To the Editor: The number of new estimated gastric cancer of “extremely elderly “ patients (aged ≥75 years) has increased in China, which was 143.2 thousand, about 21.1% of all new estimated gastric cancer cases in the year 2015.
Gastrectomy is the first choice for curative treatment of gastric cancer. Further, D2 lymphadenectomy is now used worldwide and has been verified to be effective in improving gastric cancer prognosis based on recent follow-up data in a Dutch trial. Whether gastrectomy with standard D2 lymphadenectomy is suitable for patients more than 75 years old with gastric cancer remains unclear.
The present report compared the clinicopathological characteristics and outcomes of patients with gastric cancer, aged ≥75 years, who underwent operation versus best supportive care and who underwent R0 resection with D1/D1+ versus D2 lymphadenectomy. Our aim was to identify whether gastrectomy and standard D2 lymphadenectomy for gastric cancer are acceptable for relatively healthy patients aged ≥75 years.
A total of 297 patients with gastric adenocarcinoma received radical resection between January 2004 and December 2015 in the Gastrointestinal Surgery Department in a Beijing Hospital of National Center of Gerontology. This retrospective study was approved by the Ethical Committee of the Beijing Hospital.
Patients, who had histologically proven adenocarcinoma of the stomach without evidence of distant metastasis, were aged ≥75 years and were in adequate physical condition for R0 resection (Eastern Cooperative Oncology Group performance status [PS] score 0–3) were identified. Patients were excluded if they had previous or coexisting cancer or had undergone gastrectomy for benign tumors or they received chemotherapy for gastric cancer. Patients who underwent operation were placed in operation (OP) group. Patients whose tumors were thought to be resectable; however, they did not undergo operation were placed in best supportive care (BSC) group; they received nutritional support and symptomatic treatment when necessary. Patients who underwent R0 resection were divided into D1/D1+ and D2 groups.
Physical conditions of patients were measured by the Eastern Cooperative Oncology Group PS score and the physiological and operative severity score for the enumeration of mortality and morbidity (POSSUM). R0 resections (radical resections) were confirmed after the final pathological examination when no peritoneal implantation was noted, the resection lines were microscopically tumor negative, no distant lymph node stations (beyond N2) were involved, and no distant metastases occurred.
D1/D1+ versus D2 lymphadenectomy was defined according to the 2004/2010 Japanese guidelines. Before 2011, the 2004 Japanese guidelines were followed. D2 lymphadenectomy was defined as without splenectomy in this study. Comorbidities at the time of surgery were classified according to the Charlson comorbidity score (CCS). Patients with a CCS <2 were defined as low morbidity, and patients with a CCS ≥2 were defined as high morbidity. Postoperative complication grade was according to the Clavien–Dindo classification. Patients with postoperative complication Grade ≥III were defined as a severe complication.
Pathological diagnoses and classifications were made according to the seventh edition of the International Union against Cancer tumor node metastasis (TNM) Classification of Malignant Tumors.
The information of the patients was obtained either by individual chart review or by contacting their family members. The survival situation, tumor recurrence, and dietary and activity status were recorded. The median follow-up was 28 months (range: 1–131 months), and the last follow-up date was February 2018. The overall survival (OS) and disease-specific survival (DSS) of the patients who underwent operation were calculated from the day of surgical resection until the time of death or the final follow-up. The OS and DSS of patients who did not undergo operation were calculated from the day of pathological diagnosis. Thirteen patients were excluded because of loss to follow-up or lack of sufficient data.
The statistical analyses were performed using the SPSS, version 19.0 (IBM Corp, Chicago, IL). Categorical data of patient characteristics were presented as number (%) and compared using the Chi-square test. Quantitative data of the patient characteristics were presented as mean ± standard deviation and compared using the Fisher's exact test. The OS and DSS were calculated using the Kaplan–Meier method. Survival differences were compared using the Log-rank test. P < 0.05 was considered statistically significant. Confidence intervals were calculated at the 95% level.
