Abstract
Inorganic arsenic is a human carcinogen that can target the prostate. Accumulating evidence suggests arsenic can disrupt stem cell (SC) dynamics during the carcinogenic process. Previous work demonstrated arsenic-transformed prostate epithelial (CAsE-PE) cells can recruit prostate SCs into rapidly acquiring a cancer SC (CSC) phenotype via the secretion of soluble factors. Exosomes are small, membrane-derived vesicles that contain lipids, RNA, and proteins, and actively contribute to cancer initiation and progression when taken up by target cells. Here we hypothesized that CAsE-PE cells are recruiting SCs to a CSC-like phenotype via exosomal signaling. CAsE-PE cells secreted 700% more exosomes than parental RWPE-1 cells. CAsE-PE exosomes were enriched with oncogenic factors, including oncogenes (KRAS, NRAS, VEFGA, MYB, and EGFR), inflammation-related (cyclooxygenase-2, interleukin 1B (IL1B), IL6, transforming growth factor-β, and tumor necrosis factor-A), and apoptosis-related (CASP7, CASP9, and BCL2) transcripts, and oncogenesis-associated microRNAs. When compared with SCs cultured in exosome-depleted conditioned medium (CM), SCs cultured in CM containing CAsE-PE-derived exosomes showed increased (198%) matrix metalloproteinase activity and underwent an epithelial-to-mesenchymal transition in morphology, suggesting an exosome-mediated transformation. KRAS plays an important role in arsenic carcinogenesis. Although KRAS transcript (>24 000%) and protein (866%) levels were elevated in CAsE-PE exosomes, knock-down of KRAS in these cells only partially mitigated the CSC-like phenotype in cocultured SCs. Collectively, these results suggest arsenic impacts both exosomal quantity and cargo. Exosomal KRAS is only minimally involved in this recruitment, and additional factors (eg, cancer-associated miRNAs) likely also play a role. This work furthers our mechanistic understanding of how arsenic disrupts SC dynamics and influences the tumor microenvironment during carcinogenesis.
Keywords: arsenic, cancer, exosomes, prostate, stem cells
Accumulating evidence suggests that cancer stem cells (CSCs) drive tumor initiation, progression, metastasis, and can contribute to therapy resistance and tumor recurrence (Rosen and Jordan, 2009; Visvader and Lindeman, 2008). Similar to stem cells (SCs), CSCs reside in a niche and possess the capacity to differentiate and undergo self-renewal; however, self-renewal is dysregulated in CSCs (Tang, 2012). The origin of CSCs is debated, although growing evidence suggests that mutations in key genes can promote SCs, progenitor cells, or even differentiated cells into forming CSCs (Iliopoulos et al., 2011; Wang, 2010). Additionally, the cellular microenvironment can play a critical role in promoting CSC formation, and influence CSC self-renewal and differentiation (Polyak et al., 2009).
Millions of people worldwide are chronically exposed to arsenic through consumption of contaminated drinking water (IARC, 2011). Chronic arsenic exposure is associated with the development of skin, lung, and bladder cancer (IARC, 2011), while recent evidence suggests the prostate is also a target of arsenic carcinogenesis (reviewed in Benbrahim-Tallaa and Waalkes, 2008). Despite decades of research, the mechanisms underlying arsenic carcinogenesis remain poorly defined, although accumulating evidence indicates that arsenic may induce tumorigenesis through a disruption of SC dynamics. For example, mice exposed to arsenic in utero develop squamous cell carcinomas that contain an overabundance of CSCs (Waalkes et al., 2008), while in vitro arsenic transformation of human epithelial cells also results in CSC overabundance or increase in CSC-like properties (Dreval et al., 2018; Ooki et al., 2018; Sun et al., 2011; Tokar et al., 2010b). Furthermore, arsenic can also directly transform human SCs into CSC-like cells in vitro (Tokar et al., 2010a, 2013), while SCs cocultured with arsenic-transformed malignant prostate epithelial cells (CAsE-PE) rapidly (≤3 weeks) acquire a CSC-like phenotype (Xu et al., 2012). In this model, CAsE-PE cells hyper-secrete soluble factors such as interleukin-6 (IL-6) and transforming growth factor-β1 (TGF-β1), and treatment of SCs with either IL-6 or TGF-β1 facilitates the acquisition of several traits consistent with a CSC-like phenotype in the transformed SCs (Xu et al., 2012, 2013).
Cells within the tumor microenvironment can communicate with one another by secreting extracellular vesicles (EVs), including microvesicles (MVs) and exosomes. Exosomes are smaller in diameter (approximately 40–100 nm) than MVs (up to 1000 nm), and are derived via the endo-lysosomal pathway, whereas MVs are generated by budding from the plasma membrane (Raposo and Stoorvogel, 2013). Nearly every cell type secretes exosomes, which can be loaded with lipids, proteins, and RNA (including regulatory microRNAs [miRs]). Delivery and uptake of exosomes by a target cell can have myriad functional consequences, including tumor progression (Nakano et al., 2015). Cancer cells can directly alter adult SC function through exosome signaling (Abd Elmageed et al., 2014; Cho et al., 2011, 2012; Chowdhury et al., 2015; Peinado et al., 2012). For example, exosomes released by prostate cancer cells trigger adipose-derived SCs to undergo a neoplastic transformation (Abd Elmageed et al., 2014), while others have found that prostate cancer cell exosomes can skew mesenchymal SC differentiation towards myofibroblast cells that display enhanced tumor proliferation and invasiveness (Chowdhury et al., 2015). However, it remains unknown whether environmental carcinogen-transformed cells can influence SC dynamics via exosomal signaling.
Since exosomes constitute an important intercellular signaling mechanism in the tumor microenvironment (reviewed in Suchorska and Lach, 2016), we hypothesized that exosomal signaling plays an important role in arsenic-transformed CAsE-PE cell recruitment of SCs into a CSC-like phenotype. For this, we collected and characterized exosomes from CAsE-PE cells and used the noncontiguous cell coculture model (Xu et al., 2012) to determine the potential effects of these exosomes on SC recruitment. Results indicate that CAsE-PE cells not only secrete more exosomes compared with nonmalignant parental control cells (RWPE-1), but that CAsE-PE exosomes are enriched with pro-oncogenic factors that likely play a role in recruiting SCs to a CSC-like phenotype, as depletion of exosomes from CAsE-PE conditioned media (CM) mitigates the development of several acquired CSC-like characteristics. This work furthers our mechanistic understanding of how arsenic can disrupt SC dynamics and influences the tumor microenvironment during arsenic carcinogenesis.
