Abstract
Background:
Fibroblasts were the main seed cells in the studies of tissue engineering of the pelvic floor ligament. Basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) were widely studied but at various concentrations. This study aimed to optimize the concentrations of combined bFGF and EGF by evaluating their effects on proliferation and collagen secretion of fibroblasts.
Methods:
Fibroblasts were differentiated from rat adipose mesenchymal stem cells (ADSCs). Flow cytometry and immunohistochemistry were used for cell identification. The growth factors were applied at concentrations of 0, 1, 10, and 100 ng/ml as three groups: (1) bFGF alone, (2) EGF alone, and (3) bFGF mixed with EGF. Cell proliferation was evaluated by Cell Counting Kit-8 assays. Expression of Type I and III collagen (Col-I and Col-III) mRNAs was evaluated by real-time quantitative reverse transcription-polymerase chain reaction. Statistical analysis was performed with SPSS software and GraphPad Prism using one-way analysis of variance and multiple t-test.
Results:
ADSCs were successfully isolated from rat adipose tissue as identified by expression of typical surface markers CD29, CD44, CD90, and CD45 in flow cytometry. Fibroblasts induced from ADSC, compared with ADSCs, were with higher mRNA expression levels of Col I and Col III (F = 1.29, P = 0.0390). bFGF, EGF, and the mixture of bFGF with EGF can enhanced fibroblasts proliferation, and the concentration of 10 ng/ml of the mixture of bFGF with EGF displayed most effectively (all P < 0.05). The expression levels of Col-I and Col-III mRNAs in fibroblasts displayed significant increases in the 10 ng/ml bFGF combined with EGF group (all P < 0.05).
Conclusions:
The optimal concentration of both bFGF and EGF to promote cell proliferation and collagen expression in fibroblasts was 10 ng/ml at which fibroblasts grew faster and secreted more Type I and III collagens into the extracellular matrix, which might contribute to the stability of the pelvic floor microenvironment.
Keywords: Adipose Mesenchymal Stem Cells, Basic Fibroblast Growth Factor, Epidermal Growth Factor, Fibroblasts, Tissue Engineering
摘要
背景:
在盆底韧带组织工程的研究中,成纤维细胞是主要的种子细胞。碱性成纤维细胞生长因子(bFGF)和表皮生长因子 (EGF)研究广泛、但浓度多样。本研究旨在优化bFGF和EGF的作用浓度,并探索其对成纤维细胞的增殖和胶原蛋白表达的 作用。
方法:
提取大鼠脂肪进行脂肪间充质干细胞原代培养,并将其诱导分化为成纤维细胞,采用流式细胞术和免疫组化方法进行 细胞表型鉴定。生长因子被分为三组:1)bFGF单作用组,2)EGF单作用组,3)bFGF+ EGF联合作用组,浓度均分为0,1,10, 和100 ng/ml四种进行作用。以CCK8检测法检测细胞增殖,通过RT-PCP技术检测成纤维细胞I型和III型胶原蛋白mRNA的表达 水平。数据分析采用SPSS软件和GraphPad Prism软件进行单因素方差分析和多样本t检验。
结果:
脂肪间充质干细胞提取成功并进行原代培养,并完成CD29, CD44, CD90和CD45的细胞表型流式鉴定,成纤维细胞诱 导分化成功,且伴随I型和III型胶原蛋白表达增高(F=1.29,P=0.0390)和FSP-1的表达阳性。bFGF组、EGF组和bFGF+ EGF组中 浓度为10ng/ml的实验组中促进成纤维细胞增殖作用最强(all P <0.05),10ng/mlbFGF联合 EGF作用组中的I型和III型胶原蛋白的mRNA表达水平最高(all P <0.05)。
结论:
bFGF和EGF的优化作用浓度为10ng/ml,该浓度作用下成纤维细胞增殖更快,I型和III型胶原蛋白分泌更多,有助于在体外重建组织工程中维持盆底组织细胞微环境的稳定性。
INTRODUCTION
With the development of life science, biomicroenvironments had attracted increasing attention. Research on the microenvironments of tissue engineering in the field of regenerative medicine and various growth factors in cell functions was also gaining more attention.[1] Tissue engineering was expected to mimic microenvironments in vivo.[2] In this study to construct sacral ligament tissue in vitro by tissue engineering, we focused on the role of growth factors in fibroblasts as seed cells. The fibroblasts in this study were differentiated from adipose-derived mesenchymal stem cells (ADSCs). The effects of growth factors were evaluated by measuring the proliferation and collagen secretion of fibroblasts to determine optimal growth factor concentrations.
Fibroblasts are one of the main components of pelvic floor tissue. They are important for the healing process of pelvic floor tissue defects.[3] In a previous study on the reconstruction of pelvic floor tissue defects, Ruiz-Zapata et al.[4,5] found that basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and vascular endothelial growth factor are expressed in the damaged area to promote angiogenesis and granulation tissue regeneration. In advanced defect repair, a large number of fibroblasts mature to secrete collagen, which facilitates wound healing and repair functions.[6] Therefore, the collagen secretion and proliferation of fibroblasts are important for defect healing. In this study, fibroblasts were induced from ADSCs.[7,8] In studies on the effects of bFGF to promote fibroblast functions, bFGF concentrations vary from 0.005 to 2000 ng/ml,[2,9,10,11,12] with no defined optimal concentration. EGF is a widely used growth factor to promote the repair of skin fibroblasts.[13] In this study, we found that EGF plays a synergistic role with bFGF in promoting the proliferation of fibroblasts. It is difficult to detect the physiological effects of various growth factor concentrations around the pelvic floor ligament. Therefore, this study was designed to optimize the concentrations of combined bFGF and EGF to promote pelvic floor microenvironment stability.
This study was only a part of microenvironments’ research of tissue engineering reconstruction of pelvic sacral ligament. ADSCs performed as original seed cells for the feature of low immunogenicity, widely source, high survival rate, and conducive to subsequent experiments in vivo; in addition, ADSCs could be differentiated to specific cells according to the demand of tissue engineering. In the whole tissue engineering reconstruction experiment, ADSCs were induced to differentiate into fibroblasts and smooth muscle cells, and the homology of the two kinds of cells was conducive to the development of cell culture in vitro and the transplantation of reconstruction of the sacral ligament of tissue engineering in the future. This article only introduced the experimental results of fibroblast growth microenvironment study.
