate and multivariate logistic analysis was used to evaluate the risk factors for CI-AKI. A two-sided P < 0.05 was considered to indicate statistical significance. All tests were performed using the IBM SPSS statistical software package version 22 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Study population
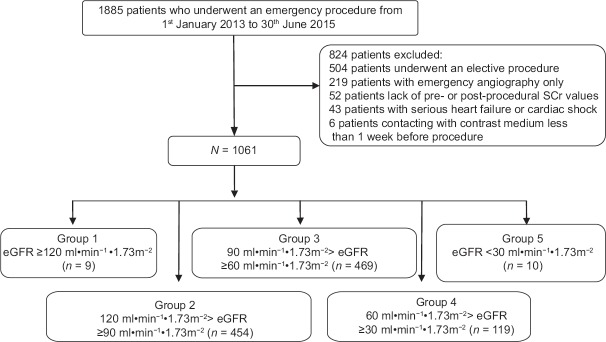
A total of 1885 consecutive patients who underwent an emergency PCI were initially enrolled in this study. Of 504 patients who underwent elective PCI operations, 219 patients underwent emergency coronary angiography only, 52 patients lacked pre- or postprocedural SCr values, 43 patients had serious heart failure or cardiac shock, and 6 patients had contact with contrast medium <1 week before the procedure. A total of 1061 patients were finally included in the study; males accounted for 80.7% (856/1061). Among the patients, there were 9 patients in Group 1 with eGFR ≥120 ml·min−1·1.73 m−2, 454 patients in Group 2 with 120 ml·min−1·1.73 m−2> eGFR ≥90 ml·min−1·1.73 m−2, 469 patients in Group 3 with 90 ml·min−1·1.73 m−2> eGFR ≥60 ml·min−1·1.73 m−2, 119 patients in Group 4 with 60 ml·min−1·1.73 m−2> eGFR ≥30 ml·min−1·1.73 m−2, and 10 patients in Group 5 with eGFR <30 ml·min−1·1.73 m−2, as displayed in Figure 1.
Figure 1.
Patient flowchart of the study. SCr: Serum creatinine; eGFR: Estimated glomerular filtration rate.
Baseline clinical and procedural characteristics of patients with emergency percutaneous coronary intervention
The baseline clinical and procedural characteristics of emergency PCI patients are shown in Table 1. The ages in Groups 1–5 were 35 (29, 39) years, 53 (46, 59) years, 63 (56, 70) years, 71 (61, 78) years, and 74 (69, 79) years, respectively, with a significant difference (H = 325.75, P < 0.001), indicating that five groups might be with five different age ranges. Besides, most of the included variables showed significant differences among groups, including male, height, weight, smoking, hypertension, diabetes mellitus (DM), history of MI, history of transient ischemic attack/stroke, LVEF, history of statin contact, white blood cell count, hemoglobin (Hb) level, platelet, alanine aminotransferase, fasting glucose, triglyceride, total cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, erythrocyte sedimentation rate (ESR), high-sensitive C-reactive protein, big endothelin-1 (ET-1), SCr, intra-aortic balloon pump, left anterior descending (LAD) stented, administration of clopidogrel, platelet glycoprotein IIa/IIIb receptor antagonist, angiotensin-converting enzyme inhibitor, β-receptor blocker, and diuretic.
Table 1.