MATERIALS AND METHODS
Cell lines and culture conditions
RWPE-1 is an immortalized, nonmalignant human prostate epithelial cell line (Bello et al., 1997). CAsE-PE is a malignant cell line generated from RWPE-1 cells chronically exposed to 5 µM arsenite (Achanzar et al., 2002). Multipotent WPE-SCs (henceforth referred to as SCs) were isolated from the RWPE-1 cell line via single cell dilution cloning, and express many characteristics of stem/progenitor cells of the prostate epithelium (Tokar et al., 2005). All cell lines were cultured at 37°C in 5% CO2 in Keratinocyte Serum Free Medium (KSFM) (Gibco, No. 10724-011) supplemented with 50 µg/ml bovine pituitary extract (Gibco, No. 13028-014), 5 ng/ml epidermal growth factor (EGF) (Gibco, No. 10450-013), and 1% antibiotic-antimycotic solution (Gibco, No. 15240062). For SCs, culture surfaces were coated with 2.5 µg/ml murine type IV collagen (Trevigen, No. 3410-010-01) and 2.5 µg/ml human fibronectin (BD Bioscience, No. 354008). SCs were cocultured with CAsE-PE cells using a noncontact coculture system (Corning Life Sciences, No. 3491) as previously described (Xu et al., 2012). This system utilizes polytetrafluoroethylene membrane inserts with 0.4 µm pores that keep cells separated by 1.0 mm, a distance equivalent to approximately 50–100 epithelial cells, but allow soluble factors to freely pass between the cocultured cells. Cells were passaged weekly, while medium was refreshed every 2 days.
Exosome isolation
Exosomes were isolated as described in Lässer et al. (2012). Briefly, exosomes were isolated from bovine pituitary extract-free media (as animal serum and extracts can contain exosomes) conditioned by 50 million RWPE-1 or CAsE-PE cells for 72 h. The CM were spun at 1800 rcf for 10 min. The supernatant was then transferred to a new tube, spun at 16 500 rcf for 20 min, and then filtered through a 0.22 µm filter membrane to remove cellular debris. The filtrate was then spun at 100 000 rcf for 1 h at 4°C using a Beckman L8-80M ultracentrifuge to pellet the exosomes. The exosome pellet was washed once with Dulbecco's phosphate buffered saline (DPBS), repelleted, and then resuspended in a buffer dependent upon downstream application (ie, in Trizol for RNA isolation, lysis buffer for western blot analysis, DPBS for size/concentration determination). The concentration of isolated exosomes was normalized to the number of viable cells, as determined by trypan blue exclusion, on the day of isolation. RWPE-1 and CAsE-PE cell lysate was also collected for molecular comparisons of exosome cargo.
Exosome size and concentration
The mean diameter and concentration of exosomes isolated from 50 million RWPE-1 or CAsE-PE cells was determined via Nanoparticle Tracking Analysis using a NanoSight LM10 instrument (System Biosciences, LLC, Mountain View, California).
Gene expression
Total RNA, including miRs, was isolated from exosomes and cell lysates using the miRNeasy Kit (Qiagen, No. 217004). RNA was quantified using a NanoDrop 2000 spectrophotometer (ThermoFisher) and converted to cDNA using the miScript II RT kit (Qiagen, No. 218160) following manufacturer’s instructions. Expression of 84 mature, cancer-associated, miRs was determined using the miScript SYBRGreen PCR Kit (Qiagen, No. 218073) and the Human Cancer Pathway-Focused PCR Array. Real-time PCR was performed on an iCycler MyIQ 2 Two Color Real-time PCR Detection System (Bio-Rad) and miR expression was determined using the delta cycle threshold (ΔΔCt) method. All miR expression data were normalized to the expression of 6 internal control genes (SNORD 61, 68, 72, 95, 96 A, and RNU6B/RNU6–2), and to passage-matched controls.
For mRNA expression, mRNA was converted to cDNA using moloney murine leukemia virus reverse transcriptase and random oligo-d primers. RT-PCR was performed on an iCycler PCR Detection System (Bio-Rad) using Absolute QPCR Mix (ThermoFisher, No. AB1163A). RT-PCR primers were designed using ABI Primer Express 3.0 Software (Applied Biosystems). Ct values for all target genes were normalized to the Ct value of the housekeeping gene, GAPDH, from matched samples. Data are expressed as percent control of the appropriate passage-matched control sample.
Protein expression
Protein expression was determined via western blot analysis. Briefly, total protein was extracted from exosomes and cell lysate using M-PER reagent (Pierce, No. 78501) following manufacturer’s instructions. Total protein content was determined using the Bradford assay (Bio-Rad, No. 5000001). 10–20 µg of total protein was loaded into and separated via 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene difluoride membranes (Invitrogen). Membranes were incubated with primary mouse antiKRAS (Santa Cruz, No. sc-30, diluted 1:250), rabbit antiGRP94 (Abcam, No. ab3674, diluted 1:1000), rabbit antiCANX (Abcam, No. ab22595, diluted 1: 1000), mouse antiHSP70 (Abcam, No. ab2787, diluted 1:1000), mouse antiCD9 (Abcam, No. Ab65230, diluted 1:1000), mouse antiCD81 (Abcam, No. ab35026, diluted 1:1000), mouse antiBCL-XL (Santa Cruz, No. sc-1690, diluted 1:250), mouse antiBCL-2 (Santa Cruz, No. sc-7382, diluted 1:500) or mouse antiβ-actin (Sigma Aldrich, No. A5316, diluted 1:20 000) antibodies. Membranes were washed, and incubated with secondary horseradish peroxidase (HRP)-conjugated goat antimouse antibodies (Santa Cruz, No. sc-2005). Finally, membranes were incubated with chemiluminescent HRP substrate (ThermoFisher, No. 34080), and exposed to Hyperfilm (Amersham, No. 28906839). Band densitometry was measured using the ImageJ analysis software (NIH).
KRAS knockdown in CAsE-PE cells
Arsenic-transformed CAsE-PE cells were transduced with lentiviral particles carrying KRAS shRNAmir, or control sham shRNAmir as previously described (Ngalame et al., 2014a). Using this method, KRAS protein levels were stably reduced to 5% of sham controls.
Matrix metalloproteinase activity
Secreted matrix metalloproteinase (MMP) activity was assessed in SCs cocultured with sham or KRAS knowckdown (KRAS KD) CAsE-PE cells. Following coculture, SCs were replated and cultured alone for 48 h. CM was then collected and MMP-2 and MMP-9 activity was assessed by zymography as previously described in Tokar et al. (2005).
Anchorage-independent growth
Anchorage-independent growth of SCs following their coculture with sham or KRAS-KD CAsE-PE cells for 3 weeks was assessed via colony formation in soft agar as previously described in Tokar et al. (2005).
Invasive capacity
Invasive capacity was determined using a modified Boyden chamber invasion assay as previously described (Bello et al., 1997).
CAsE-PE conditioned medium
CAsE-PE cells were grown in arsenic-free KSFM until they reached approximately 70% confluency. Medium was then replaced with fresh KSFM, and cells were grown for an additional 24 h (medium was conditioned for only 24 h to help avoid nutrient depletion). CM was then collected, centrifuged at 16 500 rcf for 10 min, and passed through a 0.22 µm filter membrane to remove cellular debris. Half of the CM was then centrifuged at 100 000 rcf for 1 h to remove exosomes. Fresh CM was prepared weekly and stored at 4°C until use. SCs were grown in unconditioned control KSFM, conditioned KSFM, or exosome-deplete conditioned KSFM for 3 weeks. During this period, media were refreshed daily to avoid nutrient depletion. After 3 weeks of culture, media were refreshed with basal KSFM (no EGF or bovine pituitary extract) and secreted MMP-2 activity was measured as described in “Matrix Metalloproteinase Activity” section. Cells were imaged using the IncuCyte Zoom (Essen Bioscience) at 10× magnification.
