To the Editor: Cerebral ventricular infection (CVI), also termed ependymitis, involves an inflammatory response in the cerebral ventricular system. CVI is characterized by leukocytosis in the cerebrospinal fluid (CSF), a positive culture result of pathogenic bacteria, and imaging changes in the ventricles.[1] CVI mainly occurs after traumatic brain injury or surgery and is particularly prevalent in patients receiving external ventricular drainage (EVD) or a ventricular peritoneal shunt.[2] CVI is a serious complication after neurosurgery and can affect the prognosis and quality of life of patients. Therefore, early evaluation and treatment are critical for CVI patients. Herein, we report a retrospective study examining the role of neuroendoscopic evaluation and treatment for CVI over multiple medical centers in China.
The study included clinical data from 25 patients with CVI (14 men and 11 women; average age 45.13 ± 11.4 years; range: 24.0–62.0 years) from six Chinese medical centers from March 2010 to 2017. The causes of CVI included Ommaya reservoir placement (one case), pituitary adenoma resection (one case), open craniotomy (three cases), endoscopic third ventriculostomy (four cases), EVD (six cases), and ventricular peritoneal shunt (10 cases). The clinical presentations were fever (range, 37–42°C) (25 cases), headache (22 cases), intracranial hypertension (19 cases), meningeal irritation sign (19 cases), epilepsy (11 cases), slurred speech (five cases), and consciousness disorder (five cases). CSF examinations revealed abnormal CSF appearance (21 cases), leukocytosis (25 cases), and positive Pan reaction (25 cases). CSF culture revealed positive results for all cases, with pathogens including Staphylococcus epidermidis (10 cases), Staphylococcus aureus (five cases), Enterococcus faecium (five cases), Pseudomonas aeruginosa (seven cases), Klebsiella pneumoniae (four cases), Enterobacter cloacae (three cases), and mixed pathogens (nine cases). Computed tomography and magnetic resonance imaging scans of all cases before surgery revealed linear enhancement of ventricular walls (16 cases), ventricular dilation (19 cases), intraventricular debris (12 cases), the intraventricular compartment (11 cases), and intraventricular abscesses (four cases).
All patients underwent the neuroendoscopic bilateral frontal approach or the unilateral frontal approach with septostomy under general anesthesia. The neuroendoscope was placed into the lateral ventricle and the third ventricle to evaluate the severity of CVI; to obliterate the intraventricular debris, pus, and clots; to fenestrate or incise the intraventricular compartments; and to reconstruct CSF circulation. The intrachannel endoscopic technique was converted into an extrachannel to obliterate large volumes of intraventricular debris, pus, and clots with an aspirator; to remove implanted material that is usually adhered to the choroidal plexus or the ventricular wall; and to place a 14F EVD tube into the bilateral ventricle under the guidance of a neuroendoscope. Intraventricular lavage with antibiotic saline (ILAS) was applied during intraoperative and postoperative procedures. The use of intraventricular medications, including vancomycin, gentamycin, or amikacin, was based on the CSF bacteriological culture and drug sensitivity results. The antibiotic dose of ILAS was 5–10% of that provided intravenously.