Baseline clinical and procedural characteristics of patients who underwent an emergency PCI
| Variables | Group 1 (n = 9) | Group 2 (n = 454) | Group 3 (n = 469) | Group 4 (n = 119) | Group 5 (n = 10) | Statistics | P |
|---|---|---|---|---|---|---|---|
| Age (years) | 35 (29, 39) | 53 (46, 59) | 63 (56, 70) | 71 (61, 78) | 74 (69, 79) | 325.75* | <0.001 |
| Male | 9 (100) | 395 (87.0) | 376 (80.2) | 69 (58.0) | 7 (70.0) | 53.94† | <0.001 |
| Height (cm) | 168 (165, 171) | 170 (166, 174) | 170 (164, 173) | 170 (160, 172) | 170 (157, 172) | 19.21* | 0.001 |
| Weight (kg) | 65 (63, 83) | 75 (68, 85) | 72 (65, 80) | 70 (60, 79) | 67 (58, 72) | 32.25* | <0.001 |
| Smoking | 7 (77.8) | 333 (73.3) | 319 (68.0) | 66 (55.5) | 4 (40.0) | 18.54† | 0.001 |
| Hypertension | 9 (100) | 264 (58.1) | 309 (65.9) | 78 (65.5) | 9 (90.0) | 15.01† | 0.005 |
| Hyperlipidemia | 7 (77.8) | 386 (85.0) | 398 (84.9) | 104 (87.4) | 10 (100) | 2.65† | 0.659 |
| DM | 1 (11.1) | 115 (25.3) | 131 (27.9) | 46 (38.7) | 6 (60.0) | 14.59† | 0.007 |
| History of MI | 0 (0) | 56 (12.3) | 89 (19.0) | 14 (11.8) | 3 (30.0) | 12.43† | 0.014 |
| History of TIA/stroke | 0 (0) | 46 (10.1) | 84 (17.9) | 32 (26.9) | 5 (50.0) | 34.12† | <0.001 |
| SBP (mmHg) | 125 (121, 140) | 124 (115, 138) | 123 (115, 135) | 124 (112, 136) | 135 (104, 163) | 2.90* | 0.575 |
| LVEF | 56 (48, 61) | 56 (50, 60) | 55 (49, 59) | 54 (45, 58) | 52 (39, 58) | 18.11† | 0.001 |
| History of statin contact | 0 (0) | 86 (18.9) | 134 (28.6) | 25 (21.0) | 4 (40.0) | 16.66† | 0.002 |
| Laboratory characteristics | |||||||
| WBC (×109/L) | 14.32 (12.30, 15.79) |
10.68 (8.55, 12.76) |
10.17 (8.28, 12.59) |
11.17 (8.82, 13.65) |
9.72 (8.10, 14.88) |
14.55* | 0.006 |
| Hb (×1012/L) | 155 (145, 159) | 151 (142, 158) | 145 (135, 155) | 137 (124, 153) | 119 (108, 135) | 72.58* | <0.001 |
| Platelet (×109/L) | 271 (221, 299) | 220 (188, 254) | 212 (177, 245) | 202 (169, 242) | 189 (156, 222) | 20.00* | 0.001 |
| ALT (IU/L) | 62 (33, 80) | 39 (29, 61) | 37 (24, 56) | 38 (24, 64) | 61 (30, 151) | 10.71* | 0.030 |
| Fasting glucose (mmol/L) | 5.68 (4.70, 8.32) | 6.72 (5.58, 8.79) | 6.71 (5.61, 8.50) | 7.66 (6.04, 9.92) | 8.11 (6.12, 9.21) | 13.35* | 0.010 |
| Triglyceride (mmol/L) | 1.48 (1.24, 2.54) | 1.54 (1.08, 2.28) | 1.31 (0.94, 1.80) | 1.39 (1.04, 1.89) | 0.92 (0.65, 1.46) | 34.50* | <0.001 |
| Total cholesterol (mmol/L) | 5.82 (4.36, 6.38) | 4.55 (4.00, 5.32) | 4.37 (3.73, 5.09) | 4.26 (3.62, 4.84) | 3.60 (2.90, 4.57) | 26.09* | <0.001 |
| HDL-c (mmol/L) | 0.93 (0.76, 1.13) | 0.97 (0.85, 1.17) | 1.02 (0.89, 1.21) | 0.99 (0.81, 1.21) | 0.92 (0.70, 1.21) | 9.92* | 0.042 |
| LDL-c (mmol/L) | 3.88 (2.88, 4.76) | 2.92 (2.37, 3.52) | 2.78 (2.21, 3.37) | 2.67 (2.07, 3.28) | 2.30 (1.45, 3.06) | 25.36* | <0.001 |
| HbA1C (%) | 5.6 (5.3, 7.9) | 6.0 (5.6, 7.1) | 6.1 (5.7, 6.6) | 6.2 (5.7, 7.2) | 6.8 (5.8, 8.6) | 7.35* | 0.118 |
| ESR (mm/h) | 6 (2, 11) | 6 (2, 9) | 7 (3, 13) | 10 (4, 25) | 29 (3, 42) | 41.14* | <0.001 |
| hs-CRP (mg/L) | 10.25 (7.56, 11.18) | 6.46 (2.45, 11.15) | 7.66 (3.04, 11.59) | 10.41 (4.38, 12.32) | 12.02 (3.07, 13.07) | 19.71* | 0.001 |