Statistical analysis
Data are presented as mean ± SEM from at least 2 independent experiments. All data were initially analyzed via 1 or 2 factor ANOVAs, and when warranted (p < .05) post-hoc analysis was performed using Tukey’s HSD test. Statistical analysis was performed using JMP v13.0 software (SAS Institute).
RESULTS
EVs Released by Arsenic-Transformed CAsE-PE Cells Play a Role in Converting SCs Into an Oncogenic Phenotype
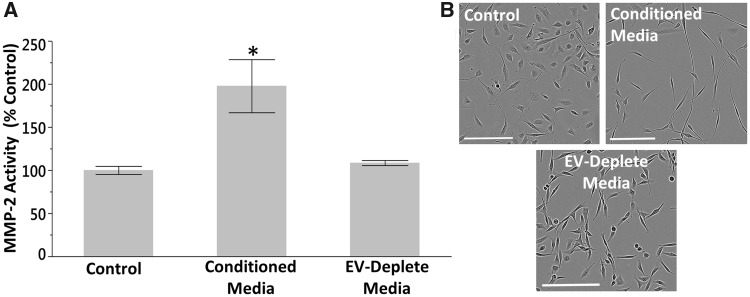
Noncontiguous coculture of SCs with arsenite-transformed CAsE-PE cells results in acquisition of a CSC-like phenotype (Xu et al., 2012). To determine if EVs play a role in this transformation, we grew SCs in CAsE-PE CM containing secreted EVs or devoid of EVs (removed via ultracentrifugation). We then assessed secreted MMP activity of SCs after 3 weeks of culture in CM. SCs grown in CM containing EVs displayed increased (198%) secreted MMP-2 activity, while the secreted MMP-2 activity of SCs cultured in EV-depleted CM was similar to control levels (109%) (Figure 1A). Furthermore, SCs cultured in EV-containing CM displayed a depolarized spindle-like morphology typical of epithelial-to-mesenchymal transition (EMT), while the morphology of SCs cultured in EV-depleted medium more closely resembled that of control cells (Figure 1B).
Figure 1.
EVs play a role in recruiting SCs to an oncogenic phenotype. A, Secreted MMP-2 activity of SCs grown in CAsE-PE CM containing EVs or devoid of EVs for 3 weeks. Asterisk denotes statistical significance (one factor ANOVA, p = .02, n = 3). Post-hoc comparisons were made using Tukey’s HSD test. Bars ± SEM. B, Morphology of SCs grown in CAsE-PE CM for 3 weeks (scale bars = 200 µm).
Arsenic Transformation Increases Secretion and Biological Molecule Cargo of Exosomes
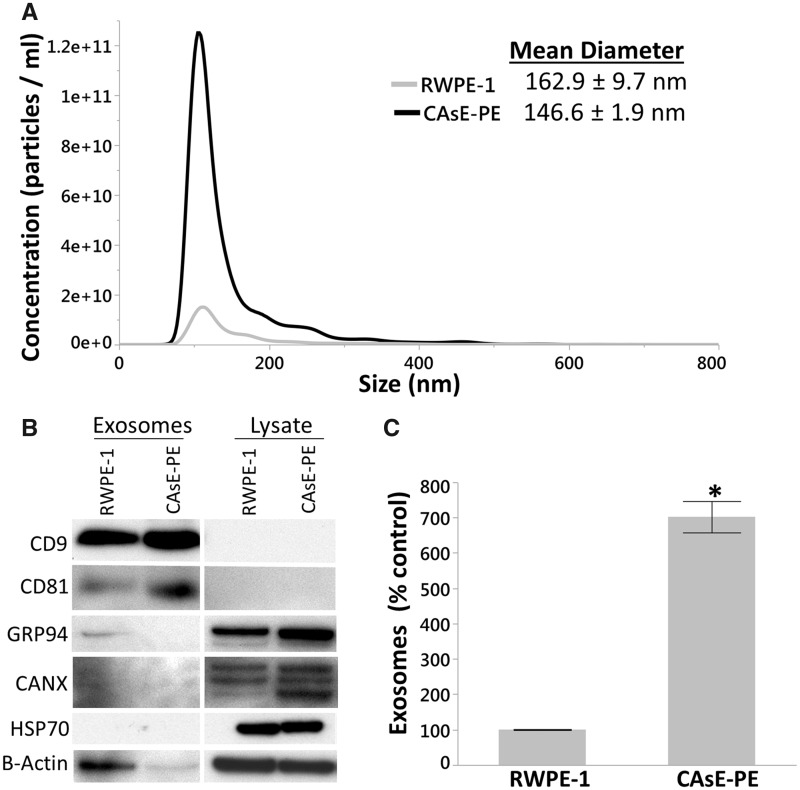
EVs isolated from parental RWPE-1 and arsenic-transformed CAsE-PE cells were characterized using a combination of nanoparticle tracking analysis (NTA) and western blot analysis. NTA identified a major peak for both RWPE-1 (162.9 ± 9.7 nm) and CAsE-PE (146.7 ± 1.9 nm) in the exosomal size range. The average diameter of secreted exosomes did not significantly differ between cell lines (Figure 2A). Western blot analysis showed expression of exosome-specific markers CD81 and CD9 in the pellets, confirming their exosomal composition (Figure 2B). These markers were not detectable in the cell lysates. Furthermore, Calnexin (CANX), an endoplasmic reticulum marker (Wada et al., 1991); HSP70, an mitochondrial-specific heat shock protein (Gambill et al., 1993); and GRP94, an MV-specific marker (Dozio and Sanchez, 2017), were highly expressed in cell lysate, but not the exosome fraction. NTA revealed that CAsE-PE cells secrete 702% more exosomes than RWPE-1 cells (Figure 2C andTable 1). Moreover, the CAsE-PE exosome pellet contained 730% more protein and 420% more RNA than the RWPE-1 pellet, despite being isolated from the same number of cells (5 × 107 cells) (Table 1). Although ultracentrifugation is the “gold standard” method for isolating exosomes, larger EVs (≤200 nm in diameter) can sometimes also be coisolated, which may explain why the mean exosome diameter of our samples exceeds the established exosome diameter upper limit of approximately 100 nm. However, given the high expression of exosome-specific markers CD9 and CD81, coupled with the absence or barely detectable expression of organelle or cytosolic proteins HSP70, CANX, and GRP94, it is reasonable to suggest that ours were highly enriched exosomal fractions with only minimal coisolation of nonexosomal EVs.
Figure 2.
Arsenite transformed CAsE-PE cells secrete more exosomes than RWPE-1 cells. A, Nanoparticle tracking analysis of isolated exosomes. B, Western blot analysis of exosomal markers CD9 and CD81 and nonexosomal markers GRP94, CANX, and HSP70. C, CAsE-PE cells secrete 702% more exosomes than RWPE-1 cells (1 factor ANOVA, p = .0002, n = 3, Bars ± SEM).
Table 1.