METHODS
Primary culture of ADSCs
Adipocytes were isolated from groin adipose tissue of Sprague-Dawley rats (8–10 weeks old). All procedures were approved by the Ethics Committee of our hospital. The adipose tissue was rinsed with phosphate-buffered saline (PBS) three times, visible blood vessels and fascial fibers were removed,[14] and the tissue was cut into 1 mm3 pieces. Digestion was performed with 0.01% Type I collagenase at 37°C in a water bath for 30 min. The digestion was terminated by adding PBS (HyClone, Logan, Utah, USA) containing 10% fetal bovine serum (Gibco, Grand Island, New York, USA) and 1% antibiotics (100 U/ml penicillin/streptomycin) (Gibco). The cells were cultured at 37°C with 5% CO2. The medium was changed after 3 days and then every 3 days. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Hospital (No. 2015-35).
Fibroblasts induced from ADSCs
According to the classic report,[14,15] the fibroblasts could be induced by ADSCs cultured in the 20 ng/ml bFGF and 20 ng/ml EGF medium. The culture medium was replaced with fibroblast-inducing medium containing 20 ng/ml bFGF (Gibco) and 20 ng/ml EGF (Gibco), and then ADSCs were cultured to 40–50% confluence.[15,16] Cells were cultured for another 2 weeks, and the medium was changed every 2–3 days. The cells were then identified by flow cytometry and immunohistochemistry.
Flow cytometric analysis
The third passage of ADSCs was identified by flow cytometric analysis of CD29, CD44, CD90, and CD45 (Abcam, San Francisco, USA).[14] Suspensions of ADSCs were prepared at 1 × 106 cells/ml. One hundred microliters of cell suspension were stained with 20 μl rat CD29-FITC (1:100, ab93759), CD44-FITC (1:100, ab157107), CD45-FITC (1:100, ab10558), or CD90-FITC (1:20, ab123511) for 30 min at 37°C while protected from light. The samples were centrifuged for 5 min at 200 ×g and then washed with PBS twice. A Becton-Dickinson FACScan flow cytometer (Becton-Dickinson, New York, USA) was used to analyze the samples after resuspension with 0.4 ml PBS.
Proliferation assay
The effects of the growth factors on fibroblast proliferation were determined by Cell Counting Kit-8 (CCK-8, MedChem Express, New Jersey, USA).[16] Fibroblasts were seeded at 2 × 102 cells/ml in 96-well plates and treated with various concentrations and combinations of growth factors for 1–7 days. The treatments were as follows: (i) bFGF at 0, 1, 10, and 100 ng/ml, (ii) EGF at 0, 1, 10, and 100 ng/ml, and (iii) both bFGF and EGF at 0, 1, 10, and 100 ng/ml each. The medium was changed every day. A CCK-8 assay was performed each day up to the 7th day. All experiments were repeated three times.
Reverse transcription quantitative real-time polymerase chain reaction of Type I and III collagen mRNAs
ADSCs and fibroblasts were cultured in six-well plates. They were cultured in normal medium and medium of the various groups of growth factors for 7 days. Total RNA of the cells was extracted before and after induction from ADSCs, and total RNA of fibroblasts was extracted at the 7th day. RNA samples were reverse transcribed, and cDNA of the following genes was amplified by reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR): Type Icollagen (Col-I), Type III collagen (Col-III), and β-actin as the reference gene. Primer sequences [Table 1] were designed using Primer Premier (version 5.0, PREMIER Biosoft, California, USA).[17] PCR samples were subjected to agarose gel electrophoresis and imaging. Expression values were analyzed using Adobe Photoshop. The experiments were repeated three times.
Table 1.
Primer sequences and molecular size of the product
| Gene | Primer sequences 5’- 3’ | Product |
|---|---|---|
| Col-I | F: ATGGTGGCAGCCAGTTTG | 328 bp |
| R: AGGAATGGCAGGCGAGAT | ||
| Col-III | F: AAGAGCGGAGAATACTGGG | 532 bp |
| R: CAATGTCATAGGGTGCGATA | ||
| R: CTCACCAGTCGGGTCTCAGTA | ||
| β-actin | F: GAGGGAAATCGTGCGTGAC | 445 bp |
| R: CTGGAAGGTGGACAGTGAG |
F: Forward; R: Reverse; Col-I: Type I collagen; Col-III: Type III collagen.
Immunohistochemistry of ADSCs
ADSCs were cultured on glass coverslips in six-well plates for 7 days. The cells were washed with PBS twice and then fixed in 4% paraformaldehyde. The samples were analyzed by immunohistochemistry of CD29, CD44, CD90, and CD45 and observed by light microscopy.[14] Fibroblasts were also cultured on glass coverslips in six-well plates for 7 days and then analyzed by immunohistochemistry of fibroblast-specific protein-1 (FSP-1).
Statistical analysis
All data are presented as mean ± standard deviation. Statistical analysis was performed with SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA) and GraphPad Prism (version 6.0, GraphPad, Inc., La Jolla, California, USA) using nonparametric and ordinary one-way analysis of variance and multiple t-test. Values of P < 0.05 were accepted as statistically significant.
RESULTS
Rat abdominal fat tissue-derived ADSCs isolation and immunophenotyping
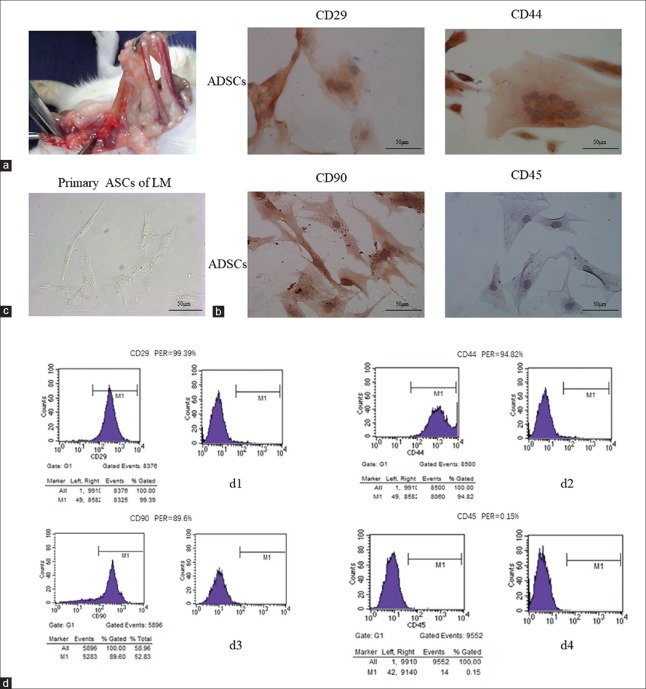
ADSCs were derived successfully from rat pelvic tissue [Figure 1a]. After 3 days of primary culture, the ADSCs morphology became stable with an irregular polygonal shape [Figure 1c]. ADSCs grew markedly fast from day 4. Immunocytochemistry revealed expression of specific surface markers of ADSCs [Figure 1b]. ADSCs were positive for CD29, CD44, and CD90 and negative for CD45. Flow cytometry results showed the typical immunophenotype of ADSCs with expression rates of CD29, CD44, CD90, and CD45 at 99.39%, 94.6%, 89.6%, and 0.15%, respectively [Figure 1d].