The clinicopathological features of all 284 registered patients with gastric cancer are listed in Supplementary Table 1. Sixty patients received best supportive care because of some reason independent of medicine. In addition, 224 patients underwent operation. Of these, 200 patients underwent curative resection (R0), and 24 patients underwent noncurative resection including 13 patients who underwent operation; however, the tumor was not cutoff, 7 patients who underwent R1 resection, and 4 patients with peritoneal implants. Significant differences were found in the PS and POSSUM between the two groups (P < 0.05). The number of patients with POSSUM <20 was higher in OP.
Supplementary Table 1.
Comparison of clinicopathological characteristics of patients classified according to treatment
| Clinicopathological characteristics | OP (n = 224) | BSC (n = 60) | P |
|---|---|---|---|
| Gender (male), n (%) | 179 (79.9) | 48 (80.0) | 0.988 |
| Age (years), mean ± SD | 78.3 ± 3.3 | 79.3 ± 4.0 | 0.060 |
| BMI (kg/m2), mean ± SD | 22.1 ± 3.7 | 22.0 ± 3.9 | 0.868 |
| Charlson comorbidity score, n (%) | |||
| <2 | 144 (64.3) | 37 (61.7) | 0.708 |
| ≥2 | 80 (35.7) | 23 (38.3) | |
| Performance status, n (%) | |||
| 0 | 7 (3.1) | 2 (3.3) | 0.018 |
| 1 | 82 (36.6) | 16 (26.7) | |
| 2 | 105 (46.9) | 23 (38.3) | |
| 3 | 30 (13.4) | 19 (31.7) | |
| POSSUM, n (%) | |||
| <20 | 136 (60.7) | 21 (35.0) | <0.001 |
| ≥20 | 88 (39.3) | 39 (65.0) | |
| Clinical stage, n (%) | |||
| I | 27 (12.1) | 6 (10.0) | 0.612 |
| II | 71 (31.7) | 23 (38.3) | |
| III | 126 (56.2) | 31 (51.7) | |
| Tumor location, n (%) | |||
| Upper one-third | 64 (28.6) | 13 (21.7) | 0.366 |
| Middle one-third | 26 (11.6) | 9 (15.0) | |
| Lower one-third | 99 (44.2) | 32 (53.3) | |
| Two-thirds or more | 35 (15.6) | 6 (10.0) | |
| Tumor size (cm), mean ± SD | 4.5 ± 1.8 | 4.8 ± 2.1 | 0.440 |
| Histology, n (%) | |||
| Differentiated | 97 (43.3) | 24 (40.0) | 0.646 |
| undifferentiated | 127 (56.7) | 36 (60.0) | |
| Histotype (Lauren), n (%) | |||
| Intestinal | 115 (51.3) | 28 (46.7) | 0.230 |
| Diffuse | 49 (21.9) | 20 (33.3) | |
| Mixed | 49 (21.9) | 11 (18.3) | |
| Other | 11 (4.9) | 1 (1.7) | |
| Grading (Bormann), n (%) | |||
| I | 34 (15.2) | 13 (21.7) | 0.550 |
| II | 57 (25.4) | 15 (25.0) | |
| III | 129 (57.6) | 30 (50.0) | |
| IV | 4 (1.8) | 2 (3.3) |
BMI: Body mass index; BSC: Patients who received best supportive care except Stage IV or performance status 4; OP: Patients who underwent a curative operation; POSSUM: Physiological and operative severity score for the enumeration of mortality and morbidity; SD: Standard deviation; BMI: Body mass index.