Comparison of Exosomes Isolated From RWPE-1 and CAsE-PE Cells
| RWPE-1 | CAsE-PE | % Control | |
|---|---|---|---|
| Exosomes (Particles/ml) | 4.8 × 1011 ± 6.0 × 109 | 3.4 × 1012 ± 2.2 × 1011 | 702% |
| Total Protein (µg) | 8.4 ± 2.5 | 61.7 ± 11.1 | 730% |
| Total RNA (ng) | 0.5 | 2.1 | 420% |
CAsE-PE Exosomes Are Enriched With Oncogenic Factors
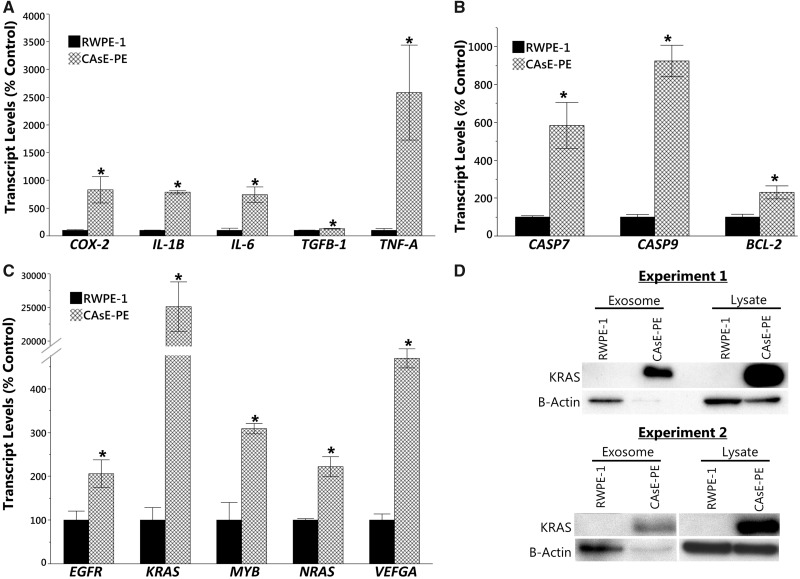
Exosomes released by cancer cells can contain elevated levels of oncogenic factors that can recruit surrounding cells, including SCs, to a malignant phenotype (reviewed in Hannafon and Ding, 2015). To determine the oncogenic potential of CAsE-PE exosomes, we measured the transcript levels of several oncogenes, inflammation-related genes, and antiapoptotic genes in exosomes isolated from RWPE-1 and CAsE-PE cells. CAsE-PE exosomes contained more mRNA for the inflammation-related genes cyclooxygenase-2 (COX-2) (831%), IL1-B (788%), IL-6 (743%), tumor necrosis factor-A (TNFA) (2, 584%), and TGFB-1 (129%) (Figure 3A), the apoptosis-related genes BCL-2 (230%), CASP7 (584%), and CASP9 (924%) (Figure 3B), and the oncogenes KRAS (24, 622%), NRAS (222%), VEFGA (470%), EGRF (206%), and MYB (309%) compared with exosomes isolated from RWPE-1 (Figure 3C). Furthermore, CAsE-PE exosomes were enriched with KRAS protein (by at least 866%) compared with RWPE-1 (Figure 3D); however, β-actin levels in CAsE-PE exosomes were low, making absolute quantification of KRAS protein levels difficult.
Figure 3.
Gene and protein expression in exosomes. mRNA expression of (A) inflammation-related genes, (B) apoptosis-related genes, and (C) oncogenes in isolated exosomes. (D) Western blot analysis of KRAS protein levels in exosomes and cell lysate. Asterisk denotes statistical significance (1 factor ANOVA, p < .05, n = 3). Bars ± SEM.
To test if miRs play a role in the transformation of normal SCs noncontiguously cocultured with CAsE-PE cells, we measured the expression of 84 cancer-related miRs in CAsE-PE and RWPE-1 cell lysates and exosomes. Expression of 61 out of 84 miRs was altered in CAsE-PE exosomes compared with RWPE-1 exosomes (Table 2). The majority (56 out of 61) of these miRs were down-regulated in CAsE-PE exosomes, with miR-34c-5p (42.3-fold), miR-138-5p (30.5-fold), miR-146b-5p (14.6-fold), miR-135b-5p (14.1-fold), miR-193a-5p (13.8-fold), miR-143-3p (13.3-fold), and miR-155-5p (12.5-fold) being the most downregulated. Alternatively, miR-9-5p (7.2-fold), let-7f-5p (2.7-fold), miR-183-5p (2.1-fold), miR-10a-5p (1.8-fold), and miR-27b-3p (1.3-fold) were the only miRs upregulated in CAsE-PE exosomes. Interestingly, expression of only 20 miRs was similarly altered in both CAsE-PE cell lysate and exosomes, while 41 were altered only in CAsE-PE exosomes (Table 2). These results demonstrate that miR cargo loaded into exosomes varies between control (RWPE-1) and arsenic-transformed (CAsE-PE) cells, and suggests that arsenic affects packaging of exosomal miR cargo or miR biogenesis.
Table 2.
Comparison Between Exosomal and Cellular miRs s Analyzed by miRs Cancer Array
| Fold Regulationa |
Fold Regulationa |
||||
|---|---|---|---|---|---|
| miRs | Exosomal | Cellular | miRs | Exosomal | Cellular |
| miR-9-5p | 7.2 up | 7.4 up | miR-19a-3p | 3.6 down | No Change |
| let-7f-5p | 2.7 up | No Change | miR-17-5p | 3.5 down | No Change |
| miR-183-5p | 2.1 up | 2.0 up | let-7b-5p | 3.4 down | 2.6 down |
| miR-10a-5p | 1.8 up | No Change | miR-146a-5p | 3.3 down | No Change |
| miR-27b-3p | 1.3 up | No Change | miR-150-5p | 3.3 down | No Change |
| miR-34c-5p | 42.3 down | 5.9 down | miR-27a-3p | 3.2 down | No Change |
| miR-138-5p | 30.5 down | 4.2 down | miR-124-3p | 3.1 down | No Change |
| miR-146b-5p | 14.6 down | 5.7 down | miR-16-5p | 3.1 down | No Change |
| miR-135b-5p | 14.1 down | 5.4 down | miR-132-3p | 3.0 down | No Change |
| miR-193a-5p | 13.8 down | No Change | miR-25-3p | 3.0 down | No Change |
| miR-143-3p | 13.3 down | No Change | miR-128-3p | 3.0 down | No Change |
| miR-155-5p | 12.5 down | 4.4 down | miR-18a-5p | 2.9 down | No Change |
| miR-205-5p | 8.7 down | 4.1 down | miR-191-5p | 2.9 down | No Change |
| miR-7-5p | 8.6 down | No Change | miR-10b-5p | 2.9 down | 2.9 down |
| miR-222-3p | 7.8 down | 5.0 down | miR-20b-5p | 2.7 down | No Change |
| miR-181d-5p | 7.6 down | 2.8 down | miR-15a-5p | 2.7 down | No Change |
| miR-181b-5p | 5.9 down | 2.6 down | miR-127-5p | 2.7 down | 13.2 down |
| miR-130a-3p | 5.4 down | No Change | miR-372-3p | 2.5 down | No Change |
| miR-181a-5p | 5.2 down | 2.0 down | miR-378a-3p | 2.4 down | No Change |
| miR-184 | 4.9 down | No Change | miR-193b-3p | 2.4 down | No Change |
| miR-144-3p | 4.9 down | No Change | miR-15b-5p | 2.1 down | No Change |
| miR-142-5p | 4.7 down | No Change | let-7g-5p | 1.9 down | No Change |
| miR-34a-5p | 4.7 down | 2.0 down | miR-29b-3p | 1.9 down | No Change |
| miR-20a-5p | 4.7 down | No Change | miR-32-5p | 1.8 down | No Change |
| miR-215-5p | 4.5 down | No Change | miR-30c-5p | 1.6 down | No Change |
| miR-149-5p | 4.4 down | No Change | miR-210-3p | 1.5 down | No Change |
| miR-203a-3p | 4.4 down | No Change | miR-373-3p | No Change | 10.6 down |
| miR-133b | 3.9 down | No Change | miR-218 | No Change | 2.7 down |
| miR-125b-5p | 3.9 down | 1.8 down | miR-96-5p | No Change | 2.7 up |
| miR-125a-5p | 3.8 down | 2.6 down | miR-98-5p | No Change | 2.4 down |
| miR-214-3p | 3.8 down | No Change | miR-196a-5p | No Change | 2.0 down |
| miR-134-5p | 3.8 down | 14.6 down | miR-181c-5p | No Change | 1.9 down |
| let-7i-5p | 3.7 down | 2.1 down | let-7e-5p | No Change | 1.9 down |
| miR-335-5p | 3.6 down | No Change | let-7c-5p | No Change | 1.8 down |
| miR-100-5p | 3.6 down | No Change | miR-126-3p | No Change | 1.6 down |
Fold regulation is compared with miRs expression in RWPE-1 exosomes or cell lysate, and are significantly different (p < .05).