Figure 1.
Isolation and identification of ADSCs. (a) ADSCs were derived from rat groin adipose tissue. (b) Immunohistochemical staining (×400) of the third passage ADSCs showed that typical ADSC surface markers CD29, CD44, and CD90 were positive and CD45 was negative. (c) Light microscopy image (×400) of the third passage ADSCS morphology. Cells were multinucleated with a polygonal irregular shape. (d) Flow cytometry for phenotypic identification of ADSCs. d1: CD29 was highly expressed (99.39%); d2: CD44 was highly expressed (94.82%); d3: CD90 was highly expressed (89.6%); d4: CD45 was weakly expressed (0.15%). ADSCs: Adipose mesenchymal stem cells; LM: Light microscope; PER: Positive expression rate.
Identification of ADSCs-induced fibroblasts
Fibroblasts were induced from the third-passage ADSCs because they had a higher cell proliferation ability compared with the sixth passage cells (day 6: 1.413 ± 0.163 [passage 3, P3] vs. 0.686 ± 0.157 [P6], t = 5.547, P = 0.0051; day 7: 2.031 ± 0.1476 [P3] vs. 1.435 ± 0.1765 [P6], t = 4.487, P = 0.0110; Figure 2a). After 2 weeks of induction, the cells grew with a long spindle morphology and were positive for FSP-1 [Figure 2b], a specific protein expressed by fibroblasts. In addition, the expression levels of Col-I and Col-III mRNAs in fibroblasts were increased after successful differentiation (Col-I: 0.640 ± 0.068 [ADSC] vs. 1.031 ± 0.102 [fibroblasts], t = 5.524, P = 0.0052; Col-III: 0.692 ± 0.131 [ADSC] vs. 0.954 ± 0.074 [fibroblasts], t = 3.016, P = 0.0390; Figure 2c).
Figure 2.
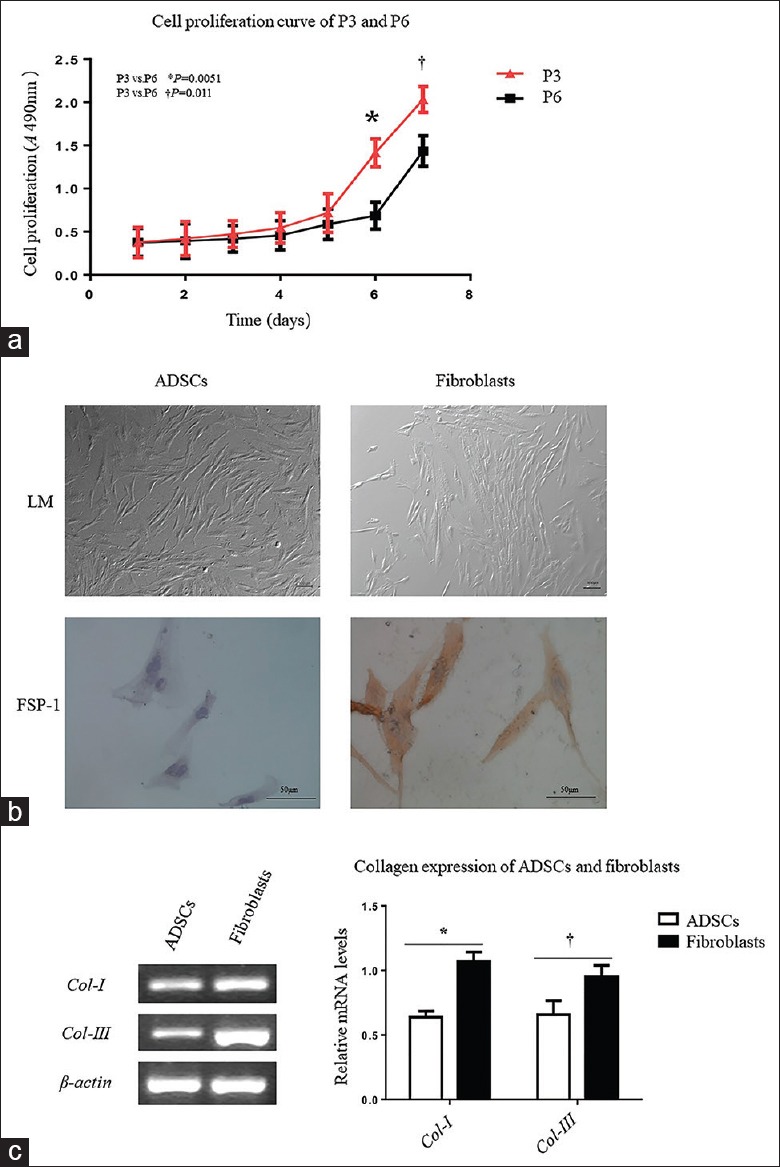
Identification of ADSCs before and after differentiation to fibroblasts. (a) Cell proliferation was evaluated by CCK8 assays in 7 days in 96-well plates by comparing the third passage and the sixth passage ADSCs. The growth curve was indicating that the third passage ADSCs had better proliferation activity (day 6: 1.413 ± 0.163 [P3] vs. 0.686 ± 0.157 [P6], t = 5.547, P = 0.0051; day 7, 2.031 ± 0.1476 [P3] vs. 1.435 ± 0.1765 [P6], t = 4.487, P = 0.0110). (b) The third passage ADSCs were cultured in induction medium for 2 weeks. The induced cells grew as long spindle cells with compact cytoplasm that was different from the loose cytoplasm of ADSCs under a light microscope (×400). FSP-1 was only expressed after ADSCs induction into fibroblasts (×400). (c) Expression of Type I and III collagens was evaluated at the mRNA level. Total RNA of cells was extracted before and after induction from ADSCs. The RNA samples were reverse transcribed, and cDNA of the following genes was amplified by RT-qPCR. The mRNA expression levels of Type I and III collagens in fibroblasts were increased after successful differentiation (Col-I: 0.640 ± 0.068 [ADSC] vs. 1.031 ± 0.102 [fibroblasts], t = 5.524, *P = 0.0052; Col-III: 0.692 ± 0.131 [ADSC] vs. 0.954 ± 0.074 [fibroblasts], t = 3.016, †P = 0.0390). ADSCs: Adipose mesenchymal stem cells; P3: The third passage ADSCs; P6: The sixth passage ADSCs; CCK8: Cell Counting Kit-8; SD: Standard deviation; LM: Light microscope; FSP-1: Fibroblast-specific protein-1; cDNA: Complementary DNA; RT-qPCR: Reverse transcription quantitative real-time polymerase chain reaction; Col-I: Type I collagen; Col-III: Type III collagen.