| Big ET-1 (pmol/L) | 0.35 (0.21, 0.48) | 0.31 (0.23, 0.40) | 0.35 (0.27, 0.50) | 0.45 (0.37, 0.73) | 1.17 (0.68, 1.61) | 117.45* | <0.001 |
| SCr | 58.8 (56.1, 62.0) | 74.0 (66.4, 79.7) | 88.8 (80.0, 96.9) | 116.3 (104.1, 130.1) | 200.4 (183.1, 211.0) | 545.93* | <0.001 |
| Procedural characteristics | |||||||
| Onset-to-balloon (h) | 8.0 (5.5, 12.0) | 7.0 (5.0, 10.0) | 7.5 (5.0, 12.0) | 7.0 (5.0, 10.0) | 8.0 (6.1, 37.5) | 7.51* | 0.111 |
| Door-to-balloon (min) | 118 (98, 202) | 120 (100, 170) | 120 (101, 169) | 120 (95, 167) | 150 (98, 220) | 1.05* | 0.903 |
| IABP | 1 (11.1) | 41 (9.0) | 61 (13.0) | 33 (27.7) | 6 (60.0) | 47.40† | <0.001 |
| LAD impaired | 7 (77.8) | 377 (83.0) | 408 (87.0) | 104 (87.4) | 9 (90.0) | 3.92† | 0.360 |
| LAD stented | 6 (66.7) | 207 (45.6) | 191 (40.7) | 36 (30.3) | 2 (20.0) | 13.68† | 0.008 |
| Volume of contrast medium >200 ml | 0 (0) | 34 (7.5) | 23 (4.9) | 4 (3.4) | 1 (10.0) | 5.19† | 0.250 |
| Medication | |||||||
| Aspirin | 9 (100) | 454 (100) | 466 (99.4) | 118 (99.2) | 10 (100) | 3.33† | 0.277 |
| Clopidogrel | 9 (100) | 454 (100) | 469 (100) | 117 (98.3) | 10 (100) | 15.86† | 0.048 |
| Heparin | 9 (100) | 440 (96.9) | 450 (95.9) | 109 (91.6) | 10 (100) | 7.69† | 0.161 |
| GPIIb/IIIa receptor antagonist | 7 (77.8) | 263 (57.9) | 246 (52.5) | 54 (45.4) | 4 (40.0) | 9.67† | 0.045 |
| Nitrates | 8 (88.9) | 405 (89.2) | 421 (89.8) | 100 (84.0) | 7 (70.0) | 6.72† | 0.124 |
| ACEI | 7 (77.8) | 376 (82.8) | 354 (75.5) | 83 (69.7) | 2 (20.0) | 31.50† | <0.001 |
| ARB | 0 (0) | 29 (6.4) | 48 (10.2) | 10 (8.4) | 1 (10.0) | 5.35† | 0.240 |
| β-blocker | 9 (100) | 402 (88.5) | 403 (85.9) | 92 (77.3) | 8 (80.0) | 11.76† | 0.022 |
| CCB | 1 (11.1) | 30 (6.6) | 31 (6.6) | 8 (6.7) | 2 (20.0) | 3.07† | 0.378 |
| Diuretic | 3 (33.3) | 161 (35.5) | 219 (46.7) | 63 (52.9) | 8 (80.0) | 23.87† | <0.001 |
| CI-AKI | 7 (77.8) | 118 (26.0) | 86 (18.3) | 26 (21.8) | 4 (40.0) | 25.19† | <0.001 |
Values are presented as count (%) or median (P25, P75). *H values; †χ2 values. 1 mmHg = 0.133 kPa. Group 1: eGFR ≥120 ml·min−1·1.73 m−2; Group 2: 120 ml·min−1·1.73 m−2> eGFR ≥90 ml·min−1·1.73 m−2; Group 3: 90 ml·min−1·1.73 m−2> eGFR ≥60 ml·min−1·1.73 m−2; Group 4: 60 ml·min−1·1.73 m−2> eGFR ≥30 ml·min−1·1.73 m−2; Group 5: eGFR <30 ml·min−1·1.73 m−2. PCI: Percutaneous coronary intervention; DM: Diabetes mellitus; MI: Myocardial infarction; TIA: Transient ischemic attack; SBP: Systolic blood pressure; LVEF: Left ventricular ejection fraction; WBC: White blood cell; Hb: Hemoglobin; ALT: Alanine aminotransferase; HDL-c: High-density lipoprotein-cholesterol; LDL-c: Low-density lipoprotein-cholesterol; HbA1C: Hemoglobin A1C; ESR: Erythrocyte sedimentation rate; hs-CRP: High-sensitive C-reactive protein; ET-1: Endothelin-1; SCr: Serum creatinine; IABP: Intra-aortic balloon pump; LAD: Left anterior descending; GPIIb/IIIa: Platelet glycoprotein IIb/IIIa; ACEI: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin II receptor blocker; CCB: Calcium channel blocker; CI-AKI: Contrast-induced acute kidney injury; eGFR: Estimated glomerular filtration rate.