KRAS KD in CAsE-PE Cells Only Partially Mitigates Transformation of Noncontiguously Cocultured SCs
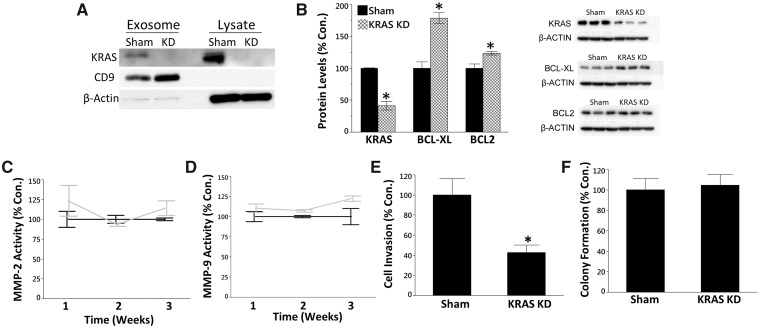
Previously, we have shown that the oncogene KRAS plays a critical role in the transformation of RWPE-1 and SCs to a malignant phenotype (Benbrahim-Tallaa et al., 2005; Ngalame et al., 2014b). Furthermore, KRAS KD in CAsE-PE and As-CSCs partially mitigates the oncogenic phenotype (Ngalame et al., 2014a). As CAsE-PE exosomes were highly enriched with KRAS at the transcript and protein levels, we hypothesized that uptake of exosomal KRAS by SCs contributes to the development of the CSC-like phenotype. To test this hypothesis SCs were noncontiguously cocultured with CAsE-PE cells transfected with sham or KRAS shRNAmir, which targets the KRAS transcript to silence KRAS expression. As previously observed (Ngalame et al., 2014a), KRAS KD dramatically reduced KRAS protein expression in cell lysate (Figure 4A), while KRAS protein expression was also reduced in CAsE-PE exosomes (Figure 4A). Next, to assess the effect of KRAS KD on the development of an oncogenic phenotype in cocultured SCs, we measured protein expression levels of the oncogene KRAS, and the antiapoptosis proteins BCL-XL and BCL2. When compared with sham control cells, KRAS protein levels were reduced 59% in SCs cocultured with KRAS KD CAsE-PE cells (Figure 4B), while BCL-XL and BCL2 protein levels were increased compared with sham control (Figure 4B). These results suggest the SCs may be resistant to apoptosis.
Figure 4.
A, Western blot analysis of KRAS protein levels in cell lysate and exosomes following KRAS KD. B, Western blot analysis of KRAS, BCL-XL, and BCL2 protein levels in SCs following 3 weeks of coculture with KRAS-KD CAsE-PE cells. C, Secreted MMP-2 (2 factor ANOVA, time (p = .34), KD (p = .23), interaction (p = .34)), and (D) MMP-9 activity (2 factor ANOVA, time (p = .38), KD (p = .013), interaction (p = .38)) in cocultured SCs. E, Invasive capacity (one factor ANOVA, p = .02) and (F) colony formation in soft agar (1 factor ANOVA, p = .8) of SCs after 3 weeks of coculture. Asterisk denotes statistical significance (p < .05, n = 3–4). Results are mean ± SEM.
To further test the role of KRAS in recruiting noncontiguously cocultured SCs to an oncogenic phenotype, we assessed several characteristics associated with malignant transformation, including secreted MMP activity, invasion capacity, and colony formation in soft agar, a metric of anchorage-independent growth. Coculturing SCs with KRAS KD CAsE-PE cells for 3 weeks reduced invasive capacity by 57% compared with SCs cocultured with sham control cells (Figure 4E). However, there was no effect on secreted MMP-2 or MMP-9 activity (Figs. 4C and 4D) or colony formation in soft agar (Figure 4F). Collectively, these results suggest exosomal KRAS may play a role in the recruitment of SCs to an oncogenic phenotype; however, it is evident that other molecular factors are also contributing to the transformation process.
DISCUSSION
Accumulating evidence shows that exosomal signaling plays a critical role in the development and progression of a wide-spectrum of cancers, including prostate cancer (Abd Elmageed et al., 2014; Chowdhury et al., 2015). However, the role of exosomal signaling in environmental toxicant-induced carcinogenesis remains poorly understood (reviewed in Harischandra et al., 2017). Prior work has shown that SCs cocultured with CAsE-PEs in a noncontact system rapidly develop a CSC-like phenotype (Xu et al., 2012). In this study, we show that arsenic-transformed CAsE-PE cells hyper-secrete exosomes compared with parental RWPE-1 cells and these CAsE-PE-secreted exosomes are highly enriched with myriad factors capable of playing a signaling role in the conversion of SCs to a CSC-like phenotype.