Basic fibroblast growth factor and epidermal growth factor promote cell proliferation of ADSCs-induced fibroblasts at an optimized concentration
The growth curves showed that cells proliferated in culture, and there were significant differences between the groups containing growth factors and groups without growth factors [Figure 3]. In groups containing bFGF, the growth of fibroblasts was enhanced markedly by 10 and 100 ng/ml bFGF, and the number of cells was increased dramatically [Figure 3a]. However, in the 100 ng/ml bFGF group, fibroblast numbers did not increase further, indicating that 10 ng/ml bFGF was a more suitable culture condition to maintain and promote the proliferation of fibroblasts (0 ng/ml, 1.710 ± 0.205; 1 ng/ml, 2.318 ± 0.260; 10 ng/ml, 2.899 ± 0.1057; and 100 ng/ml, 2.360 ± 0.2001; t0–1= 3.1806, P0–1= 0.0335, t1–10= 3.586, P1–10= 0.0230, t10–100= 4.127, and P10–100= 0.0140; F = 17.64, P = 0.0007). In groups containing 10 ng/ml EGF, the growth of fibroblasts was enhanced markedly, and the number of cells was increased dramatically [Figure 3b] compared with the 100 ng/ml EGF group, indicating that 10 ng/ml EGF was a more suitable culture condition to maintain and promote the proliferation of fibroblasts (0 ng/ml, 1.667 ± 0.232; 1 ng/ml, 2.203 ± 0.208; 10 ng/ml, 2.697 ± 0.126; 2.455 ± 0.054; t0–1= 2.9795, P0–1= 0.0408, t1–10= 3.518, P1–10= 0.0240, t10–100= 3.057, and P10–100= 0.0370; F = 11.66, P = 0.0027). The combination of 10 ng/ml bFGF and 10 ng/ml EGF had the optimal effect on promoting fibroblast proliferation among the various concentrations with the number of cells increased dramatically (0 ng/ml, 1.703 ± 0.254; 1 ng/ml, 2.550 ± 0.244; 10 ng/ml, 3.011 ± 0.102; and 100 ng/ml, 2.620 ± 0.115; t0–1= 4.165, P0–1= 0.0140, t1–10= 3.019, P1–10= 0.0390, t10–100= 4.405, and P10–100= 0.0110; F = 24.64, P = 0.0002; Figure 3c]).
Figure 3.
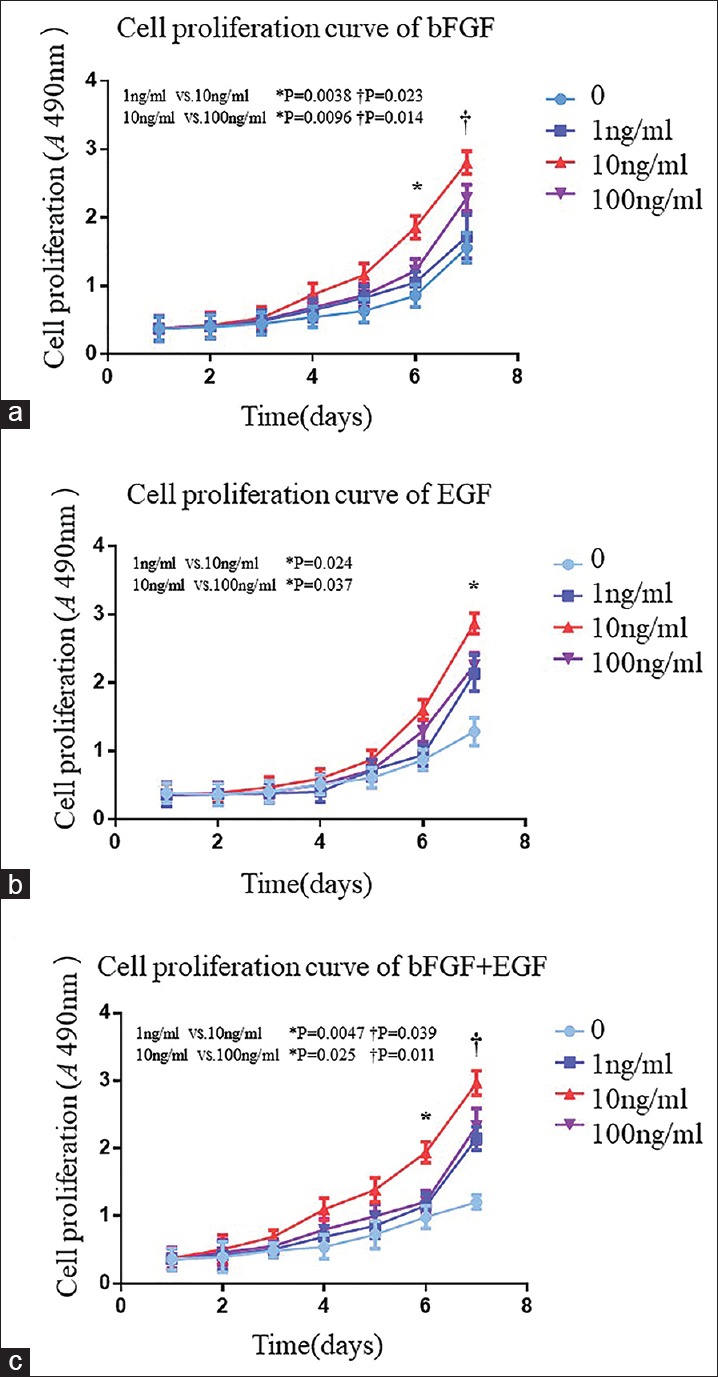
Effects of the various concentrations of bFGF and EGF on cell proliferation of fibroblasts induced from ADSCs. (a) Fibroblasts were cultured in 96-well plates and evaluated every 24 h by CCK-8 assays. OD values at 490 nm of fibroblasts were shown. Only the OD value in the 10 ng/ml bFGF group (red curve) was increased significantly (day 6: 0.754 ± 0.114, 1.069 ± 0.155, 1.855 ± 0.166, 1.216 ± 0.170, t = 2.835, P = 0.0470, t = 5.994, P = 0.0038, t = 4.658, P = 0.0096; F = 27.52, *P = 0.0001; day 7: 1.710 ± 0.205, 2.318 ± 0.260, 2.899 ± 0.106, 2.360 ± 0.200, t = 3.1806, P = 0.0335, t = 3.586, P = 0.0230, t = 4.127, P = 0.0140, t = 3.586, P = 0.0340; F = 17.64, †P = 0.0007). (b) Effect of EGF on fibroblasts. Only the OD value in the 10 ng/ml EGF group (red curve) was increased significantly during the past 2 days (1.667 ± 0.232, 2.203 ± 0.208, 2.697 ± 0.166, and 2.343 ± 0.252; t = 2.9795, P = 0.0408; t = 3.215, P = 0.0324; and t = 2.031, P = 0.1100; F = 11.66, *P = 0.0027). (c) Effects of bFGF mixed with EGF on fibroblasts. The OD value was increased obviously in the intermediate concentration group (red curve) (day 6: 1.204 ± 0.100, 2.149 ± 0.170, 2.909 ± 0.182, and 2.330 ± 0.262; t = 8.590, P = 0.0011; t = 5.678, P = 0.0047; and t = 3.457, P = 0.0250; F = 45.15, *P < 0.0001; day 7: 1.703 ± 0.254, 2.550 ± 0.244, 3.011 ± 0.102, and 2.620 ± 0.115; t = 4.165, P = 0.0140; t = 3.019, P = 0.0390; t = 4.405, P = 0.0110; F = 24.64, †P = 0.0002). ADSCs: Adipose mesenchymal stem cells; CCK8: Cell Counting Kit-8; SD: Standard deviation; OD: Optical density; bFGF: Basic fibroblast growth factor; EGF: Epidermal growth factor.