Contrast-induced acute kidney injury rates in patients who underwent an emergency percutaneous coronary intervention
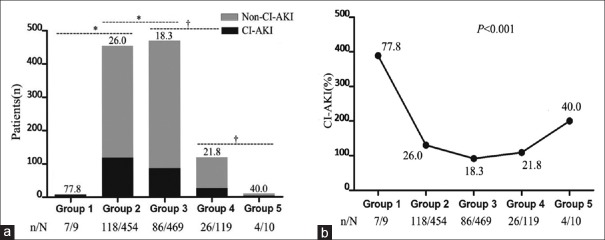
The overall incidence of CI-AKI in emergency PCI patients was 22.7% (241 of 1061 patients). From Groups 1 to 5, the CI-AKI rates were 77.8% (7/9), 26.0% (118/454), 18.3% (86/469), 21.8% (26/119), and 40.0% (4/10), respectively, with statistical significance (χ2 = 25.19, P < 0.001), as presented in Table 1 and Figure 2a. It was showing statistical significance between Groups 1 and 2 (77.8% vs. 26.0%, χ2 = 12.01, P = 0.002) and between Groups 2 and 3 (26.0% vs. 18.3%, χ2 = 7.85, P = 0.005) and showing no statistical difference between Groups 3 and 4 (18.3% vs. 21.8%, χ2 = 0.76, P = 0.433), or between Groups 4 and 5 (21.8% vs. 40.0%, χ2 = 1.70, P = 0.240). On the other hand, a U-type curve of the CI-AKI incidence was obvious as eGFR decreasing from Groups 1 to 5, and the rates of CI-AKI in Groups 1 and 5 were 2–4 times higher than those in Groups 2, 3, and 4, as shown in Figure 2b. Above all, it could be considered that the higher the level of eGFR, the higher the CI-AKI occurrence in case of eGFR ≥60 ml·min−1·1.73 m−2.
Figure 2.
The rates of CI-AKI and the trend in five groups. (a) Numbers and rates of CI-AKI in five groups as eGFR decreasing. *P < 0.05, †P > 0.05. Group 1: Group 2: 77.8% versus 26.0%, χ2 = 12.01, P = 0.002; Group 2: Group 3: 26.0% versus 18.3%, χ2 = 7.85, P = 0.005; Group 3: Group 4: 18.3% versus 21.8%, χ2 = 0.76, P = 0.433; Group 4: Group 5: 21.8% versus 40.0%, χ2 = 1.70, P = 0.240. (b) Trend of CI-AKI rates in five groups as eGFR decreasing. Group 1: eGFR ≥120 ml·min−1·1.73 m−2; Group 2: 120 ml·min−1·1.73 m−2> eGFR ≥90 ml·min−1·1.73 m−2; Group 3: 90 ml·min−1·1.73 m−2> eGFR ≥60 ml·min−1·1.73 m−2; Group 4: 60 ml·min−1·1.73 m−2> eGFR ≥30 ml·min−1·1.73 m−2; Group 5: eGFR <30 ml·min−1·1.73 m−2. CI-AKI: Contrast-induced acute kidney injury; eGFR: Estimated glomerular filtration rate.
Rates of composite endpoint events at 6- and 12-month follow-up in emergency percutaneous coronary intervention patients
The rates of composite endpoint events (including nonfatal MI, revascularization, stroke, and all-cause death) at 6- and 12-month follow-up in five groups are given in Table 2. The rates of composite endpoint events were 0, 0.9%, 2.1%, 6.7%, and 0 at 6-month follow-up from Groups 1 to 5, respectively, and 0, 3.3%, 3.4%, 16.0%, and 30.0% at 12-month follow-up, respectively, both with significant difference (χ2 = 16.26, P = 0.009 at 6-month follow-up, and χ2 = 49.05, P < 0.001 at 12-month follow-up). However, a U-type relationship of composite endpoint events at the follow-up period was not appeared as eGFR decreasing.
Table 2.
The rates of composite endpoint events at 6- and 12-month follow-up after emergency PCI procedure
| Composite endpoint events | Group 1 (n = 9) | Group 2 (n = 454) | Group 3 (n = 469) | Group 4 (n = 119) | Group 5 (n = 10) | χ2 | P |
|---|---|---|---|---|---|---|---|
| 6-month follow-up | 0 | 4 (0.9) | 10 (2.1) | 8 (6.7) | 0 | 16.26 | 0.009 |
| 12-month follow-up | 0 | 15 (3.3) | 16 (3.4) | 19 (16.0) | 3 (30.0) | 49.05 | <0.001 |
Values are presented as count (%). Composite endpoint events include nonfatal myocardial infarction, revascularization, stroke, and all-cause death. Group 1: eGFR ≥120 ml·min−1·1.73 m−2; Group 2: 120 ml·min−1·1.73 m−2> eGFR≥90 ml·min−1·1.73 m−2; Group 3: 90 ml·min−1·1.73 m−2> eGFR ≥60 ml·min−1·1.73 m−2; Group 4: 60 ml·min−1·1.73 m−2> eGFR ≥0 ml·min−1·1.73 m−2; Group 5: eGFR <30 ml·min−1·1.73 m−2. PCI: Percutaneous coronary intervention; eGFR: Estimated glomerular filtration rate.