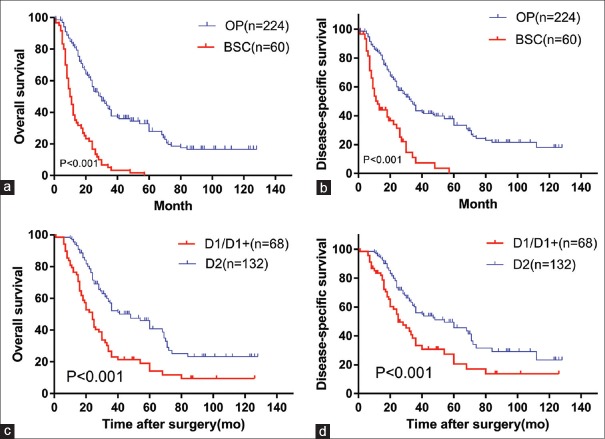
The 1-, 3-, and 5-year OS rate were 82.1%, 37.7%, and 28.0%, respectively, in OP. The 1-, 3-, and 5-year DSS rate was 84.3%, 43.5%, and 33.5%, respectively, in OP. The 1-, 3-, and 5-year OS rate were 38.3%, 3.6%, and 0%, respectively, in BSC. The 1-, 3-, and 5-year DSS rate was 48.2%, 7.3%, and 0%, respectively, in BSC. The OS and DSS are shown in Figure 1. The difference in survival rate between the two groups was statistically significant (P < 0.001). The medium OS time of OP and BSC was 29 months (1–128 months) and 10 months (1–57 months), respectively. The medium DSS time of OP and BSC was 33 months (1–128 months) and 12 months (1–57 months), respectively.
Figure 1.
Kaplan–Meier survival curve analyses for gastric cancer patients aged ≥75 years. (a) Overall survival curve and (b) disease-specific survival curve derived using the Kaplan–Meier method for OP and BSC (P < 0.001). (c) Overall survival curve and (d) disease-specific survival curve derived using the Kaplan–Meier method for the D1/D1+ and D2 groups (P < 0.001). BSC: Patients who received best supportive care except Stage IV or performance status 4; OP: Patients who underwent operation; mo: Months.
In patients who underwent operation, 24 patients were denied D1/D1+ lymphadenectomy versus D2 lymphadenectomy because they did not undergo pathological R0 resections. Of 200 patients who underwent R0 resections, 132 (66.0%) had D2 lymphadenectomy and 68 (34.0%) had D1/D1+ lymphadenectomy. The clinicopathological features of all 200 patients who underwent R0 resection are listed in Supplementary Table 2. Significant differences were noted in CCS, POSSUM, and tumor location between the two groups (P < 0.05).
Supplementary Table 2.
Comparison of clinicopathological characteristics of patients treated with curative intent classified according to the extent of lymphadenectomy
| Clinicopathological characteristics | D1/D1+ (n = 68) | D2 (n = 132) | P |
|---|---|---|---|
| Gender (male), n (%) | 56 (82.4) | 103 (78.0) | 0.473 |
| Age (years), mean ± SD | 78.8 ± 3.2 | 77.9 ± 3.2 | 0.061 |
| Charlson comorbidity score, n (%) | |||
| <2 | 37 (54.4) | 92 (69.7) | 0.032 |
| ≥2 | 31 (45.6) | 40 (30.3) | |
| BMI (kg/m2), mean ± SD | 22.0 ± 3.7 | 22.0 ± 3.8 | 0.926 |
| Performance status, n (%) | |||
| 0 | 3 (4.4) | 3 (2.3) | 0.142 |
| 1 | 17 (25.0) | 54 (40.9) | |
| 2 | 37 (54.4) | 59 (44.7) | |
| 3 | 11 (16.2) | 16 (12.1) | |
| POSSUM, n (%) | |||
| <20 | 35 (51.5) | 91 (68.9) | 0.015 |
| ≥20 | 33 (48.5) | 41 (31.1) | |
| Type of gastrectomy, n (%) | |||
| Subtotal | 55 (80.9) | 93 (70.5) | 0.111 |
| Total | 13 (19.1) | 39 (29.5) | |
| Tumor location, n (%) | |||
| Upper one-third | 31 (45.6) | 28 (21.2) | 0.001 |
| Middle one-third | 3 (4.4) | 18 (13.6) | |
| Lower one-third | 28 (41.2) | 59 (44.7) | |
| Two-thirds or more | 6 (8.8) | 27 (20.5) | |
| Tumor size (cm), mean ± SD | 4.3 ± 2.0 | 4.7 ± 1.7 | 0.145 |
| Histology, n (%) | |||
| Differentiated | 29 (42.6) | 59 (44.7) | 0.782 |
| Undifferentiated | 39 (57.4) | 73 (55.3) | |
| Histotype (Lauren), n (%) | |||
| Intestinal | 36 (52.9) | 64 (48.5) | 0.748 |