Type I and III collagens expression in ADSCs-induced fibroblasts under the optimized concentration of combined basic fibroblast growth factor and epidermal growth factor treatment
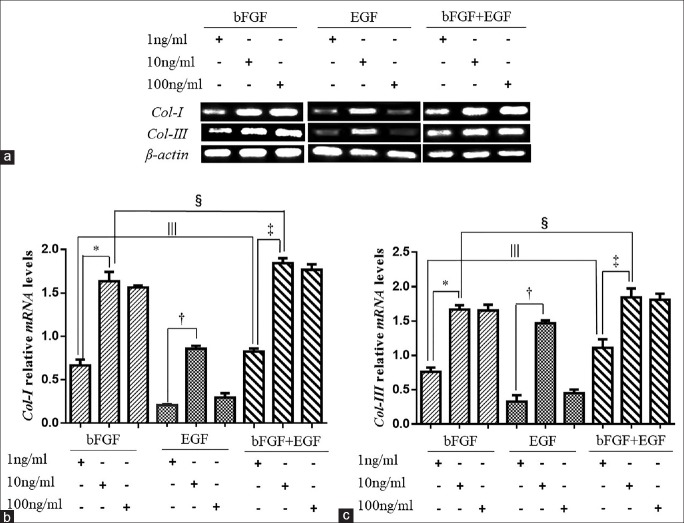
To evaluate the effects of the various experimental conditions of bFGF and EGF on the capacity of the fibroblastic cells to express Col-I and Col-III, RT-qPCR was used to measure the mRNA levels of Col-I and Col-III. As an effect of the various concentrations of bFGF and EGF, the expression of Col-I and Col-III in fibroblasts was significantly different [Figure 4a]. In the 10 ng/ml bFGF group, expression of Col-I was higher than that in the other two bFGF groups (0.699 ± 0.074 vs. 1.608 ± 0.114 vs. 1.500 ± 0.015, F = 79.13, P = 0.0025; Figure 4b). Among the EGF groups, there was slightly higher expression of Col-I in the 10 ng/ml group than that in the other concentrations (0.219 ± 0.018 vs. 0.892 ± 0.022 vs. 0.350 ± 0.048, F = 245.50, P = 0.0005; Figure 4b). The Col-I mRNAexpression of group 10 ng/ml bFGF mixed with 10 ng/ml EGF was the highest in the double growth factor groups (0.858 ± 0.023 vs. 1.883 ± 0.099 vs. 1.840 ± 0.089, F = 110.50, P = 0.0015; Figure 4b) and also the highest among all 10 ng/ml growth factor groups as well (1.608 ± 0.114 vs. 0.892 ± 0.022 vs. 1.883 ± 0.099, F = 67.45, P = 0.0032; Figure 4b). In addition, the expression of Col-I in the 1 ng/ml mixed growth factors’ group was obviously higher than the expression of Col-I in 1 ng/ml EGF group and 1 ng/ml bFGF group (0.699 ± 0.074 vs. 0.892 ± 0.022 vs. 0.858 ± 0.023, F = 104.90, P = 0.0017; Figure 4b). The increasing trend of Col-III expression in the various concentration groups was similar to that of Col-I expression. In the 10 ng/ml bFGF group, Col-III expression was higher than in the 1 ng/ml bFGF group and 100 ng/ml bFGF group (0.649 ± 0.048 vs. 1.686 ± 0.045 vs. 1.612 ± 0.080, F = 187.20, P = 0.0007; Figure 4c) but was not different compared with the 100 ng/ml bFGF group. In EGF groups, there was obviously higher expression of Col-III in the 10 ng/ml group than that in the other concentrations (0.378 ± 0.093 vs. 1.457 ± 0.023 vs. 0.490 ± 0.055, F = 173.10, P = 0.0008; Figure 4c). Col-III expression in the group of bFGF mixed with EGF was the highest at 10 ng/ml each (1.104 ± 0.110 vs. 1.894 ± 0.121 vs. 1.801 ± 0.098, F = 30.78, P = 0.0100; 1.686 ± 0.045 vs. 1.457 ± 0.023 vs. 1.894 ± 0.121, F = 16.67, P = 0.0237; Figure 4c), and Col-III expression at 1 ng/ml was still higher than that in the 1 ng/ml bFGF or EGF alone groups (0.649 ± 0.048 vs. 0.378 ± 0.093 vs. 1.104 ± 0.110, F = 35.05, P = 0.0083; Figure 4c). The ratio of Col-I expression to Col-III expression of group with10 ng/ml bFGF mixed with EGF was just less than one and compared with other groups with no significant difference.