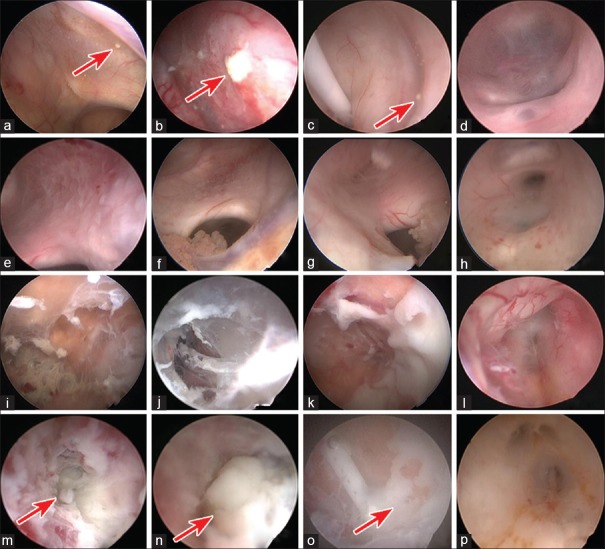
In the present study, neuroendoscopic classification of CVI including Grade I (GI) to Grade IV (GIV) was used for the evaluation of CVI. GI was characterized by a clear or yellowish CSF, a granular ependymitis, a pink or whitish choroid plexus, and identifiable anatomical landmarks [Figure 1a–1d]. GII was characterized by a yellowish or light turbid CSF, a small amount of debris, pseudomembrane, and pus, a whitish or yellowish choroid plexus, identifiable anatomical landmarks, and a thickened third ventricular floor [Figure 1e–1h]. GIII was characterized by turbid CSF, moderate debris and pus, intraventricular compartments, difficulty in identifying anatomical landmarks, a membrane-obscured choroid plexus, and a thickened, opaque third ventricular floor [Figure 1i–1l]. GIV was characterized as for GIII, but accompanied by excessive pus and an intraventricular abscess [Figure 1m–1p]. We found two GI cases, 11 GII cases, eight GIII cases, and four GIV cases. Further, two patients with GI underwent one neuroendoscopic surgery (NES), 19 patients with GII/GIII underwent two NESs, two patients with GIV underwent two NESs, and two patients with GIV underwent three NESs. Linear regression analysis revealed a significant increase in the number of NESs with increasing CVI severity (P < 0.001).
Figure 1.
Neuroendoscopic classification of CVI. (a-d) GI: Clear or yellowish CSF, granular ependymitis (red arrow), pink or whitish choroid plexus, and identifiable anatomical landmarks; (e-h) GII: Yellowish or light turbid CSF, small amount of debris and pseudomembrane, whitish or yellowish choroid plexus, identifiable anatomical landmarks; (i-l) GIII: Turbid CSF, moderate pus, intraventricular compartments, difficulty in identifying anatomical landmarks, membrane-obscured choroid plexus; (m-p) GIV: The same as GIII, but accompanied by an intraventricular abscess (red arrow). CVI: Cerebral ventricular infection; GI: Grade I; CSF: Cerebrospinal fluid; GIV: Grade IV.
In this study, one patient died of multiple organ failure, while the other 24 patients were completely cured. Intracranial hypertension was normalized in 19 patients, disturbance of consciousness was significantly diminished in three patients, and meningeal irritation sign disappeared in 18 patients. CSF appeared normal in 18 cases or light yellow in six cases. The Pan reaction was negative in all patients. The CSF leukocyte count ranged from 0 to 11 × 106/L. CSF culture was negative in all patients. Among four cases with an intraventricular abscess, three cases showed abscess disappearance, and one case showed linear enhancement with contrast-enhanced computed tomography and magnetic resonance imaging. Complications of the group of patients included seizures (three cases), subcutaneous effusion (three cases), subdural effusion (four cases), intracranial pneumatosis (seven cases), and pneumonia (three cases). At discharge, the median modified Rankin scale score was 3 (range, 0–6). After 12–60 months of follow-up, there was no recurrence of CVI. The median modified Rankin scale follow-up score was 2 (range, 0–6).
Neuroendoscopy has a range of advantages including minimal invasiveness, excellent illumination, and a panoramic surgical field, which is particularly suitable for intraventricular surgery. The application of endoscopic neurosurgical techniques also improves the evaluation and treatment strategies of CVI. Chang et al.[3] first reported the use of endoscopy for visualization of CVI caused by coagulase-negative staphylococci and confirmed that bacteria easily accumulated around the shunt. Guan et al.[4] then provided a detailed report of neuroendoscopic diagnosis and treatment of 14 CVI cases and introduced the technical characteristics for CVI treatment. Further, Kumar et al.[5] reported the importance of ILAS for complete correction of CVI. These studies provided a theoretical basis and clinical protocols for neuroendoscopic evaluation and treatment of CVI.