Previously, we have reported that SCs noncontiguously cocultured with CAsE-PE cells for 3 weeks acquire a CSC-like phenotype, but the mechanisms involved in this transformation were not fully defined (Xu et al., 2012). Exosomes are small (approximately 100 nm), membrane-derived vesicles of the endo-lysosomal pathway that can be loaded with diverse cargo, including lipids, proteins and RNA (including regulatory miRs) (Raposo and Stoorvogel, 2013). When released into the extracellular environment and taken up by neighboring cells, exosomes can influence diverse biological processes, including tumor progression (Nakano et al., 2015), SC differentiation (Chowdhury et al., 2015), and neoplastic transformation (Abd Elmageed et al., 2014). Furthermore, exosomal signaling may play a role arsenic-induced carcinogenesis, as arsenic-transformed breast and bronchial epithelial cells have been shown to secrete exosomes enriched with cancer-related miRs, although neither transformed cell line hyper-secretes exosomes compared with untransformed cells (Chen et al., 2017; Xu et al., 2015). In this study, CAsE-PE cells released dramatically higher (>700%) levels of exosomes, which were enriched with cargo that could be playing a signaling role in recruiting SCs to a CSC-like phenotype. For example, evasion of apoptosis is considered one of the hallmarks of carcinogenesis (Hanahan and Weinberg, 2011), and exosomes released by CAsE-PE cells were enriched with BCL2, a protein that prevents apoptosis by inhibiting caspase activity. Another important hallmark of cancer is chronic inflammation (Hanahan and Weinberg, 2011), and CAsE-PE exosomes were enriched with the proinflammatory cytokines IL-1B, IL-6, TGFB1, and TNFA, and the proinflammatory enzyme COX-2, which has been shown to be overexpressed in myriad cancers, including prostate cancer (Gupta et al., 2000). Finally, several oncogenes were overexpressed in CAsE-PE exosomes, including EGFR, KRAS, and NRAS, which promote cell division and survival (Boroughs and DeBerardinis, 2015), vascular endothelial growth factor A, which promotes angiogenesis (Adams and Alitalo, 2007), and MYB, which controls the differentiation and proliferation of hematopoietic progenitor cells (Sandberg et al., 2005). Collectively, these findings indicate that arsenic may be mediating the recruitment of SCs via an exosomal signaling mode of action, as the robust increases in exosome abundance and cargo could increase the signaling to the recruited cells.
Exosomes released by arsenic-transformed cells have been shown to promote inflammation and cell proliferation in parental breast or bronchial epithelial cells (Chen et al., 2017; Xu et al., 2015). Importantly, inhibiting exosome uptake or depleting exosomes from cancer-cell CM can mitigate the development of these cancer-associated phenotypes, suggesting exosomes are mediating the acquisition of the phenotypes (Chen et al., 2017; Xu et al., 2015). Similarly, here we report that SCs cultured in media conditioned by arsenic-transformed CAsE-PE cells for 3 weeks display a depolarized spindle-like morphology indicative of EMT, while also exhibiting increased secreted MMP-2 activity. EMT of SCs has been shown to be necessary for CSC formation (Mani et al., 2008; Morel et al., 2008), and is a critical mechanism for acquisition of metastatic capacity (Kang and Massagué, 2004). MMPs are a class of enzymatic proteins that play a variety of roles in carcinogenesis, including digestion of the extracellular matrix, cancer cell migration, and metastasis (Bachmeier et al., 2000). Furthermore, increased secreted MMP activity is typical of cancer cells, and is positively correlated with arsenic-induced malignant transformation (Achanzar et al., 2002; Tokar et al., 2010a). Finally, SCs cultured in exosome-depleted CAsE-PE CM did not display elevated MMP-2 activity, or a depolarized spindle-like morphology. These results suggest that exosomes released by arsenic-transformed CAsE-PE cells play an important signaling role in recruiting SCs to a CSC-like phenotype.
The oncogene KRAS was enriched in CAsE-PE exosomes at both the transcript and protein level. KRAS is a member of the RAS superfamily of oncogenes, and plays a role in numerous biological processes, including cell proliferation, differentiation, and apoptosis (Spaargaren et al., 1995). Activating mutations in KRAS have been identified in a variety of different cancers, including prostate cancer (Moul et al., 1992). Previously, we reported overexpression of KRAS in CAsE-PE cells and in arsenic-transformed CSCs (Benbrahim-Tallaa et al., 2005; Ngalame et al., 2014b). Furthermore, overexpression of KRAS precedes malignant transformation, while KRAS KD partially mitigates the oncogenic phenotype of CAsE-PE and As-CSCs, demonstrating the important role KRAS plays in the transformation process (Ngalame et al., 2014a). To test the role of exosomal KRAS in converting SCs to a CSC-like phenotype we noncontiguously cocultured SCs with CAsE-PE KRAS KD cells. Importantly, KRAS KD reduced both cellular and exosomal KRAS protein levels, thus reducing the amount of KRAS protein available to be delivered to SCs via exosomal signaling, which is demonstrated by the fact that KRAS protein levels were reduced in SCs after 3 weeks of coculture. In agreement with exosomal KRAS playing a role in recruiting SCs to an oncogenic phenotype, KRAS KD reduced the invasive capacity of cocultured SCs; however, other phenotypes associated with oncogenesis (ie secreted MMP-2 and MMP-9 activity, colony formation in soft agar) were not affected by KRAS KD. This suggests that other genetic factors or combination of factors, in addition to KRAS, are involved in the transformation of SCs to a CSC-like phenotype in this coculture model. In agreement with this, we have previously reported that CAsE-PE hypersecrete the inflammatory cytokines IL-6 and TGF-β1, and treatment of SCs with either IL-6 or TGF-β1 can induce a CSC-like phenotype (Xu et al., 2012, 2013).
miRs are small, noncoding RNAs that negatively regulate gene expression at the transcript level. We have previously demonstrated the important role miRs may play in modulating arsenic-induced transformation of CAsE-PE and CSC-like cells (Ngalame et al., 2014b). Moreover, miR-143 overexpression can partially mitigate the oncogenic phenotype associated with transformation of CAsE-PE and CSCs (Ngalame et al., 2016). When loaded into exosomes, miRs can also play an important role in intercellular signaling, regulating numerous biological processes, including immune response (Bobrie et al., 2011) and malignant transformation (Umezu et al., 2013; Yang et al., 2011). Several recent studies have provided evidence that exosomal miRs play a critical role in intercellular signaling during arsenic carcinogenesis (Chen et al., 2017; Xu et al., 2015). Arsenic-transformed human bronchial epithelial cells release exosomes enriched with miR-21 (Xu et al., 2015), while arsenic-transformed hepatic epithelial cells secrete exosomes containing elevated levels of miR-155 (Chen et al., 2017). Importantly, uptake of exosomes enriched with either miR-21 or miR-155 by cells induced cellular changes associated with arsenic carcinogenesis, demonstrating that exosomal miRs may play an important role in intercellular signaling throughout carcinogenesis. Although neither miR-155 or miR-21 were elevated in exosomes isolated from CAsE-PE cells, other cancer-related miRs were elevated, including miR-9 (7.2-fold), let-7f (2.7-fold), miR-183 (2.1-fold), miR-10a (1.8-fold), and miR-27b (1.3-fold), and several of these miRs have been shown to play a role in prostate cancer and exosomal signaling. For example, miR-183 is upregulated in prostate cancer (Ueno et al., 2013), and can promote oncogenesis by targeting the tumor-suppressor gene EGR1 (Sarver et al., 2010). Additionally, miR-183 has been shown to be enriched in exosomes isolated from renal (Grange et al., 2011) and prostate cancer cells (Sánchez et al., 2016), further implicating an extracellular signaling role for miR-183 in the tumor microenvironment. Alternatively, miR-9 has been shown to be upregulated in a wider variety of cancers, including esophageal (Song et al., 2014), breast (Ma et al., 2010), colorectal (Zhu et al., 2012), and skin cancers (Shiiyama et al., 2013). miR-9 promotes cancer metastasis by repressing the expression E-cadherin, an important cell adhesion glycoprotein frequently downregulated in transformed cells (Ma et al., 2010; Song et al., 2014). Indeed exosomal miR-9 secreted by breast cancer cells was recently shown to enhance cell motility and induce invasiveness by downregulating E-cadherin when taken up by normal breast fibroblasts (Baroni et al., 2016). Interestingly, we have previously shown that E-cadherin is downregulated in SCs grown in noncontiguous coculture with CAsE-PE cells (Xu et al., 2012), suggesting exosomal miR-9 may be another factor playing a role in converting SCs to an oncogenic phenotype. Additional work is required to test this hypothesis.