Logistic analysis for contrast-induced acute kidney injury in emergency percutaneous coronary intervention patients
From the univariate and multivariate logistic analysis for CI-AKI in patients who underwent an emergency PCI, eGFR group (odds ratio 0.487, 95% confidence interval 0.376–0.630, Wald = 29.89, P < 0.001) was confirmed to be one of independent risk factors of CI-AKI. Besides, body weight, history of MI, LVEF, ESR, big ET-1, LAD stented, heparin, and diuretic were also shown with statistical significance for CI-AKI occurrence, as shown in Table 3.
Table 3.
Univariate and multivariate logistic analysis for CI-AKI in emergency PCI patients
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| β | OR | Wald | P | β | OR | Wald | P | |
| Weight | −0.027 | 0.973 (0.962–0.985) | 18.50 | <0.001 | −0.027 | 0.973 (0.960–0.987) | 14.99 | <0.001 |
| History of MI | 0.539 | 1.715 (1.186–2.478) | 8.24 | 0.004 | 0.502 | 1.652 (1.090–2.503) | 5.59 | 0.018 |
| LVEF | −0.070 | 0.932 (0.915–0.949) | 55.67 | <0.001 | −0.032 | 0.969 (0.946–0.993) | 6.38 | 0.012 |
| ESR | 0.017 | 1.017 (1.008–1.027) | 14.07 | <0.001 | 0.015 | 1.015 (1.005–1.026) | 8.37 | 0.004 |
| Big ET-1 | 0.858 | 2.358 (1.571–3.538) | 17.17 | <0.001 | 0.957 | 2.604 (1.568–4.322) | 13.69 | <0.001 |
| eGFR group | −0.241 | 0.785 (0.638–0.967) | 5.20 | 0.023 | −0.719 | 0.487 (0.376–0.630) | 29.89 | <0.001 |
| LAD stented | 0.911 | 2.486 (1.854–3.334) | 37.03 | <0.001 | 0.454 | 1.574 (1.096–2.262) | 6.02 | 0.014 |
| Heparin | −1.043 | 0.352 (0.190–0.655) | 10.88 | 0.001 | −1.107 | 0.331 (0.168–0.651) | 10.24 | 0.001 |
| Diuretic | 1.168 | 3.215 (2.381–4.340) | 58.16 | <0.001 | 0.643 | 1.903 (1.271–2.850) | 9.75 | 0.002 |
β: Logistic correlation coefficient; OR: Odds ratio; CI-AKI: Contrast-induced acute kidney injury; PCI: Percutaneous coronary intervention; MI: Myocardial infarction; LVEF: Left ventricular ejection fraction; ESR: Erythrocyte sedimentation rate; ET-1: Endothelin-1; eGFR: Estimated glomerular filtration rate; LAD: Left anterior descending.
DISCUSSION
With wide application of PCI technology in patients with CAD, CI-AKI has become one of serious complications, especially in those with ACS. CI-AKI is reported to be associated with increased morbidity and mortality[1,2,3,4,5] and has become the third leading cause of AKI in hospitalized patients.[6] The pathogenesis behind CI-AKI has not been completely understood,[1] and there is no effective treatment to CI-AKI, emphasizing the need for clinical prevention efforts.[3,9,10,11,12] Mounts of studies have shown that low level of eGFR indicates renal dysfunction and predicts increased CI-AKI rate in patients with PCI.[13,14] However, there is no published study showing the relationship of high level of eGFR and CI-AKI development in CAD patients up to now. In the study, we explored the relationship between the baseline eGFR, especially the high level, and CI-AKI incidence in a Chinese population who underwent an emergency PCI.