| Diffuse | 13 (19.1) | 34 (25.8) | |
| Mixed | 15 (22.1) | 28 (21.2) | |
| Other | 4 (5.9) | 6 (4.5) | |
| Grading (Bormann), n (%) | |||
| I | 8 (11.8) | 26 (19.7) | 0.496 |
| II | 17 (25.0) | 33 (25.0) | |
| III | 42 (61.8) | 72 (54.5) | |
| IV | 1 (1.4) | 1 (0.7) | |
| pT, n (%) | |||
| T1 | 7 (10.3) | 15 (11.4) | 0.081 |
| T2 | 5 (7.4) | 14 (10.6) | |
| T3 | 8 (11.7) | 33 (25.0) | |
| T4 | 48 (70.6) | 70 (53.0) | |
| pN, n (%) | |||
| N0 | 21 (30.9) | 40 (30.3) | 0.933 |
| N+ | 47 (69.1) | 92 (69.7) | |
| pTNM stage, n (%) | |||
| I | 10 (14.7) | 19 (14.4) | 0.784 |
| II | 15 (22.1) | 35 (26.5) | |
| III | 43 (63.2) | 78 (59.1) |
BMI: Body mass index; D1/D1+: Patients who underwent R0 resection and D1 or D1+ lymphadenectomy; D2: Patients who underwent R0 resection and D2 lymphadenectomy; POSSUM: Physiological and operative severity score for the enumeration of mortality and morbidity; SD: Standard deviation.
The postoperative complication rate was 32.5% (65/200); however, the severe complication rate was only 12.5% (25/200). The perioperative mortality rate was 1.5% (3/200). The first three complications were pneumonia (11.0%, 22/200), ileus/anastomotic stenosis (7.0%, 14/200), and wound infection (6.0%, 12/200) [Supplementary Table 3]. Three patients died in 1 month after operation: one in the D1/D1+ group and two in the D2 group. The cause of death was pneumonia, duodenal leakage (severe infection), and heart failure. No difference was observed in postoperative and severe complications between groups.
Supplementary Table 3.
Postoperative complications by severity
| Complication type | D1/D1+ (n = 68) | D2 (n = 132) | P |
|---|---|---|---|
| Infective | |||
| Surgical site infection, n (%) | |||
| Wound infection | 4 (5.9) | 8 (6.1) | 1.000 |
| Anastomotic leakage | 0 (0) | 3 (2.3) | 0.523 |
| Intra-abdominal abscess | 1 (1.5) | 4 (3.0) | 0.848 |
| Remote site infection, n (%) | |||
| Pneumonia | 8 (11.8) | 14 (10.6) | 0.804 |
| Urinary tract infection | 3 (4.4) | 6 (4.5) | 1.000 |
| Noninfective, n (%) | |||
| Hemorrhage | 0 (0) | 4 (3.0) | 0.359 |
| Ileus/anastomotic stenosis | 5 (7.4) | 9 (6.8) | 1.000 |
| Pancreatitis/pancreatic fistula | 1 (1.5) | 3 (2.3) | 1.000 |
| Delayed gastric emptying | 2 (2.9) | 5 (3.8) | 1.000 |
| Lymphorrhea | 1 (1.5) | 1 (0.7) | 1.000 |
| Severe cardiopulmonary failure | 2 (2.9) | 9 (6.8) | 0.417 |
| Pulmonary embolism | 0 (0) | 1 (0.7) | 1.000 |
| Deep vein thrombosis | 0 (0) | 3 (2.3) | 0.523 |
| Renal dysfunction | 3 (4.4) | 8 (6.1) | 0.875 |
| Delirium | 3 (4.4) | 8 (6.1) | 0.875 |
| Clavien–Dindo classification, n (%) | |||
| 0 + I | 48 (70.6) | 87 (65.9) | 0.683 |
| II | 14 (20.6) | 26 (19.7) | |
| III + IV | 5 (7.4) | 17 (12.9) | |
| V | 1 (1.5) | 2 (1.5) | |
| Postoperative complications, n (%) | |||
| Yes | 20 (29.4) | 45 (34.1) | 0.503 |
| No | 48 (70.6) | 87 (65.9) | |
| Severe complications, n (%) | |||
| Yes | 6 (8.8) | 19 (14.4) | 0.259 |
| No | 62 (91.2) | 113 (85.6) |
D1/D1+: Patients who underwent R0 resection and D1 or D1+ lymphadenectomy; D2: Patients who underwent R0 resection and D2 lymphadenectomy.