Figure 4.
Different mRNA expression levels of Type I and III collagens in fibroblasts induced from ADSCs in various concentrations of growth factors. (a) Fibroblasts were cultured in 6-well plates. Relative mRNA expression levels of Col-I and Col-III in the various groups of fibroblasts at the 7th day were measured by RT-qPCR. Values were calculated as a percentage of expression relative to β-actin. (b) Relative mRNA expression levels of Col-I of different concentration of growth factors. In the bFGF group, expression of Col-I of 10 ng/ml was highest (0.699 ± 0.074, 1.608 ± 0.114, and 1.500 ± 0.015, F = 79.13, *P = 0.0025). Among the EGF groups, the expression of Col-I in the 10 ng/ml group was more than other two concentrations (0.219 ± 0.018, 0.892 ± 0.022, and 0.350 ± 0.048, F = 245.50, †P = 0.0005). In the groups of two kinds of growth factors, the concentration of 10 ng/ml had the most effective promoting effect on the expression of Col-I (0.858 ± 0.023, 1.883 ± 0.099, and 1.840 ± 0.089, F = 110.50, ‡P = 0.0015). At the same concentration of 10 ng/ml, the expression of Col-I of bFGF mixed with EGF group was better than the other two groups (1.608 ± 0.114, 0.892 ± 0.022, and 1.883 ± 0.099; F = 67.45, §P = 0.0032). Even at the lowest concentration, 1 ng/ml, the group with two kinds of growth factors had better effect of improving the expression of Col-I than the single growth factor group (0.699 ± 0.074, 0.892 ± 0.022, and 0.858 ± 0.023, F = 104.90, ||P = 0.0017). (c) Relative mRNA expression levels of Col-III of different concentration of growth factors. In the bFGF group, the Col-III expression of concentration of 10 ng/ml was higher than that in the 1 ng/ml bFGF group (0.649 ± 0.048, 1.686 ± 0.045, and 1.612 ± 0.080, F = 187.20, *P = 0.0007) but was not different compared with the 100 ng/ml bFGF group. In the EGF groups, only the expression of Col-III in the 10 ng/ml group was highest (0.378 ± 0.093, 1.457 ± 0.023, and 0.490 ± 0.055, F = 173.1, †P = 0.0008). Col-III expression in the group of bFGF mixed with EGF was the highest at 10 ng/ml (1.104 ± 0.110, 1.894 ± 0.121, and 1.801 ± 0.098, F = 30.78, ‡P = 0.010). The Col-III expression at 1 ng/ml of two growth factors’ group was still higher than that in the 1 ng/ml bFGF and EGF alone groups (0.649 ± 0.048, 0.378 ± 0.093, and 1.104 ± 0.110, F = 35.05, §P = 0.0083). (c) At the 10 ng/ml, the group contained two growth factors made the fibroblasts expressed more Col-III (1.686 ± 0.045, 1.457 ± 0.023, and 1.894 ± 0.121, F = 16.67, ||P = 0.0237). ADSCs: Adipose mesenchymal stem cells; RT-qPCR: Reverse transcription quantitative real-time polymerase chain reaction; Col-I: Type I collagen; Col-III: Type III collagen; bFGF: Basic fibroblast growth factor; EGF: Epidermal growth factor.
DISCUSSION
In this study, we found that the cell proliferation did not have a linear relationship with the concentrations of bFGF and EGF. However, the most effective concentration was 10 ng/ml. bFGF significantly promoted cell proliferation and collagen expression of fibroblasts. EGF mildly promoted fibroblast proliferation and had a slight effect on collagen expression. When bFGF was combined with EGF, we found significant promotion of fibroblast proliferation and expression of collagens. Therefore, bFGF plays more important roles in expression of Col-I and Col-III. This study revealed that the combined use of bFGF/EGF significantly improved fibroblast proliferation and differentiation.
Optimized concentrations of growth factors can simulate a microenvironment in tissue engineering, which is closer to the microenvironment in vivo. Growth factors such as bFGF are present in the microenvironment of tissue repair after tissue damage and play an important role in repair of the pelvic ligament. bFGF and EGF are the most widely studied growth factors.[17,18] bFGF stimulates the proliferation of fibroblasts. Moreover, Wang et al.[19] showed that bFGF promotes collagens’ expression. EGF has been shown to affect cell proliferation and collagen expression in wound repair and reconstruction of tissue.[13,17] For different organs, there may be different kinds of EGF receptors with different effects on cell proliferation and protein expression.[20,21,22] Many studies[22,23,24] have focused on how growth factors mediate signaling pathways that affect cell proliferation and protein expression, while there were few studies on the physiological concentrations of growth factors. Accumulating studies have focused on new materials and various growth factors to make the microenvironment of seed cells in tissue engineering closer to the microenvironment in vivo. Therefore, construction of the microenvironment in tissue engineering in vitro with optimal concentrations of growth factors is important. The optimized concentration of combined bFGF and EGF promoted the proliferation of fibroblasts, which are the main cells in repair of pelvic ligaments, contributing to efficient construction of tissue with seed cells for tissue engineering.