In the study, a total of 1061 patients were included and the overall CI-AKI incidence in emergency PCI patients was 22.7%. The CI-AKI rates were 77.8%, 26.0%, 18.3%, 21.8%, and 40.0% from Groups 1 to 5 as eGFR decreasing, respectively, with significant difference and showing a U-type trend. It was showing statistical significance in patients with high-level eGFRs, for example, between Groups 1 and 2 and between Groups 2 and 3, however, with no statistical difference between Groups 3 and 4 or between Groups 4 and 5. It could be considered that the higher the level of eGFR, the higher the CI-AKI occurrence in case of eGFR ≥60 ml·min−1·1.73 m−2. The rates of composite endpoint events were 0, 0.9%, 2.1%, 6.7%, and 0 at 6-month follow-up from Groups 1 to 5, respectively, and 0, 3.3%, 3.4%, 16.0%, and 30.0% at 12-month follow-up, respectively, both with significant differences but not showing the similar U-type association as eGFR decreasing.
The eGFR is a powerful predictor of adverse outcomes, but most attention has focused on studies in the setting of reduced eGFR, which is a cardinal manifestation of CKD, and may be more used in conjunction with higher risk of adverse outcomes. Several studies were linked with higher level of eGFR, and most of them were performed in patients with DM.[19,20] A Finnish study of 4201 people with type 1 DM reported an increased risk of mortality at lower and higher eGFRs, with lowest predicted mortality when eGFR was between 70 and 80 ml·min−1·1.73 m−2, [21,22] and glomerular hyperfiltration attributed the excess risk to incipient microalbuminuria – itself associated with adverse events.[23,24] Glomerular hyperfiltration was also seen in patients with type 2 DM in Yokoyama et al.'s[25] report, which indicated that a higher eGFR predicted GFR decline in Japanese normoalbuminuric patients with type 2 DM. Patients with type 2 DM with biopsy-proven diabetic nephropathy showed more GFR decrease during a 1-year interval when the patients had higher GFRs,[26] indicating that glomerular hyperfiltration is an early-phase phenomenon in diabetic nephropathy.[20] However, data about higher eGFR from nondiabetic populations are more limited. Moriya et al.[15] reported that patients with eGFR ≥120 ml·min−1·1.73 m−2 had a risk for rapid GFR decline. Tonelli et al.[16] showed that an elevated eGFR predicted all-cause mortality at the follow-up period, and they also revealed that the adjusted risk of all-cause mortality was lowest at a middle level of eGFR and increased at both lower and higher levels. These reports are compatible to the results of the study, from which patients with a higher or lower level of eGFR might be with an increased risk of CI-AKI after emergency PCI comparing to those with intermediate levels.
The causes of this phenomenon may be different in DM and non-DM populations. Among people with DM, higher levels of eGFR correlate with the presence of glomerular hyperfiltration, a pathophysiological stage of diabetic nephropathy associated with incipient loss of kidney function.[21] However, the explanation of higher eGFR associated with adverse clinical outcomes independently of diabetic status is less well documented. First, from the previous studies, the incipient DM might partially account for the higher risk of CI-AKI in patients with higher eGFR, though the status of DM, fasting glucose, or HbA1C level in this study was not shown to be linked with CI-AKI occurrence in five groups and not proved to be an independent risk factor for CI-AKI. The second possible cause might be vascular disease according to the study by Tonelli et al.[16] In the present study, the level of big ET-1 was shown as a significant risk factor of CI-AKI which might have something with vascular disease and renal hypoperfusion, though the exact mechanism has not been confirmed. Third, it is important to recognize that higher eGFR may be not corresponding to higher true eGFR, as higher eGFR correlates with lower SCr and lower muscle mass.[16] Considering about the age difference of five groups and the risk effect of body weight for CI-AKI from the results, the younger and elder patients or those with a small weight – who were with a small body mass and therefore a small production of SCr – might be with an increased risk of CI-AKI after emergency PCI. It has also been identified that a small body surface area is linked with an increased risk of CI-AKI in emergency PCI patients in our previous studies.[27,28] Forth, the present findings might be as a result of the high sensitivity of the CI-AKI definition and the special nature of the study population.[11,27] A higher eGFR usually came from a relatively lower SCr correspondingly, which was more apt to be diagnosed as CI-AKI due to a slight but ≥25% fluctuation of SCr above baseline. Moreover, the fact is that a small fluctuation is easily occurring in emergency PCI patients.[27] The last, inaccurate estimates of eGFR at very low levels of SCr might be existed.[16] We do not know, however, whether the finding at higher eGFR could be due to inadequacies of the eGFR formula at low SCr levels. Besides the possible explanations mentioned above, the rates of composite endpoint events in the follow-up period, though with significant differences, were not showing the similar U-type association as the baseline eGFR decreasing. Therefore, our data do not allow us to confirm or refute the speculation.