Here, we provide evidence that exosomes secreted by arsenic-transformed CAsE-PE cells play a signaling role in recruiting SCs to a CSC-like phenotype. Furthermore, our data indicate that delivery of exosomal KRAS from CAsE-PE cells to SCs may play a role in the development of the CSC-like phenotype, as KRAS KD in CAsE-PE cells reduced the invasive capacity of cocultured SCs. However, exosomal KRAS is not the only factor involved in this transformation process, and further research is required to understand how other components of the exosome cargo, including regulatory miRs, influence the development of a CSC-like phenotype.
ACKNOWLEDGMENTS
The authors thank Dr Sreenivasa Ramaiahgari and Dr Christopher McPherson for their careful review and insightful comments for this article.
FUNDING
This work was supported by the Division of the National Toxicology Program, National Institute of Environmental Health Sciences (NIEHS). This article may be the work product of an employee or group of employees of the NIEHS, NIH; however, the statements contained herein do not necessarily represent the statements, opinions, or conclusions of the NIEHS, NIH of the U.S. Government. The content of this publication does not necessarily reflect the views or the policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- Abd Elmageed Z. Y., Yang Y., Thomas R., Ranjan M., Mondal D., Moroz K., Fang Z., Rezk B. M., Moparty K., Sikka S. C., et al. (2014). Neoplastic reprogramming of patient‐derived adipose stem cells by prostate cancer cell‐associated exosomes. Stem Cells 32, 983–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achanzar W. E., Brambila E. M., Diwan B. A., Webber M. M., Waalkes M. P. (2002). Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J. Natl Cancer Inst. 94, 1888–1891. [DOI] [PubMed] [Google Scholar]
- Adams R. H., Alitalo K. (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464.. [DOI] [PubMed] [Google Scholar]
- Bachmeier B., Boukamp P., Lichtinghagen R., Fusenig N., Fink E. (2000). Matrix metalloproteinases-2,-3,-7,-9 and-10, but not MMP-11, are differentially expressed in normal, benign tumorigenic and malignant human keratinocyte cell lines. Biol. Chem. 381, 497–507. [DOI] [PubMed] [Google Scholar]
- Baroni S., Romero-Cordoba S., Plantamura I., Dugo M., D’Ippolito E., Cataldo A., Cosentino G., Angeloni V., Rossini A., Daidone M. G., et al. (2016). Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 7, e2312.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello D., Webber M. M., Kleinman H. K., Wartinger D. D., Rhim J. S. (1997). Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 18, 1215–1223. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L., Waalkes M. P. (2007). Inorganic arsenic and human prostate cancer. Environ. Health Perspect. 116, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L., Waterland R. A., Styblo M., Achanzar W. E., Webber M. M., Waalkes M. P. (2005). Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: aberrant genomic DNA methylation and K-ras oncogene activation. Toxicol. Appl. Pharmacol. 206, 288–298. [DOI] [PubMed] [Google Scholar]
- Bobrie A., Colombo M., Raposo G., Théry C. (2011). Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 12, 1659–1668. [DOI] [PubMed] [Google Scholar]
- Boroughs L. K., DeBerardinis R. J. (2015). Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 17, 351.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Luo F., Liu X., Lu L., Xu H., Yang Q., Xue J., Shi L., Li J., Zhang A., et al. (2017). NF-kB-regulated exosomal miR-155 promotes the inflammation associated with arsenite carcinogenesis. Cancer Lett. 388, 21–33. [DOI] [PubMed] [Google Scholar]
- Cho J. A., Park H., Lim E. H., Kim K. H., Choi J. S., Lee J. H., Shin J. W., Lee K. W. (2011). Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol. Oncol. 123, 379–386. [DOI] [PubMed] [Google Scholar]
- Cho J. A., Park H., Lim E. H., Lee K. W. (2012). Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int. J. Oncol. 40, 130–138. [DOI] [PubMed] [Google Scholar]
- Chowdhury R., Webber J. P., Gurney M., Mason M. D., Tabi Z., Clayton A. (2015). Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 6, 715.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozio V., Sanchez J.-C. (2017). Characterisation of extracellular vesicle-subsets derived from brain endothelial cells and analysis of their protein cargo modulation after TNF exposure. J. Extracell. Vesic. 6, 1302705.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreval K., Tryndyak V., Kindrat I., Twaddle N. C., Orisakwe O. E., Mudalige T. K., Beland F. A., Doerge D. R., Pogribny I. P. (2018). Cellular and molecular effects of prolonged low-level sodium arsenite exposure on human hepatic HepaRG cells. Toxicol. Sci. 162, 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambill B. D., Voos W., Kang P. J., Miao B., Langer T., Craig E. A., Pfanner N. (1993). A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J. Cell Biol. 123, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange C., Tapparo M., Collino F., Vitillo L., Damasco C., Deregibus M. C., Tetta C., Bussolati B., Camussi G. (2011). Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 71, 5346–5356. [DOI] [PubMed] [Google Scholar]
- Gupta S., Srivastava M., Ahmad N., Bostwick D. G., Mukhtar H. (2000). Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate 42, 73–78. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Hannafon B. N., Ding W. Q. (2015). Cancer stem cells and exosome signaling. Stem Cell Investig. 2, 11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra D. S., Ghaisas S., Rokad D., Kanthasamy A. G. (2017). Exosomes in toxicology: Relevance to chemical exposure and pathogenesis of environmentally linked diseases. Toxicol. Sci. 158, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC I. A f R o C. (2011). Arsenic and arsenic compounds. IARC Monogr. Eval. Carcinog. Risk Hum. 100C, 41–94. [PubMed] [Google Scholar]
- Iliopoulos D., Hirsch H. A., Wang G., Struhl K. (2011). Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc. Natl. Acad. Sci. U.S.A. 108, 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Massagué J. (2004). Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 118, 277–279. [DOI] [PubMed] [Google Scholar]
- Lässer C., Eldh M., Lötvall J. (2012). Isolation and characterization of RNA-containing exosomes. J. Vis. Exp. 59, e3037–e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Young J., Prabhala H., Pan E., Mestdagh P., Muth D., Teruya‐Feldstein J., Reinhardt F., Onder T. T., Valastyan S. (2010). miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 12, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S. A., Guo W., Liao M.-J., Eaton E. N., Ayyanan A., Zhou A. Y., Brooks M., Reinhard F., Zhang C. C., Shipitsin M., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A.-P., Lièvre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. (2008). Generation of breast cancer stem cells through epithelial-mesenchymal transition. PloS One 3, e2888.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moul J. W., Friedrichs P. A., Lance R. S., Theune S. M., Chang E. H. (1992). Infrequent RAS oncogene mutations in human prostate cancer. Prostate 20, 327–338. [DOI] [PubMed] [Google Scholar]