In the present study, there were a number of advantages of neuroendoscopic evaluation and treatment for CVI. First, neuroendoscopic classification of CVI provided objective evaluation of the severity of CVI. Indeed, we found that the neuroendoscopic classification of CVI was associated with the number of NES. With increasing number of cases of CVI, the neuroendoscopic classification of CVI will further be improved to guide effective treatment and define the association with prognosis in CVI patients. We also found that neuroendoscopic visual manipulations allowed complete removal of debris and pus in the ventricle, incision of the compartments, and reconstruction of CSF circulation. It is also difficult to treat intraventricular abscess secondary to CVI by intravenous/intrathecal medication. However, NES combined with ILAS was an effective treatment for intraventricular abscess. Further, using neuroendoscope guidance, the EVD tubes were able to be placed in an ideal position to guarantee the EVD tube fluent. Finally, inflammatory deposits, sediments, and debris were able to be obtained during NES, allowing further pathophysiological analysis of CVI.
ILAS is also an important procedure for the treatment of CVI. During NES, ILAS can displace inflammatory or bloody CSF, sediments, and debris, to provide a visualized operative field and reduce the systemic inflammatory response. Although NES completely removes the debris and pus, ependymitis is not fundamentally cured. However, ILAS maintains a stable antibiotic concentration and provides an effective sterilization level in the CSF to completely cure ependymitis. If the duration of ILAS is longer than 2 weeks, another NES should be performed to evaluate the ventricular situation and to change the EVD catheter location. During ILAS, drugs should be applied to prevent seizures.
In conclusion, neuroendoscopy is an innovative and effective method for evaluation and treatment of CVI. Neuroendoscopic classification also provides an objective and comprehensive evaluation of CVI. ILAS might be an important procedure for effective treatment of CVI.
Declaration of patient consent
The authors have obtained all appropriate patient consent forms. In the form, the patient(s) have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was supported by grants from the Capital Health Research and Development of Special Funding Support (No. 2011-2008-06) and the Capital Characteristic Clinical Application Research (No. Z1311 07002213 044).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Davies BM, Jones A, Patel HC. Surgical-site infection surveillance in cranial neurosurgery. Br J Neurosurg. 2016;30:35–7. doi: 10.3109/02688697.2015.1071321. doi: 10.3109/02688697.2015.1071321. [DOI] [PubMed] [Google Scholar]
- 2.Zheng SP, Li GP, You C. Excessive external drainage of CSF might aggravate bacterial ventriculitis. Turk Neurosurg. 2014;24:108–10. doi: 10.5137/1019-5149.JTN.7817-13.0. doi: 10. 5137/1019-5149.JTN.7817-13.0. [DOI] [PubMed] [Google Scholar]
- 3.Chang CW, Nagashima G, Fujimoto T, Suzuki R, Itokawa H, Endoh S, et al. Conspicuous endoscopic appearance of ventriculitis caused by coagulase-negative staphylococci. J Infect Chemother. 2007;13:177–9. doi: 10.1007/s10156-007-0515-x. doi: 10.1007/s10156-007-0515-x. [DOI] [PubMed] [Google Scholar]
- 4.Guan F, Hu ZQ, Huang H, Ren ZY, Wang ZY, Fu JD, et al. Strategy of neuroendoscopic diagnosis and treatment for ventricular system infection (in Chinese) Chin J Neurosurg. 2014;30:377–80. doi: 10.3760/cma.j.issn.1001-2346.2014.04.017. [Google Scholar]
- 5.Kumar A, Verma N, Agrawal D, Sharma BS. Endoscopic lavage for antibiotic unresponsive severe Acinetobacter baumanii ventriculitis: An unexplored treatment option. Acta Neurochir (Wien) 2015;157:1225–7. doi: 10.1007/s00701-015-2443-3. doi: 10.1007/s00701-015-2443-3. [DOI] [PubMed] [Google Scholar]