Even if the apparent link between eGFR and CI-AKI occurrence in the study is not causal, recognition of this association might imply for future studies and perhaps for risk stratification in clinical practice – as is the case for other clinical parameters with prognostic power, such as low SCr, younger or elder of age, and small body weight. Moreover, the present study also confirmed the effect of the risk factors for CI-AKI in emergency PCI patients, including weight, history of MI, LVEF, ESR, big ET-1, eGFR level, LAD stented, heparin, and diuretic use, similar to the previous published reports.[27,29]
There are several limitations in the study. First, the present study was based on patients enrolled from a single center and the data were collected retrospectively. Therefore, our results are subjected to limitations inherent to the observational nature of a retrospectively collected database. Second, due to the regular application of periprocedural hydration, we could not confirm the concrete and specific influence exerted on the true baseline SCr before intervention. Third, the small number of patients in Groups 1 and 5 may lead to bias or mistakes in the results, and the follow-up period may be not long enough to lead to the similar finding. Therefore, large-sized and randomized studies are expected to be performed in future to confirm the findings and explore the mechanisms.
In conclusion, besides low level of eGFR, high level might be also associated with increased risk of CI-AKI in patients with emergency PCI, which implies for future studies and risk stratification in clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–28. doi: 10.1016/j.jacc.2007.12.035. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 2.James MT, Ghali WA, Tonelli M, Faris P, Knudtson ML, Pannu N, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78:803–9. doi: 10.1038/ki.2010.258. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 3.Pyxaras SA, Sinagra G, Mangiacapra F, Perkan A, Di Serafino L, Vitrella G, et al. Contrast-induced nephropathy in patients undergoing primary percutaneous coronary intervention without acute left ventricular ejection fraction impairment. Am J Cardiol. 2013;111:684–8. doi: 10.1016/j.amjcard.2012.11.018. doi: 10.1016/j.amjcard.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation. 2010;122:2451–5. doi: 10.1161/CIRCULATIONAHA.110.953851. doi: 10.1161/circulationaha.110.953851. [DOI] [PubMed] [Google Scholar]
- 5.Weisbord SD, Palevsky PM. Contrast-induced acute kidney injury: Short – And long-term implications. Semin Nephrol. 2011;31:300–9. doi: 10.1016/j.semnephrol.2011.05.009. doi: 10.1016/j.semnephrol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gleeson TG, Bulugahapitiya S. Contrast-induced nephropathy. AJR Am J Roentgenol. 2004;183:1673–89. doi: 10.2214/ajr.183.6.01831673. doi: 10.2214/ajr.183.6.01831673. [DOI] [PubMed] [Google Scholar]
- 7.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–64. doi: 10.1161/01.cir.0000016043.87291.33. doi: 10.1161/01.CIR.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 8.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–75. doi: 10.1016/s0002-9343(97)00150-2. doi: 10.1016/S0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 9.Watabe H, Sato A, Hoshi T, Takeyasu N, Abe D, Akiyama D, et al. Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. Int J Cardiol. 2014;174:57–63. doi: 10.1016/j.ijcard.2014.03.146. doi: 10.1016/j.ijcard.2014.03.146. [DOI] [PubMed] [Google Scholar]
- 10.Valente S, Lazzeri C, Giglioli C, Margheri M, Comeglio M, Nicolaci L, et al. Contrast-induced nephropathy in urgent coronary interventions. J Cardiovasc Med (Hagerstown) 2006;7:737–41. doi: 10.2459/01.JCM.0000247320.72783.1c. doi: 10.2459/01.JCM.0000247320.72783.1c. [DOI] [PubMed] [Google Scholar]
- 11.Crimi G, Leonardi S, Costa F, Ariotti S, Tebaldi M, Biscaglia S, et al. Incidence, prognostic impact, and optimal definition of contrast-induced acute kidney injury in consecutive patients with stable or unstable coronary artery disease undergoing percutaneous coronary intervention. Insights from the all-comer PRODIGY trial. Catheter Cardiovasc Interv. 2015;86:E19–27. doi: 10.1002/ccd.25822. doi: 10.1002/ccd.25822. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, et al. Predictors of outcomes of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with chronic kidney disease. Am J Cardiol. 2014;114:1830–5. doi: 10.1016/j.amjcard.2014.09.022. doi: 10.1016/j.amjcard.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Chen YL, Fu NK, Xu J, Yang SC, Li S, Liu YY, et al. A simple preprocedural score for risk of contrast-induced acute kidney injury after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2014;83:E8–16. doi: 10.1002/ccd.25109. doi: 10.1002/ccd.25109. [DOI] [PubMed] [Google Scholar]