- Nakano I., Garnier D., Minata M., Rak J. (2015). Extracellular vesicles in the biology of brain tumour stem cells–Implications for inter-cellular communication, therapy and biomarker development. Semin. Cell Dev. Biol. 40, 17–26. [DOI] [PubMed] [Google Scholar]
- Ngalame N. N., Makia N. L., Waalkes M. P., Tokar E. J. (2016). Mitigation of arsenic-induced acquired cancer phenotype in prostate cancer stem cells by miR-143 restoration. Toxicol. Appl. Pharmacol. 312, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngalame N. N., Tokar E. J., Person R. J., Waalkes M. P. (2014a). Silencing KRAS overexpression in arsenic-transformed prostate epithelial and stem cells partially mitigates malignant phenotype. Toxicol. Sci. 142, 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngalame N. N., Tokar E. J., Person R. J., Xu Y., Waalkes M. P. (2014b). Aberrant microRNA expression likely controls RAS oncogene activation during malignant transformation of human prostate epithelial and stem cells by arsenic. Toxicol. Sci. 138, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooki A., Begum A., Marchionni L., VandenBussche C. J., Mao S., Kates M., Hoque M. O. (2018). Arsenic promotes the COX2/PGE2–SOX2 axis to increase the malignant stemness properties of urothelial cells. Int. J. Cancer. 143, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., Garcia-Santos G., Ghajar C., et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Haviv I., Campbell I. G. (2009). Co-evolution of tumor cells and their microenvironment. Trends Genet. 25, 30–38. [DOI] [PubMed] [Google Scholar]
- Raposo G., Stoorvogel W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. M., Jordan C. T. (2009). The increasing complexity of the cancer stem cell paradigm. Science 324, 1670–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez C. A., Andahur E. I., Valenzuela R., Castellón E. A., Fullá J. A., Ramos C. G., Triviño J. C. (2016). Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 7, 3993.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M. L., Sutton S. E., Pletcher M. T., Wiltshire T., Tarantino L. M., Hogenesch J. B., Cooke M. P. (2005). c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev. Cell 8, 153–166. [DOI] [PubMed] [Google Scholar]
- Sarver A. L., Li L., Subramanian S. (2010). MicroRNA miR-183 functions as an oncogene by targeting the transcription factor EGR1 and promoting tumor cell migration. Cancer Res. 70, 9570–9580. [DOI] [PubMed] [Google Scholar]
- Shiiyama R., Fukushima S., Jinnin M., Yamashita J., Miyashita A., Nakahara S., Kogi A., Aoi J., Masuguchi S., Inoue Y., et al. (2013). Sensitive detection of melanoma metastasis using circulating microRNA expression profiles. Melanoma Res. 23, 366–372. [DOI] [PubMed] [Google Scholar]
- Song Y., Li J., Zhu Y., Dai Y., Zeng T., Liu L., Li J., Wang H., Qin Y., Zeng M., et al. (2014). MicroRNA-9 promotes tumor metastasis via repressing E-cadherin in esophageal squamous cell carcinoma. Oncotarget 5, 11669.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaargaren M., BISCHOFF J. R., McCORMICK F. (1995). Signal transduction by Ras-like GTPases: A potential target for anticancer drugs. Gene Expr. 4, 345–356. [PMC free article] [PubMed] [Google Scholar]
- Suchorska W. M., Lach M. S. (2016). The role of exosomes in tumor progression and metastasis (Review). Oncol. Rep. 35, 1237–1244. [DOI] [PubMed] [Google Scholar]
- Sun Y., Tokar E. J., Waalkes M. P. (2012). Overabundance of putative cancer stem cells in human skin keratinocyte cells malignantly transformed by arsenic. Toxicol. Sci. 125, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D. G. (2012). Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 22, 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar E. J., Ancrile B. B., Cunha G. R., Webber M. M. (2005). Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differ.; Res. Biol. Divers. 73, 463–473. [DOI] [PubMed] [Google Scholar]
- Tokar E. J., Diwan B. A., Waalkes M. P. (2010a). Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ. Health Perspect. 118, 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar E. J., Person R. J., Sun Y., Perantoni A. O., Waalkes M. P. (2013). Chronic exposure of renal stem cells to inorganic arsenic induces a cancer phenotype. Chem. Res. Toxicol. 26, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar E. J., Qu W., Liu J., Liu W., Webber M. M., Phang J. M., Waalkes M. P. (2010b). Arsenic-specific stem cell selection during malignant transformation. J. Natl. Cancer Inst. 102, 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K., Hirata H., Shahryari V., Deng G., Tanaka Y., Tabatabai Z., Hinoda Y., Dahiya R. (2013). microRNA-183 is an oncogene targeting Dkk-3 and SMAD4 in prostate cancer. Br. J. Cancer 108, 1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu T., Ohyashiki K., Kuroda M., Ohyashiki J. (2013). Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 32, 2747.. [DOI] [PubMed] [Google Scholar]
- Visvader J. E., Lindeman G. J. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Liu J., Germolec D. R., Trempus C. S., Cannon R. E., Tokar E. J., Tennant R. W., Ward J. M., Diwan B. A. (2008). Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of tumor stem cell dynamics. Cancer Res. 68, 8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada I., Rindress D., Cameron P., Ou W., Doherty J. n., Louvard D., Bell A., Dignard D., Thomas D., Bergeron J. (1991). SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 266, 19599–19610. [PubMed] [Google Scholar]
- Wang J. C. (2010). Good cells gone bad: the cellular origins of cancer. Trends Mol. Med. 16, 145–151. [DOI] [PubMed] [Google Scholar]
- Xu Y., Luo F., Liu Y., Shi L., Lu X., Xu W., Liu Q. (2015). Exosomal miR-21 derived from arsenite-transformed human bronchial epithelial cells promotes cell proliferation associated with arsenite carcinogenesis. Arch. Toxicol. 89, 1071–1082. [DOI] [PubMed] [Google Scholar]
- Xu Y., Tokar E. J., Person R. J., Orihuela R. G., Ngalame N. N., Waalkes M. P. (2013). Recruitment of normal stem cells to an oncogenic phenotype by noncontiguous carcinogen-transformed epithelia depends on the transforming carcinogen. Environ. Health Perspect. 121, 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Tokar E. J., Sun Y., Waalkes M. P. (2012). Arsenic-transformed malignant prostate epithelia can convert noncontiguous normal stem cells into an oncogenic phenotype. Environ. Health Perspect. 120, 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Chen J., Su F., Yu B., Su F., Lin L., Liu Y., Huang J.-D., Song E. (2011). Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer 10, 117.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Chen H., Zhou D., Li D., Bai R., Zheng S., Ge W. (2012). MicroRNA-9 up-regulation is involved in colorectal cancer metastasis via promoting cell motility. Med. Oncol. 29, 1037–1043. [DOI] [PubMed] [Google Scholar]