In the D1/D1+ group, the 1-, 3-, and 5-year OS rate was 76.5%, 23.2%, and 14.3%, respectively. In the D2 group, the 1-, 3-, and 5-year OS rate was 95.5%, 52.2%, and 40.8%, respectively. In the D1/D1+ group, the 1-, 3-, and 5-year DSS rate was 82.0%, 33.3%, and 20.5%, respectively. In the D2 group, the 1-, 3-, and 5-year DSS rate was 95.5%, 56.0%, and 45.7%, respectively. The OS and DSS are shown in Figure 1. The difference in survival rate between groups was significant (P < 0.001). The medium OS time of all patients who underwent R0 resection was 29 months (1–128 months). The medium OS time of the D1/D1+ and D2 groups was 24 months (1–126 months) and 48 months (1–128 months), respectively. The medium DSS time of all patients who underwent R0 resection was 36 months (1–128 months). The medium DSS time of the D1/D1+ and D2 groups was 25 months (1–126 months) and 54 months (1–128 months), respectively.
This study found that 79.9% of patients who underwent operation had better OS and DSS compared with patients who did not undergo operation. The R0 resection rate was 89.3% in OP. The morbidity was relatively high (32.5%) for all patients who underwent R0 resection; however, the severe complications were only 12.5%, and the death rate was 1.5%. The patients who underwent D2 lymphadenectomy had better OS and DSS compared with the patients who underwent D1 lymphadenectomy. The data suggested that gastrectomy with D2 lymphadenectomy could be tolerable and acceptable in relatively healthy patients aged ≥75 years.
Surgery has been reported to be safe with a better surgical outcome compared with the best supportive care in elderly patients with gastric cancer.[1] The medium OS time and DSS time in OP were 29 and 33 months, respectively, in this study, which was much better than those in BSC. No patient was alive for more than 5 years after diagnosis in BSC. However, still, about 20% were alive after 5 years in OP. Only clinical TNM staging could be used for evaluating the patients who did not undergo operation, which might not be accurate. Such cases might include some patients whose tumor was unresectable or with peritoneal implants, similar to the results obtained in OP.
Differences were found in PS and POSSUM between the two groups. It seemed that some doctors or the patient family already had an inclination toward treatment before the operation. These doctors always hesitated to perform operations on the extremely elderly patients with relatively bad PS, as the risk of surgery might be higher. PS always reflects patients’ health status and tolerance for treatment. POSSUM is already being used for judging the body status and predicting morbidity and mortality risks of surgery in patients with gastric cancer; a higher score means a worse body status.[2] Individualized treatment choice is right, but for relatively healthy patients with PS score ≥3, giving up operation for resectable gastric cancer is actually not right. More evidence is needed to help patients in making appropriate decisions and think of the treatment more comprehensively.
D2 lymphadenectomy is recommended worldwide since the 15-year follow-up results of the randomized Dutch D1/D2 trial have been published. However, performing lymphadenectomy is still controversial for extremely elderly patients. An Italian study showed that D2 lymphadenectomy could only improve 5-year DSS, but not OS, and hence, D1 lymphadenectomy should be considered in elderly patients.[3] However, Japanese study results showed that the severe complication rate of D2 lymphadenectomy in patients aged more than 85 years was 16.7%, and 67.5% of these patients underwent D2 lymphadenectomy. Japanese investigators believed that the decisions to reduce the extent of lymphadenectomy during gastrectomy should not be based on advanced age alone.[4] The present data showed that the total morbidity rate in extremely elderly patients who underwent R0 resection was relatively high at 32.5%, the rate of severe complications was 12.5%, and the perioperative mortality rate was 1.5%. No difference was observed in the morbidity rate and severe complications between the D1/D1+ and D2 groups. However, the 5-year OS and DSS rates were significantly different between the two groups. Patients who underwent D2 lymphadenectomy had a longer survival. Therefore, gastrectomy with standard D2 lymphadenectomy for gastric cancer might be acceptable for relatively healthy patients aged ≥75 years.