Col-I and III play a very important role in the repair of defects in female pelvic floor tissue, which obviously influence the elasticity and toughness of ligaments in pelvic floor tissue.[25] For ideal repair of pelvic floor ligament tissue, high expression of Col-I will lead to more tenacity, and high expression of Col-III will result in greater elasticity of the ligament.[26] Both Col-I and Col-III increased in the early time after pelvic floor damaged in a very short time, while the flawed tissue stimulated and promoted the regeneration of surroundings which might gather a lot of inflammatory cells with various kinds of growth factors. However, the collagens would decrease in the next period, in which inflammatory cytokines stimulated cells to release a large amount of proteases to the extracellular matrix. Some proteases, such as fibrinolytic enzyme, kinase releasing enzyme, and matrix metalloproteinases, could inhibit the formation of collagen or promote the degradation of collagen obviously. The final result was collagens reduced in the sacrospinous ligament of patients with pelvic organ prolapse disease, and what was more significant for Col-III. The ratio of Col-I to Col-III was calculated and compared in women with pelvic floor dysfunction or not and found that the ratio of Col-I to Col-III increased.[26,27,28,29,30] Therefore, it was very important to replenish collagen in the process of pelvic floor tissue repair. By supplementing Col-I and III exogenously and resisting the degradation of local tissues, the collagen content of local tissues was improved, and local tissue mechanical properties were enhanced. In this study, certain kinds of growth factors induce fibroblasts to secrete of Col-I and Col-III to the microenvironment, which might improve the efficiency of tissue engineering.[31] By secreting collagens, surrounding cells can regenerate to repair tissue defects with a better microenvironment. The optimized concentration of combined bFGF and EGF increases the expression of Col-I and Col-III effectively. Maintaining the optimal concentrations of bFGF and EGF in culture might contribute to the stability of the tissue engineering microenvironment.
At the early stage of growth factor treatments, the various concentrations did not have obviously different effects on cell proliferation, and the small growth curve may be the result of the excessively low initial densities of cells. The effects on cell proliferation by treatment with 10 ng/ml bFGF, EGF, and both bFGF and EGF at the 7th day were similar, which might have been caused by contact inhibition of cells, and the cell densities of the three groups reached their maximum in 96-well plates. Therefore, there was no significant difference on the optical density value of the 7th day of fibroblasts among different growth factor groups. The mRNA levels of Col-I and Col-III in cells treated with 10 ng/ml bFGF, EGF, and both bFGF and EGF were similar, which might be caused by cells reaching their maximum densities in six-well plates. In addition, the reason for the significant increase of collagen expression in the 10 ng/ml EGF group may be that the number of cells was highest at this concentration. Because cell activity in vivo is regulated by interactions of several kinds of growth factors from the microenvironment, the interactions of these factors need further study at molecular and cellular levels.
In conclusion, the effects of bFGF and EGF on cell proliferation and protein expression are not stronger as their concentrations increased linearly. The optimal concentration of both bFGF and EGF for cell proliferation and collagen expression of fibroblasts was 10 ng/ml at which fibroblasts grew faster and secreted more Col-I and Col-III into the extracellular matrix, which might contribute to microenvironment stability. In tissue engineering for pelvic floor reconstruction in vitro, the effective concentrations and combination of bFGF and EGF can make the microenvironment more efficient and advantageous. The optimal concentration of growth factors should be considered in research to rebuild or repair organs.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 8157142) and Beijing Municipal Science and Technology Commission (No. D151100001915003).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Guneta V, Tan NS, Chan SK, Tanavde V, Lim TC, Wong TC, et al. Comparative study of adipose-derived stem cells and bone marrow-derived stem cells in similar microenvironmental conditions. Exp Cell Res. 2016;348:155–64. doi: 10.1016/j.yexcr.2016.09.012. doi: 10.1016/j.yexcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Cai SJ, Li CW, Weihs D, Wang GJ. Control of cell proliferation by a porous chitosan scaffold with multiple releasing capabilities. Sci Technol Adv Mater. 2017;18:987–96. doi: 10.1080/14686996.2017.1406287. doi: 10.1080/14686996.2017.1406287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi C, Chen W, Chen B, Shan T, Jia W, Hou X, et al. Bladder regeneration in a canine model using a bladder acellular matrix loaded with a collagen-binding bFGF. Biomater Sci. 2017;5:2427–36. doi: 10.1039/c7bm00806f. doi: 10.1039/c7bm00806f. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Zapata AM, Kerkhof MH, Zandieh-Doulabi B, Brölmann HA, Smit TH, Helder MN, et al. Functional characteristics of vaginal fibroblastic cells from premenopausal women with pelvic organ prolapse. Mol Hum Reprod. 2014;20:1135–43. doi: 10.1093/molehr/gau078. doi: 10.1093/molehr/gau078. [DOI] [PubMed] [Google Scholar]
- 5.Hormozi M, Assaei R, Boroujeni MB. The effect of aloe vera on the expression of wound healing factors (TGFβ1 and bFGF) in mouse embryonic fibroblast cell:In vitro study. Biomed Pharmacother. 2017;88:610–6. doi: 10.1016/j.biopha.2017.01.095. doi: 10.1016/j.biopha.2017.01.095. [DOI] [PubMed] [Google Scholar]
- 6.Vulic M, Strinic T, Tomic S, Capkun V, Jakus IA, Ivica S, et al. Difference in expression of collagen type I and matrix metalloproteinase-1 in utero sacral ligaments of women with and without pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2011;155:225–8. doi: 10.1016/j.ejogrb.2010.12.019. doi: 10.1016/j.ejogrb.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Liu Z, Jin Y, Zhu X, Wang S, Yang J, et al. Differentiation of human amniotic mesenchymal stem cells into human anterior cruciate ligament fibroblast cells by in vitro coculture. Biomed Res Int. 2017;2017:7360354. doi: 10.1155/2017/7360354. doi: 10.1155/2017/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asl RM, Ghoraeian P, Monzavi A, Bahador A. Analysis of gene expression of basic fibroblast growth factor (bFGF) following photodynamic therapy in human gingival fibroblasts. Photodiagnosis Photodyn Ther. 2017;20:144–7. doi: 10.1016/j.pdpdt.2017.09.010. doi: 10.1016/j.pdpdt.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Cha HJ, Yun JI, Han NR, Kim HY, Baek S, Lee SH, et al. Generation of embryonic stem-like cells from in vivo-derived porcine blastocysts at a low concentration of basic fibroblast growth factor. Reprod Domest Anim. 2018;53:176–85. doi: 10.1111/rda.13088. doi: 10.1111/rda.13088. [DOI] [PubMed] [Google Scholar]