- 14.Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–9. doi: 10.1016/j.amjcard.2004.03.008. doi: 10.1016/j.amjcard.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Moriya T, Tanaka S, Sone H, Ishibashi S, Matsunaga S, Ohashi Y, et al. Patients with type 2 diabetes having higher glomerular filtration rate showed rapid renal function decline followed by impaired glomerular filtration rate: Japan diabetes complications study. J Diabetes Complications. 2017;31:473–8. doi: 10.1016/j.jdiacomp.2016.06.020. doi: 10.1016/j.jdiacomp.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Tonelli M, Klarenbach SW, Lloyd AM, James MT, Bello AK, Manns BJ, et al. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. 2011;80:1306–14. doi: 10.1038/ki.2011.280. doi: 10.1038/ki.2011.280. [DOI] [PubMed] [Google Scholar]
- 17.Rich MW. Secondary prevention of cardiovascular disease in older adults. Prog Cardiovasc Dis. 2014;57:168–75. doi: 10.1016/j.pcad.2014.03.006. doi: 10.1016/j.pcad.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, et al. Comparative performance of the CKD epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis. 2010;56:486–95. doi: 10.1053/j.ajkd.2010.03.026. doi: 10.1053/j.ajkd.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silveiro SP, Friedman R, de Azevedo MJ, Canani LH, Gross JL. Five-year prospective study of glomerular filtration rate and albumin excretion rate in normofiltering and hyperfiltering normoalbuminuric NIDDM patients. Diabetes Care. 1996;19:171–4. doi: 10.2337/diacare.19.2.171. doi: 10.2337/diacare.19.2.171. [DOI] [PubMed] [Google Scholar]
- 20.Vedel P, Obel J, Nielsen FS, Bang LE, Svendsen TL, Pedersen OB, et al. Glomerular hyperfiltration in microalbuminuric NIDDM patients. Diabetologia. 1996;39:1584–9. doi: 10.1007/s001250050618. doi: 10.1007/s001250050618. [DOI] [PubMed] [Google Scholar]
- 21.Mogensen CE. Twelve shifting paradigms in diabetic renal disease and hypertension. Diabetes Res Clin Pract. 2008;82(Suppl 1):S2–9. doi: 10.1016/j.diabres.2008.09.029. doi: 10.1016/j.diabres.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Groop PH, Thomas MC, Moran JL, Wadèn J, Thorn LM, Mäkinen VP, et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651–8. doi: 10.2337/db08-1543. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin R, Turner C, van Aken S, Bahu TK, Watts A, Lindsell DR, et al. The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: The oxford regional prospective study. Kidney Int. 2005;68:1740–9. doi: 10.1111/j.1523-1755.2005.00590.x. doi: 10.1111/j.1523-1755.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 24.Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ. 1996;313:779–84. doi: 10.1136/bmj.313.7060.779. doi: 10.1136/bmj.313.7060.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoyama H, Sone H, Oishi M, Kawai K, Fukumoto Y, Kobayashi M, et al. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: The Japan diabetes clinical data management study (JDDM15) Nephrol Dial Transplant. 2009;24:1212–9. doi: 10.1093/ndt/gfn603. doi: 10.1093/ndt/gfn603. [DOI] [PubMed] [Google Scholar]
- 26.Moriya T, Tsuchiya A, Okizaki S, Hayashi A, Tanaka K, Shichiri M, et al. Glomerular hyperfiltration and increased glomerular filtration surface are associated with renal function decline in normo – And microalbuminuric type 2 diabetes. Kidney Int. 2012;81:486–93. doi: 10.1038/ki.2011.404. doi: 10.1038/ki.2011.404. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Y, Qiu H, Song L, Hu X, Luo T, Zhao X, et al. A new risk factor profile for contrast-induced acute kidney injury in patients who underwent an emergency percutaneous coronary intervention. Angiology. 2017:69:523–31. doi: 10.1177/0003319717736157. doi: 10.1177/0003319717736157. [DOI] [PubMed] [Google Scholar]
- 28.Yuan Y, Qiu H, Hu XY, Luo T, Gao XJ, Zhao XY, et al. Risk factors of contrast-induced acute kidney injury in patients undergoing emergency percutaneous coronary intervention. Chin Med J. 2017;130:45–50. doi: 10.4103/0366-6999.196578. doi: 10.4103/0366-6999.196578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan Y, Qiu H, Hu X, Luo T, Gao X, Zhao X, et al. Predictive value of inflammatory factors on contrast-induced acute kidney injury in patients who underwent an emergency percutaneous coronary intervention. Clin Cardiol. 2017;40:719–25. doi: 10.1002/clc.22722. doi: 10.1002/clc.22722. [DOI] [PMC free article] [PubMed] [Google Scholar]