The patients who underwent D1/D1+ lymphadenectomy had a relatively higher CCS. Some authors believed that high CCS[3,5] was associated with severe complications, despite no statistically significant difference. This is why some surgeons chose to perform less invasive operations for extremely elderly patients with high CCS. At the same time, patients with upper one-third gastric cancer were more likely to undergo D1/D1+ lymphadenectomy than D2 lymphadenectomy. Splenic hilar lymphadenectomy should be performed for upper one-third gastric cancer, which might be associated with more invasiveness, time, and complications for old patients. This is why some surgeons chose to reduce lymphadenectomy, but actually, no difference in complications was found between reduced lymphadenectomy and standard lymphadenectomy in extremely elderly patients.
The guidelines indicate that gastrectomy with D1/D1+ lymphadenectomy is sufficient for early-stage gastric cancer. Only computed tomography scanning was used for clinical staging in our department until endoscopic ultrasonography was initiated in the year 2014. The clinical staging was not accurate, and sometimes, D2 lymphadenectomy was performed for early-stage gastric cancer before the year 2015. This is still an actual diagnostic situation in China. This is why D1/D1+ versus D2 lymphadenectomy, including early-stage gastric cancer, was compared. Fifteen (11.4%) patients with PT1 staging underwent D2 lymphadenectomy in the present study. Fortunately, this shifting did not affect the results for D1/D1+ versus D2 groups. Most of the patients with gastric cancer in this study had advanced-stage cancer.
In conclusion, gastrectomy with standard D2 lymphadenectomy for gastric cancer may be acceptable for relatively healthy patients aged ≥75 years. Advanced age is not the only consideration to give up gastrectomy or reduce the extent of lymphadenectomy.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Endo S, Dousei T, Yoshikawa Y, Hatanaka N, Kamiike W, Nishijima J, et al. Prognosis of gastric carcinoma patients aged 85 years or older who underwent surgery or who received best supportive care only. Int J Clin Oncol. 2013;18:1014–9. doi: 10.1007/s10147-012-0482-9. doi: 10.1007/s10147-012-0482-9. [DOI] [PubMed] [Google Scholar]
- 2.Takama T, Okano K, Kondo A, Akamoto S, Fujiwara M, Usuki H, et al. Predictors of postoperative complications in elderly and oldest old patients with gastric cancer. Gastric Cancer. 2015;18:653–61. doi: 10.1007/s10120-014-0387-6. doi: 10.1007/s10120-014-0387-6. [DOI] [PubMed] [Google Scholar]
- 3.Rausei S, Ruspi L, Rosa F, Morgagni P, Marrelli D, Cossu A, et al. Extended lymphadenectomy in elderly and/or highly co-morbid gastric cancer patients: A retrospective multicenter study. Eur J Surg Oncol. 2016;42:1881–9. doi: 10.1016/j.ejso.2016.05.003. doi: 10.1016/j.ejso.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Kiyokawa T, Hiki N, Nunobe S, Honda M, Ohashi M, Sano T, et al. Feasibility of gastrectomy with standard lymphadenectomy for patients over 85 years old with gastric cancer. Ann Surg Oncol. 2015;22:3962–9. doi: 10.1245/s10434-015-4489-0. doi: 10.1245/s10434-015-4489-0. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi D, Koide N, Suzuki A, Ishizone S, Shimizu F, Tsuchiya T, et al. Postoperative complications in elderly patients with gastric cancer. J Surg Res. 2015;198:317–26. doi: 10.1016/j.jss.2015.03.095. doi: 10.1016/j.jss.2015.03.095. [DOI] [PubMed] [Google Scholar]