- 10.Chang YC, Chang MC, Chen YJ, Liou JU, Chang HH, Huang WL, et al. Basic fibroblast growth factor regulates gene and protein expression related to proliferation, differentiation, and matrix production of human dental pulp cells. J Endod. 2017;43:936–42. doi: 10.1016/j.joen.2017.01.024. doi: 10.1016/j.joen.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Erndt-Marino JD, Jimenez-Vergara AC, Diaz-Rodriguez P, Kulwatno J, Diaz-Quiroz JF, Thibeault S, et al. In vitro evaluation of a basic fibroblast growth factor-containing hydrogel toward vocal fold lamina propria scar treatment. J Biomed Mater Res B Appl Biomater. 2018;106:1258–67. doi: 10.1002/jbm.b.33936. doi: 10.1002/jbm.b.33936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SM, Lee KM, Kim HJ, Park IK, Kang HJ, Shin HC, et al. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018;66:325–34. doi: 10.1016/j.actbio.2017.11.045. doi: 10.1016/j.actbio.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 13.Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012;3:20. doi: 10.1186/scrt111. doi: 10.1186/scrt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai VD, Hsia HC, Schwarzbauer JE. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e86865. doi: 10.1371/journal.pone.0086865. doi: 10.1371/journal.pone.0086865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 16.Marquez MP, Alencastro F, Madrigal A, Jimenez JL, Blanco G, Gureghian A, et al. The role of cellular proliferation in adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2017;26:1578–95. doi: 10.1089/scd.2017.0071. doi: 10.1089/scd.2017.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MS, Song HJ, Lee SH, Lee CK. Comparative study of various growth factors and cytokines on type I collagen and hyaluronan production in human dermal fibroblasts. J Cosmet Dermatol. 2014;13:44–51. doi: 10.1111/jocd.12073. doi: 10.1111/jocd.12073. [DOI] [PubMed] [Google Scholar]
- 18.Roelofs LA, Oosterwijk E, Kortmann BB, Daamen WF, Tiemessen DM, Brouwer KM, et al. Bladder regeneration using a smart acellular collagen scaffold with growth factors VEGF, FGF2 and HB-EGF. Tissue Eng Part A. 2016;22:83–92. doi: 10.1089/ten.TEA.2015.0096. doi: 10.1089/ten.TEA.2015.0096. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Fu C, Wu Z, Chen L, Chen X, Wei Y, et al. A chitin film containing basic fibroblast growth factor with a chitin-binding domain as wound dressings. Carbohydr Polym. 2017;174:723–30. doi: 10.1016/j.carbpol.2017.05.087. doi: 10.1016/j.carbpol.2017.05.087. [DOI] [PubMed] [Google Scholar]
- 20.Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg Am. 2004;29:551–63. doi: 10.1016/j.jhsa.2004.04.020. doi: 10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Tao L, Chen B, Ren H, Hou X, Zhou S, et al. Extrahepatic bile duct regeneration in pigs using collagen scaffolds loaded with human collagen-binding bFGF. Biomaterials. 2012;33:4298–308. doi: 10.1016/j.biomaterials.2012.03.003. doi: 10.1016/j.biomaterials.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Yu A, Niiyama H, Kondo S, Yamamoto A, Suzuki R, Kuroyanagi Y, et al. Wound dressing composed of hyaluronic acid and collagen containing EGF or bFGF: Comparative culture study. J Biomater Sci Polym Ed. 2013;24:1015–26. doi: 10.1080/09205063.2012.731375. doi: 10.1080/09205063.2012.731375. [DOI] [PubMed] [Google Scholar]
- 23.Shen T, Gao K, Miao Y, Hu Z. Exogenous growth factors enhance the expression of cola1, cola3, and elastin in fibroblasts via activating MAPK signaling pathway. Mol Cell Biochem. 2018;442:203–10. doi: 10.1007/s11010-017-3204-9. doi: 10.1007/s11010-017-3204-9. [DOI] [PubMed] [Google Scholar]
- 24.Du HC, Jiang L, Geng WX, Li J, Zhang R, Dang JG, et al. Growth factor-reinforced ECM fabricated from chemically hypoxic MSC sheet with improved in vivo wound repair activity. Biomed Res Int. 2017;2017:2578017. doi: 10.1155/2017/2578017. doi: 10.1155/2017/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen SQ, Cai Q, Shen YY, Cai XY, Lei HY. Combined use of NGF/BDNF/bFGF promotes proliferation and differentiation of neural stem cells in vitro. Int J Dev Neurosci. 2014;38:74–8. doi: 10.1016/j.ijdevneu.2014.08.002. doi: 10.1016/j.ijdevneu.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Ramanathan G, Muthukumar T, Tirichurapalli Sivagnanam U. In vivo efficiency of the collagen coated nanofibrous scaffold and their effect on growth factors and pro-inflammatory cytokines in wound healing. Eur J Pharmacol. 2017;814:45–55. doi: 10.1016/j.ejphar.2017.08.003. doi: 10.1016/j.ejphar.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Roman S, Mangera A, Osman NI, Bullock AJ, Chapple CR, MacNeil S, et al. Developing a tissue engineered repair material for treatment of stress urinary incontinence and pelvic organ prolapse-which cell source? Neurourol Urodyn. 2014;33:531–7. doi: 10.1002/nau.22443. doi: 10.1002/nau.22443. [DOI] [PubMed] [Google Scholar]
- 28.Han L, Wang L, Wang Q, Li H, Zang H. Association between pelvic organ prolapse and stress urinary incontinence with collagen. Exp Ther Med. 2014;7:1337–41. doi: 10.3892/etm.2014.1563. doi: 10.3892/etm.2014.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y, Wu R, Li H, Gu Y, Wei W. Expression and significance of metalloproteinase and collagen in vaginal wall tissues of patients with pelvic organ prolapse. Ann Clin Lab Sci. 2017;47:698–705. [PubMed] [Google Scholar]
- 30.Jin M, Chen Y, Zhou Y, Mei Y, Liu W, Pan C, et al. Transplantation of bone marrow-derived mesenchymal stem cells expressing elastin alleviates pelvic floor dysfunction. Stem Cell Res Ther. 2016;7:51. doi: 10.1186/s13287-016-0308-1. doi: 10.1186/s13287-016-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding J, Han Q, Deng M, Song XC, Chen C, Ai FF, et al. Induction of human umbilical cord mesenchymal stem cells into tissue-forming cells in a murine model: Implications for pelvic floor reconstruction. Cell Tissue Res. 2018;372:535–47. doi: 10.1007/s00441-017-2781-y. doi: 10.1007/s00441-017-2781-y. [DOI] [PubMed] [Google Scholar]